#Infectious Testing Market News
Explore tagged Tumblr posts
Text
a lot of you probably knows Belphie's story, but I'll summarize just in case.
Devon Rex cats are better for people with allergies (less shed fur + less Fel d1 protein in their saliva), so on February 16, 2024, I went the breeder route and put down a deposit. before Belphie even opened his eyes, he was mine!




every Friday, the breeder sent me a new photo. I had a broken leg, and was basically rotting in bed at that point, so it was the best part of my week. then, at 12 weeks old, I BROUGHT HIM HOME!


at first, he was so alive! like a wind-up monkey that never shut off. he dangled from the wall-hangings, savaged my feet as I walked, and used my elderly cats as jumping poles to do cool acrobatics over. but all this gradually faded.
first, he stopped playing. then he stopped climbing. then he stopped moving much at all. my vet ran tests on him and found multiple pathogens (calcivrius + mycoplasma), but the medication didn't help - he kept declining.


on September 17th, I woke up to find him swollen like a balloon. we finally had an answer: he had Feline infectious Peritonitis, aka FIP. before 2017, this would've been a death sentence. he would've kept bloating until he drowned in his own fluids. and before 2024, I would've been forced to inject him with black market drugs. but thankfully, South Tower Animal Hospital in Fergus, Ontario was doing a study on the oral medication! we drove two hours, enrolled him, and left with the GS-441524 pills.

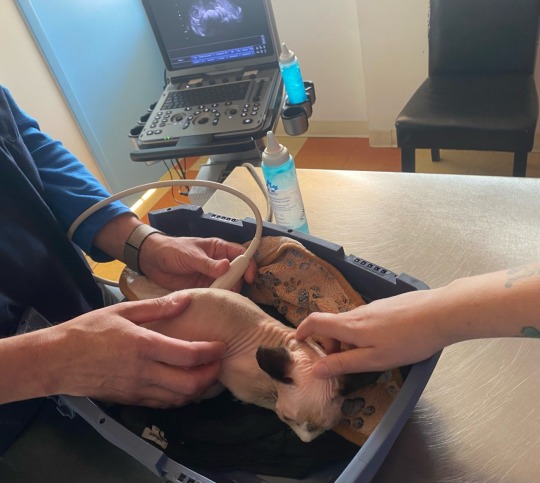
and he went from those photos above.....to this:

I thought Belphie would die as a kitten. I'd accepted that he would never grow up. but now he gets to LIVE!
and all for the low cost of $7,553.....ahhhahaha........god.
that + a recent home disaster has wiped out my savings, but I still need to pay for Belphie's medication. to remain in this study, I need to do bloodwork monthly until Feb 2025, and he'll need daily pills until March 2025.




I've put a risograph print + enamel pin set up at greerstothers.shop. I hate asking for help, but if you'd like to support Belphie's continued treatment, please consider checking them out!
#belphegor#I'm sorry that I don't have a printed version of the risograph to show you!#it's still in the process of being made#the digital preview doesn't do it justice - it will have a texture akin to pointillism and the yellow + pink inks will be practically neon
22K notes
·
View notes
Note
wait what the fuck go back why are there lambskin condoms at all

Yeah so, back in the day people didn’t like getting pregnant. And they didn’t have access to latex for a long time. So lambskin condoms are some of the OG contraceptives, they were better than nothin. The first iterations were made from animal intestines, there’s some debate but that was sometime in the 16th century, though some think it was much earlier.
When latex condoms hit the market in the 1850’s it would have been the death knell for lambskin except! People with latex allergies still needed an option that didn’t make them drop into anaphylaxis. Also animal condoms were still cheaper until the 1920’s. Fun fact: my friend Charlie is allergic to latex but decided to just use latex condoms anyway and got stuck hiding in a closet when the girls parents came home and forgot he had a biohazard on his penis until he started having a seizure and remembered to remove it.
Anyway! The downsides of current lambskin are numerous. First off, it feels an awful lot like wax paper which seems quite unpleasant for both partners. Additionally, it still transmits STIs, because lambskin is just… skin, and is permeable to infectious disease.
Lastly why it’s bad: Modern condoms are checked for microtears and breakage by running a small electrical current through them. That’s how we know they’re safe to prevent all the stuff. But lambskin can’t be subjected to this test, so it can’t even say it’s guaranteed to prevent pregnancy, if there’s microtearing semen are gonna get through.
But noooow there’s a baller new material called polyisoprene that can be tested for breakage, doesn’t smell bad like latex, and is hypoallergenic. They’re basically the best condom in every way, and lambskin should now bow out and stop existing.
411 notes
·
View notes
Text
The next pandemic is inevitable. Australia isn’t ready - Published Sept 23, 2024
(Before you Americans yell at me, It's already the 23rd in Australia. This is very late-breaking)
I thought this was a really good breakdown of the current situation given the government-approved covid denial we live in. Long, but worth a read.
By Kate Aubusson and Mary Ward
Top infectious disease and public health veterans at the nerve centre of the state’s war against COVID-19 are sounding the alarm.
NSW is less prepared today to fend off a deadly pandemic despite the lessons of COVID-19, say top infectious disease and public health veterans at the nerve centre of the state’s war against the virus.
And we won’t have another hundred years to wait.
NSW’s gold standard Test-Trace-Isolate-Quarantine and vaccination strategies will be useless if a distrusting population rejects directives, refuses to give up its freedoms again, and the goodwill of shell-shocked public health workers dries up.
A panel of experts convened by The Sydney Morning Herald called for a pandemic combat agency akin to the armed forces or fire brigades to commit to greater transparency or risk being caught off guard by the next virulent pathogen and misinformation with the potential to spread faster than any virus.
“It’s inevitable,” says Professor Eddie Holmes of the next pandemic. A world-leading authority on the emergence of infectious diseases at the University of Sydney, Holmes predicts: “We’ll have less than 100 years [before the next pandemic].
“We’re seeing a lot of new coronaviruses that are spilling over into animals that humans are interacting with,” said Holmes, the first person to publish the coronavirus genome sequence for the world to see.
“People are exposed all the time, and each time we are rolling the dice.”
The independent review of NSW Health’s response to COVID-19 opened with the same warning: “No health system or community will have the luxury of 100 years of downtime.”
Pandemic preparedness needs to be a “permanent priority”, wrote the report’s author, Robyn Kruk, a former NSW Health secretary, “rather than following the path of those that have adopted a ‘panic and forget strategy,’ allowing system preparedness to wane”.
Why we don’t have 100 years to wait for the next pandemic The World Health Organisation has declared seven public health emergencies of international concern since 2014, including the current mpox outbreak.
Climate change is turbocharging the factors that coalesce to create the perfect breeding ground for a pandemic-causing virus, including population increases, bigger cities, and better-connected global markets and migration.
“Animals will be forced into more constrained environments, and humans that rely on those environments will be again constrained in the same environments. There will be more wet markets, more live animal trade that will just increase exposure,” Holmes said.
“It was clear that we weren’t ready [for COVID],” said Jennie Musto, who, after seven years working for the World Health Organisation overseas, became NSW Health’s operations manager for the Public Health Emergency Operations Centre, the team responsible for NSW’s COVID-19 contact tracing and containment.
“Everyone had preparedness plans gathering dust on a shelf, but no one was actually ready to respond, and so everyone was on the back foot,” Musto said. “Perhaps none of us really thought this was going to happen. We were waiting 500 years.”
Who would willingly become the next doomed whistleblower? Eddie Holmes, known for his repeated assertion that SARS-CoV-2 did not come from a lab, is deeply concerned that when the next pandemic-causing virus emerges, chances are it will be covered up.
“My worry is that if the virus appeared in a small population, say, somewhere in Southeast Asia, the people involved wouldn’t blow the whistle now, given the fact that you would get blamed,” he said.
Li Wenliang, the Wuhan doctor who tried to raise the alarm about a virulent new virus, was reportedly reprimanded by police for spreading rumours and later died of COVID-19.
The global blame game, culminating in a deep distrust of China and accusations that the virus was grown in a Wuhan lab, is why Holmes believes “we’re in no better place than we were before COVID started, if not worse”.
“I work with a lot of people in China trying to keep the lines of communication open, and they’re scared, I think, or nervous about saying things that are perceived to counter national interest.”
From a vaccine perspective, our defences look strong. There have been monumental advancements in vaccine development globally, driven by mRNA technology. In Sydney this month, construction began on an RNA vaccine research and manufacturing facility.
“But the way I see it is that nothing has been done in terms of animal surveillance of outbreaks or data sharing. The [global] politics has got much, much worse,” Holmes said.
Combat force Conjoint Associate Professor Craig Dalton, a leading public health physician and clinical epidemiologist, called for a dramatic expansion of the public health workforce and the establishment of a pandemic combat force that would routinely run real-time pandemic simulations during “peacetime”.
“No one is upset with fire brigades spending most of the time not fighting fires. They train. A lot. And that’s probably how we need to move,” he said.
“We need exercise training units so that every major player in pandemic response is involved in a real-time, three to four-day pandemic response every three to five years at national, state and local [levels].”
The federal Department of Health and Aged Care recently ran a health emergency exercise focused on governance arrangements involving chief health officers and senior health emergency management officials, a spokeswoman for Health Minister Mark Butler said. The outcomes of this exercise will be tested later this year.
Dalton said desktop simulations and high-level exercises involving a handful of chiefs didn’t cut it, considering the thousands of people working across regions and states. He instead suggested an intensive training program run in the Hunter New England region before the 2009 H1N1 pandemic provided a good model.
“We were ringing people, actors were getting injections, just like a real pandemic,” said Dalton, who once ordered a burrito in a last-ditch effort to contact a restaurant exposed to COVID-19.
Our heroes have had it The expert panel was emphatic that our pandemic response cannot once again rely on the goodwill of the public health and healthcare workforce.
According to the Kruk review, what began as an emergency response ultimately morphed from a sprint into an ultra marathon and “an admirable (yet unsustainable) ‘whatever it takes’ mindset”.
They were hailed as heroes, but the toll of COVID-19 on healthcare workers was brutal. Workloads were untenable, the risk of transmission was constant, and the risk of violence and aggression (for simply wearing their scrubs on public transport in some cases) was terrifying.
“We got through this pandemic through a lot of people working ridiculous hours,” Dalton said.
“You talk to a lot of people who did that and say they could not do it again.”
Tellingly, several expert personnel who worked at the front lines or in the control centre of NSW’s pandemic defences were invited to join the Herald’s forum but declined. Revisiting this period of intense public scrutiny, culminating in online attacks and physical threats, was just too painful.
So long, solidarity Arguably, the biggest threat to our pandemic defences will be the absence of our greatest strength during COVID: the population’s solidarity and willingness to follow public health orders even when it meant forfeiting fundamental freedoms.
The public largely complied with statewide public health orders, including the stay-at-home directive that became the 107-day Delta lockdown, and other severe restrictions prevented many from being at the bedside of their dying loved ones, visiting relatives in aged care homes and attending funerals.
“My worry is that next time around when those sorts of rules come out, people may say, ‘Well, don’t worry about it.’ They relax it in the future. Why don’t we just not stick to the rules?” said Professor Nicholas Wood, associate director of clinical research and services at the National Centre for Immunisation Research and Surveillance.
“I’m not sure we quite understand whether people [will be] happy with those rules again,” he said.
Dalton was more strident.
“I tend to agree with Michael Osterholm … an eminent US epidemiologist [who] recently said the US is probably less prepared for a pandemic now than it was in 2019, mostly because the learnings by health departments in the COVID pandemic may not make a material difference if faced with a community that distrusts its public health agencies,” he said.
“If H1N1 or something else were to spill over in the next couple of years, things like masks, social distancing and lockdowns would not be acceptable. Vaccination would be rejected by a huge part of the population, and politicians might be shy about putting mandates in.”
As for the total shutdown of major industries, people will struggle to accept it unless the next pandemic poses a greater threat than COVID, said UNSW applied mathematician Professor James Wood.
The risk of the virus to individuals and their families will be weighed against the negative effects of restrictions, which are much better understood today, said Wood, whose modelling of the impact of cases and vaccination rates was used by NSW Health.
“Something like school closure would be a much tougher argument with a similar pathogen,” he said.
A previous panel of education experts convened by the Herald to interrogate pandemic decision-making in that sector was highly critical of the decision to close schools for months during NSW’s Delta lockdown.
Greg Dore, professor of infectious diseases and epidemiology at the Kirby Institute, said the public’s reluctance to adhere to restrictions again may, in part, be appropriate.
“Some of the restrictions on people leaving the country were a bit feudal and too punitive,” he said. “Other restrictions were plain stupid, [for instance] limitations on time exercising outside.”
Meanwhile, the delays to publicly recognise the benefits of face masks and the threat of airborne transmission “ate away at trust”, Dalton said.
“We shouldn’t make those mistakes again,” he said.
Transparent transgressions Uncertainty is not something politicians are adept at communicating, but uncertainty is the only constant during a pandemic of a novel virus.
Vaccines that offered potent protection against early iterations of the COVID virus were less effective against Omicron variants.
“[The public], unfortunately, got hit by a rapid sequence of changes of what was ‘true’ in the pandemic,” James Wood said.
Political distrust can be deadly if governments give the public reason to suspect they are obfuscating.
The expert panel urged NSW’s political leaders to be far more transparent about the public health advice they were given before unilaterally enforcing restrictions.
There was a clear line between public health advice and political decision-making in Victoria. The Victorian chief health officer’s written advice was routinely published online.
In NSW, that line was blurred as Chief Health Officer Kerry Chant stood beside political leaders, most notably former premier Gladys Berejiklian, at the daily press conferences.
Public health experts said that they looked for subtle cues to determine the distinction between the expert advice and the political messaging during press conferences, paying attention to body language, who spoke when and who stayed silent.
“It is fine for public health personnel to have a different view to politicians. They have different jobs. What is not OK is to have politicians saying they are acting on public health advice [when they are not],” he said.
The ‘whys’ behind the decisions being made were missing from the daily press conferences, which created “a vacuum for misinformation”, said social scientist and public health expert Professor Julie Leask at the University of Sydney.
“The communication about what you need to do came out, and it was pretty good … but the ‘why we’re doing this’ and ‘what trade-offs we’ve considered’ and ‘what dilemmas we’ve faced in making this decision’; that was not shared,” Leask said.
The infodemic In the absence of transparency, misinformation and disinformation fill the vacuum.
“We had an ‘infodemic’ during the pandemic,” said Dr Jocelyne Basseal, who worked on the COVID-19 response for WHO in the Western Pacific and leads strategic development at the Sydney Infectious Diseases Institute, University of Sydney.
“The public has been so confused. Where do we go for trusted information [when] everyone can now write absolutely anything, whether on Twitter [now called X] or [elsewhere] on the web?” Basseal said.
A systematic review conducted by WHO found misinformation on social media accounted for up to 51 per cent of posts about vaccines, 29 per cent of posts about COVID-19 and 60 per cent of posts about pandemics.
Basseal’s teenage children recently asked whether they were going into lockdown after TikTok videos about the mpox outbreak.
“There is a lot of work to be done now, in ‘peacetime’ … to get ahead of misinformation,” Basseal said, including fortifying relationships with community groups and teaching scientists – trusted and credible sources of information – how to work with media.
In addition to the Kruk review’s six recommendations to improve its pandemic preparedness, NSW Health undertook a second inquiry into its public health response to COVID-19, which made 104 recommendations.
NSW Health Minister Ryan Park said: “We are working hard to ensure the findings and recommendations from those reports are being implemented as quickly as possible.”
The expert panellists spoke in their capacity as academics and not on behalf of NSW Health or WHO.
The ‘As One System’ review into NSW Health’s COVID-19 response made six recommendations 1. Make governance and decision-making structures clearer, inclusive, and more widely understood 2. Strengthen co-ordination, communication, engagement, and collaboration 3. Enhance the speed, transparency, accuracy, and practicality of data and information sharing 4. Prioritise the needs of vulnerable people and communities most at risk, impacted and in need from day one 5. Put communities at the centre of emergency governance, planning, preparedness, and response 6. Recognise, develop and sustain workforce health, wellbeing, capability and agility.
#mask up#covid#covid 19#pandemic#wear a mask#public health#coronavirus#sars cov 2#still coviding#wear a respirator
139 notes
·
View notes
Note
hi! love your blog, i love getting to see all these cool cat colors i didnt even know were a thing LOL like i didn’t know there was such a thing as lilac or cinnamon but theyre so pretty.
ive been looking into getting a ragdoll in the future, the ones i have met have been the sweetest cats and they’re so pretty.
ive been doing research into possible health concerns they can have, and all im really seeing is the same things your typical random little guy could have when improperly cared for and things like that. kidney disease while on a bad diet, issues with hairballs when not brushed and groomed properly due to being long haired, etc. ive seen a few places say that they’re more prone to respiratory issues and heart disease, but the latter also seems to be something that breeders work to make sure their cats are safe from? at least in my research.
i guess im basically just wondering if you know anything else that can affect ragdolls specifically, or if there’s anything horribly unethical that’s totally swept under the rug or hidden about them! additional things to research and places to look would be appreciated, i never know where to research stuff like this reliably and you seem to know where to find some good info and stuff on cat breeds :)
thank you in advance for any help you can give, i hope have a good day!!
I’m glad you enjoy the blog and are learning about some new colors!
Ragdolls are definitely cool cats and they’re a pretty health breed, one of the major things to be mindful of is that this assessment only applies to well-bred individuals from good breeders.
Common breeds mean a lot of breeders which, unfortunately, also means a lot of scammers and bad breeders.
And the Ragdoll is a very common breed so there are a lot of not so good breeders out there… and the temperament and health of these backyard bred cats is a gamble, one that can end in heartbreak.
Now with that warning out of the way the big thing to worry about with the breed is Hypertrophic Cardiomyopathy. Fortunately the mutation responsible for this condition in the breed has been identified and can be tested for. Staying on top of heart health is an absolute must for a good breeder, this means regular echocardiogram’s as well as DNA testing.
The other big ones are Polycystic Kidney Disease and Progressive Retinal Atrophy, both of which can be tested for. These are common inherited disorders in the purebred population, you’ll have a hard time finding a breed which neither of these have been documented in.
Mucopolysaccharidosis VI is a storage disease that has been documented in the Ragdoll breed but it can be tested for and I’m not sure how common it is in current breeding populations.
A breeder shouldn’t cut corners when it comes to health testing, there are several commercially available tests which look for a wide variety of inherited disorders. Optimal Selection is becoming increasingly widely used, although personally I would feel most comfortable with testing submitted through somewhere like UC Davis.
But HCM is the big big one because heart disease is a silent killer. With the other conditions clinical symptoms will be apparent but a cat with HCM could appear normal and you wouldn’t know without testing. And remember - not all cardiac cases will have a heart murmur, either!
The other thing to keep in mind with Ragdolls is they seem to be more prone to developing Feline Infectious Peritonitis. This one is a little more complicated.
FIP occurs due to mutations in the feline coronavirus, which is a common viral infection in cats. Until recently FIP was considered nearly 100% fatal but thanks to Dr. Niels Pederson we now have a promising cure. One of our patients was actually one of the original study cats and has been doing well all this time, she’s amazing to work with - like a piece of living history!
One of the big problems with the treatment is the legality of it… for a long time it was only available through the black market and could easily run you thousands of dollars. Treatment is gradually becoming available through legal venues but it depends on where you live.
Anyways, the point is that we don’t know exactly why these mutations occur in some cats and not others but there’s strong evidence that there’s a genetic component - it’s common for related cats to develop FIP, we’ve seen this multiple times with littermates. We also know that it occurs at a higher incidence in some breeds than others…
And presumably your kitten wouldn’t have been exposed to feline coronavirus at the cattery but it’s not unlikely that they would be exposed at some point in their life given they don’t reside in a bubble… and if or when this happens what are the chances it’ll become the dreaded FIP?
So mitigating this risk when purchasing from a breeder can be a little more complicated… it’s not something I think should turn you off of the breed but it is something to keep in mind - and if you look into a breeder that seems good but has a lot of reviews or reports of their graduate kittens and cats developing FIP? Probably best to keep looking.
I think that’s a pretty good summary on the breed’s health but people are always welcome to chime in!
22 notes
·
View notes
Text
65,000 non - human primates are used in laboratory experiments every year in the united states
Each year, more than 110 million animals - including mice, rats, dogs, cats, rabbits, hamsters, fish and birds - are killed in U.S. laboratories for chemical, drug, food, and cosmetics testing. In order for a drug to be approved in the United States, the FDA typically requires toxicity tests on one rodent species such as a mouse or rat and one nonrodent species such as a monkey or dog.
Around 65,000 non - human primates (NHP) are used every year in the United States, and around 7,000 across the European Union. No new biomedical research projects have been approved on chimpanzees in the US since 2015.
Macaques are now the most commonly used NHP - most are imported from China and Cambodia.
The huge demand for research monkeys and their rising costs have created a market for monkey smugglers.
While most macaques imported by the US are identified as captive-bred on paper, some experts believe that many of those in US labs have been trafficked from the wild as the illegal trade in wild-caught macaques is widespread. Sources state that prices vary from $5 000 - $20 000 per monkey.
NHPs are used because of their similarities to humans with respect to genetic makeup, anatomy, physiology, and behavior which make it possible to approximate the human condition.
NHPs are used in research into HIV, neurology, behavior, cognition, reproduction, Parkinson's disease, stroke, malaria, respiratory viruses, infectious disease, genetics, xenotransplantation, drug abuse, and also in vaccine and drug testing.
The NIH is the largest public source of funding for biomedical research in the United States.
Last year new U.S. law eliminated the requirement that drugs in development must undergo testing in animals before being given to participants in human trials. It allows the U.S. Food and Drug Administration (FDA) to approve new drugs without requiring animal data.
Signed in December, the law doesn't ban the testing of new drugs on animals outright. Instead it simply lifts the requirement that pharmaceutical companies use animals to test new drugs before human trials. Companies can still test drugs on animals if they choose to.
And pro-research groups are downplaying the law, saying it signals a slow turning of the tide. Jim Newman, communications director at Americans for Medical Progress, which advocates for animal research, argues non-animal technologies are still “in their infancy” and won’t be able to replace animal models for “many, many years.” The FDA still retains tremendous discretion to require animal tests, he says.
- National Institutes of Health ( https://www.ncbi.nlm.nih.gov), Science Direct, World Animal Protection, science.org, National Anti - Vivisection Society and HSUS.
Image with kind permission from The Ethic Whisper.
@theethicwhisper

#vegan#veganism#animal rights#animal experiments#animal experimentation#animals in laboratories#ban animal experimentation
12 notes
·
View notes
Text
Flesh and Blood- [Five Hargreeves x F Reader]. Ch1 (Hard Feelings Part 3)
SUMMARY: As Christmas approaches, everything between you and Five is perfect...until a destructive temporal anomaly gets in the way. Five is convinced another permutation of himself is to blame. Nothing's simple when you're in a relationship Five Hargreeves: could your loyalties be tested in a way unique to him? On to Chapter 2 >>

Chriiissstmmaaassss.
Chapter One: Another Apocalypse
It’s Saturday morning. When you left him, the bedroom window and curtains were cracked so that pale-toned winter sunlight bathed the bed in a slanting shard. The chilly air felt pleasant on your skin and clean in your lungs, warm as you both were between the bedclothes.
He was asleep with his head turned from you, the light and shadow falling on his face. The fine hairs on his neck stood on end with the cold air. He had been snoring very lightly. The rays of light and very slight breeze tangled in his hair, fluttering it occasionally. You might have wanted him to wake, yet you could also watch him sleep for hours; you could be happy here, feeling his warmth.
But your bladder was no respecter of such sentimentality. It soon became imperative to leave the bed. After relieving yourself and taking a painkiller for a threatening headache, you’d make your way down to the kitchen and put on a pot of coffee.
You try to be quiet as you re-enter the bedroom but he stirs almost immediately.
“Mmm…coffee?” his voice is hazy.
“Yep. Good morning.”
“Morning, dear one. C’mere”
You put down the breakfast tray and rejoin him on the bed. He wraps his arms around you and you lay your head on his chest. He puts his mouth and nose against your hair and inhales.
It's been a blissful six months since the JUICED scandal. Since you started paying the (largely symbolic) rent to Reginald's estate, you'd felt better; stronger. As a result there's a new feeling between you; you can riff, harmonize and improvise around one another like a string duo- switching who plays the base notes as needed. True, it's not as if he's been seriously tested again since the JUICED scandal but, so far, it's been...nice.
Again, the breeze plays around your entwined bodies. He’s sure he can feel the rush of serotonin as he breaths in your scent…serotonin or love; call it what you want.
"How are you today?" he asks.
"Another headache."
"Really?" You can hear the worry in his voice.
"I took a painkiller: it's fine." then, to distract him, “How about we go out today?"
He grunts.
“Gonna need at least three coffees.”
You extract yourself from his arms and bring him over a cup. He takes a grateful sip.
“Ahhh. That’s good. Do I smell bagels?”
You hand him one plate and grab your own, sitting back down beside him with your own mug. For a few minutes, you eat and drink in companionable silence. Then, with your breakfast eaten, you turn to him.
“Shall we go Christmas shopping?”
He groans, “I think I'd rather scoop out my own testes with a grapefruit spoon.”
“I could arrange that for you?”
He grumbles. You kneel on the bed and swing one leg over him.
“Watch it!” he puts his coffee cup on the nightstand to avoid you knocking it out of his hands.
You sit on his knee, facing him.
“Come on,” you wheedle, “we can go and get cocoa and walk in the park and go to the German markets.”
“Kill me,” he groans but he’s smiling too, bringing his hands to your hips.
“You’re the one with the huge family to buy for. Let’s get all our gifts out of the way.”
He sighs, rolling his eyes.
“Fine.”
“Yes!” you say, pumping your fist, “but you have to promise not to be surly. Ooh, and let’s go ice skating!”
“NO ice skating!”
You laugh and kiss him. He responds enthusiastically, laughing a little into your mouth.
“I gotta draw the line at ice skating but I’ll do the rest.”

Five’s enjoying himself more than he wants to admit. Today, your joy is infectious in a way that makes you radiant.
His Christmases since arriving home had always been participated in out of obligation. He’d only really bought gifts for his nephew- he and his brothers didn’t often exchange them, although they all got together for a meal.
Though he’d been with you last year, you were still recovering in hospital from your encounter with Michael Monroe so hadn’t been able to go Christmas crazy...which he's just learning is natural to you.
Despite feeling slightly sick from the glühwein, this is undeniably pleasant. He even found himself fully engaged in picking out a gift for Lila, of all people. He'd even gone so far as to recommend one bracelet over another- and it was the bracelet he thought Lila would like more, too.
Now he’s standing in a store debating the merits of various gingerbread houses. He's laden with all your shopping bags as well as his own because you keep leaving them on the floor in your excitement to make the next purchase. If he were a less cynical man, he might call this adorable rather than annoying.
While Five valiantly tries to remain cynical, it’s hard. God knows he is not an easily led man, yet he's helplessly borne along in the wake of your excitement.
"It's style over substance," he says, indicating the giant gingerbread house you're standing beside, "if it's gonna get eaten then it's the taste that matters. Santi will demolish whatever we buy in five minutes anyway so what's the point?"
When you look at him, you're impassioned to a point that makes him want to laugh.
"Your shitty-ass gingerbread house doesn't even have a second floor. This is a gothic revival gingerbread house. Look at the windows! Look at the little wreath on the door! Look at the roof gables!"
"You're gonna eat it, not move in....and it's ninety dollars!"
"Oh fuck off. I've seen your bank balance, Five Hargreeves. This is Christmas."
He shakes his head at the absurdity of it all.
"You know, for an atheist with criticisms of capitalism, you're pretty into this."
You pout, forcing a smile from him. Despite this, he still tries to dissuade you.
"You know it will have gone stale by Christmas, anyway.
"You think I’m stupid? I'm not saying we get one now. I'm thinking to PRE-ORDER."
You give him a look of impatient, electrified enthusiasm, shining eyes bulging out of your head, eyebrows in your hairline and corners of your mouth turning down. You look entirely mad.
And then he’s impelled to take action by something stronger than his reason.
“You want to meet me on the square in an hour?” he asks
“Why?”
He tilts his head noncommittally.
“Maybe I’ve got…stuff to buy.”
“Hmm. Ok,” you say, grabbing him by the front of his coat, “maybe I got stuff to buy too.”
Then, you kiss him briefly on the lips.
And there's your smell, your soft lips, your smile….
He doesn’t consciously know where his feet are taking him until he’s there and staring in the window. How the hell has he come to this?

He’s not an easy man to buy for. His birthday back in October was tricky enough. He’s been experimenting more recently with clothing beyond suits...but you don’t just want to buy him a shirt or something: he's not your Dad. You find yourself in an antique bookstore with creaky floorboards. It smells strongly of furniture polish and beeswax. The mahogany counter and bookshelves shine with them.
It’s one of those places where the salespeople don’t fully trust you unless you look like a fellow collector. The tweed-suited man eyes you with benign suspicion as you enter. He takes his feet off his desk and stands to assist rather than letting you browse and potentially damage his stock.
“Good afternoon Ma'am."
"Hi," you smile.
"Are you looking for something in particular?”
“Uh- just a Christmas gift for my partner.”
The guy retains his polite smile, but you think you see something die behind his eyes nevertheless.
“Do you have anything in mind?”

You were overjoyed with your purchase. It had set you back a pretty penny, but it was more than worth it when you imagined his face. By the end of the encounter, the salesman had become much more unctuous.
One of the advantages of living all but rent-free in the family compound of an eccentric dead billionaire was being able to save pretty much your whole paycheck.This was aided by the fact that said paycheck had increased significantly a couple of months ago.
You'd finally achieved the promotion you privately thought you'd deserved for eighteen months. You'd like to think that the higher-ups simply noticed all your hard work but this would be optimism to the point of stupidity. You'd become a bit of an office celebrity since the JUICED scandal.
The domino mask you'd worn at the press-conference did not shield your identity from those who already knew you. You knew the news footage had been widely shared between whispering co-workers. For weeks afterwards, you'd catch people looking from you to their computers and back again. In addition, Neil from HR told a pretty convincing story about how he'd seen you meet and be driven away by 'that Hargreeves boy from the papers' in a reconditioned Corvette Stingray.
The book you'd bought for Five was a rare find and couldn’t be more perfect for him - it was beautiful, meaningful and came with that old-book smell that you’ve come to associate with him.
He collects voraciously, spending hours re-stitching broken bindings and restoring or replacing worn endsheets. Having lived most of his life in a ruined library where most of the books had been completely destroyed, he hoarded books on almost any subject. The older they were, the more he valued them. He's never confirmed this, but you think that perhaps his love of these aging survivors is a deeply personal identification.
Another headache has been threatening for the last quarter of an hour, so you sit down on a bench to wait for him. Shoppers pass with the bustle of human activity. You let your head lean forward a little and close your eyes.
And then, a rushing sound and whip-like crack.
You feel a ripple like electrical wind pass through your skin. Your stomach flips as if you’ve just missed a step walking downstairs. You and many of the people around you let out little exclamations of surprise- as you look sharply up, you see people's hair and shopping bags rustle as the almost-invisible force, (whatever it is), passes. A man standing a few feet away begins to scream. His body is caught in what looks like a film of blue light into which energy courses with a thrumming that hurts your already aching head. As he yells, fights and flails to free himself, it warps and flexes with his movements. Sparks fly with a rumbling sound like thunder.
You only have time to gasp in horror before Five blinks into being, still holding shopping bags. He raises his arms in an instinctive protective gesture, one over his own head and the other holding you back and behind him. You both watch as, in under a second, the void consumes the shrieking man and collapses in on itself with a buzz and flumping sound. Dropping the bags, Five’s hand smacks against his forehead.
“SHIT.”
People around you scream, the man who had been beside the void’s victim panics and yells:
“Kevin! KEVIN?”
Five ignores him and looks wildly around. He scans the sky, the ground, surrounding buildings and then the crowd. His body language has taken on that frenetic energy that comes over him when on the job. He pats down his own body, searching urgently.
“Pen. I need a pen. Anything.”
You pat your pockets uselessly, knowing there’s nothing there either.
“Why don’t I carry pens?”, his hand flies back to his forehead he looks around desperately, before yelling, “SHIT!” again.
“What is it?”
“I don’t…it can’t be…wait- is this stage one? No…because then I wouldn’t have asked that. Or is that what I want me to think?” he scratches his neck distractedly, his face lined with mistrust.
“Five?”
He begins to pace.
“It was me. I felt it.”
“What?”
His wild eyes find yours. He hesitates for a fraction of a second and then tells you:
“That was my power. I know the feeling. I felt it from across the street. That-” he points at the yelling man, “-was me. That was one of my temporal portals. What the hell do I think I’m doing?"
His hands come out to feel the air in front of him in the direction of the vanished portal. He draws in breath through his nose as if searching for a scent.
"It feels...like nonsense." His eyebrows contract even tighter. Again, his eyes rove your surroundings and then, finding nothing, he yells with frustration.
“WHERE ARE YOU, ASSHOLE?” he screams into the crowded street. After a few more moments of pacing, he snatches up the bags and grabs your hand.
“Come on, if he’s going to go anywhere, it'll be the Academy.”

Back at home, you sit downstairs in the living room. Five’s rapidly filling a notebook with scribbled calculations and mutters to himself compulsively. He’s been like this all the way home, speaking in random disconnected phrases that don’t mean anything and don’t seem to answer your questions: "Doesn't work with the fifth principle" or "Is this a Dallas permutation?"
“Five"
He jerks his head as if displacing an irksome fly.
"Can you explain this more?”
He holds up a finger imperiously and continues scribbling for a few seconds before looking up at you, his pen poised above the paper as if it’s taking all his self-control to pause its track across the page.
“I will. I promise. Just give me a few minutes. Get them all here- all of my brothers. Now. We need a meeting,” he holds your eyes for a second, clearly seeking affirmation that this satisfies you for now.
You nod your acceptance; your appreciation of this consideration.
Five took a lot of persuading to join the Hargreeves family group chat, but since giving in, he’s been a solid contributor. Now, as you message the group, your message appears right below one from this morning in which he joked that he used Lila’s lost razor to shave his balls.
You: Emergency meeting asap. Five says apocalypse-level shit. @all
Diego: Fuck.
Sloane: With you in 30 minutes
Viktor: Coming. 30 minutes too.
You: @Klaus??
Lila: Try the 3rd floor bathroom.
It took you having to nearly knock the door off its hinges to get Klaus to respond . He’d been listening to headphones and seemed mildly surprised when he popped his head around the door to find you looking exasperated. When, with a towel wrapped around his waist, you and he re-enter the living room, Lila and Diego are attempting to question an impatient Five, still scribbling incomprehensible math.
“Shut UP. I’m nearly done.”
Lila matches his exasperated tone.
“The hurry the fuck up!"
Finally, he throws the book down and stands.
“Okay: I’ll explain it to the others when they get here. We all need to be on the lookout for another me.”
Klaus and Diego let out sighs of frustrated weariness as Lila says:
“Oh great. Younger or older?”
“Your guess is as good as mine. All I know is, there’s a version of me running around making real shitty time portals to suck up Christmas shoppers."
“Why would you do that?” Diego asks, as if stung at Five’s behavior.
“I. don’t. know." the toes of one foot begin to tap, "I just know it was my power and the math on the relativity vector is nonsense.”
He runs his fingers through his hair, sweeping it out of his eyes.
“Time travel’s a crapshoot at the best of times but this…I’d barely even call this time travel. I doubt if that guy it caught even exists anymore. He’s probably in a thousand pieces all over the twelfth and thirteenth centuries.”
He turns to you, looking at you intensely.
“You were right there. Did you see where it came from? Did you see me?”
“No,” you say, “I felt it though. I felt it ripple.”
“Are you absolutely sure? Maybe a kid in the shorts, like in the painting? Or older, with a mustache, probably in a suit?”
You cast your mind back, “No, I didn’t see you. All I saw was the guy.”
He accepts this.
“All in all- this is not good. I know things are more flexible at the Commission now but Herb’s gotta be pissed about whatever I’m doing.”
He paces again, looking down at the last few pages of his notebook.
“This could be another apocalypse, people.”
“Really,” opines Klaus, “when I just got my hair nice?”

Again, Five sits behind his father’s desk across from Herb, whiskey poured for them both. He arrived within a second after Five used his personal pneumatic pipeline to contact him.
“I have to tell you Number Five, so far, we’re as clueless as you on this. The switchboard gave us the alert about the temporal anomaly but that’s as much as we know.”
He sips his drink, looking troubled. “Can you give me any insight on why a version of you might be running these ‘experiments’?”
“Wish I could Herb. The equations as far as I can detect them make no sense. I would have told you that I’d never try it...if I hadn’t seen it with my own eyes.”
He pushes his notebook across the desk to Herb. He scrutinizes a few pages of calculations, face the picture of confused concern. When he's seen enough, he looks back up at Five.
“You know we may have to take action on this.”
Five meets Herb’s eyes. It’s not a threat, not aggressive; he simply says it as an uncomfortable fact.
“Well it’s not me me. It’s different timeline me. I can promise you I don’t intend to start spitting out nonsense woodchipper time portals,"
Five placed his glass down on the desk, leaned back and sighed.
"He’s just likely to give you a lot of trouble.”
Herb just drinks his scotch, not meeting Five's eyes.

You lie in bed together that night.
“I need you to be vigilant,” says his voice, out of the dark, “the other versions of me…part of my power means we can exist almost independently of each other across different timestreams if we do the right math. At least...theoretically. I can’t answer for my motivations under different circumstances.”
“What do you mean? Vigilant?”
He sighs, “It might be a version of me that wouldn’t care if he hurt you.”
You stay silent.
“Maybe from before I met you. Or it could just be a me who’s traveled back. So you need to keep your eyes open.”
“Ok.”
“I need you to watch me closely too. If the other me gets too close, I’m going to develop paradox psychosis. I thought I felt a bit of it today- it’s what made me sure I was nearby. Problem is, the first stage of the psychosis is denial, so I won’t be much help when the time comes."
"Huh?
"You shouldn't really be around your doppelgangers. It's not good for you. There are seven stages you need to be on the look out for." He holds up his hands and counts them off on his fingers. "We have denial, itching, extreme thirst and urination, excessive gas, acute paranoia, uncontrolled perspiration and then homicidal rage."
You laugh nervously, "Sounds like your average Saturday night."
"Very funny." he says, though unamused, "If you see any of the warning signs, we’ll know I’m around. Then we can assess the situation and do what has to be done. I'll need you to keep a close eye on me. I might get...unmanageable but if I'll listen to anyone, I'll listen to you. ”
You lie there silently. Your overtaxed mind races. Homicidal rage? Versions of Five that could hurt you? He rolls over and turns to you, you feel his breath on your cheek.
“I know this is a lot to get your head around. I haven’t myself. But we’ll manage. Whatever it takes.”
Under the sheets, his hand strokes your hip.
Tag list: (please comment to be added or removed.) @dilfjohhny , @sunsunhe, @w4stedtr4sh, @nevbrooke-555, @theredvelvetbitch, @td-miley01, @five-hxrgreeves
On to Chapter 2 >> Masterpost
Alternatively, join me on A03. Here is a link to the whole series
#the umbrella academy smut#the umbrella academy five#the umbrella academy imagine#the umbrella academy#umbrella academy x reader#umbrella academy#umbrella academy smut#umbrella academy number five#umbrella academy five x you#umbrella academy five x reader#five hargreeves x reader#five hargreeves x you#number five imagine#five hargreeves smut#five hargreeves imagine#number five smut#number 5 imagine#number 5#fanfic#ao3 writer#read on ao3#tua fanfic#umbrella academy fanfic#five hargreaves x you#five hargreaves x reader#number 5 x reader#number 5 x you#flesh and blood#hard feelings
74 notes
·
View notes
Text
Kuiken said his concern about the risk that infected raw milk poses is not so much that the practice might somehow help the virus to mutate in ways that would allow it to spread easily to and among people — in other words, trigger a pandemic. But he believes it would likely seriously sicken people who drink raw milk from an H5N1-infected cow. Reports of the amount of virus present in infected udders is higher than anything he’s seen in studies where he’s experimentally infected animals with H5N1 to chart the illness the virus wreaked, Kuiken said.
Jürgen Richt, a veterinarian and director of the Center of Excellence for Emerging and Zoonotic Animal Diseases at Kansas State University’s College of Veterinary Medicine, spoke with a note of disbelief in his voice about the amount of dead viruses or viral particles being found in commercial milk that tested positive for the virus.
“From [results] I have seen, I wouldn’t want to drink raw milk,” Richt said. “And I wouldn’t feed it to my cats, nor my dogs, nor my calves, if I’m on a farm.”
The FDA is urging consumers not to drink raw milk or eat raw milk cheeses. That is a position the agency has long held, because of the other health risks these products hold, but it has re-emphasized it in the current context.
It has also recommended the dairy industry not “manufacture or sell raw milk or raw milk products, including raw milk cheese, made with milk from cows showing symptoms of illness, including those infected with avian influenza viruses or exposed to those infected with avian influenza viruses.”
Kuiken said he is less concerned about raw milk cheeses, saying the various processes involved in cheesemaking are “not conducive to survival of infectious virus.” He did suggest, though, that raw milk cheesemakers could be at risk, if they were inadvertently using milk laced with H5N1 virus.
Whether herds owned by farmers who sell raw milk have been infected by the virus isn’t publicly known. While authorities and scientists believe outbreaks are occurring over a much broader swathe of the country than has been detected, the U.S. Department of Agriculture has only confirmed infections of 34 herds in nine states — Texas, Kansas, Michigan, New Mexico, Idaho, Ohio, South Dakota, North Carolina, and Colorado. It has not given any details about the operations on which infected animals were found.
But the USDA has admitted some farmers have been refusing to test their animals. And analysis of the genetic sequences of viruses retrieved from cows combined with evidence of H5N1 RNA in commercial milk found in a number of U.S. markets — the FDA said Thursday that about 1 in 5 samples tested for H5N1 from across the country have been positive — bolster the argument that this has been going on for longer than has been recognized and likely involves far more herds than have tested positive.
The testing of commercial milk was done by polymerase chain reaction, or PCR. In PCR testing, the concentration of a pathogen is estimated by how many cycles the test has to run to find it. The lower the cycle threshold — known as a “Ct value” — the higher the concentration. Anything with a cycle threshold of 29 or lower is considered a strongly positive result. Some milk testing has shown a Ct value of below 10, Kuiken said.
2 notes
·
View notes
Text
The Weather
A study in Clinical Infectious Diseases reported “that the risk of developing symptomatic illness within 14 days was 5 times greater when contacts were exposed to an asymptomatic [COVID]-positive child in their household.” Nearly 11% of household contacts developed symptoms within 14 days of exposure. The study also found, during a 3-month follow-up, that 6 out of 77 asymptomatic children developed Long COVID. The likelihood of developing symptoms from asymptomatic exposure is higher than we might expect. Continue to spread awareness of asymptomatic spread and advocate for increased infection control measures at your local schools.
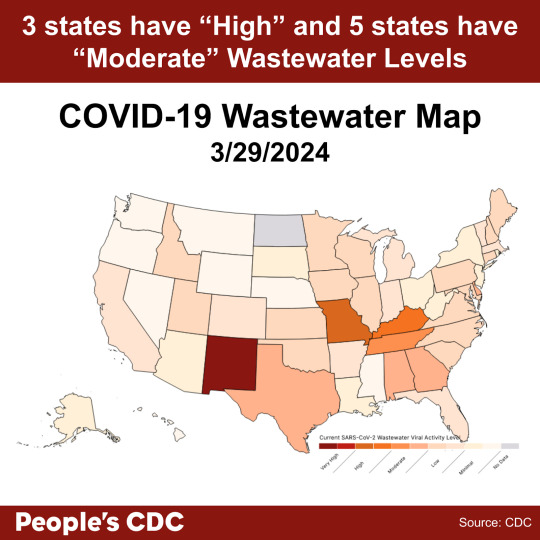
COVID wastewater levels are decreasing. As of 3/29/24, New Mexico is “Very High,” Arkansas and Kentucky are “High,” and the rest of the states are “Moderate” to “Low” levels of SARS-CoV-2 detected in wastewater.
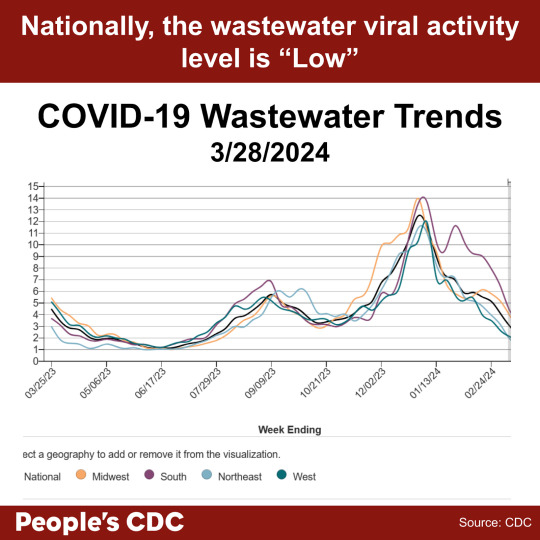
Wastewater levels continue to show a downward trend in the provisional data (gray shaded area) in all regions. The national wastewater levels are overall indicated as “Low.” While lower wastewater levels indicate decreased spread, it is important to continue to take precautions against infection. Holidays and spring breaks may bring people in closer proximity, so be sure to wear a mask to protect yourself and your community.
Wins
As we work to take more actions against the removal of vital public health measures, we remind you that you can still watch the recording of the People’s CDC press conference from March 13 and read the press release here. We would also like to remind you of the pre-proof of the People’s CDC External Review in the American Journal of Preventive Medicine Focus. The publication highlights the shortcomings of the CDC’s approach to public health and recommends a more equitable pandemic response.
News sources have published articles about the frustrations of people who continue to take COVID precautions. Time Magazine published an article presenting “both sides,” highlighting protest from people working with the CDC and concern from citizens and experts alike. While we are glad to see our voices be published in popular media, we are also saddened that “returning to normal” under economic and political pressure is so valued.
Treatments
Invyvid has received an FDA emergency use authorization for Pemgarda, a pre-exposure prophylaxis (PrEP) for people with immunocompromising conditions. Pemgarda is approved for people 12 and older with moderate to severe immunocompromise who are less likely to produce an adequate immune response to COVID vaccination alone. According to a press release from Invyvid, Pemgarda will release to market “imminently.”
Pre-exposure prophylaxis is commonly used for folks at high risk for exposure to HIV. As access to PrEP for HIV has been instrumental in keeping people safe, we hope that PrEP for COVID will be a useful tool for our community members with immune compromise. We also urge you to continue to wear high-quality masks and take other precautions to protect those most vulnerable.
Long COVID
People Magazine recently published an article highlighting an essay by Ziyad Al-Aly, physician and clinical epidemiologist, that pools data from several studies showing that COVID infection has lasting impacts on brain health. The review points out several impacts to cognitive functioning, including memory loss, spatial reasoning, and planning. Additionally, imaging studies have shown significant impact to brain tissue from inflammation, among other processes. The publication may be validating to those who experience lower cognitive function following COVID infection, including brain fog and memory dysfunction.
Take Action
We know that taking precautions–including masking, testing, and improving air quality–helps prevent the spread of airborne viral infection. Introducing more stringent precautions slowed outbreaks in the hematology ward of a hospital. The CDC recently released tips to improve ventilation. Help us urge the CDC to take other measures, including reinstating isolation periods.
Additionally, the home Test to Treat program is ending in April 2024. The program provides un-or-underinsured adults with free COVID and flu tests. If a participant in the program tests positive, they can also receive free healthcare via telehealth services. Join us to help save the program that helps so many at-risk people!
#op#covid#covid19#covid-19#covid 19#pcdc#people's cdc#long covid#covid pandemic#covid news#covid conscious#covid isn't over#pandemic#sars cov 2#coronavirus pandemic#coronavirus#sars-cov-2#cdc#prep#hiv#covid treatment#prep for covid#prep for covid-19#immunocompromised#disability#medical#uspol#img#links#described in alt text
2 notes
·
View notes
Text
Last week, in his State of the Union address, President Joe Biden told the American public that “we have broken COVID’s grip on us.” Highlighting the declines in the rates of COVID deaths, the millions of lives saved, and the importance of remembering the more than 1 million lost, Biden reminded the nation of what was to come: “Soon we’ll end the public-health emergency.”
When the U.S.’s state of emergency was declared nearly three years ago, as hospitals were overrun and morgues overflowed, the focus was on severe, short-term disease. Perhaps in that sense, the emergency is close to being over, Deeks told me. But long COVID, though slower to command attention, has since become its own emergency, never formally declared; for the millions of Americans who have been affected by the condition, their relationship with the virus does not yet seem to be in a better place.
Even with many more health-care providers clued into long COVID’s ills, the waiting lists for rehabilitation and treatment remain untenable, Hannah Davis told me. “I consider myself someone who gets exceptional care compared to other people,” she said. “And still, I hear from my doctor every nine or 10 months.” Calling a wrap on COVID’s “emergency” phase could worsen that already skewed supply-demand ratio. Changes to the nation’s funding tactics could strip resources—among them, access to telehealth; Medicaid coverage; and affordable antivirals, tests, and vaccines—from vulnerable populations, including people of color, that aren’t getting their needs met even as things stand, McCorkell told me. And as clinicians internalize the message that the coronavirus has largely been addressed, attention to its chronic impacts may dwindle. At least one of the country's long-COVID clinics has, in recent months, announced plans to close, and Davis worries that more could follow soon.
Scientists researching long COVID are also expecting new challenges. Reduced access to testing will complicate efforts to figure out how many people are developing the condition, and who’s most at risk. Should researchers turn their scientific focus away from studying causes and cures for long COVID when the emergency declaration lifts, Davids and others worry that there will be ripple effects on the scientific community’s interest in other, neglected chronic illnesses, such as ME/CFS (myalgic encephalomyelitis or chronic fatigue syndrome), a diagnosis that many long-haulers have also received.
The end of the U.S.’s official crisis mode on COVID could stymie research in other ways as well. At Johns Hopkins University, the infectious-disease epidemiologists Priya Duggal, Shruti Mehta, and Bryan Lau have been running a large study to better understand the conditions and circumstances that lead to long COVID, and how symptoms evolve over time. In the past two years, they have gathered online survey data from thousands of people who both have and haven’t been infected, and who have and haven’t seen their symptoms rapidly resolve. But as of late, they’ve been struggling to recruit enough people who caught the virus and didn’t feel their symptoms linger. “I think that the people who are suffering from long COVID will always do their best to participate,” Duggal told me. That may not be the case for individuals whose experiences with the virus were brief. A lot of them “are completely over it,” Duggal said. “Their life has moved on.”
Kate Porter, a Massachusetts-based marketing director, told me that she worries about her family’s future, should long COVID fade from the national discourse. She and her teenage daughter both caught the virus in the spring of 2020, and went on to develop chronic symptoms; their experience with the disease isn’t yet over. “Just because the emergency declaration is expiring, that doesn’t mean that suddenly people are magically going to get better and this issue is going to go away,” Porter told me. After months of relative improvement, her daughter is now fighting prolonged bouts of fatigue that are affecting her school life—and Porter isn’t sure how receptive people will be to her explanations, should their illnesses persist for years to come. “Two years from now, how am I going to explain, ‘Well, this is from COVID, five years ago’?” she said.
A condition that was once mired in skepticism, scorn, and gaslighting, long COVID now has recognition—but empathy for long-haulers could yet experience a backslide. Nisreen Alwan, a public-health researcher at the University of Southampton, in the U.K., and her colleagues have found that many long-haulers still worry about disclosing their condition, fearing that it could jeopardize their employment, social interactions, and more. Long COVID could soon be slated to become just one of many neglected chronic diseases, poorly understood and rarely discussed.
— Long COVID is the emergency that won’t end
#katherine j. wu#long covid is the emergency that won't end#current events#science#medicine#biology#human biology#disability#chronic illness#ableism#covid 19#pandemic#long covid
9 notes
·
View notes
Text
5 Points That Makes Veterinary Pathology Book by CBS Publishers & Distributors the Best
Veterinary pathology is a vital branch of veterinary medicine that deals with the study of diseases in animals. Pathology testing aids in the accurate diagnosis and treatment of your pet by your veterinarian. As a result, animals stay healthier for longer and don't experience needless delays in receiving the finest care for their disease.
Additionally, it prevents your pet from experiencing unwanted side effects from ineffective medications and enables you to learn more about how their treatment is working.
Also, veterinary pathology programs for disease screening and prevention that advance the general health and well-being of our community heavily rely on veterinary pathology. The pathology industry is contributing to a healthy future for all animals by minimising the effects of avoidable and treatable diseases in our pets.
All this hard work in veterinary pathology requires excellent knowledge and comprehension. This is why reading veterinary pathology books that help you gain in-depth knowledge regarding the same is extremely important. While there are many great books available in the market, there is a place only for one winner.

The Veterinary Pathology book by CBS Publishers & Distributors is surely it. This book is a comprehensive guide that covers all the essential aspects of veterinary pathology. Here are five points that make this book the best choice for anyone interested in veterinary pathology:
1. Comprehensive coverage: The Veterinary Pathology book covers all the major organ systems in animals, including the respiratory, cardiovascular, digestive, urinary, and reproductive systems. It surely is your one-stop solution for all the apprehensions about an animal’s body. It also covers various infectious diseases, neoplastic diseases, and inherited disorders. This is especially beneficial owing to the post covid times, where animals have been seen to undergo several side effects. This comprehensive coverage makes it an excellent resource for veterinarians, veterinary students, and animal health professionals.
2. Easy to understand: while reading any course book, the primary concern is how easily apprehensive the book is at a solitary level. The book is written in a clear and concise manner, making it easy to understand even for those with little or no knowledge of veterinary pathology. Be it a beginner or a pro, everyone can refer to this book, owing to its high standard of academic adjustments as well as basic knowledge cognizance. It uses illustrations and diagrams to explain complex concepts and processes, making it more engaging and interactive. This is especially beneficial for all the visual learners out there!
3. Up-to-date information: The book is regularly updated with the latest research and advances in the field, ensuring that the information provided is accurate and current. Many researchers and scientists are affiliated with CBS Publishers & Distributors. They make sure that every new version is equipped with the latest and most relevant knowledge. This gives an edge to veterinary pathology professionals, helping them understand everything in a more holistic manner. It also includes case studies and examples to help readers apply the concepts they have learned. A total winner for all healthcare providers.
4. Practical approach: while theoretical knowledge is very important, it cannot beat a practical setting. The Veterinary Pathology book takes a practical approach to teach, making it ideal for the students to have a practical outlook on everything they read. It helps in providing readers with the skills and knowledge they need to apply what they have learned in real-life situations. It includes chapters on diagnostic techniques, such as histopathology, cytology, and molecular diagnostics, helping readers develop the skills they need to diagnose and treat diseases in animals.
5. Comprehensive resources: In addition to the extensive information provided in the book, CBS Publishers & Distributors also offer a range of additional resources to supplement the learning experience. These include online quizzes, study guides, and practice tests, making it easier for readers to test their understanding and prepare for exams. This gives them an edge of advantage over the other available set of Veterinary Pathology books.
Overall, the Veterinary Pathology book by CBS Publishers & Distributors is the perfect one-stop solution book for anyone interested in knowing about veterinary pathology. It is an excellent resource for people who are healthcare animal professionals as well. Its comprehensive coverage, easy-to-understand writing style, and practical approach make it the best choice for veterinarians, veterinary students, and animal health professionals.
2 notes
·
View notes
Text
Lyndata a Creative Web Development Company...
●
PATHNET HEALTHCARE
NOW LIVE...
www.pathnethealthcare.com
AN INFORMATIVE WEBSITE BY LYNDATA.COM
●
"We offer Diagnostic services by performing tests on State-of -Art equipment in shortest possible Turn-around-time with cutting edge technology and easy affordability we are committed to set new standards in Preventive medicine, women’s healthcare, infectious diseases, genetics and cancer, we are advancing the course of modern medicine. In our pursuit for excellence in Diagnostic, Our well-trained technical staff strives to continuously upgrade its knowledge base & engage in accepting and implementing of new techniques on regular basis.".
●
Who are we ?
We Are Lyndata a Professional Team with more than 7 Years experienced in Web Development and Digital Marketing operating, from Lucknow , #India.
●
What are we famous for?
We provide Web Development and Digital Marketing Services with utmost customer support.
●
VISIT OUR SITE👇
www.lyndata.com
#CallUs 👇
955 949 1123

#branding#ecommerce#ecommerce web development#ecommerceportal#grapicdesign#logo design#lyndataindia#paymentintegration#trending#web development company#digital marketing#website development#tumblrpost#artists on tumblr
3 notes
·
View notes
Text
Comprehensive Overview of the Latex Agglutination Test Kits Market: Opportunities & Trends
The latex agglutination test kits global market report 2024from The Business Research Company provides comprehensive market statistics, including global market size, regional shares, competitor market share, detailed segments, trends, and opportunities. This report offers an in-depth analysis of current and future industry scenarios, delivering a complete perspective for thriving in the industrial automation software market.
Latex Agglutination Test Kits Market, 2024report by The Business Research Company offers comprehensive insights into the current state of the market and highlights future growth opportunities.
Market Size - The latex agglutination test kits market size has grown strongly in recent years. It will grow from $1.39 billion in 2023 to $1.49 billion in 2024 at a compound annual growth rate (CAGR) of 7%. The growth in the historic period can be attributed to growth in the prevalence of infectious diseases, increased funding for research and development in diagnostics, increased focus on infection control measures in healthcare facilities, and increased adoption of latex agglutination tests in water quality testing.
The latex agglutination test kits market size is expected to see strong growth in the next few years. It will grow to $1.96 billion in 2028 at a compound annual growth rate (CAGR) of 7.1%. The growth in the forecast period can be attributed to the rising prevalence of infectious and autoimmune diseases, the rising adoption of rapid and POC technology, the increasing adoption of point-of-care diagnostics, growing awareness about sexually transmitted infections, and the and the rising incidence of respiratory tract infections. Major trends in the forecast period include technological advancements, test strip technology, antigen tests, automated technologies, and point-of-care testing.
Order your report now for swift delivery @ https://www.thebusinessresearchcompany.com/report/latex-agglutination-test-kits-global-market-report
Scope Of Latex Agglutination Test Kits MarketThe Business Research Company's reports encompass a wide range of information, including:
1. Market Size (Historic and Forecast): Analysis of the market's historical performance and projections for future growth.
2. Drivers: Examination of the key factors propelling market growth.
3. Trends: Identification of emerging trends and patterns shaping the market landscape.
4. Key Segments: Breakdown of the market into its primary segments and their respective performance.
5. Focus Regions and Geographies: Insight into the most critical regions and geographical areas influencing the market.
6. Macro Economic Factors: Assessment of broader economic elements impacting the market.
Latex Agglutination Test Kits Market Overview
Market Drivers -The increasing prevalence of infectious diseases is expected to propel the growth of the latex agglutination test kit market going forward. Infectious diseases are disorders caused by pathogenic microorganisms such as bacteria, viruses, fungi, parasites, or prions. The increasing prevalence of infectious diseases can be attributed to factors such as globalization, antimicrobial resistance, urbanization, climate change, and population growth. Latex agglutination tests are commonly used for the rapid detection of various infectious diseases, such as bacterial and viral infections. For instance, in July 2023, according to UNAIDS, the Switzerland-based UN program dedicated to addressing the global HIV/AIDS epidemic, the global population living with HIV increased to 39 million in 2022, up 3.17% from 37.7 million in 2020. Moreover, there were approximately 1.3 million new HIV infections reported in 2022. Furthermore, in March 2023, according to the Centers for Disease Control and Prevention, a US-based national public health organization, in the United States, 8,331 cases of tuberculosis (TB) were reported in 2022, a rise of 5.9% from 7,874 cases in 2021. Therefore, the increasing prevalence of infectious diseases will drive the growth of the latex agglutination test kit market.
Market Trends - Major companies operating in the latex agglutination test kits market are focusing their efforts on introducing diagnostic technologies for foodborne illnesses and environmental contaminants, such as rapid and user-friendly latex agglutination assays, to enhance disease diagnosis and gain a competitive edge in the market. Rapid and user-friendly latex agglutination assays streamline pathogen detection, offering quick and straightforward identification in food and environmental samples. For instance, in March 2024, Gold Standard Diagnostics LLC, a US-based diagnostic company, launched Microgen rapid latex agglutination tests, a set of rapid, easy, and inexpensive techniques created by Gold Standard Diagnostics for the confirmation diagnosis of particular pathogens in food and environmental samples, such as Salmonella, Legionella, or Staphylococcus aureus colonies. These assays offer a very sensitive and specific substitute for conventional culture procedures, and they may be completed in as little as two minutes. They are also easily integrated into laboratory workflows. These tests are so easy to use that labs can start using them right away without the need for specific training or equipment.
The latex agglutination test kits market covered in this report is segmented –
1) By Product Type: Enzyme Linked Immunosorbent Assay, Indirect Fluorescent, Hemagglutination Inhibition, Serum Neutralization 2) By Test Type: Antibody Detection, Antigen Testing 3) By Sample Type: Blood, Urine, Cerebrospinal Fluid, Other Sample Types 4) By Application: Hospitals, Diagnostic Centers, Specialty Clinics
Get an inside scoop of the latex agglutination test kits market, Request now for Sample Report @ https://www.thebusinessresearchcompany.com/sample.aspx?id=15597&type=smp
Regional Insights - North America was the largest region in the latex agglutination test kits market in 2023. Asia Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the latex agglutination test kits market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Key Companies - Major companies operating in the latex agglutination test kits market are Cardinal Health Inc., Thermo Fisher Scientific Inc., Becton Dickinson and Company, bioMérieux SA, Bio-Rad Laboratories Inc., Hardy Diagnostics, Meridian Bioscience Inc., BioLegend Inc., ELITechGroup, Fujirebio, Sekisui Diagnostics LLC, R-Biopharm AG, Savyon Diagnostics Ltd., Creative Diagnostics, ZeptoMetrix Corporation, HiMedia Laboratories, DIALAB GmbH, Arlington Scientific Inc., Biotium Inc., Atlas Medical GmbH, Pro Lab Diagnostics Inc., Gold Standard Diagnostics Industrial Solutions
Table of Contents 1. Executive Summary 2. Latex Agglutination Test Kits Market Report Structure 3. Latex Agglutination Test Kits Market Trends And Strategies 4. Latex Agglutination Test Kits Market – Macro Economic Scenario 5. Latex Agglutination Test Kits Market Size And Growth ….. 27. Latex Agglutination Test Kits Market Competitor Landscape And Company Profiles 28. Key Mergers And Acquisitions 29. Future Outlook and Potential Analysis 30. Appendix
Contact Us: The Business Research Company Europe: +44 207 1930 708 Asia: +91 88972 63534 Americas: +1 315 623 0293 Email: [email protected]
Follow Us On: LinkedIn: https://in.linkedin.com/company/the-business-research-company Twitter: https://twitter.com/tbrc_info Facebook: https://www.facebook.com/TheBusinessResearchCompany YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ Blog: https://blog.tbrc.info/ Healthcare Blog: https://healthcareresearchreports.com/ Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
In Vitro Diagnostics Market Growth to Reach $116.28 Billion by 2031, at a CAGR of 5.3%
Increased demand for early disease diagnosis, personalized medicine, and point-of-care solutions drive IVD market growth.
According to a new report published by Meticulous Research®, the global in vitro diagnostics (IVD) market is poised for substantial growth, projected to reach $116.28 billion by 2031, expanding at a CAGR of 5.3% from 2024. The report, titled In Vitro Diagnostics (IVD) Market—Global Opportunity Analysis and Industry Forecast (2024-2031), delves into the key trends, technological advancements, and growth drivers shaping the IVD industry.
For detailed insights, request the sample report here: https://www.meticulousresearch.com/request-sample-report/cp_id=4858
Key Market Drivers and Growth Opportunities
The IVD market’s expansion is fueled by multiple factors, including a surge in chronic disease prevalence, a growing elderly population, and the rising incidence of infectious diseases. Increasing healthcare expenditure, advancements in genomics and proteomics, and rising awareness about early disease diagnosis have also significantly impacted market growth. The need for faster diagnostic solutions in clinical settings, particularly point-of-care (POC) diagnostics, has gained momentum in recent years, contributing to the rapid adoption of IVD technologies.
Furthermore, the growing interest in personalized medicine, especially in oncology and infectious disease management, has spurred demand for molecular diagnostics and advanced testing solutions. The availability of IVD products that can provide rapid, reliable results supports the increasing reliance on diagnostics for personalized treatment plans. The report offers an in-depth look at these market drivers and emerging opportunities.
For a more detailed analysis, you can download a sample report here: https://www.meticulousresearch.com/download-sample-report/cp_id=4858
Key Market Segments
Meticulous Research® segments the IVD market based on offering, technology, application, diagnostic approach, and end user:
Offering: The reagents & kits segment is projected to account for the largest share of the IVD market (81.1%) by 2024. The segment's prominence is attributed to the recurring use of assays and kits for chronic and infectious disease detection, as well as the increasing demand for self-testing solutions in emerging economies.
Technology: Molecular diagnostics is expected to be the leading technology segment, accounting for 23.1% of the market in 2024. The increasing prevalence of infectious diseases, coupled with advancements in automated diagnostic systems, has amplified the adoption of molecular diagnostics in clinical and research settings.
Application: The infectious diseases application segment is anticipated to dominate the market in 2024, with a 37.8% share. Heightened awareness around early diagnosis, government initiatives, and public health campaigns have all contributed to the growth of this segment.
For a customized view of the IVD market lndscape, tailored to specific business needs, visit: https://www.meticulousresearch.com/request-customization/cp_id=4858
Regional Market Analysis
The global IVD market spans major regions, including North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. North America is anticipated to lead the market, holding a 37.5% share in 2024. The region’s significant market share is driven by a high prevalence of chronic diseases, widespread adoption of diagnostic technologies, and robust funding for healthcare research. Advanced healthcare infrastructure and favorable government policies further support the region's market dominance.
In Asia-Pacific, the IVD market is also gaining momentum, with increasing healthcare expenditure and rising awareness about early disease detection in emerging economies. Countries like China, Japan, and India are projected to experience substantial growth due to increasing demand for POC diagnostic solutions and personalized medicine.
To access a comprehensive breakdown of regional market dynamics, you can download the https://www.meticulousresearch.com/download-sample-report/cp_id=4858
Challenges and Opportunities
While the IVD market is positioned for significant growth, challenges persist. Regulatory hurdles and technical complexities in developing high/moderate-complexity tests can impede market progress. Inconsistencies in rapid test results and shifting regulatory landscapes pose additional challenges for manufacturers. However, with the rising need for accurate, fast diagnostics, companies are investing in research and development to overcome these obstacles and improve product quality.
Opportunities exist for expanding into untapped markets in developing regions, where healthcare infrastructure is evolving, and demand for diagnostic solutions is increasing. Additionally, advances in genomics and proteomics open new pathways for targeted diagnostics and treatment, benefiting both patients and healthcare providers.
For an in-depth examination of market challenges and strategic growth opportunities, https://www.meticulousresearch.com/request-sample-report/cp_id=4858
Competitive Landscape
The report also provides a detailed analysis of the competitive landscape, highlighting key players shaping the IVD market:
Abbott Laboratories (U.S.)
Becton, Dickinson and Company (U.S.)
bioMérieux SA (France)
Danaher Corporation (U.S.)
F. Hoffmann-La Roche Ltd. (Switzerland)
Thermo Fisher Scientific Inc. (U.S.)
Siemens Healthineers AG (Germany)
These companies are at the forefront of innovation, leveraging partnerships, acquisitions, and advanced diagnostic technologies to enhance their market presence. For instance, in 2022, QIAGEN N.V. launched the therascreen EGFR RGQ PCR Kit for detecting EGFR mutations, showcasing how new products continue to drive growth within the IVD sector. Strategic alliances, regional expansions, and R&D investments are common among these market leaders as they aim to address evolving diagnostic needs.
Future Outlook for the IVD Market
The future of the IVD market is promising, with technological advancements driving innovation across applications and use cases. The increasing adoption of digital and automated diagnostic solutions, including artificial intelligence in diagnostics, is expected to enhance the accuracy and speed of diagnostic testing. These advancements, coupled with a growing emphasis on personalized medicine, position the IVD market for sustained growth over the next decade.
Furthermore, as healthcare providers worldwide prioritize early diagnosis and preventive healthcare, the demand for advanced diagnostic tools will likely continue to rise. This growth trajectory presents substantial opportunities for both established players and emerging companies seeking to innovate within the sector.
Download the full report to gain in-depth insights into the trends, drivers, and future outlook of the global in vitro diagnostics market: https://www.meticulousresearch.com/download-sample-report/cp_id=4858
Contact Us: Meticulous Market Research Pvt. Ltd. 1267 Willis St, Ste 200 Redding, California, 96001, U.S. Email- [email protected] USA: +1-646-781-8004 Europe: +44-203-868-8738 APAC: +91 744-7780008 Visit Our Website: https://www.meticulousresearch.com/ For Latest Update Follow Us: LinkedIn- https://www.linkedin.com/company/meticulous-research Meticulous Blog | Top Market Research Reports Blog - https://meticulousblog.org/
0 notes
Text
Also preserved on our archive (Daily updates!)
By Stephani Sutherland
Gentle nasal spray vaccines against COVID, the flu and RSV are coming. They may work better than shots in the arm
Alyson Velasquez hates needles. She never liked getting shots as a kid, and her anxiety only grew as she got older. “It really ballooned in my teens and early 20s,” she says. “It became a full-blown phobia.” She would panic at the sight of a needle being brought into an exam room; more than once she passed out. Velasquez says that she took an antianxiety medication before one appointment yet still ran around the room screaming inconsolably “like I was a small child; I was 22.” After that episode Velasquez, now a 34-year-old financial planner in southern California, quit needles completely. “No vaccinations, no bloodwork. For all of my 20s it was a no-go for me,” she says.
Then COVID showed up. “It finally hit a point where it wasn’t just about me,” Velasquez says. “It felt so selfish not to do this for the greater public health and the safety of our global community.” So she got vaccinated against the SARS-CoV-2 virus in 2021, although she had to sit on her husband’s lap while he held her arms. “It was a spectacle. The poor guy at CVS ... he did ask me, ‘Are you sure you want to do this?’” She very much did. “I’m very pro-vaccine. I am a rational human. I understand the necessity of [getting] them,” she insists. But today she still struggles with each injection.
Those struggles would end, however, if all her future vaccinations could be delivered by a nasal spray. “Oh, my God, amazing!” Velasquez says.
The amazing appears to be well on its way. Vaccines delivered through the nose are now being tested for several diseases. In the U.S., early clinical trials are showing success. Two of these vaccines have generated multiple immune system responses against the COVID-causing virus in people who received them through a puff up the nose; earlier this year their makers received nearly $20 million from Project NextGen, the Biden-Harris administration’s COVID medical initiative. Researchers are optimistic that a nasal spray delivering a COVID vaccine could be ready for the U.S. as soon as 2027. Although recent efforts have focused on inoculations against SARS-CoV-2, nasal vaccines could also protect us against the flu, respiratory syncytial virus (RSV), and more.
A few nasal vaccines have been introduced in the past, but they’ve been beset by problems. The flu inoculation FluMist has not gained popularity because of debates about its effectiveness, and a different vaccine was pulled from the market decades ago because some people had serious side effects. In China and India, nasal vaccines for COVID have been approved because those countries prioritized their development during the pandemic, whereas the U.S. and other wealthy nations opted to stick with arm injections. But this new crop of vaccines takes advantage of technology that produces stronger immune responses and is safer than preparations used in the past.
In fact, immunologists say these spritzes up the nose—or inhaled puffs through the mouth—can provide faster, stronger protection against respiratory viruses than a shot in the arm. That is because the new vaccines activate a branch of the immune system that has evolved for robust, rapid responses against airborne germs. “It may be more likely to really prevent infection from getting established,” says Fiona Smaill, an infectious disease researcher at McMaster University in Ontario. Such inoculations may also help reduce the enormous inequities in vaccine access revealed by the pandemic. These formulations should be cheaper and easier to transport to poor regions than current shots.
But nasal vaccines still face technical hurdles, such as how best to deliver them into the body. And unlike injected vaccines, which scientists can measure immune responses to with blood tests alone, testing for immunity that starts in nose cells is more challenging. But researchers working in this field agree that despite the hurdles, nasal formulations are the next step in vaccine evolution.
Traditional vaccines injected through the skin and into an arm muscle provide excellent protection against viruses. They coax immune cells into making widely circulated antibodies—special proteins that recognize specific structural features on viruses or other invading pathogens, glom on to them and mark them for destruction. Other immune cells retain a “memory” of that pathogen for future encounters.
Intramuscular injection vaccines are good at preventing a disease from spreading, but they do not stop the initial infection. A nasal spray does a much better job. That’s because sprays are aimed directly at the spot where many viruses first enter the body: the nose and the tissue that lines it, called the mucosa.
Mucosa makes up much of our bodies’ internal surfaces, stretching from the nose, mouth and throat down the respiratory tract to the lungs, through the gastrointestinal tract to the anus, and into the urogenital tract. Mucosa is where our bodies encounter the vast majority of pathogenic threats, Smaill says, be it flu, COVID, or bacterial infections that attack the gut. This tough, triple-layered tissue is specialized to fight off invaders with its thick coating of secretory goo—mucus—and with a cadre of resident immune cells waiting to attack. “Mucosa is really the first line of defense against any infection we’re exposed to,” Smaill says.
Mucosal immunity not only prepares the immune system for the fight where it occurs but also offers three different types of protection—at least one more than a shot does. Nasal vaccines and shots both mobilize immune messenger cells, which gather the interlopers’ proteins and display them on their surfaces. These cells head to the lymph nodes, where they show off their captured prize to B and T cells, which are members of another part of the immune system called the adaptive arm. B cells, in turn, produce antibodies, molecules that home in on the foreign proteins and flag their owners—the invading microbes—for destruction. Killer T cells directly attack infected cells, eliminating them and the microbes inside. This provides broad protection, but it takes time, during which the virus continues to replicate and spread.
That’s why a second type of protection, offered only by the mucosal tissue, is so important. The mucosa holds cells of the innate immune system, which are the body’s “first responders.” Some of these cells, called macrophages, recognize invasive microbes as foreign and swallow them up. They also trigger inflammation—an alarm sounded to recruit more immune cells.
Another part of this localized response is called tissue-resident immunity. These cells don’t have to detect telltale signs of a pathogen and make a long journey to the infected tissue. They are more like a Special Forces unit dropped behind enemy lines where a skirmish is occurring rather than waiting for the proverbial cavalry to arrive. This localized reaction can be quite potent. Its activation is notoriously difficult to demonstrate, however, so historically it’s been hard for vaccine makers to show they’ve hit the mark. But it turns out that one type of antibody, called IgA, is a good indicator of mucosal immunity because IgAs tend to predominate in the mucosa rather than other parts of the body. In an early trial of CoviLiv, a nasal COVID vaccine produced by Codagenix, about half of participants had detectable IgA responses within several weeks after receiving two doses. That trial also showed the vaccine was safe and led to NextGen funding for a larger trial of the vaccine’s efficacy.
It’s possible an inhaled vaccine may provide yet one more layer of protection, called trained innate immunity. This reaction is a bit of a mystery: although immunologists know it exists and appears also to be produced by intramuscular injections, they can’t quite explain how it works. Immune cells associated with trained innate immunity seem to have memorylike responses, reacting quickly against subsequent infections. They also have been found to respond against pathogens entirely unrelated to the intended vaccine target. Smaill and her colleagues found that when they immunized mice with an inhaled tuberculosis vaccine and then challenged them with pneumococcal bacteria, the mice were protected. In children, there is some evidence that a tuberculosis vaccine, in the arm, generates this type of broad response against other diseases.
Akiko Iwasaki, an immunologist at Yale University who is working to develop a nasal vaccination for COVID, sees two major potential benefits to nasal immunity in addition to better, faster, more localized protection. First, attacking the virus in the nose could prevent the disease from being transmitted to others by reducing the amount of virus that people breathe out. And second, Iwasaki says, the spray may limit how deeply the infection moves into the body, so “we believe that it will also prevent long COVID.” That debilitating postinfection condition, sometimes marked by signs of entrenched viral particles, disables people with extreme fatigue, chronic pain, a variety of cognitive difficulties, and other symptoms.
Making a new vaccine is hard, regardless of how you administer it. It needs to raise an immune response that’s strong enough to protect against future invasions but not so strong that the components of that response—such as inflammation and fever—harm the host.
The lining of the nose puts up its own barriers—literal, physical ones. Because the nasal mucosa is exposed to so many irritants from the air, ranging from pet hair to pollen, the nose has multiple lines of defense against invading pathogens. Nostril hair, mucus, and features called cilia that sweep the nasal surface all aim to trap small foreign objects before they can get deeper into the body—and that includes tiny droplets of vaccine.
And lots of small foreign particles—often harmless—still make it through those defenses. So the nose has developed a way to become less reactive to harmless objects. This dampened reactivity is called immunological tolerance, and it may be the biggest hurdle to successful development of a nasal vaccine. When foreign particles show up in the bloodstream, a space that is ostensibly sterile, immune cells immediately recognize them as invaders. But mucosal surfaces are constantly bombarded by both pathogens and harmless materials. The immune system uses tolerance—a complex series of decisions carried out by specialized cells—to determine whether a substance is harmful. “This is very important because we can’t have our lungs or gastrointestinal tract always responding to nonharmful foreign entities that they encounter,” says Yale infectious disease researcher Benjamin Goldman-Israelow. For example, inflammation in the lungs would make it hard to breathe; in the gut, it would prevent the absorption of water and nutrients.

These barriers may hamper the effectiveness of a nasal flu vaccine that’s been around for a while, called FluMist in the U.S. and Fluenz in Europe. The inoculation is safe, says infectious disease scientist Michael Diamond of Washington University in St. Louis, but it faces a similar problem as do injected flu vaccines: it isn’t very effective at warding off new seasonal flu strains. This might be because flu strains are so common, and people are frequently infected by the time they are adults. Their immune systems are already primed to recognize and destroy familiar flu particles. FluMist is built from a live flu virus, so immune cells probably treat the vaccine as an invader and demolish it as soon as it shows up in the nose, before it has a chance to do any good. This preexisting immunity isn’t such an issue in children, who are less likely to have had multiple flu infections. Nasal flu vaccines are routinely used to inoculate kids in Europe.
In other vaccines, researchers often use adjuvants, special agents that attract the attention of immune cells, to boost a response. Some nasal vaccines use adjuvants to overcome tolerance, but in the nose, adjuvants can pose unique dangers. In at least one case, a nasal adjuvant led to disastrous consequences. An intranasal vaccine for influenza, licensed in Switzerland for the 2000–2001 season, used a toxin isolated from Escherichia coli bacteria as an adjuvant to provoke a reaction to the inactivated virus. No serious side effects were reported during the trial period, but once the vaccine was released, Swiss officials saw a concerning uptick in cases of Bell’s palsy, a disease that causes weakness or paralysis of the facial muscles, often leading to a drooping or disfigured face. Researchers at the University of Zurich estimated that the adjuvanted flu vaccine had increased the risk of contracting Bell’s palsy by about 20 times, and the vaccine was discontinued. “We need to be cautious about using adjuvants like that from known pathogens,” says pharmaceutical formulations scientist Vicky Kett of Queen’s University Belfast in Northern Ireland.
To get around the challenges posed by the nose, some researchers are exploring vaccines inhaled through the mouth. Smaill is working on one of them. She and her McMaster colleagues aerosolized their vaccine for COVID into a fine mist delivered by a nebulizer, from which it rapidly reaches the lungs. Experiments in mice have shown promising results, with mucosal immunity established after administration of the vaccine.
Another vaccine strategy is to use a harmless virus to carry viral genes or proteins. Researchers at the Icahn School of Medicine at Mount Sinai in New York City selected a bird pathogen, Newcastle disease virus (NDV). “It’s naturally a respiratory pathogen,” so it infects nasal cells, says Michael Egan, CEO and chief scientific officer of CastleVax, a company that formed to develop the NDV vaccine for COVID. A small early clinical trial showed the CastleVax vaccine was safe and caused robust immune responses in people. “Those results were very promising,” Egan says. People who received the vaccine also produced antibodies that indicated multitiered mucosal immunity, not simply the adaptive immunity from a shot in the arm.
Following that trial, the CastleVax project received NextGen funding, and results from a trial of 10,000 people are expected in 2026. Half of those people will receive a messenger RNA (mRNA) injection, and half will get the new NDV nasal spray. The data should show whether the new nasal vaccine can do a better job of preventing infection than the mRNA injections. Egan has high hopes. “We’re expecting to see a lot fewer breakthrough infections in people who got the vaccine up the nose by virtue of having those mucosal immune responses,” he says.
Florian Krammer, one of the Mount Sinai researchers behind the vaccine, engineered NDV particles to display a stabilized version of the spike protein that’s so prominent in SARS-CoV-2. “You end up with a particle that’s covered with spike,” he says. Spike protein in the bloodstream can raise an immune response. But the NDV vaccine works in another way, too. The virus particle can also get into cells, where it can replicate enough times to cause virus particles to emerge from the cells, provoking another immune reaction. Before moving into human trials, however, researchers had to complete clinical trials to establish that the Newcastle virus is truly harmless because the nose is close to the central nervous system—it has neurons that connect to the olfactory bulb, which is part of the brain. Those trials confirmed that it is safe for this use.
Nasal sprays aim directly at the spot where most viruses first enter the body: the nose. This type of caution is one reason a COVID nasal vaccine approved in India hasn’t been adopted by the U.S. or other countries. The inoculation, called iNCOVACC, uses a harmless simian adenovirus to carry the spike protein into the airway. The research originated in the laboratories of Diamond and some of his colleagues at Washington University at the start of the pandemic, when they tested the formulation on rodents and nonhuman primates. “The preclinical data were outstanding,” Diamond says. Around the time he and his colleagues published initial animal results in Cell in 2020, Bharat Biotech in India licensed the idea from the university. In a 2023 phase 3 clinical trial in India, the nasal vaccine produced superior systemic immunity compared with a shot.
Diamond says American drug companies didn’t pursue this approach, because “they wanted to use known quantities,” such as the mRNA vaccines, which were already proving themselves in clinical trials in 2020. As the pandemic took hold, there was little appetite to develop nasal vaccine technology to stimulate mucosal immunity while the tried-and-true route of shots in the arm was available and working. But now, four years later, an inhaled vaccine using technology similar to iNCOVACC’s is being developed for approval in the U.S. by biotech company Ocugen. Both inhaled and nasal forms of the vaccine are set to undergo clinical trials as part of Project NextGen. These new vaccines are using classical vaccine methods based on the virus rather than using new, mRNA-based technology. The mRNA preparations were developed specifically for intramuscular injections and would have to be significantly modified.
Codagenix, which is developing CoviLiv, sidestepped the need for a new viral vector or an adjuvant by disabling a live SARS-CoV-2 virus. To make it safe, scientists engineered a version of the virus with 283 mutations, alterations to its genetic code that make it hard for the virus to replicate and harm the body. Without all these genetic changes, there would be a chance the virus could revert to a dangerous, pathogenic form. But with hundreds of key mutations, “statistically, it’s basically impossible that this will revert back to a live virus in the population,” says Johanna Kaufmann, who helped to develop the vaccine before leaving Codagenix for another company earlier this year.
Because most people on the planet have now been exposed to SARS-CoV-2—in the same way they’re regularly exposed to the flu—some nasal vaccines are being designed as boosters for a preexisting immune response that is starting to wane. For example, Yale researchers Iwasaki and Goldman-Israelow are pursuing a strategy in animals deemed “prime and spike.”
The idea is to start with a vaccine injection—the “prime” that stimulates adaptive immunity—then follow it a few weeks later with a nasal puff that “spikes” the system with more viral protein, leading to mucosal immunity. In a study published in 2022 in Science, Iwasaki and her colleagues reported that they primed rodents with the mRNA vaccine developed by Pfizer and BioNTech, the same shot so many of us have received. Two weeks later some of the mice received an intranasal puff of saline containing a fragment of the SARS-CoV-2 spike protein. Because the animals had some preexisting immunity from the shot, the researchers didn’t add any adjuvants to heighten the effects of the nasal puff. Two weeks later researchers detected stronger signs of mucosal immunity in mice that had received this treatment compared with mice that got only the shot.
“Not only can we establish tissue-resident memory T cells” to fight off the virus in the nose, Iwasaki says, but the prime-and-spike method also produces those vigorous IgA antibodies in the mucosal layer. “And that’s much more advantageous because we can prevent the virus from ever infecting the host,” she notes. The study suggests that this approach might also lessen the chances of transmitting the disease to others because of the lower overall viral load. Experiments in hamsters demonstrated that vaccinated animals shed less virus, and they were less likely to contract COVID from infected cage mates that had not been vaccinated themselves.
Although most of the new vaccine strategies are aimed at COVID, nasal vaccines for other diseases are already being planned. Kaufmann, formerly of Codagenix, says the company currently has clinical trials underway for nasal vaccines against flu and RSV. CastleVax’s Egan says “we have plans to address other pathogens” such as RSV and human metapneumovirus, another leading cause of respiratory disease in kids.
Vaccines that don’t need to be injected could clear many barriers to vaccine access worldwide. “We saw with COVID there was no vaccine equity,” Smaill says. Many people in low-income countries never received a shot; they are still going without one four years after the vaccines debuted.
In part, this inequity is a consequence of the high cost of delivering a vaccine that needs to stay frozen on a long journey from manufacturing facilities in wealthy countries. Some of the nasal sprays in development don’t need deep-cold storage, so they might be easier to store and transport. And a nasal spray or an inhaled puff would be much easier to administer than a shot. No health professional is required, so people could spray it into their noses or mouths at home.
For these reasons, needle-free delivery matters to the World Health Organization. The WHO is using the Codagenix nasal spray in its Solidarity Trial Vaccines program to improve vaccine equity. The CoviLiv spray is now in phase 3 clinical trials around the world as part of this effort. “The fact that the WHO was still interested in a primary vaccination trial in the geographies it’s passionate about—that’s indicative that there is still a gap,” Kaufmann says. CoviLiv was co-developed with the Serum Institute of India, the world’s largest maker of vaccines by dose. The partnership enabled production at the high volume required for Solidarity.
The CastleVax vaccine with the NDV vector provides another layer of equity because the facilities required to make it already exist in many low- and middle-income countries. “The cool thing is that NDV is a chicken virus, so it grows very well in embryonated eggs—that’s exactly the system used for making flu vaccines,” Krammer says. For example, for a clinical trial in Thailand, “we just shipped them the seed virus, and then they produced the vaccine and ran the clinical trials,” he says. Many countries around the world have similar facilities, so they will not need to depend on pharma companies based in richer places.
Even high-income countries face barriers to vaccination, although they may be more personal than systemic. For very many people, the needle itself is the problem. Extreme phobia such as Velasquez’s is uncommon, but many people have a general fear of needles that makes vaccinations stressful or even impossible for them. For about one in 10 people needle-related fear or pain is a barrier to vaccinations, says C. Meghan McMurtry, a psychologist at the University of Guelph in Ontario. Needle fear “is present in most young kids and in about half of adolescents. And 20 to 30 percent of adults have some level of fear.” A review of studies of children showed that “concern around pain and needle fear are barriers to vaccination in about 8 percent of the general population and about 18 percent in the vaccine-hesitant population,” McMurtry adds.
Some people are wary of injected vaccines even if they’re not afraid of needles, Kett says; they see injections as too invasive even if the needle doesn’t bother them. “We’re hopeful that something administered by the nasal route would be less likely to come across some of those issues,” Kett says.
In the U.S., however, sprays and puffs won’t be available until they are approved by the Food and Drug Administration, which requires clear evidence of disease protection. As Diamond points out, standards for such evidence are well established for injections, and vaccine makers can follow the rule book: regulations point to particular antibodies and specific ways to measure them with a simple blood test. But for nasal vaccines, Iwasaki says, “we don’t have a standard way to collect nasal mucus or measure antibody titers. All these practical issues have not been worked out.”
Iwasaki is also frustrated with a restriction by the U.S. Centers for Disease Control and Prevention that stops researchers from using existing COVID vaccines in basic research to develop new nasal sprays. The rule is a holdover from 2020, when COVID injections had just been developed and were in short supply; people had to wait to get vaccinated until they were eligible based on factors such as age and preexisting conditions. “That made sense back then, but those concerns are years old; things are different now,” Iwasaki says. “Now we have excess vaccine being thrown out, and we cannot even get access to the waste, the expired vaccine.”
Today scientists want to contrast the effectiveness of nasal formulations with injections already in use. “Those comparisons are really important for convincing the FDA that this is a worthy vaccine to pursue,” Iwasaki says. But the restriction has held up studies by her company, Xanadu, slowing down work. (The CDC did not respond to a request for comment.)
Despite the bureaucratic and scientific hurdles, the sheer number of nasal vaccines now in clinical trials encourages Iwasaki and other scientists pursuing the needle-free route. They say it seems like only a matter of time before getting vaccinated will be as simple as a spritz up the nose.
Velasquez, for one, can’t wait for that day to arrive. The circumstances that finally forced her to reckon with her fear of needles (a global pandemic, the prospect of parenthood and the numerous blood tests that accompanied her pregnancy) were so much bigger than her. If not for them, she might still be avoiding shots. “So having vaccines without needles—I would get every vaccine any doctor wanted me to get, ever. It would be a complete game changer for me.”
#vaccination#mask up#covid#pandemic#public health#wear a mask#covid 19#wear a respirator#still coviding#coronavirus#sars cov 2#get vaccinated#vaccinate your kids
43 notes
·
View notes
Text
Next Generation Sequencing Market 2024 Size, Share, Feasibility Status and Growth Outlook by 2032
The Next Generation Sequencing (NGS) market is poised for substantial growth, driven by advancements in genomic research, personalized medicine, and diagnostics. According to SNS Insider's latest research report, the NGS market is expected to experience significant revenue growth, propelled by the increasing adoption of NGS technologies in healthcare and life sciences. As of now, the market is valued at a notable figure, and its revenue trajectory is forecasted to expand substantially over the next few years. The increasing prevalence of genetic disorders, coupled with rising demand for precision medicine, is one of the driving factors behind this surge in NGS market revenue. Learn more about Next Generation Sequencing (NGS) Market Revenue

Next Generation Sequencing (NGS) technologies have revolutionized the field of genomics by enabling rapid and cost-effective sequencing of DNA and RNA. These innovations have significantly reduced the time and cost involved in sequencing, making it more accessible to researchers, clinicians, and healthcare providers. The integration of NGS into various healthcare applications, including disease diagnosis, drug development, and personalized treatment, has paved the way for a more precise and efficient approach to patient care. The global healthcare sector is now investing heavily in NGS platforms to support targeted therapies and enhance disease prevention.
The NGS market's rapid growth can be attributed to several key factors. The increasing focus on early diagnosis and personalized healthcare solutions is a major driver. NGS allows for a deeper understanding of an individual’s genetic makeup, enabling healthcare professionals to tailor treatment plans based on specific genetic profiles. Additionally, the growing awareness of the benefits of genetic testing, combined with an expanding pool of trained professionals, has further fueled market demand.
With the continued expansion of applications such as cancer genomics, infectious disease analysis, and rare genetic disorder diagnostics, NGS technology has become indispensable in clinical settings. Furthermore, the decreasing costs of sequencing, paired with advancements in bioinformatics, have made NGS even more accessible to a broader range of industries.
Get Free Sample Report@ https://www.snsinsider.com/sample-request/3720
The NGS market is divided into multiple segments based on technology, application, and end-users. Among these, the diagnostic application segment is expected to witness significant growth, with oncology applications being a prominent contributor. Cancer genomics, in particular, is one of the key areas benefiting from NGS technology, as it allows for more accurate detection of genetic mutations, which is crucial for targeted therapies. In addition to oncology, infectious diseases such as COVID-19, tuberculosis, and other viral infections are also being extensively studied using NGS, providing vital insights for public health monitoring and prevention strategies.
The technological advancements in NGS platforms, including single-cell sequencing, metagenomics, and CRISPR-based gene editing technologies, are further boosting market expansion. These innovations are opening up new research avenues and applications, thus propelling the growth of the NGS market. Moreover, the increasing availability of advanced bioinformatics tools, which aid in analyzing the large volumes of data generated by NGS, has further increased the technology's value in various fields of research and clinical practice.
Geographically, North America holds a significant share in the NGS market, with a strong presence of leading biotechnology and pharmaceutical companies. The region’s well-established healthcare infrastructure, along with the adoption of advanced technologies, has contributed to the dominance of North America in the NGS landscape. However, the Asia Pacific region is expected to grow at a faster rate, owing to factors such as rising healthcare investments, growing research activities, and the increasing prevalence of chronic diseases.
The competitive landscape of the NGS market is highly fragmented, with numerous key players vying for market share. Leading companies such as Illumina, Thermo Fisher Scientific, Pacific Biosciences, and Roche are at the forefront of developing cutting-edge NGS solutions. These players are focusing on technological advancements, strategic collaborations, and product innovations to strengthen their market positions. Furthermore, as the demand for NGS continues to rise, the industry is expected to see an influx of new players entering the market with novel solutions and services.
In conclusion, the Next Generation Sequencing market is on the cusp of significant growth, with advancements in technology and applications driving market dynamics. With the increasing demand for personalized medicine, early disease diagnosis, and genetic testing, the market's future looks promising. As NGS becomes more integrated into clinical settings and research labs, its potential to transform healthcare and life sciences is limitless.
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Contact Us: Akash Anand – Head of Business Development & Strategy [email protected] Phone: +1-415-230-0044 (US) | +91-7798602273 (IND)
#Next Generation Sequencing Market#Next Generation Sequencing Market Size#Next Generation Sequencing Market Share#Next Generation Sequencing Market Growth#Market Research
0 notes
Text
Liquid Biopsy Market: Trends, Growth Opportunities, and Future Insights
The liquid biopsy market is expanding rapidly, driven by advancements in precision medicine, non-invasive diagnostic methods, and growing demand for early cancer detection. This technology, which allows doctors to detect cancer and other diseases through blood samples instead of invasive tissue biopsies, is revolutionizing patient care by offering faster, less painful, and more accessible testing options. In this blog, we’ll explore the key trends, market drivers, growth opportunities, and challenges shaping the liquid biopsy industry.

Download PDF Brochure
What is a Liquid Biopsy?
A liquid biopsy is a non-invasive diagnostic test that detects genetic mutations, cancer markers, or other biomarkers in blood, urine, saliva, or other bodily fluids. Unlike traditional biopsies, which require tissue samples through surgery, liquid biopsies provide valuable information about a patient’s condition using small samples, significantly reducing discomfort and recovery time. Liquid biopsies can track a tumor’s progress, identify mutations, and monitor response to treatments, which is particularly useful for conditions that are difficult to monitor through tissue biopsies alone.
Key Drivers of Growth in the Liquid Biopsy Market
The liquid biopsy market is experiencing a surge in growth due to several factors:
1. Rising Incidence of Cancer and Demand for Non-Invasive Diagnostics
Cancer remains one of the leading causes of death worldwide, driving demand for diagnostic tools that can detect it early and with minimal invasiveness. Liquid biopsies provide a way to diagnose and monitor cancer by identifying specific biomarkers in the blood, offering a safer and less invasive alternative to surgical biopsies. This demand is particularly high in regions where the aging population and incidence of cancer are on the rise, such as North America and Europe.
2. Advances in Genomics and Precision Medicine
Advances in genomics and the trend toward precision medicine are pushing the liquid biopsy market forward. As genomic research reveals more about the genetic markers associated with diseases, liquid biopsy technologies are becoming more capable of identifying and monitoring these markers. This enables doctors to tailor treatments to individual patients based on their unique genetic profiles, resulting in more effective and personalized treatment plans.
3. Government Funding and Research Investments
Governments and research institutions are investing in cancer research and diagnostics, increasing funding for technologies that can advance early cancer detection. Many health agencies worldwide, such as the National Institutes of Health (NIH) in the United States, are allocating funds to support the development of non-invasive diagnostic tools, fostering innovation and accelerating the growth of the liquid biopsy market.
4. Growth in Companion Diagnostics
Companion diagnostics are tests that help assess a patient’s likelihood of responding to a specific treatment. These diagnostics are particularly important for cancer patients, as liquid biopsies can identify biomarkers that reveal how a patient might respond to certain therapies. This insight is invaluable in oncology, as it can guide treatment decisions and improve patient outcomes, further driving demand for liquid biopsy solutions.
Emerging Applications and Technologies
The scope of liquid biopsy applications is expanding beyond cancer diagnosis. New technologies are unlocking additional uses in areas such as:
1. Infectious Disease Monitoring
Liquid biopsies can detect circulating pathogens in the bloodstream, opening the possibility of diagnosing and monitoring infectious diseases like HIV, tuberculosis, and hepatitis. This potential is particularly valuable in areas with high rates of these infections, where liquid biopsies could improve diagnosis and treatment tracking in real time.
2. Cardiovascular Disease Detection
Research is exploring the use of liquid biopsies to identify cardiovascular biomarkers, which could assist in early diagnosis and monitoring of heart disease. By detecting certain genetic markers and proteins in the blood, liquid biopsy technology could help predict cardiovascular events, enabling preventive measures and more targeted therapies.
3. Neurological Conditions
Although still in early stages, liquid biopsy applications in neurology could revolutionize the diagnosis and monitoring of neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease. By detecting biomarkers associated with brain conditions, liquid biopsies offer a non-invasive way to diagnose neurological conditions earlier than traditional imaging techniques allow.
Competitive Landscape and Key Players
The liquid biopsy market is highly competitive, with several established companies and new entrants working to advance the technology. Key players include:
Guardant Health: Known for its Guardant360 test, which screens for cancer mutations in the blood. Guardant Health focuses on developing liquid biopsy tests for advanced-stage cancers and companion diagnostics.
Biocept: Specializes in liquid biopsy tests for both solid tumor cancers and brain metastases, with a focus on molecular diagnostics.
Foundation Medicine: Known for its FoundationOne Liquid test, which provides genomic profiling to inform cancer treatment decisions.
Natera: A leader in reproductive health diagnostics, Natera also offers liquid biopsy tests for oncology, including its Signatera test, which is used for minimal residual disease detection and monitoring.
GRAIL: Recently acquired by Illumina, GRAIL is focused on developing multi-cancer early detection tests using its proprietary liquid biopsy technology.
These companies are investing heavily in R&D, aiming to improve the accuracy, affordability, and applicability of liquid biopsies across a range of diseases.
Market Challenges
Despite its advantages, the liquid biopsy market faces several challenges:
1. Technical Limitations
Detecting circulating tumor DNA (ctDNA) and other biomarkers in blood samples is complex and requires advanced technology. While some cancers shed more ctDNA, making detection easier, others do not, which can limit the effectiveness of liquid biopsies in certain cases.
2. Regulatory and Reimbursement Hurdles
In many regions, liquid biopsy tests face stringent regulatory requirements and approval processes. Additionally, the cost of these tests and limited reimbursement options can restrict their accessibility for patients, particularly in lower-income regions or for those without sufficient healthcare coverage.
3. Data Interpretation and False Positives
Interpreting data from liquid biopsies is complex, and there is a risk of false positives, where benign mutations or low levels of ctDNA are detected as indicators of cancer. This challenge emphasizes the need for high accuracy and specificity in liquid biopsy technology to ensure reliable results.
Future Outlook and Growth Opportunities
The liquid biopsy market is expected to continue its upward trajectory, driven by technological advancements and broader applications. Key growth areas include:
1. Artificial Intelligence Integration
AI and machine learning algorithms are being integrated into liquid biopsy analysis, enhancing the accuracy of biomarker detection and interpretation. By automating complex data analysis, AI could reduce false positives and help refine liquid biopsy results, making them more reliable and accessible.
2. Expanding Beyond Oncology
While cancer detection remains the primary application, there is substantial potential for liquid biopsies in infectious disease, cardiovascular health, and neurology. The expansion into these areas could open new revenue streams for companies and significantly broaden the market’s scope.
3. Global Market Expansion
Emerging markets, particularly in Asia-Pacific and Latin America, offer high growth potential for the liquid biopsy market. Government initiatives, growing healthcare infrastructure, and a rising prevalence of cancer and other diseases are driving demand in these regions, presenting opportunities for companies to expand their global footprint.
Conclusion
The liquid biopsy market is at the forefront of a healthcare revolution, offering a less invasive, faster, and more precise way to detect and monitor diseases. With applications expanding beyond oncology, new technologies like AI integration, and increasing demand for non-invasive diagnostics, the liquid biopsy market holds immense potential for growth. As the industry continues to innovate, companies that leverage these advancements will not only drive the market forward but also play a pivotal role in shaping the future of personalized and preventive healthcare.
0 notes