#accelerating patient recruitment
Explore tagged Tumblr posts
Text
Empowering Sponsors with Compliant Data Collection and Integrity for a Variety of Clinical Studies | Jeeva Trials
Traditional clinical guidelines, and stand-alone patient study and care pathways are proven to be increasingly inadequate, especially in a post-pandemic world with low clinical adherence, disrupted workflows, and stay-at-home orders. Disrupted workflows means more time required to complete a study, fatigue of the research team, and wastage of resources.
Researchers and study teams are increasingly adopting eClinical cloud trial tools that are designed to augment researchers, study teams and clinicians to augment their complex decision-making processes with targeted clinical knowledge, patient information and computerized clinical workflows. It directly improves the quality of clinical documentation. AI technologies provide the tool capabilities for drawing insights into data beyond what humans can. CROs (Contract Research Organizations) evaluate clinical study tools largely based on speed, flexibility and cost-efficiency. However, amidst these concerns, data integrity is not to be understated or taken for granted. Data integrity is not only important for a study, it needs to be addressed throughout the product life cycle across Good Clinical Practices (GCP), Good Laboratory Practice (GLP), Good Manufacturing Practice (GMP), and other Good Practice (GxP) areas.
Data integrity is critical for studies
Good risk mitigation and management is essential to data integrity as multiple points of risk exist throughout data recording, storage, transfer, reporting and other stages of data lifecycle during a study trial. It is achieved by making data traceable throughout audit trails. Transparency is demonstrated with a chain of custody from data origin to its analysis. Without data integrity, it is not possible to regenerate a previous clinical trial result reliably. Data integrity cannot be validated by point-to-point interfaces of individual systems alone, it requires a more holistic approach towards validation and quality management as these systems need to work together across corporate borders and multi-site systems.
Quality of data can affect the quality of decision support because if data collection is not standardized, the study trial data is effectively corrupted and increases the risk of failure during the submission procedure for approval by Food and Drug Administration (FDA), Medicines and Healthcare Products Regulatory Agency (MHRA) and other regulators. Regulators in the US and across the world continue to stress the criticality of data integrity in clinical trials.

Points to consider while choosing an eClinical study solution:
A good system should not delete or obscure previously-recorded audit trail information and prevent modification by the user.
It should record complete audit trail records including identification of the data element that was changed, who authorized the change and implemented them.
It should provide early visibility to reliable data to quickly make sound decisions and bring life-enhancing treatments to life.
Cyber security is a mission-critical consideration for electronic clinical outcomes assessment (eCOA) risk management for any eClinical solution.
Regulatory-minded study teams will have data integrity plans in place as regulators can raise questions about data collection compliance, warranting rescue action. By utilizing the Jeeva Informatics eClinical cloud, study teams can have regulation-compliant risk mitigation with complete transparency, traceability, and documentation. Jeeva is a flexible bring your own device (BYOD), SaaS (Software as a Service) solution that is designed to maintain data integrity with features and protocols that fit the specific trial protocol, ensuring reliability and authenticity of the study data by adhering to the most current compliance regulations in force.
Shortening the Distance from Study Data to Action
Jeeva’s highly scalable SaaS architecture provides a cost-effective approach to support trials for multiple studies, phases and therapeutic areas. Its intuitive interface eliminates the multi-step process to navigate reports and shortens the distance from study data to action. In clinical research, data integrity and reliability of trial results are paramount. The value of a comprehensive and compliant eClinical tool is absolute. Data integrity continues to be a major theme across inspection results. The collaborative technology used in Jeeva automates high-value clinical trials recruitment and retention tasks and provides insightful retrieval of information. Adherence to the International Council on Harmonization (ICH) GCP is a core tenet for data integrity at Jeeva.
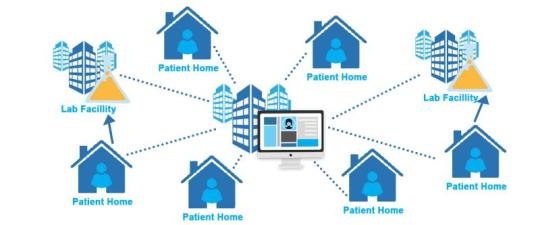
Leading research organizations have consistently been using Jeeva for compliant and adaptive research with access to immediately actionable patient data. It provides researchers personalized clinical study documentation across solutions, platforms and devices, anytime and anywhere, regardless of physical location.
Enabling Clinical Research at Scale
A failed trial not only sinks investment into the early stages of the trial itself but also results in dissatisfied sponsor clients and impacts your long and fruitful business relationship with them. Jeeva is designed to support the conduct of clinical trials utilizing validated functionality and processes. The modular software enables clinical research at scale and saves more than 70% time and logistic burden on the study teams. Utilizing the platform-agnostic software with advanced features like bi-directional communications, scheduling and touch-less electronic informed consent, investigators can rapidly enroll participants in the study, and investigators can safely review the study material remotely and conveniently from their own mobile device.
Complying with the Current Regulations
Jeeva Trials follows a human-centric approach with a deep understanding of the perspectives and requirements of various stakeholders including regulatory compliance specialists, IT security and privacy professionals, auditors and coordinators. The burden of ensuring regulatory compliance of technology solutions, GDPR (General Data Protection Regulation), Institutional Review Boards (IRBs), human subjects protection guidelines, GCP (Good Clinical Practice) guidelines by ICH (International Council of Harmonization) of Technical Requirements for Pharmaceuticals for Human use, and other regional guidelines lies with the study sponsor. Jeeva adheres to the current federal, state, and international regulations or guidelines for conducting clinical trials using electronic patient data such as the FDA 21 CFR (Code of Federal Regulations) Part 11, SOC 2 (System Organ Classes), Amazon Well (AWS) Architected Framework Review, AWS Foundational Technical Review, GDPR privacy policies, and others. Avoid having to validate multiple a la carte tools as you can now achieve the same goal with a single all-in-one integrated SaaS platform.

Saving on Costs of Failed Trials
It takes on average 10-15 years and USD 1.5-2.0 billion to bring a new drug to the market. Approximately half of this expenditure covers testing, preclinical compound discovery and regulatory processes. The high failure rate of clinical trials due to regulatory issues, patient non-adherence, low retention and high drop-offs during long-term studies is a major stumbling block in drug development. Less than one-third of all Phase II compounds advance to Phase III, with more than one third of all Phase III compounds failing to advance to approval. The most complex Phase III trials carry nearly 60% of the overall trial costs, resulting loss per failed clinical trial to the order of 0.8-1.4 billion USD.
Flexible Platform to Accelerate Patient Recruitment
Study build delays cause timelines to drag on, as such CROs face not only dissatisfied sponsor clients but they could lose a fruitful business relationship. There are major regulatory implications as well, as unverified, disintegrated and dubious data quality can land organizations in court. Jeeva Informatics Solutions is designed to reduce timelines for study startup and participants by up to more than 50%, while complying with data integrity regulations of the federal and state governments. Jeeva makes it easy for longitudinal cohort studies to collect validated data from participants in real-world settings over extended periods of time. The flexible platform accelerates patient recruitment and retention, and enables long-term engagement for 5, 10, or 15-year follow-up studies for long-term trials, such as cell and gene therapy.
Affordable subscription-based pricing of Jeeva makes it easier for the study teams to plan budgets with predictable expenses. The modular SaaS subscription model helps clinical researchers, Contract Research Organizations (CROs), and sponsors manage a clinical study’s annual budget on a simple, per participant basis.
#eclinical cloud#clinical trial cloud software#eclinical platform#mobile eclinical#clinical trial recruitment challenges#decentralized clinical trials software#software for clinical trials#clinical research software#clinical study software#software used in clinical research#clinical trials software#software used in clinical trials#clinical solutions research platforms#accelerating patient recruitment#patient retention in clinical trials
1 note
·
View note
Text
https://kitsa.ai/the-future-is-now-ai-innovations-in-the-pharmaceutical-industry/
#ai in clinical trials#ai in pharma#clinical trial companies#clinical trials recruitment ai#accelerating clinical trials#how ai is transforming clinical trials#using ai to match patients with clinical trials#clinical trials blogs#becoming a principal investigator
0 notes
Text
never underestimate the power of telling someone "come on now, you're better than that", "that's not it", "I expect better of you" and similar. it has worked exceedingly well in many scenarios where telling someone to go fuck themselves or whatnot would have just terminated the conversation or our entire relationship.
it really is a flawed practice to evaluate a person's Goodness/receptiveness based on if they'll rise above someone hurling endless vitriol, even if they are expressing a reactionary idea. human beings are just not that rational. all of us have a bias to rationalize ourselves as good and correct, and those who are other as wrong and bad. and though systemic material interest certainly exists, it is only one (albeit considerable!) force which affects a person's decision making. we must have a critical understanding of ideology and its function in these matters.
ultimately, different tactics are more effective to different people. sometimes the nuclear option, so to speak, is what is called for. in other scenarios, expressing disappointment or mere expectations can be more effective and nurture (as such things are not often immediate) long term yields.
it's a mistake to always resort to the very western and liberal tactic of "deny, push away and otherize." especially on an individual level, we have more power to change people by bringing them closer rather than by pushing them away. living up to the expectations of the people you care for or depend on is a powerful persuasive base.
we must understand very clearly that individuals have no fixed character. this should be clear as day from how many famous revolutionaries have emerged from hegemonic and reactionary classes. even looking at ourselves we will understand that nearly none of us can claim to have never held liberal, reactionary or idealistic positions in our past. if we embrace rather than reject that, then we can scientifically (experimentally) undertake the task of learning how to cause and accelerate these developments in those we are most likely to be able to affect.
at the end of the day, we can only change so many minds. not every enemy can become an ally, clearly, and after a certain point it becomes a folly to waste our time trying to remediate stubborn reactionaries. however, by honing our methods and developing a clear and strategic practice, it becomes possible to recruit more individuals into a revolutionary programme; just as reactionaries have done with no qualms whatsoever to broaden their own base!
it is clear in this regard that relying too heavily on individual morality and other lazy or idealistic notions stands to limit our capabilities. it is hard, hard work to slowly and patiently challenge people—certainly less satisfying than telling someone to kill themselves out of rage and spite. but we must engage in this work in all effective areas in order to expand our base.
#azazaza#there happens to be a tangentially related discourse but this topic has been at my attention all year essentially
18 notes
·
View notes
Text
Does Long COVID Lead to Alzheimer’s? A New Study Took an Unexpected Turn - Published Aug 29, 2024
More than one in ten people who catch COVID don’t fully recover — developing a chronic condition called Long COVID which causes a variety of debilitating symptoms, including brain fog. Since some studies have found that COVID infections are associated with overall brain shrinkage, altered brain structure, and an increased risk of developing Alzheimer’s, researchers have been investigating the links between COVID and Alzheimer’s.
“There was a lot of discussion about whether COVID or even Long COVID would lead to a sudden onset form of Alzheimer’s disease so we set out to determine whether that was the case,” Dr. William Hu, director at the Center for Healthy Aging Research at Rutgers University told Being Patient.
Hu’s new study, published in Cell Reports Medicine, analyzed the cerebrospinal fluid and immune cells of Long COVID patients with brain fog. Rather than finding the telltale signs of Alzheimer’s, he discovered the patient’s immune system was still trying to fight off the COVID infection, which occurred about nine months prior. The patients whose immune cells mounted an antiviral response started to feel better — opening the door to new potential treatments for Long COVID that boost the body’s antiviral response.
What the study found The researchers looked at a group of participants from COVID recovery clinics, comparing 100 without any cognitive complaints, 79 who had abnormal results on a cognitive assessment indicating cognitive impairment, and 57 who complained about cognitive issues even though they scored normally on a cognitive test.
Hu and his colleagues took cerebrospinal fluid and blood from both groups of people with cognitive complaints to measure protein biomarkers and look at what genes the immune cells are turning on or off to see whether there was an overlap with Alzheimer’s disease. “We did not find significant numbers of people with Alzheimer’s disease markers in the cerebrospinal fluid,” Hu said. “The many molecular pathways being active in Long COVID do not correspond to Alzheimer’s disease.”
But nine months after the initial infection, what the researchers did notice was that the immune cells behaved as if they were still fighting off a viral infection. About 50 percent of the cognitively impaired participants showed slow improvement after two years. The participants whose immune cells mounted an interferon response — a pathway used by the immune system to fight viruses — showed cognitive improvement.
“One of the key findings is that we see the immune cells in the cerebrospinal fluid, recruiting cells to fight infection,” Hu said. “So that tells me that the infection is in the brain.”
One limitation of the study is that it may not capture the experience of people with more severe Long COVID impairments — since Long COVID leads to extreme fatigue, some might not be able to participate in these studies.
Long COVID and Alzheimer’s This study suggests that the mechanisms of Long COVID and Alzheimer’s disease are distinct. COVID-19 doesn’t seem to increase the levels of Alzheimer’s biomarkers.
“I think we can convincingly say right now that COVID does not cause acute Alzheimer’s disease,” Hu said. “Now whether it increases the risk for future Alzheimer’s disease is an open question.”
According to Hu, “there are many people walking around their 60s and 70s, with [asymptomatic] Alzheimer’s disease,” which means they have amyloid and tau in the brain but no symptoms. “A systemic illness [like COVID] can accelerate the manifestation of what previously was asymptomatic Alzheimer’s disease,” he said.
Although COVID-19 doesn’t directly cause Alzheimer’s disease, like other viral infections it may increase the risk of developing symptoms. This may explain why vaccines against the flu and other viral illnesses decrease the risk of developing Alzheimer’s disease.
Interferon and antiviral drugs for treating long COVID There are currently no treatments available for Long COVID. While the National Institutes of Health has poured more than $1 billion into testing new treatments, the program has been criticized by scientific experts and patients as many of these studies are testing treatments like “exercise” and “cognitive behavioral therapy” which they say are ineffective and potentially harmful ((The National Institutes of Health’s Long COVID initiative RECOVER revised its exercise and exertion trials to reduce the risk of harm to participants.)
Hu said that patients should contact their elected representatives, senators, and congresspeople to ask them to accelerate new trials focused on developing antiviral therapies that might move the needle.
“Based on our data, it looks like a successful mounting of interferon-related pathways was associated with faster recovery,” he said. “Interferon itself can be tried and there are multiple forms of the drug.”
Interferon is already approved for treating multiple sclerosis, hepatitis C, non-Hodgkin’s lymphoma, and other autoimmune diseases. Interferon is also available in subcutaneous forms, which means that getting the drug wouldn’t require traveling to an infusion center for treatment.
How to prevent long COVID COVID-19 vaccines may prevent severe illness and death, but Hu said that so far large studies suggest that vaccines do not prevent Long COVID in particular.
“What prevents Long COVID is not catching COVID,” said Hu. Experts suggest using multiple layers of protection to reduce the chances of catching COVID-19. “Two things that have consistently worked is good air ventilation, and masking,” Hu said. “They’ve consistently shown to be effective in preventing infection.”
High-quality surgical masks and respirators effectively reduce the transmission of airborne diseases like COVID-19 and can also protect your lungs and brain during wildfire season. Ensuring proper air ventilation by opening windows, using HEPA filters, and improving airflow with fans makes it harder for infectious particles or pollutants to linger in the air.
Link to study: https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(24)00253-2
#covid#mask up#pandemic#wear a mask#covid 19#coronavirus#sars cov 2#public health#wear a respirator#still coviding#long covid
8 notes
·
View notes
Text

BBMCT: Begin Medical Research at AIIMS Hospital

## BBMCT: Your Trusted Partner for Advanced Clinical Research at AIIMS Hospital
British Biomedicine Clinical Trials (BBMCT) is a renowned name in the field of clinical research, offering unparalleled expertise in advancing medical therapies. Through our partnership with **AIIMS Hospital** — one of India’s most prestigious healthcare institutions — we are able to deliver advanced clinical research services across a wide array of therapeutic areas. This collaboration is focused on ensuring the highest standards of scientific integrity, patient safety, and regulatory compliance, all while accelerating the development of innovative medical solutions.
In this blog post, we explore how BBMCT serves as your trusted partner for clinical trials at AIIMS, highlighting the institution’s strengths, advanced facilities, and our commitment to delivering reliable and scientifically robust outcomes.
### 1. **BBMCT: Begin Medical Research at AIIMS Hospital**
AIIMS Hospital has long been a global leader in medical education, research, and patient care. Its world-class infrastructure and commitment to scientific excellence make it an ideal setting for conducting cutting-edge clinical trials. Through our partnership with AIIMS, BBMCT ensures that each clinical study is conducted under the highest standards of care, bringing advanced therapies closer to market.
AIIMS offers a comprehensive research environment, housing various specialized departments and providing access to a vast network of clinical specialists, researchers, and medical professionals. This allows for thorough examination and testing of new treatments, ensuring that every phase of the clinical trial is executed seamlessly.
### 2. **Distinguished Institution for Clinical Research**
AIIMS Hospital is internationally recognized for its significant contributions to medical research, particularly in the fields of oncology, cardiology, neurology, and infectious diseases. As a government-funded institution, AIIMS not only provides state-of-the-art medical facilities but also offers a platform for translational research that bridges the gap between laboratory discoveries and clinical applications.
BBMCT leverages this environment of academic excellence and research rigor to conduct advanced clinical trials. The hospital’s collaboration with leading global pharmaceutical companies and researchers further solidifies its position as a trusted institution for clinical research. By partnering with AIIMS, BBMCT enhances its ability to conduct trials with unmatched scientific precision, leading to the discovery of groundbreaking treatments.
### 3. **Access to Diverse Patient Groups**
One of the key advantages of conducting clinical trials at AIIMS Hospital is its diverse and extensive patient base. AIIMS serves a wide demographic, providing access to patients from various socio-economic backgrounds, geographical regions, and ethnicities. This diversity is crucial for clinical trials, as it ensures the robustness of data and the generalizability of trial results.
BBMCT’s partnership with AIIMS enables researchers to recruit patients who meet the specific requirements for each clinical trial while maintaining a focus on patient safety and care. This access to a diverse patient group not only accelerates recruitment but also ensures that the outcomes of the trials reflect the needs of a broad population, enhancing the relevance and impact of the research.
### 4. **Cutting-Edge Research Facilities Available**
AIIMS Hospital is equipped with some of the most advanced medical and research facilities in the world. From state-of-the-art diagnostic tools to modernized laboratory equipment, AIIMS offers an unparalleled infrastructure for conducting clinical trials. The institution’s focus on integrating new technologies and maintaining world-class facilities ensures that every clinical trial conducted within its walls adheres to the highest standards of scientific excellence.
BBMCT takes full advantage of AIIMS’ advanced capabilities, utilizing cutting-edge technology for accurate data collection, patient monitoring, and clinical analysis. Whether it’s conducting Phase I trials for new drugs or large-scale Phase III trials, AIIMS’ research facilities support the entire spectrum of clinical research, providing an ideal environment for rigorous study.
### 5. **Experienced Researchers Ensure Reliable Outcomes**
At the heart of every successful clinical trial are the researchers who guide the study from initiation to completion. BBMCT works with a team of highly qualified and experienced researchers, clinicians, and medical experts at AIIMS. These professionals bring their wealth of knowledge to every trial, ensuring that rigorous standards are upheld throughout the study process.
The collaboration between BBMCT and AIIMS Hospital ensures that research is conducted with precision and care. Our team of experts is committed to delivering reliable, reproducible, and scientifically sound results. This expertise is a critical factor in maintaining the credibility of clinical trials and ultimately bringing safe and effective treatments to market.
### 6. **Commitment to Ethical Research Standards**
Ethics form the cornerstone of all clinical research, and at BBMCT, we place a strong emphasis on conducting trials with the highest ethical standards. Partnering with AIIMS, we adhere to strict guidelines such as the **International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH-GCP)**, ensuring patient rights, confidentiality, and safety at every stage of the trial.
Our commitment to ethical research practices extends to transparent communication with patients, ensuring informed consent and offering patients the opportunity to ask questions and understand the potential risks and benefits of participation. At BBMCT, we prioritize patient welfare alongside scientific progress, maintaining the integrity of every clinical trial.
### 7. **Strong Regulatory Compliance and Guidance**
Clinical trials must comply with stringent regulatory standards set by national and international health authorities, including the **U.S. Food and Drug Administration (FDA)**, **European Medicines Agency (EMA)**, and **India’s Central Drugs Standard Control Organization (CDSCO)**. BBMCT, in partnership with AIIMS, ensures that every trial meets these regulatory requirements, ensuring patient safety and scientific rigor.
Our team works closely with regulatory bodies throughout the trial process, from protocol design to data submission, to ensure that all compliance standards are met. This robust regulatory oversight ensures that the clinical trials we conduct are ethically sound and legally compliant, contributing to the success and credibility of our research.
### 8. **Efficient Management and Execution of Trials**
Managing and executing a clinical trial involves many intricate steps, from patient recruitment and site management to data collection and analysis. BBMCT has refined its approach to clinical trial management, ensuring efficient execution at every stage. Through our partnership with AIIMS, we leverage the hospital’s advanced infrastructure, experienced staff, and research expertise to streamline every aspect of the clinical trial process.
From protocol design and regulatory submission to patient recruitment and data analysis, BBMCT’s team ensures that trials are conducted efficiently, on time, and within budget. Our meticulous management approach minimizes delays, optimizes resources, and ensures that the research is completed successfully.
### 9. **Established Track Record of Success**
BBMCT has an established track record of successfully managing clinical trials across a variety of therapeutic areas. From early-phase studies to large-scale multi-center trials, our experience and expertise enable us to consistently deliver reliable results that meet the highest scientific and regulatory standards.
The successful completion of clinical trials at AIIMS Hospital, backed by our expert team and robust infrastructure, further enhances our reputation as a trusted partner in advancing medical research. Our track record not only demonstrates our ability to execute trials effectively but also our commitment to improving patient outcomes and bringing innovative therapies to market.
/media/7326bcfa7a349bf482791ea71163376c
— -
## FAQs About British Biomedicine Clinical Trials (BBMCT)
### 1. **What makes BBMCT a trusted partner for clinical trials?**
BBMCT’s partnership with AIIMS Hospital provides a unique combination of scientific expertise, advanced infrastructure, and access to diverse patient groups. Our commitment to patient safety, ethical research practices, and strong regulatory compliance ensures that every trial we conduct is scientifically robust and reliable. With an experienced team and a history of successful clinical trials, BBMCT is a trusted partner for pharmaceutical companies and medical device developers.
### 2. **What types of clinical trials does BBMCT conduct at AIIMS?**
BBMCT conducts clinical trials across various therapeutic areas, including oncology, cardiology, neurology, endocrinology, and infectious diseases. Our trials range from early-phase studies to large-scale Phase III trials. We offer comprehensive support from trial design and patient recruitment to data analysis, ensuring that every trial is executed with precision and meets the highest scientific and regulatory standards.
### 3. **How does BBMCT ensure patient safety during clinical trials?**
BBMCT, in partnership with AIIMS, follows strict ethical guidelines and regulatory standards to ensure patient safety throughout the trial process. Patients are fully informed about the risks and benefits of participating in the trial, and their safety is closely monitored throughout the study. Our experienced research team ensures that trials are conducted with the utmost care, adhering to **ICH-GCP** standards and other relevant safety protocols.
### 4. **What are the benefits of conducting clinical trials at AIIMS Hospital?**
AIIMS Hospital offers cutting-edge research facilities, a diverse patient population, and a team of highly experienced researchers and clinicians. This combination makes AIIMS an ideal environment for conducting reliable and scientifically sound clinical trials. The hospital’s reputation for excellence in healthcare and research further enhances the credibility and success of trials conducted under its roof.
### 5. **How can I collaborate with BBMCT for my clinical trials?**
Pharmaceutical companies, medical device developers, and research organizations interested in collaborating with BBMCT can contact us through our website or customer support. We offer comprehensive clinical trial services, including study design, regulatory guidance, patient recruitment, and data analysis. Our team will work closely with you to tailor a research strategy that meets your specific needs and objectives.
— -
### Conclusion
BBMCT’s collaboration with AIIMS Hospital offers an exceptional opportunity for conducting advanced clinical trials in a scientifically rigorous and ethically sound environment. With cutting-edge research facilities, a diverse patient base, experienced researchers, and a commitment to regulatory compliance, BBMCT ensures that each clinical trial is conducted with precision, care, and the highest standards of safety. Whether you are a pharmaceutical company, biotech firm, or healthcare organization, BBMCT provides the expertise and infrastructure needed to bring innovative medical treatments to market, helping shape the future of healthcare. Partner with BBMCT today and make a significant impact in the world of clinical research.
Subscribe to BBMCLINICALTRIALS YouTube channel for Research Insights
Be sure to subscribe to the **BBMCLINICALTRIALS YouTube channel** for exclusive access to the latest updates and in-depth insights into British Biomedicine Clinical Trials (BBMCT). Stay informed on cutting-edge research, clinical trial advancements, patient safety protocols, and breakthrough therapies being tested at AIIMS Hospital. Our channel provides expert discussions, industry trends, and detailed videos on the clinical trial process across various therapeutic areas. Whether you’re a healthcare professional, researcher, or simply interested in biomedical innovation, subscribing will keep you at the forefront of clinical research developments. Don’t miss out — join our community today!
#anya mouthwashing#artists on tumblr#batman#captain curly#bucktommy#cats of tumblr#agatha harkness#911 abc#agatha all along#dan and phil
2 notes
·
View notes
Text
Top Futuristic AI Based Applications by 2024
2024 with Artificial Intelligence (AI) is the backdrop of what seems to be another revolutionary iteration across industries. AI has matured over the past year to provide novel use cases and innovative solutions in several industries. This article explores most exciting AI applications that are driving the future.
1. Customized Chatbots
The next year, 2024 is seeing the upward trajectory of bespoke chatbots. Google, and OpenAI are creating accessible user-friendly platforms that enable people to build their own small-scale chatbots for particular use cases. These are the most advanced Chatbots available in the market — Capable of not just processing text but also Images and Videos, giving a plethora of interactive applications. For example, estate agents can now automatically create property descriptions by adding the text and images of listings thatsurgent.
2. AI in Healthcare

AI has found numerous applications in the healthcare industry, from diagnostics to personalized treatment plans. After all, AI-driven devices can analyze medical imaging material more accurately than humans and thus among other things help to detect diseases such as cancer at an early stage. They will also describe how AI algorithms are used to create tailored treatment strategies personalized for each patient's genetics and clinical past, which helps enable more precise treatments.
3. Edge AI
A major trend in 2024 is Edge AI It enables computer processing to be done at the edge of a network, rather than in large data centers. Because of its reduced latency and added data privacy, Edge AI can be used in applications like autonomous vehicles transportations, smart cities as well as industrial automation. Example, edge AI in autonomous vehicles is able to get and process real-time data, increasing security by allowing faster decision-making.
4. AI in Finance

Today, the financial sector is using AI to make better decisions and provide an even stronger customer experience. Fraud detection, risk assessment and customised financial advice have introduced insurance into the AI algorithm. AI-powered chatbots and virtual assistants are now common enough to be in use by 2024, greatly assisting customers stay on top of their financial well-being. Those tools will review your spending behavior, write feedback to you and even help with some investment advices.
5. AI in Education
AI is revolutionizing education with individualized learning. These AI-powered adaptive learning platforms use data analytics to understand how students fare and produces a customised educational content (Hoos, 2017). This way, students get a tailored experience and realize better outcomes. Not only that, AI enabled tools are also in use for automating administrative tasks which shortens the time required by educators on teaching.
6. AI in Job Hunting

This is also reverberating in the job sector, where AI technology has been trending. With tools like Canyon AI Resume Builder, you can spin the best resumé that might catch something eye catchy recruiter among a dozen others applications he receives in-between his zoom meeting. Using AI based tools to analyze Job Descriptions and match it with the required skills, experience in different job roles help accelerating the chances of a right fit JOB.
7. Artificial Intelligence in Memory & Storage Solutions
Leading AI solutions provider Innodisk presents its own line of memory and storage with added in-house designed AI at the recent Future of Memory & Storage (FMS) 2024 event. Very typically these are solutions to make AI applications easier, faster and better by improving performance scalability as well on the quality. This has huge implications on sectors with substantial data processing and storage demands (healthcare, finance, self-driving cars).
Conclusion
2024 — Even at the edge of possible, AI is revolutionizing across many industries. AI is changing our lives from tailored chatbots and edge AI to healthcare, finance solutions or education and job search. This will not only improve your business profile as a freelancer who create SEO optimized content and write copies but also give your clients in the writing for business niche some very useful tips.
#ai#ai in healthcare#ai in finance#ai in wealth management#ai in business#AI trends#artificial intelligence#advanced technologies#innovation#technological advancements
2 notes
·
View notes
Text
Clinical Development Solutions
In the rapidly evolving field of healthcare, clinical development plays a crucial role in bringing novel treatments and therapies to patients worldwide. Clinical Development Solutions (CDS) is at the forefront of this exciting journey, pioneering innovative approaches to accelerate the development and approval of life-saving drugs and medical devices. With a dedicated team of experts and cutting-edge technologies, CDS is committed to transforming the landscape of clinical research and improving patient outcomes.
At CDS, we understand the challenges and complexities of clinical development. Our comprehensive suite of solutions is designed to address these challenges head-on, providing tailored strategies and support throughout the entire drug development lifecycle. From early-phase clinical trials to post-marketing studies, we offer a wide range of services that enable pharmaceutical and biotech companies to navigate the regulatory landscape efficiently and effectively.
One of the key strengths of CDS lies in our expertise in clinical trial design and optimization. We work closely with our clients to design robust and scientifically rigorous trials that generate high-quality data while minimizing risks. By leveraging our extensive knowledge and experience, we can identify the most appropriate patient populations, endpoints, and study designs to maximize the chances of success. Our statistical and data management teams ensure that the collected data is accurate, reliable, and compliant with regulatory requirements.
In addition to trial design, CDS also excels in patient recruitment and retention strategies. We understand the importance of enrolling a diverse and representative patient population to ensure the generalizability of study results. Through our innovative patient-centric approaches, such as digital recruitment platforms and targeted engagement campaigns, we connect with potential study participants and enhance their overall trial experience. By fostering strong relationships with patients and investigators, we improve retention rates and reduce dropout rates, ultimately leading to faster and more reliable study results.
CDS is at the forefront of adopting emerging technologies to drive efficiency and innovation in clinical development. We harness the power of big data analytics, artificial intelligence, and machine learning to uncover valuable insights from complex datasets. These advanced analytics enable us to identify trends, predict outcomes, and optimize trial protocols, thus accelerating the development timeline and reducing costs. Our investment in digital health technologies and wearable devices further enhances data collection and remote monitoring capabilities, enabling more flexible and patient-friendly trial designs.
In the realm of regulatory affairs, CDS provides comprehensive support to ensure compliance with global regulations and standards. Our regulatory experts have in-depth knowledge of regional requirements, including those of the FDA, EMA, and other regulatory authorities worldwide. From preparing regulatory submissions to managing post-marketing safety surveillance, we guide our clients through every step of the regulatory process, ensuring timely approvals and post-approval compliance.
CDS is also committed to fostering collaboration and knowledge sharing within the clinical research community. We organize scientific symposia, webinars, and training programs to facilitate the exchange of ideas and best practices. By promoting interdisciplinary collaboration and staying up to date with the latest industry advancements, we continuously enhance our capabilities and stay at the forefront of clinical development.
In conclusion, Clinical Development Solutions is a leading provider of innovative solutions in clinical development. Through our expertise, technology-driven approaches, and commitment to patient-centricity, we strive to transform the drug development landscape and improve patient outcomes. By partnering with CDS, pharmaceutical and biotech companies can navigate the complexities of clinical research with confidence, bringing new therapies to patients faster and more efficiently. Together, let us shape the future of healthcare through innovation and collaboration.
#clinical development#development solutions#biometric solution providers#clinical development consultant#clinical development service#drug development solutions#clinical product development#clinical development solution company#clinical development specialist#Clinical Development Services in Hyderabad#Clinical Services in Hyderabad#clinical development services agency in hyderabad india#best clinical development agency in india#project management solutions provider#project management service provider#biometric service provider near me#Clinical Trial Services In Hyderabad#specialized clinical pharmacist#clinical pharmaceutical company
2 notes
·
View notes
Text
Chicago Biomedical Consortium names UIC grad among incoming fellows

The Chicago Biomedical Consortium (CBC) has announced its 2023 class of the CBC Entrepreneurial Fellows, which includes a graduate from the University of Illinois Chicago’s College of Pharmacy.
The Entrepreneurial Fellows program provides mentorship to bio-entrepreneurial minded junior researchers who will gain real-world experience by helping Chicago’s university researchers, including those at UIC, develop academic science into biomedical applications.
The fellows were recruited through a nationwide search of graduating PhD scientists. Incoming fellow Ahmed Disouky earned his PhD from UIC, where he studied the extent of hippocampal neurogenesis in Alzheimer’s disease patients and its impact on their learning and memory. He will join three other fellows as part of this second cohort in the program.
The CBC’s mission is to stimulate collaboration among scientists at UIC, Northwestern University, The University of Chicago, and other area institutions to accelerate discovery and expand the life sciences ecosystem here.
The Entrepreneurial Fellows receive a full-time, paid, two-year position that offers them professional and career development, as well as a curriculum in early-stage drug development and the business of biotech. They will work with a network of industry mentors including venture capitalists, biotech executives, Chicago-area entrepreneurs, member institution tech transfer offices, and senior advisors.
This network, combined with guidance from CBC staff, helps the fellows evaluate technologies sourced from commercially promising research projects from the three CBC member universities. The best of these projects will receive up to $250,000 in funding through the CBC Accelerator Award to advance the science.
“UIC is committed to helping our scientists translate their ideas into medicines through our Proof of Concept Awards. The CBC has been a wonderful collaborator by partnering their Environmental Fellows with our faculty to provide guidance, strategic feedback and follow-on funding after the POC awards,” said Joanna Groden, vice chancellor for research at UIC.
The other three fellows are Owen Shelton, a neuroscientist who has studied how the nervous system generates movement; Sonal Rangnekar, a nanomaterials researcher; and Rachel Wallace, an immunoengineer whose expertise lies at the intersection of immunology, materials science and nanotechnology.
3 notes
·
View notes
Text
Blockchain in Biotech: Redefining Trust in Research
The biotech industry, critical for innovation, faces challenges such as data breaches, irreproducible clinical trials, and intellectual property disputes. Blockchain technology offers a groundbreaking solution through immutability, decentralization, and transparency, reshaping trust in biotech R&D.
Challenges in Biotech R&D
Data Integrity: Clinical studies are frequently scrutinized because of worries regarding data manipulation or falsification. According to one study, up to 15% of clinical trial data may be corrupted, resulting in mistrust and slowed approvals. Intellectual Property Risks: With increased competition, protecting ground-breaking innovations is more difficult than ever. Unauthorized leaks and disputes over ownership rights are prevalent. Decentralized Collaboration: Cross-border R&D necessitates secure and efficient data sharing, yet current methods fall short of protecting privacy and regulatory compliance.
How Blockchain Solves Biotech Challenges
1. Data Security and Transparency
Blockchain’s decentralized ledger records every transaction or data entry immutably. For clinical trials, this means:
Every data point, from patient recruitment to results, is time-stamped and verifiable.
Researchers can trace the origin of every dataset, ensuring accountability.
2. Streamlining clinical trials
Clinical trials involve multiple stakeholders—pharma companies, regulators, patients, and researchers. Blockchain-enabled smart contracts automate processes such as:
Ensuring payments upon milestone completions.
Monitoring compliance in real-time.
3. Protecting Intellectual Property (IP)
Blockchain allows timestamped proof of IP ownership. By recording innovations on the blockchain, researchers can:
Safeguard their discoveries against unauthorized usage.
Establish clear ownership, reducing disputes.
4. Decentralized Data Sharing
Blockchain enables secure and anonymous sharing of large datasets, vital for genomic research and patient confidentiality.
Real-World Success Stories
1.MediLedger Network
Focus: blockchain for pharmaceutical supply chains.
MediLedger ensures end-to-end transparency, preventing counterfeiting and ensuring compliance with regulations like the Drug Supply Chain Security Act (DSCSA).
Result: Improved drug traceability and patient safety.
2. Insilico Medicine
Focus: AI-driven drug discovery integrated with blockchain.
Blockchain secures sensitive algorithms and datasets, enabling transparent collaborations between AI models.
Result: Accelerated drug discovery timelines and more secure data-sharing protocols.
3. Stanford’s Blockchain Health Initiative
Focus: blockchain for clinical data sharing and patient consent.
The system records patient consent on a blockchain ledger, ensuring compliance with ethical standards.
Result: enhanced patient trust and improved trial participation rates.
Future of Blockchain in Biotech
Blockchain is still in its infancy in biotech, but its potential is undeniable. Here’s what the future might hold:
Decentralized R&D Platforms Blockchain can foster open innovation networks where researchers worldwide collaborate seamlessly. Tokenization of research contributions could incentivize participation.
Integration with Emerging Technologies Combining blockchain with AI and IoT could lead to precision medicine breakthroughs. Imagine a smart wearable device that collects patient data, encrypts it using blockchain, and uses AI to offer real-time insights.
Regulatory Evolution Governments and regulatory bodies, like the FDA, are beginning to pilot blockchain systems. These efforts may lead to industry-wide adoption within the next decade.
Challenges to Overcome
While blockchain offers immense promise, challenges remain:
Scalability: Biotech generates vast amounts of data, and blockchain systems must evolve to handle this efficiently.
High Costs: Initial setup and integration costs can be prohibitive for smaller organizations.
Regulatory Hurdles: Ensuring compliance with global laws like GDPR and HIPAA is crucial for blockchain adoption.
Despite these hurdles, opportunities abound. Collaborations between blockchain startups and biotech firms, along with consortia like PharmaLedger, are paving the way for solutions.
Conclusion: A Revolution in Trust
Blockchain is revolutionizing the biotech industry, addressing critical challenges in data security, transparency, and collaboration. By ensuring trust in clinical trials, protecting patient data, and streamlining R&D, blockchain paves the way for a transformative future in biotechnology.
As adoption grows, we’ll see biotech companies harness blockchain to innovate faster, reduce costs, and earn public trust. The question for industry leaders is not whether to adopt blockchain but how quickly they can embrace this transformative technology.
The future of biotech begins today—with blockchain at its core.
#Tech4bizsolutions #BlockchainInBiotech #ClinicalTrialsInnovation #BiotechDataSecurity #DecentralizedResearch #PharmaBlockchain #R&DInnovation #FutureOfBiotech #DataIntegrity
0 notes
Text
Driving Clinical Development with Haleus: Excellence in Clinical Trial Management, Operations, and Monitoring

In the ever-evolving world of clinical research, ensuring the seamless progression from discovery to market approval is paramount. Haleus excels in this realm by expertly aligning with both scientific and regulatory standards, making it a trusted partner in clinical development.
Clinical Trial ManagementEffective clinical trial management is the backbone of successful drug development. Haleus employs robust strategies to design, plan, and oversee clinical trials, ensuring they are conducted with precision and compliance. By integrating innovative tools and streamlined processes, Haleus minimizes risks, optimizes timelines, and enhances data integrity.
Clinical Trial OperationsOperational excellence is key to executing clinical trials efficiently. Haleus’s dedicated teams oversee every operational aspect, from site selection and patient recruitment to protocol adherence and resource allocation. With a focus on collaboration and adaptability, Haleus ensures that each phase of the trial progresses smoothly, meeting regulatory and sponsor requirements.
Clinical Trial MonitoringMonitoring is critical to maintaining trial quality and participant safety. Haleus employs advanced monitoring techniques, including risk-based approaches and real-time data analysis, to ensure compliance with protocols and standards. Their experienced monitors work closely with sites to identify and address issues promptly, safeguarding the trial's integrity and outcomes.
By harmonizing scientific rigor with regulatory demands, Haleus empowers sponsors to navigate the complexities of clinical trials. Their expertise in clinical trial management, operations, and monitoring ensures efficient progression, ultimately accelerating the delivery of innovative therapies to patients.
#Clinical Trial Monitoring#Clinical Trial Operations#Clinical Trial Management#clinical research#clinical monitoring#haleus#clinical trial
0 notes
Text
Accelerate Your Career: Exploring the Benefits of a 2-Year Online Nursing Degree
Accelerate Your Career: Exploring the Benefits of a 2-Year Online Nursing Degree
In today’s rapidly evolving healthcare landscape, pursuing a nursing degree is not just a career path but a gateway to endless possibilities. For aspiring nurses looking to jumpstart their careers, a 2-year online nursing degree offers a unique blend of flexibility, comprehensive education, and career growth. This article delves into the numerous benefits of obtaining an online nursing degree, practical tips for success, and real-life experiences from those who have taken the plunge!
Why Choose a 2-Year Online Nursing Degree?
Online nursing programs have gained immense popularity for several reasons:
Flexibility: Balance your studies with work and life commitments.
Cost-Effective: Save on commuting and housing costs.
Time Efficient: Complete your degree in a fraction of the time of traditional programs.
Quality Education: Accredited online programs maintain high standards to prepare competent nurses.
Benefits of a 2-Year Online Nursing Degree
1. Enhanced Career Opportunities
With a nursing shortage prevalent across many regions, obtaining a nursing degree opens doors to various opportunities:
Career Opportunities
Average Salary
Registered Nurse (RN)
$75,000
Nursing Educator
$78,000
Clinical Nurse Specialist
$90,000
Nurse Manager
$85,000
2. Develop Critical Skills
A 2-year nursing degree not only provides theoretical knowledge but also hones critical nursing skills such as:
Clinical Skills: Hands-on training prepares you for real-world patient care.
Communication: Critical for patient interaction and collaboration with healthcare teams.
Critical Thinking: Evaluate situations and make informed decisions quickly.
3. Networking Opportunities
Online nursing programs often include virtual networking sessions, allowing you to connect with:
Fellow nursing students.
Experienced faculty members.
Healthcare professionals and recruiters.
4. Improved Job Stability
The healthcare industry is one of the most stable employment sectors, with registered nurses in high demand. According to the Bureau of Labor Statistics, the employment of RNs is projected to grow 9% from 2020 to 2030. A 2-year online nursing degree boosts your job security and opens up various career paths.
Practical Tips for Success in Online Nursing Programs
Stay Organized: Utilize digital planners or apps to keep track of deadlines.
Create a Study Schedule: Consistency leads to better retention of information.
Engage Actively: Participate in discussions and collaborate with peers.
Seek Help When Needed: Utilize academic support services offered by your institution.
Case Studies: Success Stories
Case Study 1: Sarah’s Journey
Sarah, a mother of two, found traditional nursing programs challenging due to her family commitments. Enrolling in a 2-year online nursing degree allowed her to manage her responsibilities while gaining the skills necessary to become a registered nurse. Today, she works in a busy hospital and shares her love for nursing with her children, inspiring them to pursue healthcare careers.
Case Study 2: John’s Transition
After working in healthcare administration for several years, John realized his passion for patient care. He enrolled in an online nursing program to transition into nursing. Within two years, he earned his degree, gained hands-on experience through clinicals, and secured a position as a clinical nurse where he thrives in providing quality healthcare services.
First-Hand Experiences from Nursing Students
Here’s what some students have said about their experiences:
“I loved that I could study at my own pace, and the support from instructors was amazing!” – *Amanda, RN*
“My online program gave me the freedom to keep my job while learning. It was a game-changer.” – *Marcus, RN*
Conclusion
A 2-year online nursing degree is an invaluable asset for those ready to take their healthcare career to the next level. With flexibility, quality education, and numerous opportunities for career advancement, aspiring nurses should consider this path. Whether you’re starting fresh or transitioning from another profession, the benefits of an online nursing degree can help you accelerate your career and make a meaningful impact in healthcare. Embrace the journey and become part of a rewarding profession that makes a difference each day!
youtube
https://nursingcertificationcourses.com/accelerate-your-career-exploring-the-benefits-of-a-2-year-online-nursing-degree/
0 notes
Text
Why Improving Diversity, Equity, and Inclusion in Clinical Trials should be a Research Priority? | Jeeva Trials
Disparities related to diversity, equity, and inclusion (DEI) are common and well-known in clinical trials. It is well-documented that racial minorities, underprivileged, and non-white ethnic groups are much less represented in clinical trials. Historically, the numbers of clinical trial participants from diverse populations have not reflected real-world populations. Minorities often underrepresented in clinical trials include women, members of the LGBTQ+ community, indigenous populations, older adults, Native Americans, pediatric patients, and people living in hard-to-reach geographies.
In the United States, socio-economic and geographic divides persistently limit patient diversity in clinical trials. As a result, we have only partial understanding of how safe and effective therapies are when they launch. Without diverse communities, researchers run the risk of making assumptions about drug safety and effectiveness that may not be accurate. There is a need to increase participation and retention among diverse patients who may otherwise not be invited to participate in clinical trials for new drug development. Not only would these measures provide pivotal data for a variety of backgrounds, but it would also provide these study participants with first access to new precision therapies at no cost, a privilege of the few.
Why are inclusive clinical trials important?
Addressing the challenges of diversity, equity and inclusion in clinical trials is important because there are many occurrences when drugs behaved differently from one population to another. Failing to understand these differences at the clinical trial stage, in which patients are monitored most closely, could result in suboptimal drug efficacy and potentially avoidable safety issues due to overexposure and underexposure to the drugs in many future patients. Having representative patient populations in clinical trials helps ensure the safety and effectiveness of therapies for everyone.

How to increase diversity, equity and inclusion in clinical trials
Do not select a site merely because of familiarity or convenience, especially if these sites have no demonstrable reach in that community beyond their location. Clinical Trial sites should include locations with a higher concentration of racial and ethnic minority patients. Factor in relevant disease prevalence data in those areas when designing protocols or planning recruitment initiatives.
Do not treat Black and Brown communities as monolithic groups that have the same life experiences. Do not set people of color into shallow narratives and stereotypes, such as Black people can only be reached through the church. Similarly, defaulting to do business with majority-owned (read: White) firms simply because they are familiar, and you feel comfortable to communicate and connect with them is not the right practice.
It is important to carefully examine exclusion and inclusion criteria to ensure they are necessary to achieve study objectives and that they do not pose an unnecessary barrier for would-be enrollees. When possible, reducing the frequency of study visits, collaborative strategies, expanded access, flexibility in visit windows, and electronic communication tools should be employed to make trials more inclusive. Clinical trial participation should be made less burdensome for the volunteers and caregivers.
Legal frameworks and recent initiatives to improve diversity and inclusion in clinical trials
Improvements in DEI initiatives have of late come from recognition by drug developers, lawmakers, sponsors, patient advocates and regulatory authorities of the importance of DEI in clinical trials, and how sociocultural variables reverberate in clinical research. In reality, the void in diversity, equity and inclusion in clinical trials and research is an old problem as it only represents a disproportional disease burden. What is unprecedented is the widespread attention that diversity, equity and inclusion has gotten in clinical trials recently. The fierce urgency to develop effective coronavirus solutions means that these inequities in clinical trials are finally getting the attention long needed.
Indeed, there is a need to address the issues of diversity, equity, and inclusion in clinical trials if innovators are to fulfill their promise of precision medicines for each individual. Information flow, data sharing, and reducing the logistical burden to participate are high-priority areas to improve access for underrepresented populations. This is also true in research laboratories where the greater the diversity of the participating patient population, the higher the chances that certain breakthroughs from clinical trials may be achieved.
Overcoming barriers and achieving DEI in clinical trials with technology
Systematic change in how we approach the issue of diversity, equity and inclusion in clinical trials is needed for the real clinical trial diversity to transpire. The Jeeva eClinical Cloud (Jeeva) is a modular Software as a Service (SaaS) subscription model that is designed to help a clinical study’s annual budget on a simple per participant basis, while ensuring that the study participants are truly represented to include diversity, equity, and inclusion. The platform has many features such as eConsent, pre-screening, automated enrollment workflow, adverse event reporting and more to maximize diversity, equity, and inclusion for the participants, such as women and minorities that are less likely to participate in clinical trials due to logistical burdens and special needs such as childcare, transportation and loss of pay.

Walking the talk of patient-centricity
Jeeva considers patients as critical partners, not merely subjects of study, and walks the talk of patient-centricity. The cloud platform incorporates patient voices early during clinical trial protocol development and logistical planning. Jeeva believes that humanizing the workflows leaves room for humanizing the patient experience, and creates an atmosphere of trust, especially among the communities of color and ethnic minorities that have traditionally been underrepresented in studies. Jeeva is developed by researchers with empathy who listen to help clinical researchers, hospitals, academia, CROs and biopharmaceutical sponsors to address the issues of diversity, equity and inclusion in clinical trials, and accelerate patient recruitment by three times faster.
Minimizing regulatory risk and maximizing compliance
Jeeva’s bring your own device (BYOD) platform makes it easy for study investigators to onboard, retain and engage participants with an appropriate focus on diversity, equity, and inclusion. Jeeva’s experienced coordinators are trained to manage trial operations to minimize burden, reduce dropouts, and improve compliance meeting regulatory requirements at various levels, such as Good Clinical Practice (GCP) guideline by the international code of harmonization (ICH), human subjects’ protection guidelines, data protection guidelines such as GDPR, and institutional review boards (IRBs) that help in accelerating the development of therapies.
The platform is designed to enhance geographic and demographic diversity and reduces 70% burden on study teams and participants to collect data that are representative of the population. Jeeva supports multi-site studies with centralized monitoring dashboard, and centralized study management & monitoring.
As researchers seek to accelerate regulatory approvals, Jeeva eClinical SaaS can help achieve this goal while also increasing diversity, equity, and inclusion in clinical trials by enabling wider access to participants irrespective of their zipcode.
#eclinical cloud#clinical trial cloud software#eclinical platform#mobile eclinical#clinical trial recruitment challenges#decentralized clinical trials software#software for clinical trials#clinical research software#clinical study software#software used in clinical research#clinical trials software#software used in clinical trials#clinical solutions research platforms#accelerating patient recruitment#patient retention in clinical trials#Remote Consent#clinical trial protocol software#clinical trials recruitment and retention#clinical trial software platform
1 note
·
View note
Text
https://kitsa.ai/team/
#ai in clinical trials#ai in pharma#clinical trials recruitment ai#accelerating clinical trials#clinical trial companies#how ai is transforming clinical trials#using ai to match patients with clinical trials
0 notes
Link
#datasecurity#EMR/EHRsystems#HealthInformatics#Healthcare#healthcareapplications#HIPAAcompliance#interoperability#MedicalSoftware#mobilehealth#patientportals#softwaredevelopment#telehealth
0 notes
Text
Work from Home CRA Job vacancies at Syneos Health Syneos Health is hiring Clinical Research Associates for remote positions in Hyderabad/Bangalore. Minimum 03 year of CRA experience required. Apply now for a chance to work with a leading biopharmaceutical solutions organization. Job Title: Clinical Research Associate I (CRA I) Job ID: 24005945 Location: India-Asia Pacific - IND-Home-Based Description: Syneos Health® is a leading fully integrated biopharmaceutical solutions organization focused on accelerating customer success. We are currently seeking Clinical Research Associates for remote positions in Hyderabad/Bangalore. Job responsibilities Performs site qualification, site initiation, interim monitoring, site management activities and close-out visits (performed on-site or remotely) ensuring regulatory, ICH-GCP and/or Good Pharmacoepidemiology Practice (GPP) and protocol compliance. Uses judgment and experience to evaluate overall performance of site and site staff and to provide recommendations regarding site-specific actions; immediately communicates/escalates serious issues to the project team and develops action plans. Maintains a working knowledge of ICH/GCP Guidelines or other applicable guidance, relevant regulations, and company SOPs/processes. Verifies the process of obtaining informed consent has been adequately performed and documented for each subject/patient, as required/appropriate. Demonstrates diligence in protecting the confidentiality of each subject/patient. Assesses factors that might affect subject/patient’s safety and clinical data integrity at an investigator/physician site such as protocol deviation/violations and pharmacovigilance issues. Per the Clinical Monitoring/Site Management Plan (CMP/SMP): Assesses site processes Conducts Source Document Review of appropriate site source documents and medical records Verifies required clinical data entered in the case report form (CRF) is accurate and complete Applies query resolution techniques remotely and on site, and provides guidance to site staff as necessary, driving query resolution to closure within agreed timelines Utilizes available hardware and software to support the effective conduct of the clinical study data review and capture Verifies site compliance with electronic data capture requirements May perform investigational product (IP) inventory, reconciliation and reviews storage and security. Verifies the IP has been dispensed and administered to subjects/patients according to the protocol. Verifies issues or risks associated with blinded or randomized information related to IP. Applies knowledge of GCP/local regulations and organizational procedures to ensure IP is appropriately (re)labelled, imported and released/returned. Routinely reviews the Investigator Site File (ISF) for accuracy, timeliness and completeness. Reconciles contents of the ISF with the Trial Master File (TMF). Ensures the investigator/physician site is aware of the requirement of archiving essential documents in accordance with local guidelines and regulations. Documents activities via confirmation letters, follow-up letters, trip reports, communication logs, and other required project documents as per SOPs and Clinical Monitoring Plan/Site Management Plan. Supports subject/patient recruitment, retention and awareness strategies. Enters data into tracking systems as required to track all observations, ongoing status and assigned action items to resolution. For assigned activities, understands project scope, budgets, and timelines; manages site-level activities / communication to ensure project objectives, deliverables and timelines are met. Must be able to quickly adapt to changing priorities to achieve goals / targets. May act as primary liaison with study site personnel, or in collaboration with Central Monitoring Associate. Ensures all assigned sites and project-specific site team members are trained and compliant with applicable requirements.
Prepares for and attends Investigator Meetings and/or sponsor face to face meetings. Participates in global clinical monitoring/project staff meetings (inclusive of Sponsor representation, as applicable) and attends clinical training sessions according to the project specific requirements. Provides guidance at the site and project level towards audit readiness standards and supports preparation for audit and required follow-up actions. Maintains a working knowledge of ICH/GCP Guidelines or other applicable guidance, relevant regulations, and company SOPs/processes; completes assigned training as required. For Real World Late Phase, the CRA II will use the business card title of Site Management Associate II. Additional responsibilities include: Site support throughout the study lifecycle from site identification through close-out Knowledge of local requirements for real world late phase study designs Chart abstraction activities and data collection Collaboration with Sponsor affiliates, medical science liaisons and local country staff The SMA II may be requested to train junior staff Identify and communicate out of scope activities to Lead CRA/Project Manager Proactively suggest potential sites based on local knowledge of treatment patterns, patient advocacy and Health Care Provider (HCP) associations Qualifications What we’re looking for Minimum 3yrs of experience in onsite monitoring and remote monitoring. Should have Neurology, immunology, rare diseases, CVD and Oncology therapeutic area experience preferred. Candidate from Mumbai, Delhi, Bengaluru, Hyderabad, Ahmedabad preferred. Bachelor’s degree or RN in a related field or equivalent combination of education, training and experience Knowledge of Good Clinical Practice/ICH Guidelines and other applicable regulatory requirements Must demonstrate good computer skills and be able to embrace new technologies Excellent communication, presentation and interpersonal skills Ability to manage required travel of up to 75% on a regular basis [caption id="attachment_66251" align="aligncenter" width="1200"] Work from Home Clinical Research Associate Opportunities at Syneos Health[/caption] Get to Know Syneos Health: Over the past 5 years, Syneos Health has worked with 94% of all Novel FDA Approved Drugs and 95% of EMA Authorized Products across 73,000 sites and 675,000+ trial patients. Join us in a highly competitive and ever-changing environment. Additional Information: Tasks, duties, and responsibilities as listed in this job description are not exhaustive. The company may assign other tasks as needed. Equivalent experience, skills, and education will also be considered. The company is committed to complying with applicable legislation and providing reasonable accommodations as needed. Apply now for a rewarding career opportunity with Syneos Health in the clinical research field! Apply online
0 notes
Text
BBMCT: Begin Medical Research at AIIMS Hospital

## BBMCT: Your Trusted Partner for Advanced Clinical Research at AIIMS Hospital
British Biomedicine Clinical Trials (BBMCT) is a renowned name in the field of clinical research, offering unparalleled expertise in advancing medical therapies. Through our partnership with **AIIMS Hospital** — one of India’s most prestigious healthcare institutions — we are able to deliver advanced clinical research services across a wide array of therapeutic areas. This collaboration is focused on ensuring the highest standards of scientific integrity, patient safety, and regulatory compliance, all while accelerating the development of innovative medical solutions.
In this blog post, we explore how BBMCT serves as your trusted partner for clinical trials at AIIMS, highlighting the institution’s strengths, advanced facilities, and our commitment to delivering reliable and scientifically robust outcomes.
### 1. **BBMCT: Begin Medical Research at AIIMS Hospital**
AIIMS Hospital has long been a global leader in medical education, research, and patient care. Its world-class infrastructure and commitment to scientific excellence make it an ideal setting for conducting cutting-edge clinical trials. Through our partnership with AIIMS, BBMCT ensures that each clinical study is conducted under the highest standards of care, bringing advanced therapies closer to market.
AIIMS offers a comprehensive research environment, housing various specialized departments and providing access to a vast network of clinical specialists, researchers, and medical professionals. This allows for thorough examination and testing of new treatments, ensuring that every phase of the clinical trial is executed seamlessly.
### 2. **Distinguished Institution for Clinical Research**
AIIMS Hospital is internationally recognized for its significant contributions to medical research, particularly in the fields of oncology, cardiology, neurology, and infectious diseases. As a government-funded institution, AIIMS not only provides state-of-the-art medical facilities but also offers a platform for translational research that bridges the gap between laboratory discoveries and clinical applications.
BBMCT leverages this environment of academic excellence and research rigor to conduct advanced clinical trials. The hospital’s collaboration with leading global pharmaceutical companies and researchers further solidifies its position as a trusted institution for clinical research. By partnering with AIIMS, BBMCT enhances its ability to conduct trials with unmatched scientific precision, leading to the discovery of groundbreaking treatments.
### 3. **Access to Diverse Patient Groups**
One of the key advantages of conducting clinical trials at AIIMS Hospital is its diverse and extensive patient base. AIIMS serves a wide demographic, providing access to patients from various socio-economic backgrounds, geographical regions, and ethnicities. This diversity is crucial for clinical trials, as it ensures the robustness of data and the generalizability of trial results.
BBMCT’s partnership with AIIMS enables researchers to recruit patients who meet the specific requirements for each clinical trial while maintaining a focus on patient safety and care. This access to a diverse patient group not only accelerates recruitment but also ensures that the outcomes of the trials reflect the needs of a broad population, enhancing the relevance and impact of the research.
### 4. **Cutting-Edge Research Facilities Available**
AIIMS Hospital is equipped with some of the most advanced medical and research facilities in the world. From state-of-the-art diagnostic tools to modernized laboratory equipment, AIIMS offers an unparalleled infrastructure for conducting clinical trials. The institution’s focus on integrating new technologies and maintaining world-class facilities ensures that every clinical trial conducted within its walls adheres to the highest standards of scientific excellence.
BBMCT takes full advantage of AIIMS’ advanced capabilities, utilizing cutting-edge technology for accurate data collection, patient monitoring, and clinical analysis. Whether it’s conducting Phase I trials for new drugs or large-scale Phase III trials, AIIMS’ research facilities support the entire spectrum of clinical research, providing an ideal environment for rigorous study.
### 5. **Experienced Researchers Ensure Reliable Outcomes**
At the heart of every successful clinical trial are the researchers who guide the study from initiation to completion. BBMCT works with a team of highly qualified and experienced researchers, clinicians, and medical experts at AIIMS. These professionals bring their wealth of knowledge to every trial, ensuring that rigorous standards are upheld throughout the study process.
The collaboration between BBMCT and AIIMS Hospital ensures that research is conducted with precision and care. Our team of experts is committed to delivering reliable, reproducible, and scientifically sound results. This expertise is a critical factor in maintaining the credibility of clinical trials and ultimately bringing safe and effective treatments to market.
### 6. **Commitment to Ethical Research Standards**
Ethics form the cornerstone of all clinical research, and at BBMCT, we place a strong emphasis on conducting trials with the highest ethical standards. Partnering with AIIMS, we adhere to strict guidelines such as the **International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH-GCP)**, ensuring patient rights, confidentiality, and safety at every stage of the trial.
Our commitment to ethical research practices extends to transparent communication with patients, ensuring informed consent and offering patients the opportunity to ask questions and understand the potential risks and benefits of participation. At BBMCT, we prioritize patient welfare alongside scientific progress, maintaining the integrity of every clinical trial.
### 7. **Strong Regulatory Compliance and Guidance**
Clinical trials must comply with stringent regulatory standards set by national and international health authorities, including the **U.S. Food and Drug Administration (FDA)**, **European Medicines Agency (EMA)**, and **India’s Central Drugs Standard Control Organization (CDSCO)**. BBMCT, in partnership with AIIMS, ensures that every trial meets these regulatory requirements, ensuring patient safety and scientific rigor.
Our team works closely with regulatory bodies throughout the trial process, from protocol design to data submission, to ensure that all compliance standards are met. This robust regulatory oversight ensures that the clinical trials we conduct are ethically sound and legally compliant, contributing to the success and credibility of our research.
### 8. **Efficient Management and Execution of Trials**
Managing and executing a clinical trial involves many intricate steps, from patient recruitment and site management to data collection and analysis. BBMCT has refined its approach to clinical trial management, ensuring efficient execution at every stage. Through our partnership with AIIMS, we leverage the hospital’s advanced infrastructure, experienced staff, and research expertise to streamline every aspect of the clinical trial process.
From protocol design and regulatory submission to patient recruitment and data analysis, BBMCT’s team ensures that trials are conducted efficiently, on time, and within budget. Our meticulous management approach minimizes delays, optimizes resources, and ensures that the research is completed successfully.
### 9. **Established Track Record of Success**
BBMCT has an established track record of successfully managing clinical trials across a variety of therapeutic areas. From early-phase studies to large-scale multi-center trials, our experience and expertise enable us to consistently deliver reliable results that meet the highest scientific and regulatory standards.
The successful completion of clinical trials at AIIMS Hospital, backed by our expert team and robust infrastructure, further enhances our reputation as a trusted partner in advancing medical research. Our track record not only demonstrates our ability to execute trials effectively but also our commitment to improving patient outcomes and bringing innovative therapies to market.
/media/7326bcfa7a349bf482791ea71163376c
— -
## FAQs About British Biomedicine Clinical Trials (BBMCT)
### 1. **What makes BBMCT a trusted partner for clinical trials?**
BBMCT’s partnership with AIIMS Hospital provides a unique combination of scientific expertise, advanced infrastructure, and access to diverse patient groups. Our commitment to patient safety, ethical research practices, and strong regulatory compliance ensures that every trial we conduct is scientifically robust and reliable. With an experienced team and a history of successful clinical trials, BBMCT is a trusted partner for pharmaceutical companies and medical device developers.
### 2. **What types of clinical trials does BBMCT conduct at AIIMS?**
BBMCT conducts clinical trials across various therapeutic areas, including oncology, cardiology, neurology, endocrinology, and infectious diseases. Our trials range from early-phase studies to large-scale Phase III trials. We offer comprehensive support from trial design and patient recruitment to data analysis, ensuring that every trial is executed with precision and meets the highest scientific and regulatory standards.
### 3. **How does BBMCT ensure patient safety during clinical trials?**
BBMCT, in partnership with AIIMS, follows strict ethical guidelines and regulatory standards to ensure patient safety throughout the trial process. Patients are fully informed about the risks and benefits of participating in the trial, and their safety is closely monitored throughout the study. Our experienced research team ensures that trials are conducted with the utmost care, adhering to **ICH-GCP** standards and other relevant safety protocols.
### 4. **What are the benefits of conducting clinical trials at AIIMS Hospital?**
AIIMS Hospital offers cutting-edge research facilities, a diverse patient population, and a team of highly experienced researchers and clinicians. This combination makes AIIMS an ideal environment for conducting reliable and scientifically sound clinical trials. The hospital’s reputation for excellence in healthcare and research further enhances the credibility and success of trials conducted under its roof.
### 5. **How can I collaborate with BBMCT for my clinical trials?**
Pharmaceutical companies, medical device developers, and research organizations interested in collaborating with BBMCT can contact us through our website or customer support. We offer comprehensive clinical trial services, including study design, regulatory guidance, patient recruitment, and data analysis. Our team will work closely with you to tailor a research strategy that meets your specific needs and objectives.
— -
### Conclusion
BBMCT’s collaboration with AIIMS Hospital offers an exceptional opportunity for conducting advanced clinical trials in a scientifically rigorous and ethically sound environment. With cutting-edge research facilities, a diverse patient base, experienced researchers, and a commitment to regulatory compliance, BBMCT ensures that each clinical trial is conducted with precision, care, and the highest standards of safety. Whether you are a pharmaceutical company, biotech firm, or healthcare organization, BBMCT provides the expertise and infrastructure needed to bring innovative medical treatments to market, helping shape the future of healthcare. Partner with BBMCT today and make a significant impact in the world of clinical research.
Subscribe to BBMCLINICALTRIALS YouTube channel for Research Insights
Be sure to subscribe to the **BBMCLINICALTRIALS YouTube channel** for exclusive access to the latest updates and in-depth insights into British Biomedicine Clinical Trials (BBMCT). Stay informed on cutting-edge research, clinical trial advancements, patient safety protocols, and breakthrough therapies being tested at AIIMS Hospital. Our channel provides expert discussions, industry trends, and detailed videos on the clinical trial process across various therapeutic areas. Whether you’re a healthcare professional, researcher, or simply interested in biomedical innovation, subscribing will keep you at the forefront of clinical research developments. Don’t miss out — join our community today!
#anya mouthwashing#artists on tumblr#batman#captain curly#agatha harkness#cats of tumblr#bucktommy#agatha all along#911 abc#dan and phil
1 note
·
View note