#anthocyanin food color
Explore tagged Tumblr posts
Text
The Multifaceted Uses of Anthocyanin Food Colour in Modern Food Processing
Anthocyanins, derived from plants like grapes, berries, and black carrots, are natural pigments widely used in the food industry. Their ability to change color based on pH levels—from red to blue to purple — makes them a versatile and valuable ingredient for enhancing the visual appeal of food products. This article explores the multifaceted uses of anthocyanin food color, its production, and its benefits.
Production and Types of Anthocyanin
Anthocyanins are extracted from the aqueous extracts of various plants, such as grapes, black carrots, blueberries, red cabbage, and elderberries. These extracts are then spray-dried to form a free-flowing powder, often with maltodextrin as a carrier. The final product is available in both liquid and powder forms, making it adaptable for different food processing needs.
The anthocyanin color is classified as E163 (ii-v) and includes alternative names like grape skin extract and red cabbage extract. The strength of the color can vary, typically ranging from 1% to 12%.
Applications in the Food Industry
Anthocyanin food color is used in a wide range of food products, including:
Desserts and Ice Creams: Providing natural, vibrant colors to enhance the appeal of sweets and frozen desserts.
Beverages: Used in fruit juices, soft drinks, and alcoholic beverages to offer an attractive and natural appearance.
Confectionery and Baked Goods: Adds color to candies, jams, jellies, yogurts, bakery fillings, and toppings.
The ability of anthocyanins to change color based on pH levels is particularly advantageous in the food industry. This characteristic allows for creative culinary applications, where the color of a product can be modified to suit different tastes and preferences.
Health Benefits and Safety
Anthocyanins are not just colorants; they also offer several health benefits. They are rich in antioxidants, which help protect the body against oxidative stress and inflammation. Regular consumption of anthocyanin-rich foods can promote cardiovascular health, improve vision, and reduce the risk of chronic diseases.
Anthocyanin food color is considered safe for consumption and is approved by food safety authorities globally. Its natural origin and health benefits make it a preferred choice over synthetic dyes, which may carry health risks.
Environmental Sustainability
The production of anthocyanin food color is environmentally sustainable. The use of natural plant sources and eco-friendly extraction processes align with the increasing consumer demand for sustainable and natural food ingredients. This makes anthocyanin food color an attractive option for food manufacturers committed to environmental stewardship.
Future Prospects
As consumer preferences continue to shift towards natural and health-conscious products, the demand for anthocyanin food color is expected to grow. Innovations in extraction and production techniques are likely to enhance the quality and consistency of anthocyanin color, further expanding its applications in the food industry.
For more information visit us:
0 notes
Text
Faulkner presses the tips of his fingers together in a succession of small bounces while considering Agent Hemingway’s prediction. “You are apt with your observation, Agent,” Faulkner recognizes and drops his hands to his sides, ever the fitting sentinel, continuing in his mellowed cadence, “Your suggestion would be the best method of sensible action.”
This Agent will excuse himself and pick up a box of bottled water in the building’s communal kitchen storage after this conversation. Nodding along to Hemingway as his plan unfolds, Faulkner adds, “For the record, I do not expect obeisance from my reminder. After all, our nation did not do so well with Temperance, did it?” He lowly chuckles a rumbly three beats, which fade in and fade out. One could mistake it for a bass drop from the song playing in the background.
Standing to attention, hands behind his back, Faulkner’s fingers gently knead the back of his blazer while Hemingway looks into the gift bag. Fine wrinkles imprint onto the polyester-blend fabric. Faulkner answers quickly, “Yes, I’m aware, Agent. I chose to. Your date of birth is worth celebration, and I noticed the last time I had visited that your cupboard was sparse of this type of glassware…”
Faulkner’s explanation trails when he catches Agent Hemingway’s eyes watering. Had Faulkner misspoke and offended Hemingway by pointing out the lack of stout glasses? He hadn’t criticized too harshly, did he? Should he reach for his handkerchief? A hand hovers up and slinks into the inner pocket of his jacket and pauses —
Agent Hemingway is smiling. Faulkner’s chest warms up. His hand drops down and burrows into his pants pocket.
Usually, water would be Faulkner’s go-to, or seltzer with a slice of citrus in a simulated attempt of letting one’s hair down — figuratively, as Faulkner would never be without orderly style in the workplace — but as Hemingway rinses the glasses, Faulkner decides to take a chance. “Sī fuerīs Rōmae, Rōmānō vīvitō mōre; sī fuerīs alibī, vīvitō sīcut ibī.”
Agent Hemingway knows his Latin, and Faulkner smiles, hoping the agent will approve his response. When in Rome. “I wouldn’t mind what you were making, Agent Hemingway.” He leans slightly, almost on the side of a counter, but doesn’t commit. An inch of air separates his hip from what appears to be a granite-like stone cut. Rome wasn’t built in a day, and so, too, are Agent Faulkner’s casual informalities.
“Your hypothesis is humorous, Agent. The price of fame would be quite a toll, though, wouldn’t you say,” Faulkner inquires, already picturing Hemingway and Fitzgerald as a two-man jazz band reminiscent of the Bebop 40s. “Yes, I can see that you two would be some hepcats… Heh, then we, speaking unofficially for the group, will take you up on your offer. The stage is yours, Agent.”
Faulkner spreads his arm and gestures to a small set-up with a TV and a plugged-in karaoke machine. This Agent is sure that Agent Hemingway has gone through the correct channels and alerted his neighbors of noise before the commencement of this event. Stepping forward and waiting for his drink, Faulkner adds, “Then we can coordinate who goes next and corral the group for the True Colors ensemble piece later. I shall organize it for you, so please enjoy yourself… Birthday Man.”

this is exactly the kind of answer he was expecting to hear and, to be fair, this is the energy hemingway probably should be sharing. supposedly, he's one of the responsible ones so instead of playing bartender, he should be telling everyone to watch it, maybe keep tabs on who's just got their first drink down and who's about to get cut off. and any other day, he probably would but it's his birthday and so hemingway's allowed a little leeway. a night off from all the babysitting that nobody even asked for.
"you could try but i'm not sure how enthusiastic everyone's gonna be about the reminders. maybe we're better off being sneaky about it. just put water bottles everywhere," he says, only half-joking. "or we can just leave them to their own devices. it's a party after all."
when faulkner acknowledges the elephant in the room, namely the giftbag hemingway noticed the second the other agent walked into the kitchen, his smile grows even brighter. he did say that gifts aren't necessary when he invited everyone but who doesn't love a birthday present, come on. "ah, shit, you really didn't have to," he still says as he takes the glasses out of the bag. "these are beautiful, thank you." and just like that, he's starting to feel like he might shed a tear or two—they'd be happy tears, obviously, but he feels like any sort of crying would ruin the whole thing. so he looks up, blinks the tears away. works well enough.
hemingway gives faulkner another smile, just one more way of saying thank you and then clears his throat. "what are you drinking, though?" he asks as he sets the glasses down in the sink so he can rinse them. "i can make you a non-alcoholic something or just ... well, water."

"why, thank you. in another life, i'm a world famous performer. sold-out shows, all the time," he jokes as he dries the gifted glasses. "or me and fitz start that jazz band he's always talking about." hemingway looks over faulkner's shoulder, his eyes scanning the room until they land on fitzgerald, deep in conversation with another one of their teammates; hemingway smiles and then turns his attention back to faulkner. "but either way, the audience here is worth more than any music career could get me. i'll give you guys the opening act."
"oh, you're onto something here," he laughs. hemingway's still talking to faulkner and tending to his gift at the same time—he's finally arrived at the last step, which is pouring his drink into the crystal glass in one, swift motion. there. he gives faulkner a self-satisfied smile. "glad that you didn't suggest girls just wanna have fun, that's my song. and for true colors we should do an ensemble. and i will cry."
#event; ch0#post; thread#thread; hemingway#thread; bday#( ok so this the directors cut on red velvet cake )#( as requested 🫡💕 )#( Red Velvet Cake historically and perhaps ironically is a testament of the Great Depression. )#( Entering the households of the privileged in the late 30s largely thanks to red food coloring developed by Adams Extracts and then )#( beet juice during the rations of the Second World War the humble red and white dessert has been on many American plates. )#( However unlike its retro cousins Lemon Cheese Layer Cake or a Lady Baltimore; Red Velvet's resurgence from the Waldorf-Astoria Hotel's )#( menu to the big screens in 1989's Steel Magnolias has kept this cake from disappearing into the end pages of many a home cook's book. )#( The “velvet” in Red Velvet cake is simply a descriptor of its softer and finer crumb; the reaction of acidic buttermilk combining with )#( the alkaline baking soda and the batter's color formed through anthocyanins in cocoa powder turning brown in the presence of acids. )#( The white frosting by the year 1995 most likely sports a buttercream or cream cheese foundation; it's less time-consuming than the )#( original ermine frosting; a cooked flour and sugar paste similar to a roux whipped with softened butter until airy and fluffy. )#( In the progress of efficiency and speed these adaptations have brought the modern state of the Red Velvet Cake. )
7 notes
·
View notes
Text


eat the rainbow! 🌈 reaching a total of 4-1/2 cups of colorful fruits and vegetable a day is the goal for a powerful plate.
🍓🍒🍎 red: rich in the carotenoid lycopene, a potent scavenger of gene-damaging free radicals that seems to protect against prostate cancer as well as heart and lung disease.
🍊🍌🍍 orange and yellow: provide beta cryptothanxin, which supports intracellular communication and may help prevent heart disease.
🥝🍏🥦 green: rich in cancer-blocking chemicals like sulforaphane, isothiocyanates, and indoles, which inhibit the action of carcinogens
🫐🍆🍇 blue and purple: have powerful antioxidants called anthocyanins believed to delay cellular aging and help the heart by blocking the formation of blood clots.
🥔🧄🧅 white and brown: contains allicin, which has anti-tumor properties. other foods in this group contain antioxidant flavonoids like quercetin and kaempferol.
#health and wellness#wellness#healthy girl#healthy eating#becoming that girl#dream life#self improvement#wellness girl#glow up#that girl
131 notes
·
View notes
Text

Berry Magic: Create Natural Purple Dye with Blackberries and Blueberries
Blackberries and blueberries are not only delicious and nutritious, but they are also amazing sources of natural purple dye 💮✨ Thanks to anthocyanins, we can easily extract a rich and beautiful dye from them. Simply crush the berries and boil them with water, then strain the liquid to get a perfect purple dye for coloring food, fabrics, and more.💜
#aesthetic#colors#purple#beauty#fashion#home & lifestyle#dye#nature#berry#berries#blackberries#blueberries#anthocyanins
15 notes
·
View notes
Text
Study on the storage stability of phycocyanin from Spirulina obtusususiae
Abstract: The effects of temperature, sunlight and different additives on the stability of aqueous solutions of phycocyanin were studied. It was concluded that phycocyanin should be stored at 40 ℃ and protected from light, and should be stored under neutral conditions; glucose, sodium chloride and sorbitol could effectively improve the stability of phycocyanin, and the pigment preservation rate of phycocyanin increased from 50.90% to 78.10%, 67.02% and 69.08% after 72 h at room temperature, respectively; the stabilizers of phycocyanin were compounded with glucose, sodium chloride and sorbitol in the mass ratio of 1 : 1 : 0.3 and left at 4 ℃ for 14 days. After adding glucose, sodium chloride and sorbitol as stabilizers in the mass ratio of 1:1:0.3, the pigment retention rate of the alginate was increased by 54.4% compared with that of the unadded alginate after being placed at 4 ℃ for 14 d. The pigment retention rate of the alginate added with the additive was increased by 16.1% compared with that of the unadded one after being placed at 25 ℃.

Spirulina (English name spirulina), also known as "spirulina", belongs to the family of Cyanobacteria, Chlamydomonas; at present, there are three types of large-scale cultivation at home and abroad, namely, Spirulina major, Spirulina obtususus and Spirulina indica. Spirulina obtususus is a blue-green seaweed (cyanobacteria) belonging to the Candida family.
It is a non-branched, multicellular spiral mycelium with a length of about 200 μm~300 μm and a width of about 5 μm~10 μm [1]. The amino acid composition of the proteins contained in Spirulina obtusususiformis is very uniform and reasonable, which suggests that it can be used as a potential health food for human beings [2].
Phycocyanin is one of the photosynthesizing proteins in the phycobilins, which are chromophore polypeptides consisting of α and β subunits with a molecular weight of about 20,000 daltons [3]. The phycobilisome in the cyanobacterium Spirulina obtususus is composed of an alpha and beta subunit in the center and a phycocyanin in the periphery. Phycocyanin is the most important bile protein in Spirulina, accounting for about 20 % of the dry weight [4-6]. It has a blue color in aqueous solution and fluoresces in purple. The UV-Vis spectra of phycocyanin in Spirulina obtusususiformis show characteristic absorption peaks at 278, 360 and 620 nm [7]. It has also been shown that the maximum absorption peak of L. obtususus is at 620 nm and its fluorescence emission peak at room temperature is at 645 nm [8].
Natural pigments are very rich in variety and are classified according to a variety of bases. According to solubility can be divided into fat-soluble pigments, water-soluble pigments; according to the source can be divided into animal pigments, microbial pigments and phytochromes; in order to classify the different chemical structures for anthocyanins, carotenoids and other five categories [9-10].
Alginin is a natural blue pigment with high application value. It has been shown to be anticancer[11-12] and can be used as a health food for patients with enteritis[13] . It is highly water-soluble and can be easily extracted from Spirulina. In the process of extraction and purification, the control of pH value and ionic strength is very crucial for the stability of algal blue protein. The discoloration and denaturation of phycocyanin is determined by the grade of protein polymers, and its polymer form is mainly affected by light intensity, light time, temperature, pH value, irradiation and protein concentration [14-17].
It has been studied that the higher concentration of sodium chloride can protect the stability of alginate, and the appropriate amount of sodium benzoate can protect the color and preservation of alginate to a certain extent [18-19], but the stability of alginate is still low. Therefore, on the basis of previous studies, this experiment was carried out to investigate the effects of different food-grade additives as well as glucose, sodium chloride and sorbitol additives on the stability of alginate.
1 Materials and Methods
1.1 Materials and Main Instruments
Spirulina obtususifolia powder: Inner Mongolia Wuxingzhao Ecological Industry Development Co.
FD-10 Freeze Dryer: Beijing DTY Technology Development Co., Ltd; 756PC UV Spectrophotometer: Tianjin Prius Instrument Co., Ltd; DK-98-II Electric Thermostatic Water Bath: Tianjin Taiste Instruments Co.
1.2 Extraction and purification of algal blue protein
1.2.1 Extraction of algal blue proteins[19]
Appropriate amount of spirulina powder was dissolved in distilled water according to the material-liquid ratio of 1:40 (mass ratio), and then stirred with a stirring rotor at a speed of 1,000 r/min for 1.5 h. It was frozen at -18 ℃, and then thawed rapidly in a 37 ℃ water bath for 24 h. After repeating this procedure for four times, it was centrifuged at a high speed for 10 min at 10,000 r/min, and the absorbance at 620 and 280 nm was measured after taking the supernatant and diluting it with appropriate multiplicity.
1.2.2 Purification of algal blue proteins[17]
Take the crude extract of algal blue protein with the concentration of 5 mg/mL, slowly add ammonium sulfate solid to the saturation degree of 40%, and at the same time, carry out magnetic stirring until complete dissolution, stand at 4 ℃ for 2 h, then centrifuged at 10 000 r/min for 15 min, collect the precipitate, dissolve it in an appropriate amount of distilled water, and then freeze-dried after dialysis and set aside.
1.3 Research on storage stability of algal blue protein
1.3.1 Effect of temperature on the stability of phycocyanin[19]
30 mg of alginate was dissolved in 30 mL of citrate phosphate buffer at pH 5.0, 6.0 and 7.0, and incubated in 6 temperature gradients (20, 30, 40, 50, 60 and 70 ℃) for 30 min. The absorbance was measured at 620 nm after appropriate dilution, and the pigment retention rate was calculated. The pigment retention rate was calculated according to equation (1):
Pigment retention rate/% = ×100 Equation (1)
1.3.2 Effect of daylight illumination on the stability of phycocyanin [19]
Two groups of 1 mg/mL aqueous phycocyanin solution were taken, one group was irradiated under a single light source (sunlight) and the other group was stored away from light, and then diluted appropriately after 12, 24, 36, 48, 60, and 72 hours, respectively, and the absorbance value was measured at 620 nm to compare the changes in the retention rate of phycocyanin pigments.
1.3.3 Effect of pH on the stability of algal blue protein
Take 0.1 g of alginate powder and dissolve it in 100 mL of citrate phosphate buffer with pH value of 5.0, 5.5, 6.0, 6.5 and 7.0 respectively, there are 5 groups in total, and take samples at 30 min intervals to dilute appropriately, and measure the absorbance value at the wavelength of 620 nm, and then compare the changes of the preservation rate of the alginate pigment.
1.3.4 Effect of food additives on the stability of algal cyanoproteins [20-21]
Take 100 mL of algal blue protein solution with a concentration of 1 mg/mL, and add the following additives in order according to the maximum additive amount of food additives stipulated in GB 2760-2011 Standard for the Use of Food Additives: glucose (5 g), sucrose (5 g), sodium chloride (5 g), sorbitol (0.003 g), sodium benzoate (0.000 2 g), ascorbic acid (0.002 g), and sodium benzoate (0.000 2 g), and the following additives are added to the solution. 0.002 g). After 24, 48 and 72 hours of exposure to sunlight at room temperature and appropriate dilution, the absorbance value at 620 nm was measured to compare the changes in the retention rate of phycocyanin pigments. The effects of different concentrations of glucose and sodium chloride on the stability of algal blue protein were measured according to the above method. Select appropriate concentrations of glucose, sodium chloride and sorbitol and add them into the aqueous solution of phaeocyanin, and carry out the test according to the above method to observe the change of pigment retention rate.
2 Results and analysis
2.1 Effect of temperature on the stability of phycocyanin
The effect of temperature on the stability of phycocyanin is shown in Fig. 1.
As can be seen from Fig. 1, the pigment retention rate of algal blue protein decreased with the increase of temperature when it was placed at different temperatures for 30 min. When the temperature was 20 ℃
The pigment retention rate of alginate stored at 40 ℃ was almost unchanged; the pigment retention rate of alginate stored at 50 ℃ and 60 ℃ decreased by 11.68% and 20.71%, respectively, compared with that of the initial one after 30 min, and the pigment retention rate of alginate stored at 70 ℃ showed the greatest decrease, which was 58.58% lower than that of the initial one.
High temperature will destroy the structure of algal blue protein and cause its denaturation, resulting in a decrease in the pigment retention rate of algal blue protein. It can be seen from the results that phycocyanin has the highest and most stable pigment retention rate between 20 ℃ and 40 ℃. Therefore, high temperature storage should be avoided below 40 ℃.
2.2 The effect of light on the stability of phycocyanin
The effect of sunlight illumination on the stability of phycocyanin is shown in Fig. 2.
As can be seen from Fig. 2, under the irradiation of room temperature and single sunlight source, the pigment retention rate of the algal blue protein solution decreased greatly from 48 h. At the same time, the color fading was obvious, and the color gradually changed from sapphire blue to light blue from 48 h, and became almost colorless and transparent at 60 h. The pigment retention rate decreased by 59.31% compared with that at 0 h, and the rate of the pigment retention rate was only 29.26% of the initial one at 72 h. The color retention rate of the solution decreased from 0 h to 60 h, and the color retention rate of the solution was only 29.26% of the original one at 72 h. After 72 h, the pigment retention rate was only 29.26%. The pigment retention rate of phycocyanin stored at room temperature under the condition of light protection was higher than that of sunlight, but the effect was not great, and the pigment retention rate of phycocyanin at 72 h was 13.51% higher than that of sunlight. It can be concluded that the sensitivity of phycocyanin to heat is greater than that to light, but light also has a certain effect on the pigment stability of phycocyanin. Therefore, phycocyanin should be stored under light-proof conditions.
2.3 Effect of pH value on the stability of algal blue protein
The effect of pH on the stability of phycocyanin is shown in Fig. 3.
Figure 3 shows that the pigment retention rate of phycocyanin solution at pH 5.0, 5.5, 6.0, 6.5 was small, and the pigment retention rate was kept in the range of 95.49%~102.19%; and it can be seen that the phycocyanin was the most stable and the highest pigment retention rate was found at pH 6.0. At pH 7.0, the pigment retention rate decreased greatly, from 100 % to 87.46 % gradually. This may be due to the fact that the alkaline condition damaged the structure of phycocyanin, so it should be preserved in neutral condition instead of alkaline condition.
2.4 Effect of additives on the stability of algal blue proteins
The effect of food additives on the stability of algal blue proteins is shown in Fig. 4.
Additive type
Fig. 4 Effect of food additives on the stability of algal blue proteins
Fig.4 The influence of food additives on stability of phycocyanin
Figure 4 shows that the retention rate of phycocyanin pigments in phycocyanin solutions with different additives increased and then decreased during 72 h of storage at room temperature under sunlight. This may be due to the incomplete dissolution of phycocyanin at the beginning. The highest pigment retention was observed in the alginate with glucose, sorbitol and ascorbic acid, which decreased from the initial 100 % to 78.10 %, 69.08 % and 67.24 %, respectively, which was significantly higher than that of the blank group (50.90 %). This may be attributed to the fact that the additives can protect the color of the algal blue protein and increase its pigment retention rate. However, the solution of phycocyanin with ascorbic acid produced a large amount of precipitation. Therefore, glucose, sodium chloride and sorbitol were selected for further study.
2.5 Effect of glucose concentration on the stability of algal blue proteins
The effect of glucose concentration on the stability of phycocyanin is shown in Fig. 5.
As shown in Fig. 5, the color retention rate of glucose-added phaeocyanin increased after 24 h, and then decreased with time. This may be due to the color protection effect of glucose on phycocyanin. The pigment retention rate of the alginate without glucose did not change much after 24 h at room temperature. When the concentration of glucose was 10 mg/mL, the absorbance value of phycocyanin increased greatly after 24 h, and the pigment retention rate of phycocyanin increased by 16.15%, which was 12.62% higher than that of phycocyanin without added glucose; the pigment retention rate of phycocyanin with added glucose at 10 mg/mL reached 78.09%, which was 27.19% higher than that of phycocyanin without glucose. After 72 h, the color retention rate of the solution with 10 mg/mL glucose reached 78.09%, which was 27.19% higher than that of the solution without glucose, and then the retention rate of alginate color tended to slow down as the concentration of glucose solution increased. Therefore, for the purpose of cost saving, 10 mg/mL of glucose was chosen for the next study.
2.6 Effect of sodium chloride concentration on the stability of algal blue proteins
The effect of NaCl concentration on the stability of algal blue protein is shown in Fig. 6.
Fig. 6 Effect of sodium chloride concentration on the stability of algal blue protein
As can be seen from Fig. 6, the pigment retention rate of the alginate without NaCl remained almost unchanged after 24 h, while the absorbance values of the alginate with NaCl increased, which was attributed to the protective effect of NaCl on the color of the alginate to inhibit the denaturation of the alginate. The color retention rate of the solution with 10 mg/mL NaCl was significantly higher than that of the blank group after 72 h, reaching 75.90%, and then leveled off. Therefore, in order to save the cost of the experiment, 10 mg/mL NaCl was chosen for the next study.
2.7 Effects of sorbitol, sodium chloride and glucose on the stability of phycocyanin
The effects of sorbitol, NaCl and glucose on the stability of phycocyanin are shown in Figure 7.
Figure 7 shows the complex color protection effect of the three additives on phycocyanin. The pigment retention rate of the alginate solutions increased to different degrees after 24 h at room temperature under sunlight, which was attributed to the color protection effect of the additives. In the blank group, the pigment content of the alginate solution remained almost unchanged after 24 h, and then decreased rapidly; the absorbance value of the alginate solution with the addition of sorbitol, dextrose and sodium chloride increased the most obviously, which was 41.29% higher than that at 0 h, and 38.38% higher than that of the alginate solution without the addition of the additives; and the color preservation was 23.01% higher than that of the blank group at 72 h. The effect of color preservation was very obvious. After 72 h, the color preservation rate was higher than that of the blank control group by 23.01%, and the color preservation effect was obvious. The stability of sorbitol-added phycocyanin was second, and its pigment preservation rate was 19.09% higher than that of the blank control group after 72 h at room temperature under sunlight. This is due to the compound effect of sorbitol, glucose and sodium chloride on alginate to play a good role in color protection and preservation, which is better than several other combinations of additives. Therefore, sorbitol, dextrose and sodium chloride can be added as compound stabilizers in alginate at a mass ratio of 1 : 1 : 0.3.
2.8 Effect of three additives on the stability of algal blue proteins
The initial pictures of phycocyanin (without additive) and phycocyanin (with additive) at (4±5)°C and (25±5)°C are shown in Fig. 8, and the pictures of phycocyanin (without additive) and phycocyanin (with additive) at (4±5)°C and (25±5)°C after 14 d are shown in Fig. 9, and the effects of three additives on the stability of phycocyanin are shown in Fig. 10.
Figures 8, 9 and 10 show the changes in pigment content of phycocyanin after the addition of glucose, sodium chloride and sorbitol as stabilizers for 14 d. The pigment retention rate of phycocyanin solutions decreased with the increase of storage days and varied under different conditions. The pigment retention of phycocyanin solutions decreased with the increase of storage days, and the pigment retention varied under different storage conditions. The most suitable storage condition for phycocyanin solution was 4 ℃ with preservative, and its pigment retention rate only decreased by 30.21% after 14 d of storage, which was 54.5% higher than that of phycocyanin stored at 4 ℃ without additive. However, the pigment retention rate of the unadditive alginate solution was almost zero after 14 d of storage at 25 ℃, with almost total loss of phycocyanin, and the pigment retention rate of the additive solution was 16.1% higher than that of the unadditive one. The pigment retention rate of the additive solution was significantly higher than that of the unadditive one at 25 ℃ and 4 ℃, which was attributed to the excellent color protection and anticorrosive effect of the three additives on the phycocyanin. This is due to the fact that the combination of the three additives has a good effect on the color protection and preservation of phycocyanin. Therefore, alginate is suitable for storage at low temperature with additives.
3 Conclusion
Differences in temperature, sunlight and pH all affect the storage stability of phycocyanin, with temperature having the most pronounced effect on the stability of phycocyanin and sunlight having a lesser effect on the stability of phycocyanin.
Appropriate concentrations of sorbitol, dextrose and sodium chloride can obviously protect the color of alginate and preserve it, and do not affect its properties. In this experiment, the three additives were added into the aqueous solution of phycocyanin, and it was found that they had obvious improvement effects on the storage stability of phycocyanin pigments. The compound additives added to phycocyanin can be widely used in food, cosmetics and other fields, and has high application value.
References:
[1] Hedenskog G, Hofsten A V. The Ultrastructure of Spirulina platensis -A New Source of Microbial Protein[J].Physiologia Plantarum, 1970, 23(1):209- 216
[2] Belay A, Ota Y, Miyakawa K, et al. Current knowledge on potential health benefits of Spirulina[J]. Journal of Applied Phycology, 1993, 5(2):235-241
[3] Serena Benedetti, Sara Rinalducci, Francesca Benvenuti, et al. Pu - rification and characterization of phycocyanin from the blue-green alga Aphanizomenon flos-aquae [J]. Journal of Chromatography B, 2006, 833(1):12-8
[4] Jaouen P, Lépine B, Rossignol N, et al. Clarification and concentra- tion with membrane technology of a phycocyanin solution extracted from Spirulina platensis[J]. Biochemical Society Transactions, 1999, 13(12):877-881
[5] Cohen Z. Spirulina platensis (Arthrospira), Physiology, Cell-Biology and Biotechnology [J]. Quarterly Review of Biology, 1997 (3):353 - 354
[6] Jespersen L, Stromdahl L D, Olsen K, et al. Heat and light stability of three natural blue colorants for use in confectionery and bever- ages[J]. European Food Research & Technology, 2005, 220 (3/4): 261-266
[7] Yin Gang, Li Chen. Separation and purification of algal bile proteins and polysaccharides from Spirulina and product characterization [J]. Fine Chemical Industry, 1999, 16(2):10-13
[8] PENG Weimin, SHANG Shutian, FU Youlan, et al. Studies on the nature of bile protein in Spirulina obtususus[J]. Journal of China Agricultural University, 1999, 4(C00):35-38
[9] Hui Qiusha. Research overview of natural pigments[J]. Northern Pharmacology, 2011, 8(5):3-4
[10] GUO Fenghua,WANG Hui . Research on the extraction and application of natural pigments[J]. Shandong Food Fermentation , 2007, 36(4):36-38
[11] Ch R,González R,Ledón N,et al. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects[J]. Cur- rent Protein & Peptide Science, 2003, 4(3):207-216
[12] Eriksen N T. Production of phycocyanin--a pigment with applica - tions in biology, biotechnology, foods and medicine[J]. Applied Mi- crobiology & Biotechnology, 2008, 80(1):1-14
[13] Fretland D J, Djuric S W, Gaginella T S. Eicosanoids and inflamma DOI: 10.3969/j.issn.1005-6521.2017.12.008
#phycocyanin #Spirulinaobtusususiae #phycocyaninpowder
9 notes
·
View notes
Text
Removing chlorophyll from a leaf has several significant effects on the plant. Firstly, photosynthesis stops because chlorophyll is essential for absorbing sunlight and converting carbon dioxide and water into glucose and oxygen. Without this process, the plant cannot produce its own food.
Secondly, the color of the leaf changes. Chlorophyll gives leaves their green color, so its removal results in the leaves turning yellow or whitish, as other pigments like carotenoids and anthocyanins become more visible.
Thirdly, the plant's growth and development come to a halt. Photosynthesis is crucial for energy production, and without it, the plant cannot grow or develop properly.
Lastly, the overall health of the plant deteriorates. Without the energy produced from photosynthesis, the plant cannot sustain itself and will eventually die.
6 notes
·
View notes
Text
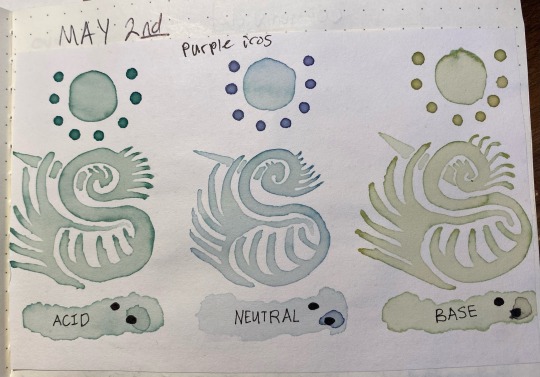
I've been meaning to make more posts about this, but there's so much i have to say/images to show that this will be a part 1 out of ??? and this one focuses mainly on the first batch of Purple Iris watercolors and the pH mystery they made me unravel through chaotically-organized researching.
Basically, I've been messing around with anthocyanins, a common class of plant pigments that are pH sensitive/can be used as a pH indicator. The first source I've tried has been purple irises, which i've only vaguely been familiar with in the past. The ones I picked were the ones that had begun to shrivel slightly, to the point where they were still a deep purple but picking them they would almost be leaking a purple liquid that stained my hands. I put them in a thing of hot tap water (not boiled, just the hot setting on the faucet), enough to cover the flowers, and let them steep. they began changing the color of the water almost immediately, with the fresher ones not losing their color as quickly as the ones that had begun to wilt on the plant. within 30 minutes i decided it was extracted enough.

This left a strong purple in the water, which i then poured off into three other containers, two of which i would alter the pH of.
The purple is due to delphinidin, a type of anthocyanidin that forms the building blocks of anthocyanins. Note i italicize the word anthocyanidin just so it's easier to tell apart the two.
there are anywhere from 16-31 anthocyanidins depending on what source you find, but they are basically the backbone structure of anthocyanins, of which there are over 600 something. The main thing that turns an anthocyanidin (aglycon) into an anthocyanin (glycoside form) is a sugar attached to it.
Realistically, that distinction isn't useful when simply extracting things from flowers in hot water, but i thought it was a fun fact to note. Anthocyanidins also come in handy for knowing what builds the anthocyanins in your flowers/plant part;
cyanidin (30%), delphinidin (22%), and pelargonidin (18%) make up the base for a good majority of all the anthocyanins in plants (~60% collectively),
peonidin, malvidin, and petunidin being runnerups (20% collectively)
the 20-something remaining anthocyanidins make up the rest
So basically, they all have slightly different colors that are pH reactive, and can provide anything from red to pink to orange to purple to blue. But, for our purposes, if you have a blue/purple flower, that likely means it has some amount of delphinidin-based anthocyanins in it! there can also be more than one anthocyanidin type present in the same plant.
Other well-known sources of anthocyanins are grape skins, red cabbage, red onions, butterfly pea tea, and purple violets. However, they're also very abundant in many many other plants, these are just the common ones i can think of that lots of people are probably familiar with to some degree.
Fun fact, grape skins are actually really well-studied as far as anthocyanins go (i believe they mainly have malvidin-based ones) because they're so important for the coloration of wine! Anthocyanins as a whole are also studied as a natural source of food dyes, along with other flavonoids such as carotenoids.
As for why it turns colors, this is because of the way the anthocyanin changes structure in different pHs. The short answer is it turns red/pink in low pH (acidic) conditions, purple in slightly acidic/neutral conditions, and blue/green in slightly high pH conditions.
The long answer is something I'll explain in a moment, but for now here's the acid/base colors:


(on the left, i altered it with vinegar and it became a bright magenta color; on the right, i altered it with baking soda and it became a sea green/blue cyan that refused to show up accurately on camera). That's one thing I've noticed, and others have too, is that when working with pigments (especially natural ones) the color accuracy of the camera often just completely fails. there's only so many colors a digital camera can capture!
Here's a slightly more accurate color due to different lighting, note how it's more a malachite green than a pure blue. off the bat this was interesting becuase I wasn't expecting as green of a liquid as i got.

Anyways, the first thing i did with them was use them as-is, no alterations past the addition of the respective vinegar and baking soda. I painted with them just as one would paint with watercolors, and interestingly enough, when i put them onto paper, they began to change from their pink/purple/malachite colors to a teal/indigo/emerald set instead.
This seems to be the result of something in the paper itself, likely calcium carbonate (which i only recently learned is added to "buffer" paper against acidic substances; the cellulose in paper is more stable long term when there's no acid present, and the calcium carbonate neutralizes any acids applied to a degree).
It's still interesting that even though the acids are neutralized, they give a unique color when compared to the basic paint.
I also tried soaking some of the same paper in vinegar water, which got rid of that buffer and let me paint with the pinks intact, but that's for another post.
Also, note that i said "neutral" for the middle, this is just what i wrote down for the tap water sample; in actuality the tap water is actually a bit closer to pH 6 instead of a true neutral 7, which i only found out after i had gotten this far. So whenever i say "neutral," i mean "tap water that's slightly acidic"
Here's something interesting that happened, though. Overnight i left the jars on my desk, and while the acid and neutral colors were the same when I came back ~24 hours later, the basic had degraded into a murky brown. this was interesting since that meant the instability was pH-dependent.
So, i made another color swatch with the acid/neutral/base, and used that as a comparison to look at how it had changed. Surprisingly, it painted out a yellowy-green instead of a murky gray-brown
here's the murky water that the once-malachite-green turned into:

I also poured off a bit into another container, and shifted it back into a low pH with a bit of vinegar to see if it would still change color. Surprisingly, it turned a slight pink, like pink lemonade, which means there were still anthocyanins in there but they were likely a lot less concentrated than they used to be.
Here's the pink, with a few leftover bubbles from the baking soda/vinegar reaction:

Here's the results of painting with these:
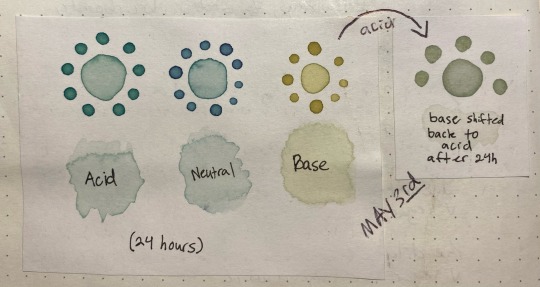
the acid was basically unchanged after sitting in a jar for 24 hours, the neutral had lost a bit of its purple color but was still about the same, and the base was now a lot yellower/tanner with a bit of green still showing through. The shifted sample was a pale stormy gray that ended up taking on a green color as it dried, as though following the trend of pink shifting to a bluer color but on a much more muted scale.
Now obviously, i wanted to figure out what caused this, so i dug around both on wikipedia and other sites but found myself eventually reading into scientific papers on the topic, at which point it became very clear that i would need to learn like, organic chemistry and such to be able to say for sure what was happening.
I did eventually manage to figure out a few things despite the dense terminology; for one thing, anthocyanins are more unstable than other plant pigments such as carotenoids. there are plenty of things that can affect their stability, including the pH of the substance they're stored in. Any higher than pH 7 (basic pHs) and theyll begin to degrade. This explains why the high pH sample lost its blue/green color, and why there was very little left to be shifted back to a pink color.
I also found out that the pH color shift isn't as simple as it seemed. Rather, there are multiple chemical forms of anthocyanins.
At the lowest pHs, basically all of them are in the "flavylium cation" state, which basically means it's positively charged and this is what gives a red color
still at a low pH (2-4), there's anothe chemical form that appears, the "quinoidal" structure that gives a blue color. Note that the red cation is still present, just no longer the only form
the more the pH rises, the more forms start to coexist, with some of those forms being colorless (one of which is a "colorless carbinol"
so, between 4 and 6 there are the cations (red) quinoidal (blue) carbinol (colorless) and something called a chalcone that gives a pale yellow
and then past that, I'm unsure, but of course that's around when the anthocyanins begin to degrade
There are also a lot more than these that i've encountered in various contexts but these seem to be the basic ones.
Do note that i do not fully understand these terms (flavylium, quinoidal, carbinol, chalcone, etc.) and have only recently begun to actually try to learn what they mean and the context surrounding them as i only had a class of basic high school chemistry under my belt prior to this. The main paper i combed over to try to find info on it seems to be behind a paywall but the DOI is:
doi.org/10.1016/j.foodchem.2008.09.001
for anyone curious and able to access it, whether through legit means or what have you.
That being said, to me, the takeaway here seems to be that there's a yellow form that appears around the time that other color forms begin to disappear, and as those degrade it makes sense that the resulting degraded forms also contribute to a murky color. This helps explain why it changed color in the jar and also retained a bit of yellow and green.
This also explains why the blue form seems to also be slightly green, it's got the blue quinoidal chemical form as well as the yellow chalcones.
There are also interesting things of note that I will get into at a later date, such as the texture/reflectivity of the way it dries, the differences in extraction ease between this and purple violets, the addition of a genuinely neutral/pH 7 sample later, a sample from a plant that doesnt seem to have delphinidins, and sample the seems to genuinely sparkle??? Much more of interest to come soon!
#art#traditional art#anthocyanins#watercolors#purple irises#painting#science#natural dyes#pigments#dyes#pH indicators
68 notes
·
View notes
Text

Antioxidant-rich foods
1. Berries: Berries such as blueberries, strawberries, raspberries, and blackberries are rich in antioxidants such as vitamin C, anthocyanins, and flavonoids.
2. Dark chocolate: Dark chocolate is high in antioxidants called flavonoids, which have been shown to have numerous health benefits.
3. Nuts: Nuts like almonds, walnuts, and pecans are high in antioxidants such as vitamin E and polyphenols.
4. Leafy greens: Dark leafy greens like spinach, kale, and Swiss chard are rich in antioxidants like vitamin C, beta-carotene, and lutein.
5. Green tea: Green tea is a rich source of antioxidants called catechins, which have been linked to various health benefits.
6. Red grapes: Red grapes contain antioxidants such as resveratrol, which has been shown to have anti-inflammatory and heart-protective effects.
7. Tomatoes: Tomatoes are high in the antioxidant lycopene, which has been linked to a reduced risk of certain types of cancer.
8. Turmeric: Turmeric contains the antioxidant curcumin, which has powerful anti-inflammatory properties.
9. Beans: Beans such as black beans, kidney beans, and pinto beans are high in antioxidants like flavonoids and anthocyanins.
10. Dark-colored fruits: Fruits like cherries, plums, and pomegranates are rich in antioxidants such as vitamin C and polyphenols.
#food for thought#food fight#comfort food#fast food#healthy food#food photography#foodie#food#foodpics#foodlover#japanese food#foodmyheart#tw food#lunch recipes#pasta recipes#pasta recipe#salad recipes#soup recipe#recipe#reciprocity#recipies#healthy salad recipes#recipes#autumn cozy#cozy fall#cozyhome#cozy cozy#cozy living#cozy art#cozy mystery
7 notes
·
View notes
Text





Red Velvet Cake Day
National Red Velvet Cake Day, also known as the 'Sweetest Day of the Year'- no seriously, somebody once called it that on Twitter and we've been tickled pink (or should we say, red?) ever since. It's the day where diet rules are thrown out of the window and the world unites in their love for the heavenly hybrid of chocolate and vanilla, draped in a seductively deep red hue.
When is Red Velvet Cake Day?
It's national red velvet cake day on the 18th September.
The Decadent History
The origins of the red velvet cake are as rich and velvety as the cake itself. Thought to have originated during the Victorian era, the red velvet cake got its 'red' demeanor from anthocyanin, a pigment found in cocoa that reacts to acidic ingredients, like vinegar and buttermilk, in the cake but with the Cockaigne revolution, red food coloring took centre stage and hence, the iconic red was birthed.
Let's Talk Internet
Surprisingly, there's not much evidence for National Red Velvet Cake Day predating the rise of the internet. The earliest mention we found was on 18th September 2015, with 19 occurrences until now. And since then, it's been all about sharing mouth-watering images of red-tinged slices smeared with cream cheese frosting and even adventurous red velvet-themed desserts like pancakes and cookies, turning the internet into a literal sweet shop!
How to Celebrate?
Go all out on National Red Velvet Cake Day! Bake your own red velvet cake at home, visit your favorite bakery for a fresh slice or even treat yourself (and your waistline) to a red velvet smoothie or a healthy red velvet mug cake. It's about immersing yourself in the red madness for a day and showing your solidarity to fellow red velvet cake lovers across the globe.
History behind the term 'Red Velvet Cake'
1873
The Birth of Red Velvet Cake
Red velvet cake is believed to have originated in the 19th century, with the first known recipe appearing in 1873. This rich and indulgent dessert quickly gained popularity due to its distinctive vibrant red color and velvety texture. The exact origin of the cake is unclear, but it is often associated with the American South.
1930s
Addition of Cocoa and Buttermilk
In the 1930s, red velvet cake recipes began to include cocoa powder and buttermilk. These ingredients not only intensified the cake's red color but also added a subtle chocolate flavor and moistness to the crumb. The reaction between the acidic buttermilk and cocoa creates a chemical reaction that enhances the cake's reddish hue.
1940s
Waldorf Astoria's Red Velvet Recipe
Red velvet cake gained further popularity in the 1940s when the luxurious Waldorf Astoria hotel in New York City popularized their own version of the cake. The hotel claimed their red velvet cake recipe as a closely guarded secret, and it became a sought-after dessert for their guests. The Waldorf Astoria's version accentuated the cake's red color with a rich cream cheese frosting.
1990s
Red Velvet Revival
After a few decades of relative obscurity, red velvet cake experienced a resurgence in popularity during the 1990s. Many bakeries and dessert shops began offering red velvet as a specialty item, incorporating modern twists, such as red velvet cupcakes and layer cakes. The cake's distinct appearance and indulgent flavor made it a beloved choice for special occasions and celebrations.
Present
Red Velvet Worldwide
Today, red velvet cake has become a beloved dessert worldwide. It has transcended its Southern roots and has become a staple in bakeries and restaurants across the globe. The unique combination of the vibrant red color, subtle chocolate flavor, and creamy frosting continues to captivate dessert enthusiasts. Red velvet cake is often associated with celebrations, particularly Valentine's Day and Christmas.
Source
#Ultimate Red Velvet Cake Cheesecake#Red Velvet Donut#Red Velvet Cheesecake#travel#original photography#vacation#USA#Canada#food#dessert#snack#18 September#restaurant
2 notes
·
View notes
Text
White, brown or red? A guide to choosing the healthiest rice for your meals

Rice is a staple food for millions of people around the world, providing a versatile and comforting base for countless dishes. With so many varieties available, choosing the healthiest rice for your meals can be overwhelming. Whether you're looking for a quick side dish, a hearty main course, or a gluten-free option, understanding the nutritional benefits and culinary uses of white, brown and red rice can help you make the best choice for your diet and lifestyle.
In this guide you'll explore the unique properties of each rice type, providing valuable information about their health benefits, nutritional profiles and ideal cooking methods. In the end, you'll be armed with the knowledge to choose the right rice for your meals, enhancing both your health and culinary experience.
Nutritional Comparison: White, Brown and Red Rice
When it comes to nutritional content, not all rice is the same. Each variety offers different benefits that meet different dietary needs and preferences.
White rice: Often considered the most popular, white rice is milled and polished, removing the bran and germ. This process results in a softer texture and longer shelf life but also reduces its nutritional content. White rice is typically enriched with iron and B vitamins, making it a robust choice. However, it has a higher glycemic index (GI) than other varieties, which can more significantly impact blood sugar levels.
Brown rice: A whole grain, brown rice retains its bran and germ, preserving essential nutrients such as fiber, magnesium, and phosphorus. Its high fiber content aids digestion and may contribute to heart health by lowering cholesterol levels. Brown rice has a lower GI than white rice, making it a more suitable choice for those managing blood sugar levels.
Red rice: Known for its distinctive color and nutty flavor, red rice is rich in anthocyanins, powerful antioxidants that give it its red color. These antioxidants are believed to reduce inflammation and support heart health. Red rice is also rich in fiber, iron, and other essential nutrients, making it a nutrient-rich option for any meal.
Health Benefits and Considerations
Benefits of white rice: Although often overshadowed by its nutrient-rich counterparts, white rice remains a good choice for those with digestive sensitivities. Its mild flavor and soft texture make it easily digestible, and it is often packed with essential nutrients. Additionally, white rice is a quick-cooking grain, ideal for busy weeknights.
Benefits of brown rice: The fiber in brown rice can aid digestion and promote a feeling of fullness, which can be beneficial for weight management. Its low GI also makes it a better choice for individuals with diabetes or those looking to stabilize blood sugar levels. The nutrient profile of brown rice supports heart health and provides sustained energy.
Benefits of Red Rice: The high antioxidant content in red rice provides unique health benefits, including anti-inflammatory properties and potential protection against chronic diseases. Its robust flavor pairs well with a variety of dishes, making it a versatile addition to your pantry. The iron content in red rice may also be a valuable nutrient for those at risk for anemia.
Choosing the Right Rice for Your Dietary Needs
Choosing the right rice depends on your dietary preferences and health goals. For those following a gluten-free diet, all three types of rice are safe choices. If you are focused on weight management or blood sugar control, brown or red rice may be more suitable due to its low GI and high fiber content. For athletes or individuals who need an instant energy boost, white rice may be a practical option.
Also consider the type of food you're preparing. The neutral flavor of white rice pairs well with a wide range of dishes, from stir-fries to desserts. The chewy texture and nutty taste of brown rice is perfect for grain bowls and salads, while the rich flavor of red rice can enhance soups, stews, and hearty main dishes.
You can also order large quantities of healthy Basmati rice from Vi Exports India.
2 notes
·
View notes
Text

During the first three days of Whole Detox, we immerse in the color red.
🟥
Red is a color of urgency, emergency, and response. It evokes a reaction.
🆘⛔️🛑❌
In a similar way, red foods in nature contain protective compounds that assist in immune response: anthocyanins, fisetin, lycopene, phloretin, and quercetin, to name a few.
🍎🍉🍒🍓🌶️🍅
To the healing of red! ♥️

29 notes
·
View notes
Text
The World of Pink: Unveiling the Magic Behind Your Favorite Food Colors

Pink food coloring takes center stage in countless treats, from bubblegum frosting to rosy macarons. But have you ever wondered how this vibrant hue transforms your favorite dishes? Delving deeper, we'll explore the fascinating world of pink food colour manufacturer, uncovering the science and creativity behind these delightful pigments.
Natural vs. Synthetic: A Spectrum of Pinks Helvetica Neue
Pink food colour manufacturer offer a spectrum of options, catering to both health-conscious consumers and those seeking bold hues. Here's a breakdown of the two main categories:
Natural Pink Colors: Derived from fruits, vegetables, and minerals, these colorings offer a subtler, softer pink. Beetroot juice, hibiscus extract, and anthocyanins (found in berries) are some popular natural sources.
Synthetic Pink Colors: Manufacturers create these vibrant pinks through a controlled chemical process. They are often more cost-effective and produce a wider range of intense shades. However, some consumers prefer natural options due to potential concerns about artificial ingredients.
The Art and Science of Pink Perfection
Creating the perfect pink shade requires a delicate balance of science and artistry. Manufacturers employ sophisticated equipment and expertise to ensure consistent color, stability, and safety for consumption. Here's a peek behind the curtain:
Selection of Raw Materials: Whether natural or synthetic, the starting materials undergo rigorous testing to ensure purity and suitability for food applications.
Color Formulation: Food scientists meticulously blend different colorants to achieve the desired pink shade. Factors like lightfastness (resistance to fading) and shelf life are crucial considerations.
Quality Control: Throughout the production process, stringent quality checks guarantee consistent color and adherence to food safety regulations.
Beyond the Bakery: A World of Pink Applications
Pink food coloring isn't limited to frosting and candy. It plays a vital role in various food applications, including:
Dairy Products: Yogurt, ice cream, and pink-hued milkshakes often incorporate pink food coloring.
Meats and Seafood: Some processed meats and seafood may utilize pink colorings to enhance their appearance.
Beverages: Pink drinks like fruit punches and sports drinks often rely on food coloring for a visually appealing hue.
The Future of Pink: Innovation and Sustainability
As consumer preferences evolve, Pink food colour manufacturer are constantly innovating. are some exciting trends to watch:
Natural Color Focus: The demand for natural colorings is on the rise. Manufacturers are exploring new and sustainable ways to extract vibrant pinks from natural sources.
Clean Label Movement: Consumers are increasingly interested in products with recognizable ingredients. Manufacturers are responding by developing "clean label" pink colorings with simpler formulations.
A Touch of Pink: Adding Vibrancy to Your Plate
Pink food colour manufacturer plays a significant role in creating visually appealing and delightful food experiences. From the vibrant hues in candies to the subtle blush in yogurt, these colorings add a touch of magic to our plates. As the industry continues to evolve, we can expect even more innovative and sustainable ways to bring the world of pink to life!
2 notes
·
View notes
Text
Health Importance of Black Rice
Introduction
Black rice is also known as forbidden rice or purple rice, primarily cultivated in the northeastern states of India, with Manipur being a significant producer. its is a G.I Tag Crop of Manipur and here it is known as “Chak-Hao”, this rice that has gained attention in recent years due to its unique color, distinct flavor, and impressive nutritional profile. Historically, it was once considered a rare and precious grain reserved for royalty in ancient China. The nutritional value of black rice (100 g, cooked rice)
· Calories: 150 kcal · Carbohydrates: 34 g · Protein: 3 g · Fat: 1.5 g · Fiber: 2 g · Vitamin E: 0.8 mg · Iron: 1.5 mg · Magnesium: 43 mg · Phosphorus: 101 mg · Potassium: 84 mg · Zinc: 1.2 mg Black rice is also a good source of various vitamins and minerals, as well as dietary fiber & a powerful antioxidant, Anthocyanin. These values are approximate & can vary slightly depending on factors such as the variety, growing conditions, and processing methods. Health Benefits of Black Rice:
1. Rich in Antioxidants, as anthocyanins, reducing oxidative stress and inflammation 2. Improved Heart Health by reducing cholesterol levels, improving blood vessel function, and regulating blood pressure. 3. Improved Digestion by its high fiber content which aids in digestion and can help prevent constipation. 4. Blood Sugar Regulation, due to the slow digestibility of black rice, beneficial in diabetes. 5. Cancer Prevention, anthocyanins have potential anti-cancer properties and may help inhibit the growth of cancer cells 6. Weight Management due to high fiber & slow digestion 7. Nutrient rich food, maintain overall health & improve immune system 8. Allergen-Free, free from gluten sensitivity or celiac disease. 9. Neuroprotective effect improve Brain Health
Black rice has a slightly nutty flavour & chewy texture. It requires longer cooking time and more water than white rice due to its bran layer. It can be used in both sweet and savoury dishes. In Asian cuisines, black rice is often used in traditional desserts, rice-based puddings, and porridge. It can also be incorporated into salads, side dishes, and even main courses for a visually striking and nutrient-rich meal

2 notes
·
View notes
Photo

June is a joyfully colorful month in this region for several reasons! For starters, by the time June’s warmer temperatures seep into downstate New York, the growing season is entering full swing with a bright palette of fresh produce arcing through the farmstands in our markets. Plus, June is of course Pride Month – symbolized by the six-striped rainbow flag that cheerfully adorns flag poles, government buildings, store fronts and many other public spaces throughout the course of this month. Rainbows have risen to become a symbol of good fortune, positivity, diversity and inclusiveness in our popular culture, so it’s no coincidence that the phrase “eat the rainbow” is used by dieticians and other medical professionals to encourage people to put more fresh produce on their plates. In fact, consuming a variety of colorful fruits and veggies is the best way to introduce the most nutrients into your diet, without adding excessive calories. The naturally occurring pigments in these foods indicate different compounds with different properties that have been shown to provide a wide range of unique health benefits. So, to aid in the quest to taste and embrace the many seasonal colors of this month, here are some rainbow-hued items to look out for in the farmers market this weekend: Purple daikon Purple daikon radishes are at their peak during winter and spring months and are easily spotted at farmstands thanks to their bright violet skins offset by emerald leaves. This exotically hued root vegetable is native to Asia where daikon has been cultivated for thousands of years. Purple daikon is a specialty hybrid belonging to the same Brassicaceae family as the red radish. Their globular roots are oblong and cylindrical in shape with a thin tapered “tail.” The striking purple pigment is due to a type of flavonoid called anthocyanin -- the same antioxidant that gives blueberries their color, which will be entering the markets soon. If you don’t see purple daikon at the farmers market, look for watermelon radish, a round, green-white radish that reveals a dazzling hot pink interior when sliced open. Daikon radish can be enjoyed raw thanks to its crispy, crunchy texture and spicy, peppery profile that will add a splash of color and extra dimension to summer salads, grain bowls, pasta dishes and crudité platters. It can also be cooked using a variety of methods, including tossing the roots in olive oil, salt and pepper and roasting them at 375 °F for 10-15 minutes or until easily pierced with a fork. Don’t toss your leaves though! If they are still fresh and unblemished, radish greens of all kinds are delicious pan sautéed with thinly sliced garlic and olive oil then spritzed with a little freshly squeezed lemon juice. Red beets Beets grow in a rainbow of colors from gold to magenta to stunning candy cane-striped Chioggia beets. They derive their jewel-like hues from betalain, a type of natural plant pigment that has antioxidant and anti-inflammatory properties. The most common kind of beets you’ll find in the farmers market are the reddish/purple variety, but you can generally locate them in every color. Beets burst back into season this month so grab a big bunch of the spherical beauties this weekend and “get down to the beet”: * Beet salad with Goat Cheese and Balsamic * Balsamic and Fresh Thyme Roasted Beets * Easy, Homemade Pickled Beets Green peas Peas are cool weather-loving plants which means that June is a prime month for these exploratory climbers before they become sapped by the arrival of hotter temps. Peas contain a variety of minerals including magnesium, potassium and calcium and are also rich in antioxidant nutrients like vitamin C, carotenoids and flavanols. Snow, snap, and garden peas are all members of the legume family, but there are subtle differences between the three. Though they may look similar, each has a different texture and level of sweetness:
Garden (aka sweet or English) peas: Pods are firm and rounded and are shelled then discarded (toss them in your compost!) to retrieve the sweet peas inside which can be eaten raw or cooked. These are the common green peas that are sold shelled and frozen.
Snow peas: Snow peas are often used in stir-fries. The whole pod is edible and they are flat with very small peas inside. The tough “strings” along the seams are usually peeled back and removed before eating. Snow peas are mildly flavored and can be served raw or cooked.
Snap peas: A cross between snow peas and garden peas, the whole pod is eaten and has a crunchy texture and sweet flavor. Snap peas may be eaten raw or cooked. Stringless varieties are now available.
Of course, there are many more ways to shop the rainbow at the farmers market this week: orange carrots, canary yellow oyster mushrooms, blue potatoes, carmine red strawberries and the list goes on! And, if you want to eat your way to some extra credit, pick up a bunch of beautiful rainbow chard! The stalks come in an array of bright hues and pretty pastels from white, yellow, red, purple, pink and striped topped with vibrant, leafy greens.
2 notes
·
View notes
Text

Unveil the Beauty of Purple with Red Hibiscus
Discover the magic of red hibiscus! 🌺✨ This plant, scientifically known as "Hibiscus sabdariffa", is not only beautiful but also contains a treasure of natural purple dye. 🌿 The crimson calyces, rich in anthocyanins, give this plant its unique ability to produce a stunning purple dye. Simply boil the calyces and extract the liquid to obtain the natural purple color, which can be used to dye food, fabrics, and more.
#purple#beauty#aesthetic#colors#dye#home & lifestyle#nature#hibiscus#red hibiscus#plants#flowers#hibiscus sabdariffa#natural dyes#anthocyanins
3 notes
·
View notes
Text
Eating healthy food at night before bed can be a good choice as long as you make mindful selections. The key is to opt for foods that are light, easy to digest, and won't cause discomfort during sleep. Here are some healthy bedtime snack options:

Greek Yogurt
Greek yogurt is a popular and nutritious dairy product that has gained widespread acclaim for its many health benefits and versatile culinary uses. It is made by straining regular yogurt to remove most of the whey, resulting in a thicker and creamier texture. Here are some key characteristics and benefits of Greek yogurt:
High Protein Content: Greek yogurt is an excellent source of protein, making it a popular choice among those looking to increase their protein intake. It typically contains around twice the protein of regular yogurt, which can aid in muscle maintenance and appetite control.
Probiotics: Many varieties of Greek yogurt contain live probiotic cultures, which support gut health and digestion. These beneficial bacteria can help maintain a healthy balance of microorganisms in the digestive system.
Cottage Cheese
Cottage cheese is a fresh cheese made from the curds of cow's milk. It is a versatile and nutritious dairy product that has been enjoyed for centuries. Here are some key characteristics and benefits of cottage cheese:
High Protein Content: Cottage cheese is renowned for its high protein content. It is an excellent source of complete protein, providing all the essential amino acids necessary for muscle growth and repair. This makes it a favorite among athletes and those looking to increase their protein intake.
Low in Fat: Cottage cheese comes in various fat content options, including low-fat and non-fat varieties. It's a good choice for individuals looking to maintain a lower-fat diet.
Berries
Berries are small, colorful, and delicious fruits that are not only a treat for the taste buds but also packed with nutrients and health benefits. There are many different types of berries, including strawberries, blueberries, raspberries, blackberries, and cranberries, among others. Here's a look at some of the key features and benefits of berries:
Rich in Antioxidants: Berries are known for their high antioxidant content, particularly anthocyanins, which give them their vibrant colors. Antioxidants help protect cells from damage caused by free radicals and contribute to overall health.
Low in Calories: Berries are generally low in calories, making them a healthy and guilt-free addition to your diet. They can be a satisfying and sweet option for those looking to manage their calorie intake.
Conclusion.
selecting the right healthy food to eat before bed is essential for promoting a good night's sleep and overall well-being. While individual dietary needs and preferences may vary, some key considerations for bedtime snacks include:
Lightness and Digestibility: Opt for foods that are easy to digest, as heavy or greasy foods can lead to discomfort and sleep disturbances.
Balanced Nutrients: A balanced bedtime snack should ideally include a combination of protein, healthy fats, and complex carbohydrates to help stabilize blood sugar levels and promote a feeling of fullness.
Electric Lady (woohoo)
4K notes
·
View notes