#Physics Science
Explore tagged Tumblr posts
Text




45K notes
·
View notes
Text
By weaving popsicle sticks together in a specific pattern, there is a build up potential energy (stored energy) in the bent and twisted sticks. When released from one end, this stored potential energy is converted into kinetic energy (energy of motion) as the sticks rapidly unfurl and fly through the air in a chain reaction.
34K notes
·
View notes
Text
Okay so a guy in my solid state physics class was telling us about this muon scanning startup he worked at, GScan, and I'm going insane. I don't work there and I have no stake in the company, financial or otherwise, I just need to tell you about it.
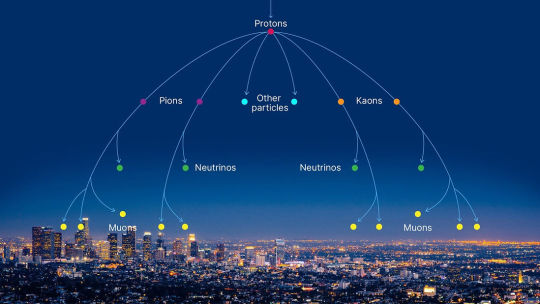
Muons are short-lived subatomic particles, same charge as an electron but ~200 times more massive. On Earth, they're produced by cosmic rays colliding with the upper atmosphere, and they hit the ground at a rate of about ten thousand per minute per square meter.
They're moving extremely fast at ground level, like 0.99 c. So they careen right through matter, deflecting only very slightly around heavy atomic nuclei – they'll penetrate like a hundred meters into solid rock.
What do you do with this continuous shower of deep-penetrating charged particles, constantly blanketing every square inch of the Earth's surface?
(source)
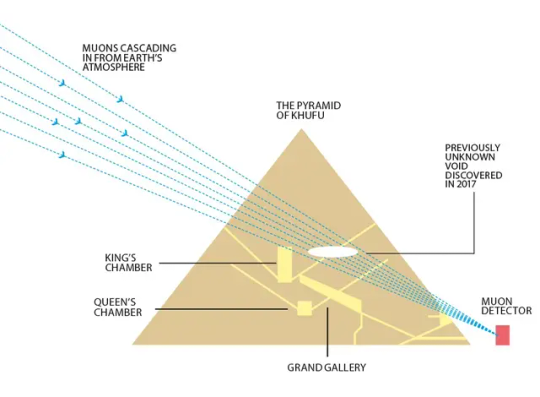
The classic thing is use them to image the inside of massive structures, like we use x-rays to look inside living tissue – except instead of generating them yourself, you just use atmospheric muons. Muon archeology is a whole thing, they've used it to find hidden chambers in pyramids and stuff. Neat!
But this one Estonian company is doing some crazy bullshit and I love it.


Sandwich anything between a pair of portable muon detectors and get full 3D imaging of the interior, with sub-millimeter accuracy, by tracking the minute deflection of muons between them. Samples that are WAY too thick for x-rays, made of literally anything. Just put some muon detectors on some two by fours in a warehouse and call it a day.
You can just. Image anything??? Anything you want?? Completely passively!! Just detectors! No particle source! Put them anywhere. The detectors themselves are a mature technology, the company's tech is in the algorithms they use to get this level of spatial and elemental resolution.

You can detect failures inside cable-reinforced concrete bridges without cutting open the bridges.


Decommissioned Soviet nuclear submarine filled with concrete, with no drawings or documentation, that may or may not have spent fuel canisters in it? And you need to cut it up for storage? Just look at the muons.
One of the wackiest ideas is to put one detector under your bed and one on the ceiling, so you get a full 3D scan of your body every night, passively. I want one.
7K notes
·
View notes
Text
IF NOT FOR EAT WHY CAKE?

IF NOT FOR MOUTH WHY HONEYCOMB?

IF NOT FOOD WHY LOOK LIKE FORBIDDEN SNACK?

#science#chemistry#physics#nuclear#uranium#nuclear physics#stem#stemblr#science side of tumblr#forbidden snack
9K notes
·
View notes
Text
look, I know I've talked about this essay (?) before but like,
If you ever needed a good demonstration of the quote "Any sufficiently advanced technology is indistinguishable from magic", have I got an exercise for you.
Somebody made a small article explaining the basics of atomic theory but it's written in Anglish. Anglish is basically a made-up version of English where they remove any elements (words, prefixes, etc) that were originally borrowed from romance languages like french and latin, as well as greek and other foreign loanwords, keeping only those of germanic origin.
What happens is an english which is for the most part intelligible, but since a lot everyday english, and especially the scientific vocabulary, has has heavy latin and greek influence, they have to make up new words from the existing germanic-english vocabulary. For me it kind of reads super viking-ey.
Anyway when you read this article on atomic theory, in Anglish called Uncleftish Beholding, you get this text which kind of reads like a fantasy novel. Like in my mind it feels like it recontextualizes advanced scientific concepts to explain it to a viking audience from ancient times.
Even though you're familiar with the scientific ideas, because it bypasses the normal language we use for these concepts, you get a chance to examine these ideas as if you were a visitor from another civilization - and guess what, it does feel like it's about magic. It has a mythical quality to it, like it feels like a book about magic written during viking times. For me this has the same vibe as reading deep magic lore from a Robert Jordan book.
#off topic#literature#language#linguistics#science#science history#science fiction#fantasy#physics#atomic theory#anglish#chemistry#robert jordan#the wheel of time#uncleftish beholding
44K notes
·
View notes
Text
The US military has done thousands of unconscionable things, each one more evil than the last, but on top of all of those things, on a minor but more spiteful level, I'll also never forgive them for what they did to Lithium
3K notes
·
View notes
Text
The long wavelengths of the light spectrum—red, yellow, and orange—can penetrate to approximately 15, 30, and 50 meters (49, 98, and 164 feet), respectively, while the short wavelengths of the light spectrum—violet, blue and green—can penetrate further, to the lower limits of the euphotic zone. Blue penetrates the deepest, which is why deep, clear ocean water and some tropical water appear to be blue most of the time. Moreover, clearer waters have fewer particles to affect the transmission of light, and scattering by the water itself controls color. Water in shallow coastal areas tends to contain a greater amount of particles that scatter or absorb light wavelengths differently, which is why sea water close to shore may appear more green or brown in color.
Follow @scienceisdope for more science and daily facts.
Video credit: Kendall Roberg
48K notes
·
View notes
Text

My latest cartoon for New Scientist
9K notes
·
View notes
Text
The whole “scientists use big words on purpose to be exclusive” is such a bunch of anti-intellectual bullshit. Specific and concise language exists for a reason; you need the right words to convey the right meaning, and explaining stuff right is a hugely important part of science. Cultures that live around loads of snow have loads of words to describe different types of snow; cultures that live in deserts have loads of words to describe different types of sand. Complex language is needed for complex meaning.
25K notes
·
View notes
Text
Hey, so--we cooled your boyfriend down to a hundredth of a kelvin above absolute zero. Yeah, it was so cold that all of the chemical reactions in his body ceased. Sorry. We, uh, yeah, we used him as a dielectric material in a tiny qubit. And then we quantum-entangled him with another qubit, just to see if we could. Sorry. Yeah, anyway, we thawed him out after two weeks and apparently he's doing fine now. Didn't really teach us anything about how quantum processes work in biological systems, but it sure was, uh, cool. If you'll pardon the pun.
9K notes
·
View notes
Text
I did a post asking about this ages ago, but now that there's polls, I'd like to do another round for this question, since it always has me curious:
Also, I am not including stuff like "I just stay up thinking about things that make me anxious", since this is specifically about things that help you sleep, not things that keep you up!
Just curious how widespread this practice is!
#poll#hopefully this goes over much better than the last one lol#I really wish someone would do a science with this#it seems like the necessity of fiction to our physical health could be a neat thing to study#sleep#imagination#storytelling
3K notes
·
View notes
Text

does anyone see my vision
#you got biology chemistry and physics all in one#please ignore how the lighting is everywhere#THE SCIENCE BROTHERS 🔥🔥🔥#house md#house md fanart#house md art#dr house#brba#brba fanart#brba art#breaking bad fanart#breaking bad art#breaking bad#walter white#half life#half life fanart#half life art#hl#hl fanart#hl art#half life 2#half life 2 fanart#half life 2 art#hl2#hl2 fanart#hl2 art#gordon freeman
1K notes
·
View notes
Text
6K notes
·
View notes
Text
I love when engineers write papers and get to make fun of physicists a bit. Don't get me wrong, I love physicists, some of my closest friends are physicists, but some of y'all need to log off and touch some actual materials before you get too far into spherical cow territory.
1K notes
·
View notes
Text
"Dew Point" Deposits Droplets

Artist Lily Clark loves to work in water. One of her recent sculptures, "Dew Point," uses superhydrophobic ceramic to grow and manipulate water droplets over and over and over. (Video credit: L. Turczan; artwork by: L. Clark; via Colossal) Read the full article
2K notes
·
View notes
Text
I need people to understand that Uranium is an eldritch horror
I'm not talking about radiation, or nuclear weapons, or anything that you can do with uranium, I mean its mere existence on Earth is a reminder of cosmic horrors on a scale you can barely conceive of.
When a nuclear power plant uses Uranium to boil water and spin steam turbines to keep the lights on, they're unleashing the fossilized energy of the destroyed heart of an undead star.
Allow me to elaborate:
In the beginning, there were hydrogen and helium. The primordial fires of the Big Bang produced almost exclusively the two lightest elements, along with a minuscule trace of lithium. It was a start, but that's not much to build a universe out of. Fortunately, the universe is full of element factories. We call them "stars".
Stars are powered by nuclear fusion, smooshing light elements together to make heavier elements, and releasing tremendous amounts of energy in the process, powering the star and making it shine. This goes on for millions to billions of years depending on the stars mass (although not how you might think, the bigger stars die young), the vast majority of that time spent fusing hydrogen into yet more helium. Eventually, the hydrogen in the core starts to run low, and if the star is massive enough it starts to fuse helium into carbon, then oxygen, neon, and so on up through successively heavier elements.
There's a limit to this though:

This chart shows how much energy is released if you were to create a given element/isotope out of the raw protons and neutrons that make it up, the Nuclear Binding Energy. Like in everyday life, rolling downhill on this chart releases energy. So, starting from hydrogen on the far left you can rapidly drop down to helium-4 releasing a ton of energy, and then from there to carbon-12 releasing a fair bit more.
But, at the bottom of this curve is iron-56, the most stable isotope. This is the most efficient way to pack protons and neutrons together, and forming it releases some energy. But once its formed, that's it. You're done. Its already the most stable, you can't get any more energy out of it, and in fact if you want to do anything to it and make it into a different element you're going to have to put energy in.
So, when a massive star's core starts to fill up with iron, the star is doomed. Iron is like ash from the nuclear fire that powers stars, its what's leftover when all the fuel is used up. When this happens, the core of the star isn't producing energy and can't support itself anymore and catastrophically collapses, triggering a supernova explosion which heralds the death of the star.
What kind of stellar-corpse gets left behind depends again on how massive the star is. If its really big, more than ~30 times the mass of the sun and its probably going to form a black hole and whatever was in there is gone for good. But if the star is a bit less massive, between 8-25 solar masses, it leaves behind a marginally less-destroyed corpse.
The immense weight of the outer layers of the star falling down on the core compresses the electrons of the atoms into their nuclei, resulting in them reacting with protons and turning them all into neutrons, which creates a big ball of almost pure neutrons a couple miles across, but containing the entire mass of the star's core, 3-5 sun's worth.
This is the undead heart of the former star: a neutron star.
If, like many stars, this one wasn't alone but had a sibling, it can end up with two neuron stars orbiting each other, like a pair of zombies acting out their former lives. If they get close enough together, their intense gravity warps the fabric of spacetime as they orbit, radiating away their orbital energy as gravitational waves, slowing them down and bringing them closer together until they eventually collide.
The resulting kilonova explosion destroys both of the neutron stars, most likely rendering the majority of what's left into a black hole, but not before throwing out a massive cloud of neutron-rich shrapnel. This elder-god blood-splatter from the collision of the undead hearts of former stars contains massive nuclei with hundreds to thousands of neutrons, the vast majority of which are heinously unstable and decay away in milliseconds or less. Most of their decay products are also unstable and decay quickly as well, eventually falling apart into small enough clusters to be stable and drift off into the universe becoming part of the cosmic dust between the stars.
However,
Some of the resulting massive elements are merely almost stable. They would like to decay, but for quantum-physics reasons decaying is hard and slow for them, so they stick around much longer than you might expect. Uranium is one such element, with U-238 having a half-life of around 4.5 billion years, about the same as the age of the Earth, and its spicier cousin U-235 which still has a respectable 200 million year half life.
These almost-stable isotopes were only able to be created in the fiery excess of energy in a neutron star collision, and are the only ones that stick around long enough to carry a fraction of that energy to the era where hairless apes could figure out that a particular black rock made of them was emitting some kind of invisible energy.
So as I said at the beginning, Uranium is significant because it stores the fossilized energy of the destroyed heart of an undead star, and we can release that energy at will if we set it up just right.
When you say it like that, is it any shock that the energy in question will melt your face off and rot your bones from the inside if you stay near it too long?
#nuclear physics#nucleosynthesis#stellar nucleosynthesis#neutron star#uranium#radiation#supernova#kilonova#cosmic horror#physics#science#space#astrophysics#stars#stellar evolution
1K notes
·
View notes