#Metabolic pathway
Explore tagged Tumblr posts
Text
Amino Acid Metabolism: The Crossroads of Cellular Nutrient Use
Amino Acid Metabolism: The Crossroads of Cellular Nutrient Use In the intricate symphony of cellular processes, amino acids stand as versatile players, serving as the building blocks of proteins, precursors for various molecules, and even energy sources. Their metabolic fate is determined by the body’s needs and energetic demands, making amino acid metabolism a crucial pathway that ensures the…

View On WordPress
0 notes
Text
A comparison of metabolic pathways that are either stimulated (by SAGs) or repressed (by SDGs) during sequential leaf senescence in Arabidopsis is shown in Figure 22.17.
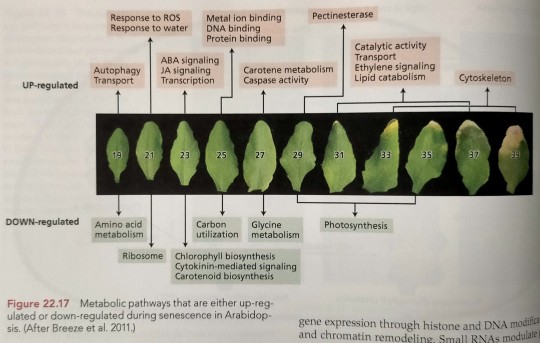
"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#metabolic pathway#sag#sdg#comparison#senescence associated genes#senescence down regulated genes#leaf senescence#arabidopsis
0 notes
Text
The major metabolic pathway in the starchy endosperm is, as the name implies, starch biosynthesis: the precursor molecule, ADP-glucose, is synthesized in the cytosol and then imported into the amyloplast, where it is enzymatically polymerized into amylose and amylopectin.
"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quote#plant physiology and development#nonfiction#textbook#metabolic pathway#starch#endosperm#biosynthesis#adp#glucose#cytosol#amyloplast#amylose#amylopectin#enzymes
0 notes
Text
when i'm at the schizophrenic incoherent rambling competition and an embryologist shows up
#people complain about clinical biochemistry but the metabolic pathways make sense#they do click#nothing in embryology is coherent
6 notes
·
View notes
Text
Finally discovered a combination of supplements to help me get 4h of sleep: .05 Estradiol, 100mg micronized progesterone (sometimes additional progesterone cream), DIM, 400mg magnesium citrate, 50mg allithiamine, a B Complex. That’s it.
#it’s been a month 🤞#the complicated science of body chemistry…#estrogen kept metabolizing down the wrong pathways
4 notes
·
View notes
Text
For work, I’m reading the article where Moderna shows how different ionizable lipids they synthetised work when tested on mice and non-human primates, to optimise them to serve as safe and effective carriers for mRNA vaccines (”We sought lipids that enabled high levels of protein expression, demonstrated rapid tissue clearance, and resulted in a toxicity profile that would support chronic therapeutic indications”).
This is your regular reminder that all the We Can Replace ALL Animal Testing With Simulations, AI and Testing On Human Volunteers are, and will be for the next decades at the very least, naively hopeful sci-fi at best, maliciously misleading misinformation at worst.
#me and my lovely protein#you can't simulate liver clearence#you can't simulate metabolic pathways#you can't simulate endosomal escape#you can't simulate - let alone train AI on - biological processes that are not fully described and understood#large scale testing on humans will just lead to people 'volunteering' because they a) need the money b) can't really say no
2 notes
·
View notes
Text
The Hypothetical Loss of Long-Lifespan Adaptations Post-Noah

If we hypothetically consider that humans once possessed adaptations for 1000-year lifespans, the shift from a fruit-based diet to a meat-based diet after Noah's time could have played a role in the loss of those long-lifespan adaptations. Increased metabolic stress, altered gut microbiome, nutrient deficiencies, reduced detoxification, genetic selection, and organ strain might have contributed to cumulative cellular damage, inflammation, and accelerated aging, ultimately leading to the loss of those remarkable cellular adaptations.
Metabolic Overload & Oxidative Stress: A carnivorous diet, characterized by increased fatty acid oxidation and gluconeogenesis, may lead to elevated reactive oxygen species (ROS) production due to inefficiencies in the electron transport chain. This can perturb redox homeostasis, contributing to mitochondrial dysfunction, genomic instability, and accelerated cellular senescence.
Gut Microbiome & Inflammation: The high protein and lipid content of a carnivorous diet can induce gut dysbiosis, potentially decreasing beneficial butyrate-producing bacteria and increasing pro-inflammatory taxa such as certain Bacteroides species. This dysbiosis can lead to increased production of endotoxins (lipopolysaccharides), triggering systemic low-grade inflammation and contributing to immune senescence, a process known as 'inflammaging'.
Nutrient Imbalances & Signaling Disruption: A carnivorous diet, often deficient in micronutrients like ascorbic acid (vitamin C), and phytochemicals found in plant-based foods, can disrupt signal transduction pathways crucial for DNA repair, telomere maintenance, and autophagy. These deficiencies can impair the insulin/IGF-1 and sirtuin/FOXO signaling networks, which are known to play a role in longevity.
Reduced Fiber & Detoxification: The lack of dietary fiber in a carnivorous diet impairs the synthesis of beneficial short-chain fatty acids (SCFAs) by gut bacteria and reduces enterohepatic detoxification. This can lead to the accumulation of uremic toxins (e.g., indoxyl sulfate), advanced glycation end products (AGEs), and lipofuscin, all of which contribute to cellular senescence and accelerated organismal aging.
Genetic & Epigenetic Changes: While long-term dietary changes might exert selective pressures on alleles related to protein and lipid metabolism, a carnivorous diet can also induce epigenetic modifications, such as DNA methylation and histone acetylation. These modifications can repress the expression of pro-longevity genes like SIRT and FOXO, potentially compromising adaptive stress responses and reducing organ resilience.
#Post-Apocalyptic Landscape#Eden-like Fruit-Based World#Meat-Centric Dystopia#Metabolic Overload & Oxidative Stress#Pro-Inflammatory Gut Microbiome#Nutrient Imbalance & Aging#Mitochondrial Dysfunction & ROS#Telomere Attrition & DNA Damage#Epigenetic Modifications#Longevity Pathway Suppression#SIRT#FOXO Pathway#Genomic Instability & Senescence#Organ Strain & Immune Dysregulation#Reactive Oxygen Species (ROS)#Advanced Glycation End Products (AGEs)#Short-Chain Fatty Acids (SCFAs)#Mitochondria & Telomeres Symbols#DNA Strands & Histone Acetylation
0 notes
Text
youtube
#Metabolite regulation of epigenetics in cancer involves key areas such as cancer metabolism#tumor microenvironment#epigenetic modifications#DNA methylation#histone acetylation#chromatin remodeling#oncogene activation#tumor suppressor silencing#metabolic pathways#tumor progression#hypoxia-induced epigenetics#acetyl-CoA dynamics#S-adenosylmethionine (SAM)#alpha-ketoglutarate (α-KG)#fumarate accumulation#lactate influence#therapeutic targeting#Youtube
0 notes
Text
Mitochondrial Dysfunction in Endometriosis
A Technical Overview of Cellular Mechanisms
Endometriosis, a common gynecological condition affecting approximately 10% of women during their reproductive years, is characterized by the presence of endometrial-like tissue outside the uterine cavity, most frequently in the ovaries, fallopian tubes, and peritoneal cavity. This ectopic tissue leads to a chronic inflammatory environment, pain, and infertility. While the pathophysiology of endometriosis is not fully understood, recent studies have increasingly highlighted mitochondrial dysfunction as a central feature of the disease. This technical article provides a detailed exploration of the role of mitochondria in endometriosis, examining the molecular and cellular mechanisms through which mitochondrial dysfunction contributes to disease progression.
Mitochondrial Function and Metabolism
Mitochondria are dynamic organelles responsible for numerous vital cellular processes, most notably ATP production through oxidative phosphorylation (OXPHOS). ATP is generated within the mitochondrial matrix by the electron transport chain (ETC), which involves the transfer of electrons from NADH and FADH2 to oxygen molecules, ultimately producing ATP. In addition to ATP production, mitochondria are involved in the regulation of calcium signaling, the maintenance of cellular redox balance, apoptosis, and the synthesis of key metabolites, including lipids and steroids. Mitochondria also contain their own genome (mitochondrial DNA or mtDNA), which encodes essential components of the ETC and mitochondrial protein synthesis machinery.
Mitochondria maintain their function through a balance of fusion and fission, processes that help ensure the organelle's shape, distribution, and response to stress. Mitochondrial dysfunction can arise from an imbalance in these processes, as well as from damage to mitochondrial DNA (mtDNA), excessive reactive oxygen species (ROS) production, and impaired bioenergetic functions. In the context of endometriosis, these disruptions have profound implications for cellular homeostasis and tissue function.
Mitochondrial Dysfunction in Endometriosis
In endometriosis, altered mitochondrial function contributes significantly to the disease's pathology. The following mechanisms are central to understanding how mitochondrial dysfunction drives the progression of endometriosis:
1. Altered Metabolic Shifts: The Warburg Effect
A hallmark of cancerous and proliferative cells is a shift in cellular metabolism, often referred to as the Warburg effect, in which cells preferentially utilize glycolysis over oxidative phosphorylation for ATP production, even in the presence of oxygen. This metabolic reprogramming is also observed in endometriotic cells, particularly in ectopic lesions, where cells exhibit increased glycolytic activity. In these lesions, endometrial cells rely less on mitochondrial OXPHOS and instead preferentially use glycolysis for ATP production, generating lactate as a byproduct.
This metabolic shift supports enhanced cell proliferation and survival under suboptimal conditions, characteristic of the hyperplastic nature of endometriosis. Glycolysis is less efficient in terms of ATP production compared to OXPHOS, yet it provides the necessary metabolic intermediates for cell division and biosynthesis. Additionally, the accumulation of lactate in the extracellular space lowers the local pH, which can exacerbate tissue inflammation and create a microenvironment conducive to the growth and persistence of ectopic lesions.
2. Mitochondrial DNA Damage and Instability
Mitochondria are highly susceptible to damage due to their proximity to ROS-producing processes in the electron transport chain. ROS, which are byproducts of cellular respiration, can damage mitochondrial lipids, proteins, and most notably, mitochondrial DNA (mtDNA). Unlike nuclear DNA, mtDNA is not protected by histones, making it particularly vulnerable to oxidative damage. In endometriosis, there is compelling evidence that mtDNA is significantly damaged in ectopic endometrial tissue. Studies have shown mtDNA deletions, mutations, and increased levels of mtDNA fragmentation in these tissues, which suggest a breakdown in the integrity of mitochondrial function.
The damaged mtDNA further exacerbates mitochondrial dysfunction, impairing the ability of mitochondria to generate ATP through OXPHOS. This, in turn, results in an increased reliance on anaerobic glycolysis, fueling the Warburg effect. Furthermore, mtDNA mutations can impair mitochondrial protein synthesis, leading to dysfunctional mitochondrial complexes and altered cellular bioenergetics, perpetuating a cycle of cellular dysfunction in endometriotic lesions.
3. Oxidative Stress and Inflammation
One of the critical roles of mitochondria is the regulation of cellular redox balance. Under normal conditions, mitochondria produce ROS as part of the electron transport chain. However, when mitochondrial function is compromised—whether due to damage, oxidative stress, or metabolic reprogramming—excess ROS are produced, leading to a state of oxidative stress. In endometriosis, ectopic endometrial tissue exhibits elevated levels of ROS, contributing to a persistent inflammatory environment.
Oxidative stress in endometriotic lesions is amplified by mitochondrial dysfunction and is further exacerbated by the Warburg effect, which generates additional ROS during glycolysis. ROS directly activate inflammatory pathways, particularly through the nuclear factor-kappa B (NF-κB) signaling pathway, leading to the production of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α. These cytokines perpetuate the inflammatory response, recruiting immune cells to the site of ectopic lesions, which leads to pain, fibrosis, and the development of adhesions.
Moreover, ROS play a critical role in sensitizing nociceptors, contributing to the chronic pain experienced by women with endometriosis. The interplay between oxidative stress and inflammation forms a vicious cycle that fuels the progression of endometriosis and promotes the growth and persistence of ectopic lesions.
4. Impaired Mitochondrial Dynamics: Fragmentation and Dysfunction
Mitochondria undergo constant fusion and fission, processes that regulate mitochondrial morphology, quality control, and function. Fusion allows for the mixing of mitochondrial contents, which can help dilute damaged components, while fission helps eliminate dysfunctional mitochondria through mitophagy. In endometriosis, there is evidence of disrupted mitochondrial dynamics, particularly an increase in mitochondrial fragmentation. Fragmented mitochondria are less efficient at ATP production and more prone to accumulating damaged proteins and lipids, which further impairs mitochondrial function.
The imbalance between mitochondrial fusion and fission in endometriosis is linked to altered expression of key proteins such as mitofusins (MFN1/2) and dynamin-related protein 1 (DRP1). DRP1-mediated mitochondrial fission is upregulated in endometriotic lesions, contributing to the generation of fragmented mitochondria. These fragmented organelles are associated with increased oxidative stress, apoptosis resistance, and enhanced cell proliferation—features that contribute to the pathogenesis of endometriosis.
5. Apoptosis Resistance and Cell Survival
Mitochondria play a pivotal role in regulating apoptosis through the release of pro-apoptotic factors, such as cytochrome c, from the mitochondrial intermembrane space. These factors initiate the caspase cascade, leading to cell death. However, in endometriosis, ectopic endometrial cells exhibit resistance to apoptosis, allowing them to survive and proliferate abnormally.
Mitochondrial dysfunction in endometriosis leads to alterations in key apoptotic proteins, including Bcl-2 family members, which regulate mitochondrial outer membrane permeabilization (MOMP). The overexpression of anti-apoptotic proteins, such as Bcl-2 and Bcl-xL, and the downregulation of pro-apoptotic proteins, such as Bax and Bak, result in the persistence of damaged cells. This resistance to apoptosis allows for the survival of endometriotic lesions in hostile environments, contributing to the chronic nature of the disease and complicating treatment strategies.
Therapeutic Implications: Targeting Mitochondrial Dysfunction
Given the central role of mitochondrial dysfunction in endometriosis, therapeutic approaches targeting mitochondrial function hold promise for improving disease management. Several potential strategies include:
Antioxidant Therapies: Reducing oxidative stress through antioxidants such as N-acetylcysteine (NAC), Coenzyme Q10 (CoQ10), and vitamin E could help restore mitochondrial function and reduce inflammation in endometriotic tissues.
Modulation of Mitochondrial Dynamics: Targeting proteins involved in mitochondrial fusion and fission, such as DRP1 and MFN2, may help restore mitochondrial morphology and improve bioenergetic function in endometriotic lesions.
Inhibition of Glycolysis: Given the shift toward glycolysis in endometriotic cells, inhibiting key glycolytic enzymes, such as hexokinase or lactate dehydrogenase, may help reduce lesion growth and metabolic reprogramming.
Mitochondrial Biogenesis Stimulation: Activators of PGC-1α, a central regulator of mitochondrial biogenesis, could promote the generation of healthy mitochondria and improve overall cellular metabolism in endometriotic tissue.
Conclusion
Mitochondrial dysfunction is a key contributor to the pathogenesis of endometriosis. Alterations in mitochondrial metabolism, oxidative stress, mitochondrial DNA damage, and impaired apoptotic regulation are central to the disease's progression. Understanding the molecular mechanisms underlying mitochondrial dysfunction in endometriosis provides novel insights into potential therapeutic strategies. Targeting mitochondrial function and bioenergetics could lead to more effective treatments for endometriosis, alleviating its symptoms and improving outcomes for affected women.

#Mitochondrial Dysfunction#Endometriosis#Mitochondrial DNA (mtDNA)#Oxidative Stress#Reactive Oxygen Species (ROS)#Energy Metabolism#Glycolysis#Warburg Effect#Mitochondrial Dynamics#Mitochondrial Fusion and Fission#Mitochondrial Fragmentation#ATP Production#Oxidative Phosphorylation (OXPHOS)#Cell Proliferation#Apoptosis Resistance#Inflammation#Chronic Pain#Mitophagy#Bcl-2 Family Proteins#Cytokine Production#NF-κB Pathway#Mitochondrial Biogenesis#PGC-1α#Pro-inflammatory Cytokines#Fibrosis#Endometrial Tissue#Antioxidant Therapy#Mitochondrial Targeted Therapies#Fertility Impairment#Cellular Metabolism
1 note
·
View note
Text
MTHFR Gene Mutation: Why It Matters & How to Get Tested
Discover the Role of the MTHFR Gene, the Impact of Its Mutations on Your Health, & How You Can Get Tested to Understand Your Genetic Risk Factors You may not have heard of MTHFR, but this enzyme plays a vital role in our body’s ability to process folate (vitamin B9) and maintain your DNA. Related to another critical B vitamin, I recently wrote a story about the global B12 epidemic. Analyzing…
#23andMe#C677T and A1298C polymorphisms#deleterious mutations#DTC Testing vs. Healthcare-Ordered Tests#elevated homocysteine levels#Folate deficiency and MTHFR#folate metabolism and methylation pathways#genetic profile#Genetic testing for MTHFR#Getting Checked to Prevent Cardiovascular Issues#holistic health strategy#homocysteine and poor B12 absorption#How to test for MTHFR mutation#Literature Review on MTHFR#methylated B12 (methylcobalamin)#Methylation and MTHFR#MTHFR and health risks#MTHFR C677T polymorphism and autism#MTHFR gene mutation#MTHFR gene mutation and pregnancy#MTHFR mutation symptoms#MTHFR mutation treatment#MTHFR Polymorphism#MTHFR testing#MTHFR’s thermolability#NVAF cardiometabolic stroke have MTHFR gene mutations#Vitamin B12 deficiency and MTHFR
0 notes
Text
Stanford Reverses Cognitive Decline in Alzheimer’s With Brain Metabolism Drug
Neuroscientists at Stanford have Linked Alzheimer’s Disease to the Disruption of Brain Metabolism via the Kynurenine Pathway, which is Affected by Amyloid Plaque and Tau Proteins.
— By Stanford University | August 22, 2024

Stanford Researchers Have Found That Blocking the Kynurenine Pathway in the Brain Can Reverse the Metabolic Disruptions Caused By Alzheimer’s Disease, Improving Cognitive Functions in Mice. Credit: SciTechDaily.com
Alzheimer’s Disease and Brain Energy Metabolism
Their research has demonstrated that drugs blocking this pathway can restore cognitive function in Alzheimer’s mice by improving brain metabolism. This discovery not only bridges the gap between neuroscience and oncology but also provides a fast track to repurposing existing drugs for Alzheimer’s treatment.
Neuroscientists believe one of the key mechanisms by which Alzheimer’s disease impairs brain function is through the disruption of glucose metabolism, which is essential for energizing a healthy brain. Essentially, a decrease in metabolism deprives the brain of vital energy, thereby hindering cognitive functions and memory.
Against that backdrop, a team of neuroscientists at the Knight Initiative for Brain Resilience at Stanford’s Wu Tsai Neurosciences Institute have zeroed in on a critical regulator of brain metabolism known as the kynurenine pathway. They hypothesize that the kynurenine pathway is overactivated as a result of amyloid plaque and tau proteins that accumulate in the brains of patients with Alzheimer’s disease.
Restoring Cognitive Function in Lab Mice
Now, with support from research and training grants from the Knight Initiative, they have shown that by blocking the kynurenine pathway in lab mice with Alzheimer’s Disease, they can improve, or even restore, cognitive function by reinstating healthy brain metabolism.
“We were surprised that these metabolic improvements were so effective at not just preserving healthy synapses, but in actually rescuing behavior. The mice performed better in cognitive and memory tests when we gave them drugs that block the kynurenine pathway,” said senior author, Katrin Andreasson, a neurologist at the Stanford School of Medicine and member of the Wu Tsai Neurosciences Institute.
The study, which included collaborations with researchers at the Salk Institute for Biological Studies, Penn State University, and others, was published on August 22, 2024, in the journal Science.
Hungry Neurons
In the brain, kynurenine regulates production of the energy molecule lactate, which nourishes the brain’s neurons and helps maintain healthy synapses. Andreasson and her fellow researchers specifically looked at the enzyme indoleamine-2,3-dioxygenase 1 — or IDO1, for short — which generates kynurenine. Their hypothesis was that increases in IDO1 and kynurenine triggered by accumulation of amyloid and tau proteins would disrupt healthy brain metabolism and lead to cognitive decline.
“The kynurenine pathway is over activated in astrocytes, a critical cell type that metabolically supports neurons. When this happens, astrocytes cannot produce enough lactate as an energy source for neurons, and this disrupts healthy brain metabolism and harms synapses” Andreasson said. Blocking production of kynurenine by blocking IDO1 restores the ability of astrocytes to nourish neurons with lactate.
Potential Fast-Tracking of IDO1 Inhibitors
Best of all for Andreasson, and for Alzheimer’s patients, IDO1 is well known in oncology and there are already drugs in clinical trials to suppress IDO1 activity and production of kynurenine. That meant Andreasson could circumvent the time-intensive work of identifying new drugs and to begin testing in lab mice almost immediately.
In those tests, in which mice with Alzheimer’s Disease must navigate an obstacle course before and after drug intervention, Andreasson and team found that the drugs improved hippocampal glucose metabolism, corrected deficient astrocytic performance, and improved the mice’s spatial memory.
Promising Results Across Different Pathologies
“We also can’t overlook the fact that we saw this improvement in brain plasticity in mice with both amyloid and tau mice models. These are completely different pathologies, and the drugs appear to work for both,” Andreasson noted. “That was really exciting to us.”
Better yet, this intersection between neuroscience, oncology, and pharmacology could help speed drugs to market if proved effective in ongoing human clinical trials for cancer.
“We’re hopeful that IDO1 inhibitors developed for cancer could be repurposed for treatment of AD,” Andreasson stressed.
A Glimpse into the Future of Alzheimer’s Treatment
The next step is to test IDO1 inhibitors in human Alzheimer’s patients to see if they show similar improvements in cognition and memory. Prior clinical tests in cancer patients tested the effectiveness of IDO1 inhibitors on cancer but did not anticipate or measure improvements in cognition and memory. Andreasson is hoping to investigate IDO1 inhibitors in human trials for Alzheimer’s disease in the near future.
Reference: “Restoring hippocampal glucose metabolism rescues cognition across Alzheimer's disease pathologies” 22 August 2024, Science. DOI: 10.1126/science.abm6131
Stanford Wu Tsai Neurosciences Institute / Knight Initiative for Brain Resilience Authors:
Paras S. Minhas (co-lead), Amira Latif-Hernandez (co-lead), Aarooran S. Durairaj, Qian Wang, Siddhita D. Mhatre, Takeshi Uenaka, Joshua Crapser, Travis Conley, Hannah Ennerfelt, Yoo Jin Jung, Yeonglong Albert Ay, Matthew Matrongolo, Edward N. Wilson, Tao Yang, Marius Wernig, Frank M. Longo, and Katrin I. Andreasson (corresponding).
Other Contributing Institutions
The Salk Institute for Biological Studies (including co-lead author Jeffrey R. Jones), Keio University, Princeton University, Penn State University, UC San Francisco, and the Banner Sun Research Institute.
Wu Tsai Neurosciences Institute / Knight Initiative for Brain Resilience Support:
The research was supported by an Innovation Award and a Brain Resilience Scholar Award from the Knight Initiative for Brain Resilience at the Wu Tsai Neurosciences Institute. The study made use of Wu Tsai Neurosciences Institute Community Laboratories: the Stanford Behavioral and Functional Neuroscience Laboratory and the Stanford Neuroscience Microscopy Service, as well as the Stanford Mass Spectroscopy Core.
— Competing Interests: Andreasson is a Co-Founder, Board Member, and Consultant for Willow Neuroscience, Inc. Longo is a Founder of, Board Member of, and Consultant for and has Financial Interest in PharmatrophiX, a Company Focused on Small-Molecule Development for Treatment of Neurodegenerative Disorders.
#Stanford University#Reverses | Cognitive | Decline#Alzheimer#Brain Metabolism | Drug#Neuroscientists#Disruption | Brain Metabolism#Kynurenine Pathway#Amyloid Plaque | Tau Proteins#Cognitive Functions#Restoring | Cognitive Function | Lab Mice#Hungry Neurons#Fast-Tracking | IDO1 Inhibitors#Pathologists | Pathologies
1 note
·
View note
Text
Gluconeogenesis: The Backbone of Cellular Metabolism
In the intricate symphony of cellular processes, glucose stands as the maestro, providing energy for a diverse array of cellular operations. However, when blood glucose levels plummet, a backup plan kicks in, ensuring that energy demands are met. This remarkable process is known as gluconeogenesis. A Journey from Non-Carbohydrates to Glucose Gluconeogenesis revolves around the conversion of…
View On WordPress
0 notes
Text
NAD-Linked Glycerol Dehydrogenase
-- part of oxidoreductase family
-- catalyst is NAD+
-- oxidizes glycerol
-- forms glycerone
#studyblr#notes#my notes#medblr#biochemistry#biochem#biochem notes#biochemistry notes#science#scienceblr#biology#enzymes#cell biology#enzyme mechanisms#enzyme pathways#enzyme notes#medical notes#medical chemistry#chemistry#molecular biology#molecular bio#enzyme science#specific enzymes#enzyme reactions#metabolism#anabolism#catabolism#metabolic pathways
1 note
·
View note
Text
AAAGGGH i should have majored in microbio but i will utilize my biochem degree to work in microbio EVENTUALLY
#personal#currently thinking i wanna work with elucidating microbial biosynthetic pathways or microbial metabolism thingys#so excited#jumps around#also rlly like archaea but that’s like later phd if i end up doing a phd#i don’t know a lot abt it yet but it’s just 💥💥💥💥💥 RAAAAGH so exciting#i heart stem
0 notes
Text
youtube
#m6A modification#28S rRNA#oncogenic mRNA translation#tyrosine catabolism#RNA epigenetics#ribosomal biology#cancer metabolism#tumor progression#mRNA regulation#epitranscriptomics#oncogene expression#translational control#metabolic pathways#RNA modification#ribosome recruitment#cancer research#mRNA stability#oncogenic signaling#molecular oncology#targeted cancer therapy.#Youtube
0 notes
Text

Hmm
Might have found my niche. Maybe I should start posting.
hate what people did to the dead dove tag
#last Thursday I gave an intro to high scho students about vitamins and amino acids#and because therr were no curriculum and no one could stop me#i of course made it all about genes and metabolic pathways#i love a good metabolic pathway
77K notes
·
View notes