#Omicron BA.5 Subvariant
Explore tagged Tumblr posts
Link
1 note
·
View note
Text

A detail view of a face mask on September 24, 2021, in Kohler, Wisconsin. Donald Trump and conservatives across social media are heightening awareness to potential mask mandates due to new cases stemming from coronavirus variants. Richard Heathcote/Getty Images
United States: Mask Mandate Comeback Sparks 'We Will Not Comply' Movement
— By Nick Mordowanec | August 31, 2023
will not comply' movement is slowly formulating across social media, spurred by Donald Trump's renewed focus on mask mandates and COVID-affiliated lockdowns that he initiated at the pandemic's inception.
Trump, in a video posted Wednesday on X, formerly Twitter, vowed to reject any "fearmongering" of new coronavirus variants and if elected president pledged to cut federal funding for entities like schools and airlines that follow such protocols.
Trump was the individual who set the original mandates and lockdowns in motion, however, when coronavirus cases escalated exponentially starting in March 2020. At the time, he urged individuals to avoid bars, restaurants and other areas where 10 or more people were gathered in the hope that the virus would dissipate by that summer.
"'Do not comply' means your [sic] not going to go to work if your employer requires a mask as part of the 'mandate' not law; your [sic] not going to wear one at the Dr, Dentist, restaurant or stores," wrote one Facebook user. "Imagine if everyone did not comply how that would hurt our government or economy.
"If every American did not go to work or buy anything at all for one or two days things would get real. We are all slaves to our Government until we stop conforming to the demands and dollar."
New coronavirus variants now emerging with case spikes in certain parts of the United States include EG.5 and BA.2.86. Major companies like Pfizer and Moderna who were highly involved in the swift rollout of vaccines at the height of the pandemic are scheduled to release a new vaccine in mid-September to combat the omicron subvariant XBB.1.5, pending approval from the Centers for Disease Control and Prevention (CDC) and Food and Drug Administration (FDA).
A CDC spokesperson told Newsweek on Thursday via email that the center's advice for individual and community actions around COVID-19 is tied to hospital admission levels, which are currently low for more than 97 percent of the country.
"CDC continues to recommend that all people are up to date on their COVID-19 vaccines and take steps to themselves and others," the spokesperson said. "Anyone may choose to wear a mask at any time."
Time may tell whether the discussion around mandates and lockdowns is alarmist considering that very few places in the country have COVID-related measures currently in place.
One, for example, is Morris Brown College, a small Atlanta-based historically Black college, which told students to adhere to mask-wearing for a two-week period due to an influx of COVID-related cases.
"Dear Atlanta College, Regarding your precautionary mask mandate... I have a precautionary Foot I'd like to shove up you're a**!" wrote comedian and former Saturday Night Live actor Rob Schneider on X, in response to the Morris Brown mandate. "But don't worry, it's just for the next 14 days! For your own protection! Ps. Students WAKE UP, SHEEPLE! SAY NO!"
Former Alaska Governor Sarah Palin released a video on X of her literally shaking her head when confronted with hypothetical mandates, even burning some masks outdoors.
Libs of Tik Tok, which has 2.4 million followers on X, is encouraging individuals to ignore all mandates and pledges to support impacted businesses—and even pay any fines for noncompliance.
One X user posted that she would ignore mandates instituted by Trump, President Joe Biden or anyone else.
"I won't mask again," the user wrote. "I don't care what Trump or Fauci or Birx or Biden or any other governmental agency try and push again. I won't deal with the anxiety mask wearing brings me again. Not going to cover my daughter's beautiful face or force her to deal with the frequent painful breakouts again. Nope. For my child, I say, never again."
#United States 🇺🇸#Mask Mandate#'We Will Not Comply' Movement#Nick Mordowanec | Newsweek#Donald Trump#COVID-19#Fearmongering#New Coronavirus Variants#EG.5 and BA.2.86#Omicron Subvariant XBB.1.5#Centers for Disease Control and Prevention (CDC)#Food and Drug Administration (FDA)#Morris Brown College | Atlanta Georgia | Blacks’ College#Rob Schneider#Former | Alaska Governor | Sarah Palin#Tik Tok#Donald J. Trump | President Joe Biden#Trump | Fauci | Birx | Biden
0 notes
Text
Updated vaccines against Covid-19 are coming, just as hospitalizations and deaths due to the virus are steadily ticking up again.
Today, the US Food and Drug Administration authorized new mRNA booster shots from Moderna and Pfizer, and a panel of outside experts that advises the Centers for Disease Control and Prevention voted to recommend the shots to everyone in the United States ages 6 months and older. Once Centers for Disease Control and Prevention director Mandy Cohen signs off on the recommendations and the vaccines are shipped, people can start getting the boosters.
The recommendation is projected to prevent about 400,000 hospitalizations and 40,000 deaths over the next two years, according to data presented at the meeting by CDC epidemiologist Megan Wallace.
This year’s mRNA vaccines are different from the 2022 booster in a key way. Last year’s shot was a bivalent vaccine, meaning it covered two variants: the original one that emerged in China in 2019, plus the Omicron subvariant BA.5, which was circulating during much of 2022. This fall’s booster drops the original variant, which is no longer circulating and is unlikely to return. It targets just the Omicron subvariant XBB.1.5, which was dominant throughout much of 2023.
Pfizer and Moderna’s vaccines work by introducing a tiny piece of genetic material called messenger RNA, or mRNA, that carries instructions for making SARS-CoV-2’s characteristic spike protein. Once it is injected, cells in the body use those instructions to temporarily make the spike protein. The immune system recognizes the protein as foreign and generates antibodies against it. Those antibodies stick around so that if they encounter that foreign invader again, they will mount a response against it.
Since the start of the Covid-19 pandemic, the virus has acquired new mutations in its spike protein and elsewhere. These mutations result in new variants and subvariants that diverge from the original virus. When enough mutations accumulate, these new versions can more easily evade the antibodies created by previous vaccine doses or infections.
The constantly evolving nature of the virus is the reason health regulators decided last year to update the original mRNA vaccines, which were designed against the version of the virus that first appeared in 2019. This year, once again, the virus has changed enough to warrant an updated booster.
In June, an advisory committee to the FDA recommended that this fall’s booster be a monovalent vaccine—targeting only the then-dominant XBB.1.5 subvariant.
At that meeting, committee members reviewed evidence suggesting that the inclusion of the original variant may hamper the booster’s effectiveness against newer offshoots. “The previous bivalent vaccine contained the ancestral spike and thus skewed immune responses to the old spike,” says David Ho, a professor of microbiology at Columbia University whose research, which is not yet peer-reviewed, was among the evidence the FDA panel reviewed. “This is what we call immunological imprinting, and it results in lack of immune responses to the new spike.” He thinks taking out the old variant should optimize the immune response.
But over the past few months, even newer Omicron offshoots have arrived. Currently, EG.5.1, or Eris, is the dominant one in the United States, United Kingdom, and China. Meanwhile, a variant called BA.2.86, or Pirola, has been detected in several countries. Pirola has raised alarm bells because it has more than 30 new mutations compared to XBB.1.5.
Even though the new boosters were formulated against XBB.1.5, they’re still expected to provide protection against these new variants. “The reason is, while antibodies are important in protection against mild disease, the critical part of the immune response that’s important for protecting against severe disease is T cells,” says Paul Offit, a professor of vaccinology at the University of Pennsylvania and member of the FDA’s vaccine advisory committee.
These cells are a different part of the immune response. Unlike antibodies, which neutralize a pathogen by preventing it from infecting cells, T cells work by eliminating the cells that have already been invaded and boosting creation of more antibodies. Both the Moderna and Pfizer-BioNTech Covid vaccines produce long-lasting T cells in addition to antibodies.
It’s why, Offit says, when the Omicron wave hit in late 2021 and peaked in January 2022, the US didn’t see a dramatic increase in hospitalizations and deaths even as cases rose significantly: People’s T cells kicked into gear, even when their antibodies didn’t recognize the Omicron variant.
“In some ways,” says Offit, when it comes to vaccine booster development, “it almost doesn’t matter what we pick to target” because the coronavirus has yet to evolve away from T cell recognition. “Everything works.”
Scientists think T cells are able to protect against severe Covid because they’re recognizing parts of the virus that have remained unchanged throughout the pandemic. “I suspect that as we continue to vaccinate, there are some conserved regions [of the virus],” says Jacqueline Miller, Moderna’s head of infectious diseases. “So even with the accumulation of mutations, we’re still building on previous immunity.”
People who have hybrid immunity—that is, have had a Covid infection and have also been vaccinated—seem to have the best immune responses to new variants, she says, which suggests that previous exposure shapes and improves immune responses to new variants. Preliminary studies show that antibodies generated by previous infections and vaccinations should be capable of neutralizing Pirola.
Earlier this month, Moderna issued a press release saying that clinical trial data showed that its updated booster generated a strong immune response against Pirola, as well as the more prevalent Eris variant.
In a statement to WIRED, Pfizer spokesperson Jerica Pitts said the company continues to closely monitor emerging variants and conduct tests of its updated monovalent booster against them. Data presented at Tuesday’s CDC meeting showed that Pfizer-BioNTech’s updated booster elicited a strong neutralizing antibody response against both Eris and Pirola.
The FDA expects that Covid-19 vaccines will continue to be updated on an annual basis, unless a completely new variant emerges that requires a different approach. “We will always be a little behind the virus,” says Ho. “In this instance, we won’t suffer too much, but that might not be the case going forward. Surveillance is imperative.”
813 notes
·
View notes
Text
Reference archived on our website
Another study showing the lack of lasting covid immunity from mRNA vaccination. 2 main takeaways: if you get mRNA, keep boosted, and if you can get novavax or another protein-based vaccine, get it. Studies on protein covid vaccines show much higher lasting immune effects even if their antibody titres aren't quite as high as the mRNA peaks (which very rapidly drop as shown in this study among others).
Highlights • These are the first phase four randomised trial data of the immunogenicity, reactogenicity and safety of second booster (fourth doses) of mRNA and protein subunit COVID-19 vaccines in adults previously primed with two doses of AZD1222. • BNT162b2, mRNA-1273 and NVX-CoV2372 were well tolerated and boosted humoral immune responses until Day 84. • Higher binding and neutralising antibodies against Ancestral SARS-CoV-2 were observed following boosting with mRNA vaccines (BNT162b2 and mRNA-1273) compared to NVX-CoV2372 at all time points. • Lower neutralising antibody responses were observed against Omicron subvariants BA.5 and XBB.1.5 following all vaccines until Day 84 highlighting the need for boosting with vaccines with greater specificity for Omicron subvariants.
Abstract Objectives PICOBOO is a randomised, adaptive trial evaluating the immunogenicity, reactogenicity, and safety of COVID-19 booster strategies. We report data for second boosters among individuals 50-<70 years old primed with AZD1222 (50-<70y-AZD1222) until Day 84.
Methods Contributed equally as first authors.Immunocompetent adults who received any first booster >three months prior were eligible. Participants were randomly allocated to BNT162b2, mRNA-1273 or NVX-CoV2373 1:1:1. The concentrations of ancestral anti-spike immunoglobulin was summarised as the geometric mean concentrations (GMC). Reactogenicity and safety outcomes were captured. Additional analyses including neutralising antibodies were performed on a subset. ACTRN12622000238774.
Results Between Mar 2022-Aug 2023, 743 participants were recruited and had D28 samples; 155 belonged to the 50-<70y-AZD1222 stratum. The mean adjusted GMCs (95% credible intervals) were 20,690 (17,555-23,883), 23,867 (20,144-27,604) and 8,654 (7,267-9,962) U/mL at D28 following boosting with BNT162b2, mRNA-1273 and NVX-CoV2372, respectively, and 10,976 (8,826-13,196), 15,779 (12,512-19,070) and 6,559 (5 220-7 937) U/mL by D84. IgG against Omicron BA.5 was 2.7–2.9 times lower than the ancestral strain. Limited neutralisation against Omicron subvariants was found following all vaccines. Severe reactogenicity events were <4%.
Conclusions All vaccines were immunogenic with more rapid waning after mRNA vaccines. These data support boosting with vaccines with greater specificity for circulating Omicron subvariants.
#covid vax#covid vaccines#covid vaccine#get vaccinated#mask up#covid#pandemic#covid 19#wear a mask#public health#sars cov 2#coronavirus#still coviding#wear a respirator#covid pandemic#covid conscious#covid is airborne#covid isn't over#covid19#covidー19
41 notes
·
View notes
Text
FAQ on COVID-19 subvariant XBB.1.5

- By Sameer Elsayed , Western University , The Conversation -
Despite intensive public health efforts to grind the COVID-19 pandemic to a halt, the recent emergence of the highly transmissible, extensively drug-resistant and profoundly immune system-evading XBB.1.5 SARS-CoV-2 subvariant is putting the global community on edge.
What is XBB.1.5?
In the naming convention for SARS-CoV-2 lineages, the prefix “X” denotes a pedigree that arose through genetic recombination between two or more subvariants.
The XBB lineage emerged following natural co-infection of a human host with two Omicron subvariants, namely BA.2.10.1 and BA.2.75. It was first identified by public health authorities in India during summer 2022. XBB.1.5 is a direct descendent, or more accurately, the “fifth grandchild” of the original XBB subvariant.
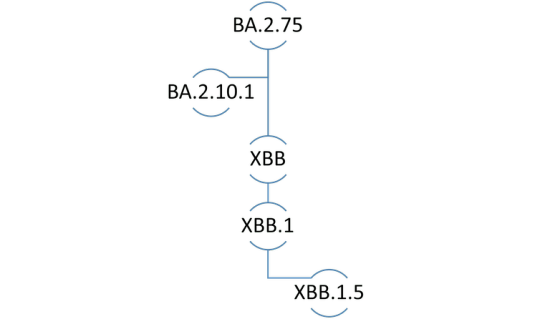
Genetic lineage of COVID-19 subvariant XBB.1.5. (Sameer Elsayed), Author provided
How does XBB.1.5 differ from Omicron?
XBB.1.5 is one of many Omicron subvariants of concern that have appeared on the global pandemic scene since the onset of the first Omicron wave in November 2021. In contrast to other descendants of the original Omicron variant (known as B.1.1.529), XBB.1.5 is a mosaic subvariant that traces its roots to two Omicron subvariant lineages.
XBB.1.5 is arguably the most genetically rich and most transmissible SARS-CoV-2 Omicron subvariant yet.
Where is XBB.1.5 prevalent?
According to the World Health Organization, XBB.1.5 is circulating in at least 38 countries, with the highest prevalence in the United States, where it accounts for approximately 43 per cent of COVID-19 cases nationwide. Within the U.S., there is wide geographic variation in the proportion of cases caused by XBB.1.5, ranging from seven per cent in the Midwest to over 70 per cent in New England.
XBB.1.5 has also been officially reported by governmental agencies in Australia, Canada, the European Union, Japan, Kuwait, Russia, Singapore, South Africa and the United Kingdom. Real-time surveillance data reveals that XBB.1.5 is rapidly spreading across the globe and will likely become the next dominant subvariant.
XBB.1.5 has also been detected in municipal wastewater systems in the United States, Europe and other places.
How likely is XBB.1.5 to cause serious illness?
There is limited data about the ability of XBB.1.5 to cause serious illness. According to the World Health Organization, XBB.1.5 does not have any specific mutations that make it any more dangerous than its ancestral subvariants.
Nonetheless, XBB.1.5 is perceived as being equally capable of causing serious illness in elderly and immunocompromised persons compared to previous Omicron subvariants of concern.
Are current mRNA vaccines effective against XBB.1.5?
XBB.1.5 and XBB.1 are the Omicron subvariants with the greatest immune-evasive properties. Therefore, one of the most contentious issues surrounding XBB.1.5 relates to the degree of protection afforded by currently available mRNA vaccines, including the latest bivalent booster formulations.
Researchers from the University of Texas determined that first-generation and bivalent mRNA booster vaccines containing BA.5 result in lacklustre neutralizing antibody responses against XBB.1.5. A report (yet to be peer reviewed) from investigators at the Cleveland Clinic found that bivalent vaccines demonstrate only modest (30 per cent) effectiveness in otherwise healthy non-elderly people when the variants in the vaccine match those circulating in the community.
Furthermore, some experts believe the administration of bivalent boosters for the prevention of COVID-19 illness in otherwise healthy young individuals is not medically justified nor cost-effective.
In contrast, public health experts from Atlanta, Ga. and Stanford, Calif. reported that although the neutralizing antibody activity of bivalent booster vaccines against XBB.1.5 is 12 to 26 times less than antibody activity against the wild-type (original) SARS-CoV-2 virus, bivalent vaccines still perform better than monovalent vaccines against XBB.1.5.
However, investigators from Columbia University in New York found that neutralizing antibody levels following bivalent boosting were up to 155–fold lower against XBB.1.5 compared to levels against the wild-type virus following monovalent boosting.
This suggests that neither monovalent nor bivalent booster vaccines can be relied upon to provide adequate protection against XBB.1.5.
How can you protect yourself against XBB.1.5?
The rapid evolution of SARS-CoV-2 continues to pose a challenge for the management of COVID-19 illness using available preventive and therapeutic agents. Of note, all currently available monoclonal antibodies targeting the spike protein of SARS-CoV-2 are deemed to be ineffective against XBB.1.5.
Antiviral medicines such as remdesivir and Paxlovid may be considered for the treatment of eligible infected patients at high risk of progressing to severe disease.
Standard infection control precautions including indoor masking, social distancing and frequent handwashing are effective measures that can be employed for personal and population protection against XBB.1.5 and other subvariants of concern.
Although bivalent boosters may be considered for elderly, immunocompromised and other risk-averse individuals, their effectiveness in preventing COVID-19 illness due to XBB.1.5 remains uncertain.
Why is XBB.1.5 nicknamed ‘Kraken’?
Some scientists have coined unofficially-recognized nicknames for XBB.1.5 and other SARS-CoV-2 subvariants of concern, arguing that they are easier to remember than generic alphanumeric designations.
The ‘Kraken’ label for XBB.1.5 is currently in vogue on social media sites and news outlets, and the nicknames ‘Gryphon’ and ‘Hippogryph’ have been used to denote the ancestral subvariants XBB and XBB.1, respectively. Kraken refers to a mythological Scandinavian sea monster or giant squid, Gryphon (or Griffin) refers to a legendary creature that is a hybrid of an eagle and a lion, while Hippogryph (or Hippogriff) is a fictitious animal hybrid of a Gryphon and a horse.
Notwithstanding their potential utility as memory aids, the use of nicknames or acronyms in formal scientific discussions should be avoided.

Sameer Elsayed, Professor of Medicine, Pathology & Laboratory Medicine, and Epidemiology & Biostatistics, Western University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
--
Read Also
Study: 30% of COVID patients develop ‘long COVID’
3 notes
·
View notes
Text
The population’s antibody response to SARS2 is hanging by a thread
October 28, 2024 Radagast Uncategorized 54
I’ve said before that immunity to SARS-COV-2 in most people who were vaccinated now depends on a small number of loops in the N-Terminal Domain of Spike. This is why I wish to briefly go over this study:
Omicron-specific ultra-potent SARS-CoV-2 neutralizing antibodies targeting the N1/N2 loop of Spike N-terminal domain A multitude of functional mutations continue to emerge on the N-terminal domain (NTD) of the spike protein in SARS-CoV-2 Omicron subvariants. Understanding the immunogenicity of Omicron NTD and the properties of antibodies elicited by it is crucial for comprehending the impact of NTD mutations on viral fitness and guiding vaccine design. In this study, we find that most of NTD-targeting antibodies isolated from individuals with BA.5/BF.7 breakthrough infection (BTI) are ancestral (wildtype or WT)-reactive and non-neutralizing. Surprisingly, we identified five ultra-potent neutralizing antibodies (NAbs) that can only bind to Omicron but not WT NTD. Structural analysis revealed that they bind to a unique epitope on the N1/N2 loop of NTD and interact with the receptor-binding domain (RBD) via the light chain. These Omicron-specific NAbs achieve neutralization through ACE2 competition and blockage of ACE2-mediated S1 shedding. However, BA.2.86 and BA.2.87.1, which carry insertions or deletions on the N1/N2 loop, can evade these antibodies. Together, we provided a detailed map of the NTD-targeting antibody repertoire in the post-Omicron era, demonstrating their vulnerability to NTD mutations enabled by its evolutionary flexibility, despite their potent neutralization. These results revealed the function of the indels in the NTD of BA.2.86/JN.1 sublineage in evading neutralizing antibodies and highlighted the importance of considering the immunogenicity of NTD in vaccine design.
You don’t have to be a genius, to understand why this is bad news. This is an antibody response that quite literally hangs by a thread: Only the light chain of these new antibodies that emerged in the Omicron era still binds the receptor binding domain, the heavy chain has to bind to the N-Terminal Domain. This is not how any of this is supposed to work, as illustrated by the fact that it only happened as a way to deal with the Omicron variants.
To explain what we’re reading, let’s go back to the very beginning. People were vaccinated, to encourage the production of neutralizing antibodies. A neutralizing antibody blocks a viral particle from attaching to the receptor of a cell in which it can replicate itself.
The part of a Spike protein that binds to the receptor is called the Receptor-Binding domain. That’s where you expect to find neutralizing antibodies to bind that serve to develop immunity against a virus. Some vaccines against SARS2 didn’t even contain the rest of the Spike protein, they only injected people with the Receptor-Binding Domain.
Over time however, this part of the virus mutates. The immune system is then forced to use the antibodies it already developed against the RBD and change them, to fit the new RBD (somatic hypermutation). These tend to be of lower quality, than if it had the opportunity to develop a whole new antibody response from scratch.
This is what we call Original Antigenic Sin, a term that is exactly as intimidating as it should be, to warn humans that you have to be really sure you know what you’re doing, when you want to intervene in a complex system like this.
In most of the human population, a second problem has by now emerged. These antibodies against the RBD are no longer part of the IgG3 class, which is able to bind very strongly. They class-shifted to IgG2 or IgG4. IgG antibodies have two identical arms (except IgG4), that can move separately and bind identical looking targets. Those two arms both contain a light chain and a heavy chain. IgG3 is unique in that it can form cross-links: It’s a very bendy molecule, so its two arms can easily find two separate Spike proteins, thereby offering much stronger neutralization.
This means the immune system of most people is now stuck with antibodies against the Receptor Binding Domain, that have poor affinity. You can think of this as a bunch of magnets floating in water, that very slowly move towards a piece of metal (the Spike protein) in the water. If it happens before the metal reaches its destination (generally the ACE2 receptor), the protein has been neutralized.
In the lab this works pretty well. You put these antibodies in your Petri dish, let them soak together with the Spike proteins. You wait for a while, then you add your cells, see if any get infected and then you say to yourself: “Well, good news, the Spike proteins are still being neutralized!” In the human lung, it doesn’t work like that of course. The Spike protein may find the ACE2 receptor before the antibody gets a chance to bind. In that case, the antibodies will look neutralizing to you in your petri dish, but in practice fail at neutralization in the body.
So the immune system can no longer rely on antibodies against the RBD, to neutralize viral particles. In a sense, you could say that it already “spent its ammunition”, on the receptor binding domain, so it’s now left with antibodies binding there that are just too slow to neutralize. And in response to that, the immune system becomes forced to come up with different antibodies, that still manage to achieve neutralization. This is the underlying problem.
So that’s what this study found. There are five loops in another part of the Spike protein, the N-Terminal Domain. Those loops are very immunogenic, that is, they look very different from our own proteins, so our body can produce antibodies against them without causing trouble for us. But just because they are immunogenic, does not necessarily mean that antibodies against these regions will be neutralizing. Antibodies could bind to this part of the Spike protein, without blocking the Spike protein from performing its job.
In this case, they found that the vast majority of the antibodies made against the N-Terminal Domain, are not neutralizing. They found one exception however. The immune system is able to make antibodies against the N1 and N2 loops of the N-Terminal Domain, that bind with one part to these loops and with the other part, to the Receptor Binding domain.
This way, the immune system can still produce neutralizing antibodies: Instead of finding an entire region of the Receptor Binding to bind to, like a normal neutralizing antibody would, these new antibodies find a small region of the Receptor Binding Domain and a small region of the start of the N-Terminal Domain.
These are strange new antibodies, an abnormal way of neutralizing a viral particle. The immune system only started developing these antibodies once it ran out of ways to neutralize through the Receptor Binding Domain. We know this, because they’re only made against the Omicron variants, there are none of these antibodies seen against the original pre-Omicron variants of the virus. If it was a normal way to neutralize the virus, we would have seen it emerge before Omicron.
The result of course, is that huge pressure is placed on the virus, to change the region where these antibodies still manage to bind. Change that region and people are again left without potent neutralizing antibodies, that is, antibodies that will actually manage to beat the virus in a real human body, instead of just in a Petri dish.
So that’s what happened. The virus responded by changing the N1 loop, with a new very different variant, called BA.2.86. This happened about a year ago. It added four new amino acids at position 16, right at the start of this N1 loop. This made the N1 loop look so different that most of these new antibodies would have become useless. All strains now circulating descend from this new variant.
The immune system responds to this, with new antibodies, that find some other part of the N1 and N2 loops, to bind to the new version of the virus. But those new versions of the antibodies, are forcing the virus to mutate yet again. And this time, things are looking very different.
Why? Because now the virus is adding glycans, sugar molecules, to its N1 and N2 loops. When glycans are added to a part of a protein, it becomes very hard if not impossible, to neutralize a virus through that part of the protein, even with new antibodies.
Here’s what happened: One version put a glycan on S:30, just next to the N1 loop, by deleting amino acid S:31. This is what made most people sick this summer.
But another version, XEC, which is now taking over the world, went with a different solution: It puts a glycan on S:22, right in the middle of the N1 loop. These glycans are called N-linked glycans, they’re very big sugar molecules. There’s clearly huge pressure to get rid of these antibodies, because almost everything now has a glycan on either S:22 or S:30.
But there are other glycans, called O-linked glycans (because they attach to an Oxygen atom in an amino acid), that are smaller. In addition to S:22, on S:59, XEC put Serine, which should allow the virus to add an O-linked glycan there. This most likely blocks antibodies from binding to the N2 loop, which runs from amino acid 67 to 79.
You can see in the past few months, that the virus is under huge pressure to add these sugar molecules in this region, as a consequence of these strange new “last ditch effort” antibodies produced by the immune system.

If it changes amino acid 72, that seems to be sufficient to interfere in the neutralizing antibodies that depend on the N2 loop. What it changes the amino acid into matters less, either R or D works.
It’s clear from these numbers, that this XEC variant is extremely eager to change the N2 loop, as we see mutations pop up at position 70, 72 and 75, all in recent weeks.
So people can’t make antibodies anymore that neutralize by binding the N1 loop and the RBD, the glycans have dealt with that. Antibodies that use the N2 loop and the RBD are now starting to suffer the same problem with the XEC variant, which is clearly very rapidly changing N2.
The question of course is: What happens after that, when all these antibodies using this exotic mechanism become unable to neutralize Spike?
It seems the immune system is running out of new ways to produce neutralizing antibodies. There might be some other mechanism for antibodies to neutralize viral particles that nobody is currently aware of. But the obvious target (RBD) has been exhausted and the new exotic mechanism that emerged with Omicron, is now being exhausted too.
So instead, people are now stuck with a wide range of low-affinity non-neutralizing antibodies. Those non-neutralizing antibodies won’t stop the viral particles from entering a new cell. Without neutralizing antibodies, the adaptive immune system can’t stop the cycle anymore, it can only clean up after the fact. These non-neutralizing antibodies also interfere in the innate immune system recognizing the virus, especially if they are IgG2 or IgG4 , but I have explained that before.
The incentives normally don’t favor the development of complete neutralizing antibody resistance in respiratory viruses. Consider the glycans a virus needs to achieve such resistance. They tend to make it easier for the innate immune system to deal with a virus. Because in most people, the innate immune system would normally be doing most of the job, adding glycans would not have a clear benefit.
But in our case, the virus is now continually under huge abnormal antibody pressure in most of the population, against a very small part of its Spike protein. It then mutates that small part of the protein, the antibody pressure then shifts to another part of the protein, so then it mutates that small part. Right now, that small part is the N2 loop of the N-Terminal Domain, the N1 loop has already been dealt with and the RBD was dealt with long ago.
So the virus has by now dealt with the N1 loop and versions of the virus that have in addition dealt with the N2 loop are already here.
The question is really: What’s left after this?
A situation in which most of humanity can’t develop a neutralizing antibody response anymore, would be new. But that seems to be where we’re now at, with the newest XEC lineages.
To summarize, what happened is roughly as following:
-Vaccination
-People develop antibodies against the RBD of Wuhan
-Delta emerges, which is faster
-Antibodies concentrations have to rise to stop Delta breakthrough infections (compensatory response #1)
-High concentrations of IgG3 antibodies lead to excessive inflammation, so class shift happens (compensatory response #2)
-Omicron emerges
-Antibodies undergo somatic hypermutation to bind the Omicron RBD (compensatory response #3)
-Omicron avoids those too.
-Unusual new antibodies develop that only bind partly to the RBD and partly to the N1 or N2 loops (compensatory response #4)
-BA.2.86 emerges and shakes most of those antibodies off.
-New antibodies develop that bind to the new version of the loops. (Compensatory response #5)
-The new version of the loops now starts adding glycans to N1, making it impossible for antibodies to bind here.
-Antibodies that use N2 have to do all the work now.
-XEC begins changing N2. (Compensatory response #6)
The question that’s now left for us, is whether there is any real way left, to develop new potently neutralizing antibodies against these new XEC lineages.
But it seems unlikely. The normal route (antibodies against the RBD) has been exhausted. This is the route the immune system normally pursues and it’s also the route that all the vaccines sought to use back in 2021. But that option has now been exhausted in most people.
So the immune system found an abnormal route, as part of a compensatory response. And that compensatory response is now nearing its end.
So what’s left after this?
You might think I’m “doom-mongering”. But the evidence is just pretty clear: The immune system was forced to develop an abnormal antibody response once Omicron emerged, because the original response could no longer achieve neutralization. That abnormal antibody response is now easily avoided by new mutations. There’s no real way to dispute this.
Human beings have an innate immune system that normally handles most of the viral load of respiratory viruses. When the innate immune system can’t handle such a virus on its own, the adaptive immune system has to join and develop antibodies that neutralize the virus. It normally picks small regions where it develops antibodies with high affinity. As time progresses, it improves those antibodies further (affinity maturation).
That puts a virus under certain molecular constraints, by disadvantaging the variants that provoke these antibodies. That’s for example why the 1918 flu never returned: The survivors had very potent antibodies, that only worked against the 1918 influenza, but no other influenza viruses.
We didn’t let this process run its natural course with SARS-COV-2. Rather, we wanted the whole population to have very high concentrations of antibodies against this virus. We used our vaccines for this. The effect becomes that antibodies can’t discriminate against virulent variants of SARS-COV-2.
But more importantly, it means the innate immune system does not get to improve itself through successive reinfections. Instead, the body depends on these antibodies to protect itself. And because this is the case for most of the population, the virus is forced to evolve to get rid of these antibodies. It has been very successful at this, forcing the immune system to develop a strange compensatory response.
But the effect is that most of humanity is now about to lose the mechanism that normally allows the immune system to put a break on viral respiratory infections that the innate immune system can not handle on its own: Neutralizing antibodies.
In hindsight, it should have been obvious something went terribly wrong, when it became clear that the mRNA vaccinated had an IgG4 response to the receptor binding domain, with no IgG3 left, whereas all(!) of the unvaccinated still had a normal IgG3 response. That’s when all the alarm bells should have gone off.
But what people did instead, back when there was still time left to come up with a solution, was to come up with excuses. People said that there’s an IgG4 response seen to measles too. That merely illustrates the problem. Measles is known to interfere in the body’s immune system, by discouraging inflammation. That’s how it causes the IgG4 class shift. In practice it doesn’t matter, because you’re never reinfected by measles.
So now you have clear evidence that the immune system became forced to develop an abnormal compensatory antibody response that binds the N1/N2 loops and the RBD together, that has already been overcome by new XEC lineages.
What’s the answer to that going to be? More excuses of course. Once you have the needle in your own arm, or worse still, your children’s arms, you no longer want to see what you did.
#covid-19 vaccine#covid-19#rintrah radagast#NTD#glycan shields#immune escape#viral escape#original antigenic sin#glycans#virology#vaccinology
1 note
·
View note
Text
Is COVID-19 Really Over? What's Going On?
Written by: Amanda Diallo
Date: May 23, 2024
Is COVID-19 really over? Has it gone away? The answer is no. But the pandemic, yes but not the virus. Sorry ladies and gentlemen, but it's here to stay. It's not only here to stay but it's still evolving (more variants).
What is Covid-19 and what are its symptoms? Well, it is the disease caused by the SARS-CoV-2 coronavirus. It usually spreads between people in close contact. Anyone can get sick with Covid-19 and become seriously ill or die, but most people will recover without treatment. Symptoms also includes loss of taste or smell, sore throat congestion or runny nose, nausea or vomiting, and diarrhea.
Researchers in China initially named it 2019-nCoV. On February 11, 2020, it was renamed SARS-CoV-2, and the disease was named Covid-19.
From the beginning of the pandemic (early 2020) we had the first variant (the Alpha), then the Beta, Gamma, Delta, and then the Omicron (2022) which was named the worst yet at that time by doctors and the media. It made most of us worry by getting tested before traveling for the holidays, continue our stay-at-home work, and kept our mask on (not all of us).
By 2023 a few more variants made its way, after the first wave of the Omicron, the European Centre for Disease Prevention and Control announced the BA.2, BA.4, BA.5 subvariants, by Spring '23 it became the XBB series, we also had the EG.5, then by late '23 the XBB, HV.1, and the FL.1.5.1.
Fast forward to now, we are in 2024. There was the JN.1 variant from late '23 into 2024, but now there's a new Omicron subvariant. What to know about the FLiRT variant.
Well, 28.2% of Covid infections in the US by the third week of May, making it the dominant variant in the nation right now. The FLiRT strains have since been identified in several other countries, including Canada and the United Kingdom.
There are also concerns of a summer uptick as we enter the season.
The point is Covid is NOT over. It's unfortunately here to stay. As far as these variants go, it will keep mutating. So, the best way is to get your booster vaccine and go about your day. Masking is a choice now, I don't mask up as I used to, especially if I'm outside. Most are not, but once in a while I see older individuals doing it indoors, and that's okay. When I enter hospitals and my doctor's office, I masked up. The doctor does too. It's a courtesy and safety type of thing.
I receive my vaccine at least twice a year and try my best to dodge the "RONA". I specifically take the Moderna vaccine for better results. Along with the Covid-19 vaccine, one can also take the flu shot the same day as well.
In the city of Wuhan (China), where it all started in late 2019 (not in a wet market by the way), the WHO declared it a global health emergency in March 2020 right before lockdowns.
In February 2021, the World Health Organization (WHO), in a joint mission with China, attempted to investigate the origins of the pandemic. By 2022, the WHO urged more investigation. The recommendation came after a theory that the virus started elsewhere, and not the marketplace in Wuhan.
Another thing to cover about Covid is long Covid. What is it? Click here to read more.
Brain fog is one of the most common, persistent complaints in patients with long COVID.
In conclusion, COVID-19 is not done with us. So, try your best not to catch it (if haven't by now) and yes, you can get the virus more than once.
Recently Dr. Fauci's (retired) former top adviser Dr. David Morens testified about the origins of COVID-19.
It's also known that the COVID-19 pandemic was the deadliest disaster in the country's (US) history. Over 1.1 million US deaths alone have been reported.
Read more on the FLiRT subvariant.
#tumblr#writers on tumblr#covid 19#long covid#coronavirus#vaccines#viruses#decade: 2020s#blog#tumblog
1 note
·
View note
Text
Water, Vol. 16, Pages 318: Rapid Spread of Omicron Sub-Lineage as Evidence by Wastewater Surveillance
The search for better tools for interpreting and understanding wastewater surveillance has continued since the beginning of the coronavirus disease 2019 (COVID-19) pandemic. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has continued to mutate, thus complicating the interpretation of surveillance results. We assessed the Omicron variants (BA.1, BA.2, and BA.5) associated with wastewater-derived SARS-CoV-2 #RNA trends by estimating the effective reproduction number (Reff) using an epidemic model that integrates explicitly the SARS-CoV-2 N2 gene concentration detected in wastewater through rt-qPCR quantitative analysis. The model inferred COVID-19 cases based on wastewater data and compared them with the ones reported by clinical surveillance. The variant of the SARS-CoV-2 associated with the wastewater-derived viral #RNA was monitored through wastewater whole-genome sequencing. Three major waves between January and September 2022 were associated with the Omicron subvariants (BA.1, BA.2, and BA.5). This work showed that disease trends can be monitored using estimates of the effective reproduction number which is simple and easy to understand. https://www.mdpi.com/2073-4441/16/2/318?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
21 cases of Covid-19 subvariant JN. 1 detected in India, authorities on high alert
With the fresh cases of coronavirus subvariant JN.1 emerging in India, Indian authorities have been on high alert. The subvariant JN. 1 is being investigated by the scientific community as the authorities work on curbing its spread already. As per NITI Aayog member (health) Dr V K Paul, as of now, 21 cases of COVID-19 JN.1 sub-variant have been detected in the country. The Indian Council of Medical Research (ICMR) is focused on the genome sequencing of the variant.
Out of the 21 cases, 19 cases have been detected in Goa while Kerala and Maharashtra have recorded one each.
First found in late 2023, the JN.1 (BA.2.86.1.1) variant of COVID-19 is a descendant of the BA.2.86 lineage (Pirola) of SARS-CoV-2. The BA.2.86 lineage (Pirola) first emerged in August 2023 and showed more than 30 mutations in the spike (5) protein unlike the SARS-CoV-2 Omicron XBB lineages. The mutations make the variant highly risky with a high potential for immune evasion.
The JN.1 subvariant has been classified as a variant of interest by the World Health Organization.
Head of Pulmonary Medicine at Safdarjung Hospital, Dr. Rohit Kumar said, “COVID is an RNA virus that changes its form from time to time, and new variants of it emerge. And now a new variant has emerged, which has been named JN.1. However, not a single case has come to light in the capital, Delhi yet.”
"We are on alert, keeping an eye on the Corona cases. Testing of patients is also being done, and the patients who are coming positive are also being sent for genome sequencing. So that new variants can also be detected, but till now no case of new variants has been reported in Delhi," Dr Kumar said.
"If there is a sore throat, cough, cold, chest pain, or difficulty breathing, consult the doctor immediately. Especially those already suffering from respiratory diseases and asthma patients need to take special care. The doctor mentioned that during this season, individuals with serious diseases should be more careful, as those dying due to Covid often have pre-existing serious conditions such as heart disease and diabetes," he added.
For more COVID-19 news India in Hindi, subscribe to our newsletter.
#werindia#top news stories#leading india news source#top news headlines#top news of the day#latest national news#national news#coronavirus#covid19#JN.1 Covid variant
0 notes
Text
"Unveiling the Elusive Omicron Subvariant: BA.5's Startling Revelation of Increased Virulence in Groundbreaking Mouse Study"
Scientists at Cornell University in the US have used genetically modified mice to study the SARS-CoV-2 Omicron subvariants. The research, published in the journal Science Advances, aimed to understand the behavior of different variants and subvariants of concern in people. The engineered mice, known as K18-hACE2 mice, expressed a human receptor that allowed the virus to enter inaccessible mouse…
View On WordPress
0 notes
Text
"Unveiling the Elusive Omicron Subvariant: BA.5's Startling Revelation of Increased Virulence in Groundbreaking Mouse Study"
Scientists at Cornell University in the US have used genetically modified mice to study the SARS-CoV-2 Omicron subvariants. The research, published in the journal Science Advances, aimed to understand the behavior of different variants and subvariants of concern in people. The engineered mice, known as K18-hACE2 mice, expressed a human receptor that allowed the virus to enter inaccessible mouse…
View On WordPress
0 notes
Text
"Unveiling the Elusive Omicron Subvariant: BA.5's Startling Revelation of Increased Virulence in Groundbreaking Mouse Study"
Scientists at Cornell University in the US have used genetically modified mice to study the SARS-CoV-2 Omicron subvariants. The research, published in the journal Science Advances, aimed to understand the behavior of different variants and subvariants of concern in people. The engineered mice, known as K18-hACE2 mice, expressed a human receptor that allowed the virus to enter inaccessible mouse…
View On WordPress
0 notes
Text
By • Olalekan Fagbade COVID-19 Again; NCDC clears air on presence of new subvariants in Nigeria The Nigeria Centre for Disease Control and Prevention (NCDC) is closely monitoring the emergence of new subvariants of the Omicron variant of the SARS-CoV-2 virus. The Director-General, NCDC, Dr Ifedayo Adetifa, gave the assurance in an interview with the News Agency of Nigeria (NAN) on Saturday in Abuja. These subvariants, named EG.5 and BA.2.86, have been reported in several countries. While the EG.5 variant has been classified as a “variant of interest” with a low global risk, the BA.2.86 variant is currently under monitoring due to its substantial genetic differences from other circulating variants. NAN reports that an update from the U.S. Centre for Disease Control and Prevention (CDC) shows that at least two cases subvariant BA.2.86 have been identified in the U.S. This prompted the centre to issue a risk-assessment summary on Aug. 23, explaining what is known about it so far. Adetifa said that the NCDC, along with its partners, was actively conducting surveillance and implementing enhanced testing measures to gather more information about these emerging variants. “It is important for the public to stay informed with verified information and continue practising preventive measures to protect themselves and their loved ones,” he said. He noted that the subvariants, EG.5 and BA.2.86, had been reported in countries such as China, U.S., Republic of Korea, Japan, Canada, Australia, Singapore, United Kingdom, France, Portugal and Spain. “The EG.5 variant, which is a descendant of XBB.1.9.2, has been identified in 51 countries. The World Health Organization (WHO) has classified EG.5 as a Variant of Interest (VoI). “However, a risk assessment conducted by the WHO has determined that this new variant poses a low risk at the global level. “It is important to note that EG.5 has not been associated with any change in symptoms or clinical manifestations, nor has it resulted in an increase in the severity of illness, hospitalizations, or death rates. “The symptoms caused by EG.5 are similar to those seen with other COVID-19 variants, including fever, cough, shortness of breath, fatigue, muscle aches, headache and sore throat,” he said. According to him, on the other hand, the BA.2.86 variant, a descendant of BA.2, has been reported in a handful of countries, including the United Kingdom, Israel, Denmark, South Africa and U.S. The director-general said that WHO had classified BA.2.86 as a Variant under Monitoring (VuM) due to its multiple genetic differences from its ancestor – BA.2, and other currently circulating XBB-derived SARS-CoV-2 variants. “As there are only a few reported cases of BA.2.86, there is not enough information to make conclusive assessments of its virulence, transmission and severity. “However, it is expected to be similar to other Omicron descendants currently circulating. “It is worth noting that while the ancestor BA.2 has been previously found in Nigeria, no BA.2.86 variant has been identified in the country,” he said. He said that the NCDC’s COVID-19 Technical Working Group (COVID-19 TWG) was actively monitoring COVID-19 epidemiology at the local, regional, continental and global levels, including the emergence of new variants. “Influenza sentinel surveillance sites continue to provide information on COVID-19 prevalence in patients with influenza-like illness and severe acute respiratory illness. “So far, there has been no observed increase in the trend of COVID-19 in this patient group,” he said. He said that the NCDC was also carrying out genomics surveillance, in spite of the low testing levels, and encourages testing locations in states to send positive samples for sequencing. “Additionally, the NCDC and its partners are working on implementing an enhanced COVID-19 testing exercise in four states to obtain more detailed information about circulating variants in the coun
try. “The distribution of COVID-19 rapid diagnostic kits is also underway to improve bi-directional COVID-19 testing,” he said. He urged members of the public to act responsibly and share only verified information to avoid unnecessary panic. “It is emphasized that COVID-19 is here to stay and mainly affects those at high risk, such as the elderly and individuals with underlying chronic illnesses. “The actions required to protect oneself and others remain the same, including getting tested for any febrile illness and respiratory symptoms, getting vaccinated against COVID-19, practising good hand hygiene, and wearing masks in high-risk situations,” he said. He said that the NCDC would continue to monitor the situation globally, especially in countries where the new variants had been confirmed. He added that the centre would provide Nigerians with scientifically-sound and evidence-based information on any changes in SARS-CoV-2 epidemiology and genomics that may pose a threat to public health.(NAN) www.nannews.ng AIR/ISHO/IGO #COVID19 #Nigeria #Subvariant
0 notes
Text
Fourth dose bivalent COVID-19 vaccines outperform monovalent boosters in eliciting cross-reactive memory B cells to Omicron subvariants - Published Aug 8, 2024
Remember when that kid called me an uneducated idiot when I suggested that monovalent vaccines provide less long-lasting broad protection from all covid strains? I present Scientific Catharsis
Abstract Bivalent COVID-19 vaccines comprising ancestral Wuhan-Hu-1 (WH1) and the Omicron BA.1 or BA.5 subvariant elicit enhanced serum antibody responses to emerging Omicron subvariants. Here, we characterized the RBD-specific memory B cell (Bmem) response following a fourth dose with a BA.1 or BA.5 bivalent vaccine, in direct comparison with a WH1 monovalent fourth dose. Healthcare workers previously immunized with mRNA or adenoviral vector monovalent vaccines were sampled before and one-month after a fourth dose with a monovalent or a BA.1 or BA.5 bivalent vaccine. Serum neutralizing antibodies (NAb) were quantified, as well as RBD-specific Bmem with an in-depth spectral flow cytometry panel including recombinant RBD proteins of the WH1, BA.1, BA.5, BQ.1.1, and XBB.1.5 variants. Both bivalent vaccines elicited higher NAb titers against Omicron subvariants compared to the monovalent vaccine. Following either vaccine type, recipients had slightly increased WH1 RBD-specific Bmem numbers. Both bivalent vaccines significantly increased WH1 RBD-specific Bmem binding of all Omicron subvariants tested by flow cytometry, while recognition of Omicron subvariants was not enhanced following monovalent vaccination. IgG1+ Bmem dominated the response, with substantial IgG4+ Bmem only detected in recipients of an mRNA vaccine for their primary dose. Thus, Omicron-based bivalent vaccines can significantly boost NAb and Bmem specific for ancestral WH1 and Omicron variants, and improve recognition of descendent subvariants by pre-existing, WH1-specific Bmem, beyond that of a conventional, monovalent vaccine. This provides new insights into the capacity of variant-based mRNA booster vaccines to improve immune memory against emerging SARS-CoV-2 variants and potentially protect against severe disease.
One-sentence summary Omicron BA.1 and BA.5 bivalent COVID-19 boosters, used as a fourth dose, increase RBD-specific Bmem cross-recognition of Omicron subvariants, both those encoded by the vaccines and antigenically distinct subvariants, further than a monovalent booster.
41 notes
·
View notes
Text
[ad_1] The BA.2 sub-variant of Omicron has been present in 5 African international locations, a Global Well being Group scientist stated on Thursday (Dado Ruvic, Reuters) Estimated learn time: 2-3 minsJOHANNESBURG, South Africa — The BA.2 sub-variant of omicron has been present in 5 African international locations, a Global Well being Group scientist stated on Thursday, including she used to be involved concerning the construction as a result of samples of BA.2 might not be noticed as a type of omicron.The BA.2 sub-variant has begun to switch omicron's extra commonplace "authentic" BA.1 variant in international locations corresponding to Denmark. Knowledge from there suggests no distinction in illness severity, in line with any other WHO reliable.The BA.2 variant used to be first known in Utah on Jan. 12, in line with the Utah Division Of Well being."BA.2 ... has been reported in 5 international locations, this is Botswana, Kenya, Malawi, Senegal in addition to South Africa," Dr. Nicksy Gumede-Moeletsi informed a web based media briefing."We're very involved," she stated, including that BA.2 used to be proving exhausting to spot as it used to be no longer at all times picked up through the S-Gene Goal Failure criterion, which is used to tell apart the unique omicron from different variants.Gumede-Moeletsi stated the WHO used to be running very intently with laboratories, asking them to ahead samples that had come again with out being flagged as omicron for additional research, to be able to acquire a extra exact image of the unfold of BA.2.The BA.1 model of omicron has been rather more straightforward to trace than prior variants. This is as a result of BA.1 is lacking one in every of 3 goal genes utilized in a commonplace PCR take a look at. Circumstances appearing this trend have been assumed through default to be brought about through BA.1.BA.2, now and again referred to as a "stealth" sub-variant, does no longer have the similar lacking goal gene as the unique omicron variant.As a substitute, scientists are tracking it the similar method they've prior variants, together with delta, through monitoring the choice of virus genomes submitted to public databases corresponding to GISAID.As with different variants, an an infection with BA.2 may also be detected through coronavirus house assessments kits, even though they can't point out which variant is accountable, mavens stated.×Similar TalesEstelle Shirbon and James Macharia ChegeExtra tales you will be eager about [ad_2] #Omicron #subvariant #BA2 #tougher #establish
0 notes