#Drug registration
Explore tagged Tumblr posts
Text
Drug License Online for Pharma Companies in Delhi

In India, obtaining a drug license is mandatory for individuals or entities involved in the pharmaceutical industry, including pharmaceutical companies, chemists, and suppliers of medicines. There are two main types of drug licenses:
Wholesale Drug License: This is required for pharmaceutical manufacturers and suppliers who distribute medicines in bulk to retail stores or other businesses.
Retail Drug License: This license is for businesses like chemist shops and pharmacies that sell medicines directly to consumers.
To apply for a Drug license in India or for more information, please contact us at +91 8178731176. We assist with the complete application process, ensuring compliance with the regulatory requirements.
#drug license in delhi#drug license in india#drug registration#wholesale drug license#retail drug license#drug license renewal
0 notes
Text
Drug License in Haryana
Drug License in Haryana is mandatory for businesses involved in the sale, distribution, or manufacture of drugs, ensuring compliance with the Drugs and Cosmetics Act. This license upholds safety and quality standards for public health. The application process requires essential documents like premise details and pharmacist qualifications. PSR Compliance offers expert guidance to simplify the Drug License application in Haryana, making it quick and hassle-free. Secure your license today reach out to us
https://www.psrcompliance.com/blog/drug-license-in-haryana

0 notes
Text
Drug registration process in South Korea
The drug registration process in South Korea can be complex and time-consuming, but it is essential for pharmaceutical companies looking to bring their products to market. At DDReg Pharma, our expert team has extensive knowledge and experience in regulatory compliance and approval and can provide comprehensive solutions to help you streamline the process.

0 notes
Text
there's still two days left but. guys. i think it might be time to find out what it feels like to flunk an exam at last
#fuck pharmacology#all my homies hate pharmacology#and we only had 10 lectures to cover all drugs ever and their mechanisms and side effects and other assorted nonsense#like. this is so important so why is it only one third of a subject (the other modules are biophys and biochem) if it's gonna be half my jo#i could still cancel my registration for the exam and take it in january but I Don't Wanna cause i promised myself i'd at least try#not to be whiny on main but pls i want to sleep#noodle rambles#'
4 notes
·
View notes
Text

Also- I dislike how much influence the fucking Delhi Police has over the DTC and less so but over the DMRC, the Delhi Police has started an anti drugs campaign (psssttt most certainly not an excuse to mass incarcerate the people who use drugs and the poor people who sell them). I, for one, hope the Delhi Police can shove some Suppositories where they belong within their bodies.
@delhi-transport-corporation totally promotes recreational drug usage, and hopes the actual Delhi government would cater more for rehabilitation programs for people who actually abuse drugs instead of incarceration and police violence.
TO PROVE MY POINT, I shall be doing drugs tonight (in Minecraft).
#drug abuse is a serious problem that should not be handled by the police#the cops need not apply#on the rear displays on the bus they now have “anti drug” propoganda.#The ECI using them to promote election registrations was good#democracy good authoritarianism bad#pol#politics#indian politics#I really wish they just left the public transit out of it#Punitive justice isn't going to stop drug abuse#please help people with drug issues by actually helping them
0 notes
Text

Complete the Drug License Registration Application to legally obtain authorization for manufacturing, distributing, or selling pharmaceutical products. Ensure compliance with regulatory standards by submitting required documents and meeting necessary safety guidelines for your business or practice
0 notes
Text
Registration of Cosmetics in India
Cosmetics are utilized to improve a person’s appearance. These are used for various beauty treatments such as skin tightening, hair removal, spot reduction, achieving radiant skin, and many more. They play a critical role in boosting an individual’s self-confidence and positive outlook. Consequently, there has been a significant increase in demand for cosmetics in the Indian market, resulting in substantial growth in the cosmetic industry in recent years. However, ensuring the highest quality and safety of cosmetics remains a major concern for the industry.
For this reason, it is mandatory to register every cosmetic in India. The registration process must be compliant with the Drugs and Cosmetics Act of 1940 and the Cosmetic Rules of 2020. The Central Drug Standard Control Organisation (CDSCO), under the Ministry of Health and Family Welfare, is the regulatory authority responsible for overseeing these regulations. All cosmetics manufactured in or imported into India must be registered with the CDSCO.
Definition of Cosmetics as per the Drugs and Cosmetics Act, 1940
Under section 3(aaa) of the Drugs and Cosmetics Act, 1940, cosmetics is defined as, “any article intended to be rubbed, poured, sprinkled or sprayed on, or introduced into, or otherwise applied to, the human body or any part thereof for cleansing, beautifying, promoting attractiveness or altering the appearance, and includes any article intended for use as a component of cosmetic”.
Under the provisions of the aforesaid Act, the manufacture of cosmetics is regulated by the State Licensing Authorities appointed by the respective State Governments, while the import of cosmetics is regulated by the Central Licensing Authority appointed by the Central Government. The Drugs Controller General (India) is the Central Licensing Authority who grants registration certificate for import.
Key Requirements for Cosmetics in India
To ensure the safety, quality, and efficacy of cosmetics in India, key requirements under the Cosmetic Rules, 2020 are as follows:
All cosmetics manufactured in or imported to India must comply with the Cosmetic Rules, 2020.
All manufacturers must obtain a license or loan license from the State Licensing Authority to manufacture cosmetics for sale and distribution in India.
All importers must obtain an import registration certificate from the Central Licensing Authority to import cosmetics to India.
All the manufacturers of cosmetics in India must label and pack the cosmetics in accordance with the Cosmetic Rules, 2020 and Legal Metrology (Packaged Commodities) Rules, 2011, before selling or distributing the product.
Additional Regulatory Requirements for Cosmetics in India
Cosmetics should not contain any of the raw materials listed in Indian Standard IS: 4707.
Cosmetic products should not contain dyes, colours, or pigments other than those specified by the Bureau of Indian Standards (IS: 4707).
Cosmetic products that contain permitted synthetic organic and natural organic colours should not contain arsenic trioxide, lead, mercury, or heavy metals in excess of the quantities specified in the Cosmetic Rules, 2020.
Hexachlorophene should not be an ingredient in any cosmetic.
Manufacturers should not use animals for testing cosmetics.
Process to get Import Registration Certificate
Under Sections 12 and 13 of the Cosmetic Rules, 2020, a foreign manufacturer's authorised agent or authorised subsidiary may obtain import registration certification through the following process:
Apply to register cosmetics intended for import into India through the central government's online portal, Form COS-1.
The Form COS-1 can be submitted either by the manufacturer himself or his authorized agent or the importer or an Indian subsidiary authorized by the manufacturer.
If the Central Licensing Authority deems the documents provided with the application satisfactory, it may grant the applicant the Import Registration Certificate. The Central Licensing Authority may also reject an application, documenting its reasons in writing within six months of the application date.
If the Central Licensing Authority rejects the application, the applicant has forty-five days to appeal to the Central Government. If the government considers it necessary, it can pass orders in relation thereto within a period of ninety days from the date of appeal.
Before registering the import of a new cosmetic into India, the applicant must obtain prior permission from the Central Licensing Authority in Form COS-3 before registration of the cosmetic.
Process to get Licence or Loan Licence to Manufacture Cosmetics for Sale or Distribution
Under Section 23 of the Cosmetic Rules, 2020, anyone intending to manufacture cosmetics for sales and distribution should obtain a license from the State Licensing Authority through the following process:
Apply for a license through an identified online portal, (can apply offline if online portal is not operational) in Form COS-5 for a license or in Form COS-6 for a loan license.
For a new cosmetic, the manufacturer must obtain prior approval in Form COS-3 from the Central Licensing Authority.
In addition to the required documents, the applicant must also submit a self-declaration in Form COS-7 conforming to Good Manufacturing Practices and additional manufacturing related requirements.
Upon receipt of the application, within a period of forty-five days, the State Licensing Authority will grant a license or loan license after confirming that all requirements have been met or will inform the applicant if it determines that the applicant has not fulfilled the requirement.
Within thirty days from the date of grant of the license or loan license, the manufacturing site will be inspected by the subordinate officer delegated by the State Licensing Authority to verify the information given in the self-certificate in Form COS-7.
Requirements for Registration of Cosmetics for Import
Following is the list of main documents/details that need to be submitted at the time of applying for a cosmetic registration for import.
Authorization from Manufacturer as per First Schedule
Product details and undertaking as per Second Schedule Part I
Regulatory Certificates (manufacturing license/Free Sale Certificate)
Non-Animal Testing Declaration
Declaration for Heavy Metal and Hexachlorophene content
Applicable Government Fees to be paid

Conclusion
The regulations for registration and import of cosmetics in India are crucial for ensuring the quality of cosmetics and safeguarding the well-being of the consumers. Therefore, any manufacturer or importer/authorized agent involved in the cosmetics industry must follow these regulations to ensure the quality and safety of all.
At Regulatory Solution India (RSI), we specialize in providing regulatory consulting services for cosmetics. If you need assistance navigating the submission process or ensuring compliance with the latest regulations, Contact us.
#Drugs and Cosmetics Act#Registration of Cosmetics in India#Cosmetics#Requirements for Registration of Cosmetics for Import
0 notes
Text

Obtain Your Drug License with Corpseed: Hassle-Free Application Process
Secure your drug license in India with Corpseed's expert assistance. Our comprehensive services ensure a hassle-free process, from documentation to approval. Start your pharmaceutical business legally and efficiently with our support.
0 notes
Text
0 notes
Text
Delhi Drug License Registration: Types, Fees, Eligibility and Process
Learn all about the different types of drug license registrations available in Delhi, along with the associated fees and eligibility criteria. Get all the information you need to successfully apply for a drug license in the capital city.
0 notes
Text
Ensuring Food Safety Through the Safe Food for Canadians License Regulations

In today's world, food safety is a paramount concern for governments, businesses, and consumers alike. In Canada, ensuring the safety of food products is a top priority, and this responsibility falls under the purview of the Safe Food for Canadians License Regulations. This comprehensive regulatory framework governs all aspects of food safety, from Food License Registration to Food Safety Canada, Food Safety Certificate Canada, and more.
The Safe Food for Canadians License Regulations: An Overview
The Safe Food for Canadians License Regulations is a vital piece of legislation in Canada aimed at safeguarding the nation's food supply. These regulations cover a wide range of areas, ensuring that food and beverage manufacturers, importers, and distributors adhere to stringent food safety standards. Let's delve into some of the key aspects of these regulations and how they contribute to the safety of our food supply.
Food License Registration: To operate a business in the food industry in Canada, you need to obtain a Food License Registration. This license is a critical requirement, and it ensures that businesses are accountable for the quality and safety of the food they produce, distribute, or import. Food License Registration is a clear demonstration of a commitment to food safety.
Food Safety Regulations: The core of the Safe Food for Canadians License Regulations revolves around food safety. These regulations establish safe food handling, processing, and transportation requirements. They encompass everything from the cleanliness of facilities to the safe storage of food products.
Canada Food Labelling: Proper labeling of food products is essential for consumers to make informed choices. The regulations mandate clear and accurate Canada Food Labelling, ensuring that consumers have access to critical information about the products they purchase, including ingredient lists, allergen information, and nutritional content.
Food and Drug Regulations Canada: Food and Drug Regulations Canada is a comprehensive set of rules that govern the safety, efficacy, and quality of food and drug products. These regulations are an integral part of the broader framework of food safety in the country, ensuring that not only food but also pharmaceuticals and medical devices meet stringent standards.
HACCP Process: Hazard Analysis and Critical Control Points (HACCP) is a systematic approach to identifying, evaluating, and controlling food safety hazards. The Safe Food for Canadians License Regulations require food businesses to implement HACCP processes to identify potential risks and take preventive measures to mitigate them. This proactive approach is key to preventing foodborne illnesses and other safety issues.
Food Safety Certificate Canada: A Food Safety Certificate Canada is a mark of compliance with the regulations. It is evidence that a business has met the necessary requirements to ensure food safety, from proper handling to labeling. This certificate not only builds consumer trust but also allows businesses to expand and export their products to international markets.
Food Safety Canada: Food Safety Canada is a collective effort by government agencies, industry stakeholders, and consumers to maintain the highest standards of food safety. The Safe Food for Canadians License Regulations are a cornerstone of these efforts, providing a legal framework for food safety practices across the country.
The Importance of Food Safety Regulations
The significance of robust food safety regulations cannot be overstated. Foodborne illnesses are a real and present danger, and unsafe food can severely affect public health. The Safe Food for Canadians License Regulations play a crucial role in minimizing these risks and ensuring that the food we consume is safe.
For businesses in the food and beverage industry, compliance with these regulations is not just a legal requirement; it's also a matter of responsibility. Failing to adhere to these regulations can result in serious consequences, including fines, business closures, and damage to a company's reputation.
Furthermore, food safety is not limited to domestic concerns. In an increasingly globalized world, food products are frequently traded across borders. To maintain Canada's reputation for safe and high-quality food products, these regulations are essential. They ensure that Canadian food products meet international standards, allowing businesses to access international markets and enhancing the nation's global standing in the food industry.
The Role of Food License Registration
One of the first and most crucial steps for any business in the food industry is obtaining Food License Registration. This process involves submitting an application that outlines the type of food activities the business will engage in, including manufacturing, processing, importing, or exporting. The information provided in the application is critical in determining the appropriate level of oversight and regulation for the business.
Food License Registration is not a one-time process. It requires ongoing compliance with the Safe Food for Canadians License Regulations. This includes maintaining records, providing access to inspectors, and ensuring that facilities and equipment meet established standards. The purpose of these ongoing requirements is to ensure that businesses maintain a consistent commitment to food safety.
Canada Food Labelling and Transparency
Accurate and clear Canada Food Labelling is a fundamental component of food safety regulations. Food labels provide consumers with vital information, allowing them to make informed choices about the products they purchase. This includes ingredient lists, nutritional information, allergen warnings, and safe handling instructions.
Proper labeling also extends to the presentation of food products. Misleading packaging or labeling can not only deceive consumers but can also have serious health consequences. Food safety regulations dictate that labels must be truthful, easy to understand, and properly placed on the product.
Food and Drug Regulations Canada
The Safe Food for Canadians License Regulations is part of a broader regulatory framework that includes the Food and Drug Regulations Canada. These regulations govern not only food but also pharmaceuticals and medical devices. This comprehensive approach reflects Canada's commitment to public health and safety.
The Food and Drug Regulations Canada specify standards for product quality, efficacy, and safety. They provide the foundation for ensuring that food and drug products in Canada are of the highest quality and pose minimal risks to consumers. This comprehensive regulatory approach is crucial for maintaining public trust in the safety and efficacy of products.
HACCP Process for Preventive Control
The HACCP process is a preventive control system that focuses on identifying and mitigating potential food safety hazards. It is a proactive approach that involves identifying critical control points where hazards can be controlled, monitoring these points, and taking corrective action when necessary. This systematic approach is designed to prevent food safety issues before they occur.
Businesses in the food and beverage industry are required to implement HACCP systems as part of their compliance with the Safe Food for Canadians License Regulations. This ensures that they have processes in place to monitor and address potential risks, reducing the likelihood of foodborne illnesses and other safety issues.
Food Safety Certificate Canada: A Mark of Quality
A Food Safety Certificate Canada is a testament to a business's commitment to food safety. It is issued to businesses that have demonstrated compliance with the Safe Food for Canadians License Regulations. This certificate not only enhances consumer trust but also opens doors to international markets.
For businesses looking to export their food products, a Food Safety Certificate Canada is often a requirement for entry into foreign markets. It demonstrates that the products meet rigorous food safety standards, allowing Canadian businesses to compete on the global stage.
The Safe Food for Canadians License Regulations are a comprehensive framework designed to ensure the safety and quality of food products in Canada. From Food License Registration to Food Safety Certificate Canada, these regulations cover all aspects of the food and beverage industry. They provide a foundation for maintaining public trust, expanding into international markets, and, most importantly, safeguarding public health.
Food safety is a shared responsibility, and these regulations provide the necessary structure and guidance for businesses to meet their obligations. By adhering to these regulations, businesses not only ensure their success but also contribute to the overall safety of the Canadian food supply. In a world where food safety is paramount, the Safe Food for Canadians License Regulations stand as a beacon of assurance for both businesses and consumers.
#Food and Drug Regulations Canada#Food License Registration#Food Safety Regulations#Food Safety Canada#Canada Food Labelling#Food Safety Certificate Canada#HACCP Process#Safe Food for Canadians Regulations#Drug Establishment Registration#Food and Beverage Certifications
0 notes
Text
How to get Drug License in Delhi?
Getting a drug license in Delhi is required for anyone planning to sell, distribute, or manufacture medicines. Agile Regulatory simplifies the process by guiding you through every step, from document preparation to application submission. Our experts ensure compliance with Delhi's drug control regulations, making the process quick and hassle-free. Trust us for reliable assistance in obtaining your drug license without any stress.
0 notes
Text
What are documents required for Drug License UP?

To get a Drug License in Uttar Pradesh, you need to provide these documents:
A cover letter
An application form
A key plan and site plan of your location
Proof that you own or lease the property
An incorporation certificate
Proof of the applicant's qualifications
An appointment letter for the registered pharmacist or qualified person
An affidavit stating that the applicant has no criminal record.
To apply for a wholesale drug license, click on the link: https://www.psrcompliance.com/blog/what-is-wholesale-drug-license.
0 notes
Text

South Korea is one of the largest pharmaceutical markets in Asia, making it an attractive destination for pharmaceutical companies looking to expand their market reach. However, like most countries, South Korea has a complex and regulated drug registration process that must be followed before any new drug can be marketed and sold. In this infographic, we will explore the drug registration process in South Korea.
#Drug registration#South Korea#Regulatory Compliance#drug registration process#Ministry of Food and Drug Safety#MFDS#ddreg pharma
0 notes
Text
🗣️THIS IS WHAT INCLUSIVE, COMPASSIONATE DEMOCRACY LOOKS LIKE
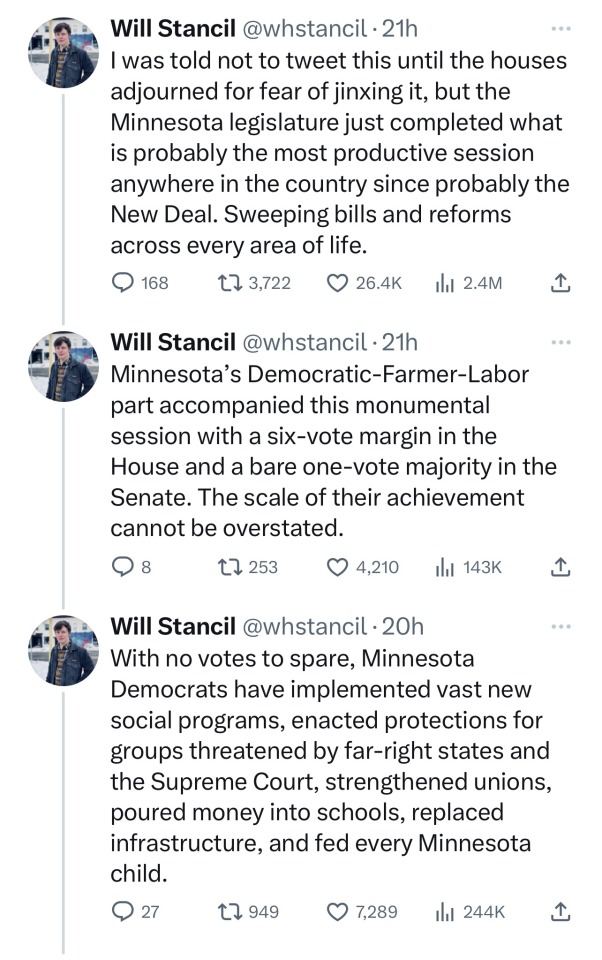
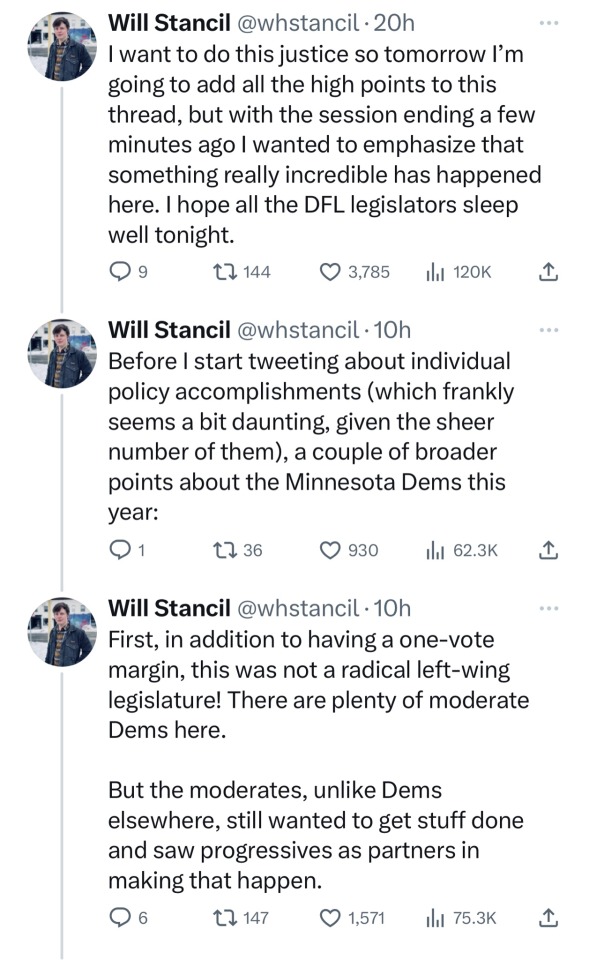
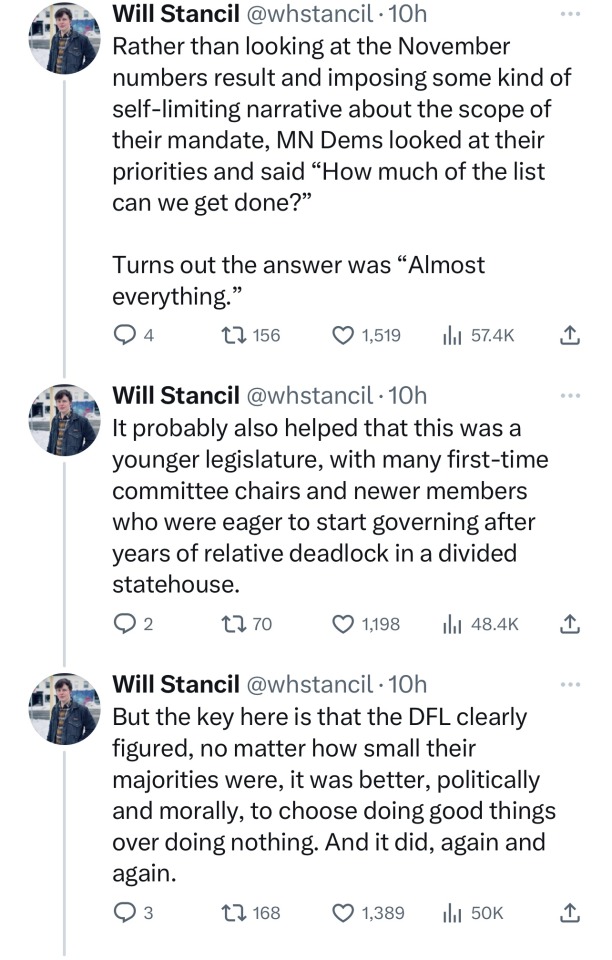
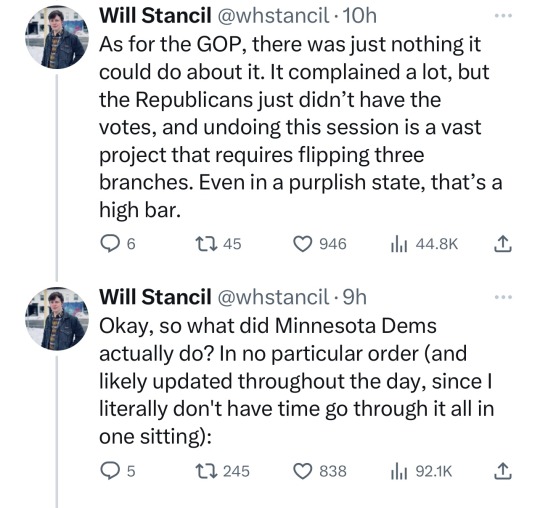
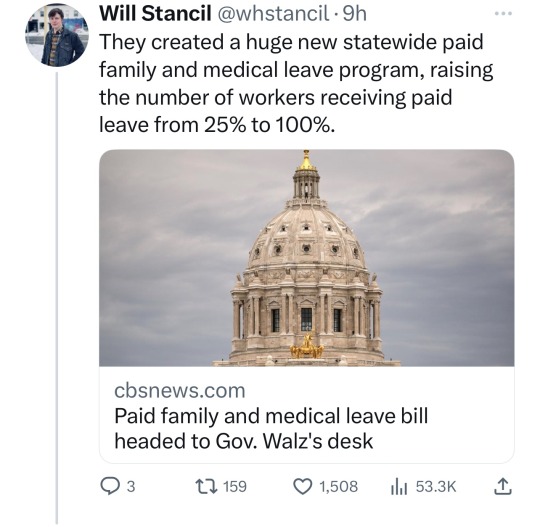
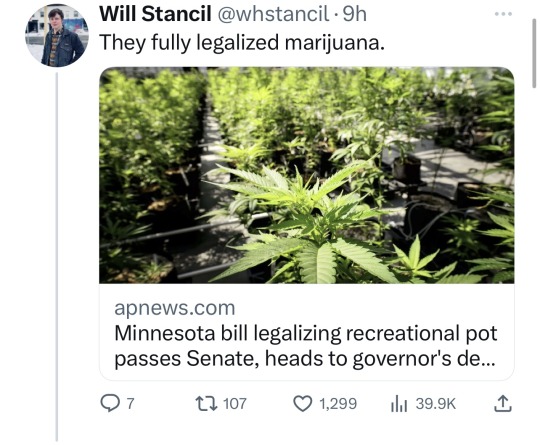

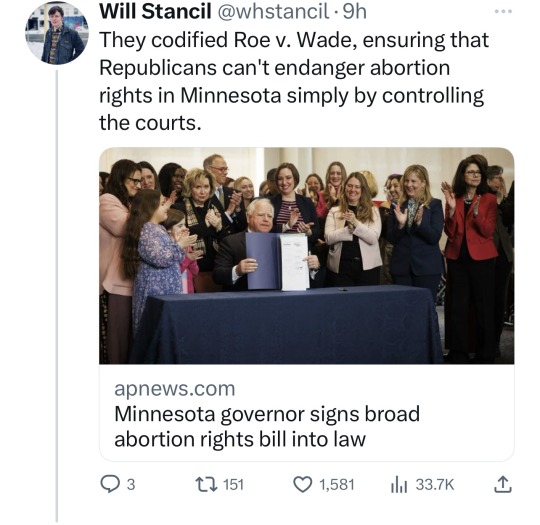

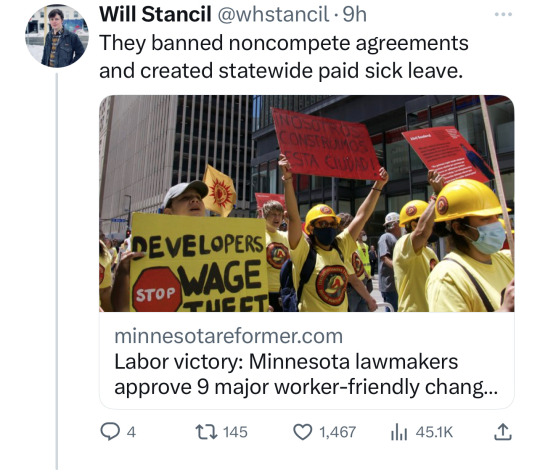
Minnesota Dems enacted a raft of laws to make the state a trans refuge, and ensure people receiving trans care here can't be reached by far-right governments in places like Florida and Texas. (link)
Minnesota Dems ensured that everyone, including undocumented immigrants, can get drivers' licenses. (link)
They made public college free for the majority of Minnesota families. (link)
Minnesota Dems dropped a billion dollars into a bevy of affordable housing programs, including by creating a new state housing voucher program. (link)
Minnesota Dems massively increased funding for the state's perpetually-underfunded public defenders, which lets more public defenders be hired and existing public defenders get a salary increase. (link)
Dems raised Minnesota education spending by 10%, or about 2.3 billion. (link)
Minnesota Dems created an energy standard for 100% carbon-free electricity by 2040. (link)
Minnesota already has some of the strongest election infrastructure (and highest voter participation) in the country, but the legislature just made it stronger, with automatic registration, preregistration for minors, and easier access to absentee ballots. (link)
Minnesota Dems expanded the publicly subsidized health insurance program to undocumented immigrants. This one's interesting because it's the sort of things Dems often balk at. The governor opposed it! The legislature rolled over him and passed it anyway. (link)
Minnesota Dems expanded background checks and enacted red-flag laws, passing gun safety measures that the GOP has thwarted for years. (link)
Minnesota Dems gave the state AG the power to block the huge healthcare mergers that have slowly gobbled up the state's medical system. (link)
Minnesota Dems restored voting rights to convicted felons as soon as they leave prison. (link)
Minnesota Dems made prison phone calls free. (link)
Minnesota Dems passed new wage protection rules for the construction industry, against industry resistance. (link)
Minnesota Dems created a new sales tax to fund bus and train lines, an enormous victory for the sustainability and quality of public transit. Transit be more pleasant to ride, more frequent, and have better shelters, along more lines. (link)
They passed strict new regulations on PFAS ("forever chemicals"). (link)
Minnesota Dems passed the largest bonding bill in state history! Funding improvements to parks, colleges, water infrastructure, bridges, etc. etc. etc. (link)
They're going to build a passenger train from the Twin Cities to Duluth. (link)
I can't even find a news story about it but there's tens of millions in funding for new BRT lines, too. (link)
A wonky-but-important change: Minnesota Dems indexed the state gas tax to inflation, effectively increasing the gas tax. (link)
They actually indexed a bunch of stuff to inflation, including the state's education funding formula, which helps ensure that school spending doesn't decline over time. (link)
Minnesota Dems made hourly school workers (e.g., bus drivers and paraprofessionals) eligible for unemployment during summer break, when they're not working or getting paid. (link)
Minnesota Dems passed a bunch of labor protections for teachers, including requiring school districts to negotiate class sizes as part of union contracts. (Yet another @SydneyJordanMN special here. (link)
Minnesota Dems created a state board to govern labor standards at nursing homes. (link)
Minnesota Dems created a Prescription Drug Affordability Board, which would set price caps for high-cost pharmaceuticals. (link)
Minnesota Dems created new worker protections for Amazon warehouse workers and refinery workers. (link)
Minnesota Dems passed a digital fair repair law, which requires electronics manufacturers to make tools and parts available so that consumers can repair their electronics rather than purchase new items. (link)
Minnesota Dems made Juneteenth a state holiday. (link)
Minnesota Dems banned conversion therapy. (link)
They spent nearly a billion dollars on a variety of environmental programs, from heat pumps to reforestation. (link)
Minnesota Dems expanded protections for pregnant and nursing workers - already in place for larger employers - to almost everyone in the state. (link)
Minnesota Dems created a new child tax credit that will cut child poverty by about a quarter. (link)
Minnesota Democrats dropped a quick $50 million into homelessness prevention programs. (link)
And because the small stuff didn't get lost in the big stuff, they passed a law to prevent catalytic converter thefts. (link)
Minnesota Dems increased child care assistance. (link)
Minnesota Dems banned "captive audience meetings," where employers force employees to watch anti-union presentations. (link)
No news story yet, but Minnesota Dems forced signal priority changes to Twin Cities transit. Right now the trains have to wait at intersections for cars, which, I can say from experience, is terrible. Soon that will change.
Minnesota Dems provided the largest increase to nursing home funding in state history. (link)
They also bumped up salaries for home health workers, to help address the shortage of in-home nurses. (link)
Minnesota Dems legalized drug paraphernalia, which allows social service providers to conduct needle exchanges and address substance abuse with reduced fear of incurring legal action. (link)
Minnesota Dems banned white supremacists and extremists from police forces, capped probation at 5 years for most crimes, improved clemency, and mostly banned no-knock warrants. (link)
Minnesota Dems also laid the groundwork for a public health insurance option. (link)
I’m happy for the people of Minnesota, but as a Floridian living under Ron DeSantis & hateful Republicans, I’m also very envious tbh. We know that democracy can work, and this is a shining example of what government could be like in the hands of legislators who actually care about helping people in need, and not pursuing the GOP’s “culture wars” and suppressing the votes of BIPOC, and inflicting maximum harm on those who aren’t cis/het, white, wealthy, Christian males. BRAVO MINNESOTA. This is how you do it! And the Minnesota Dems did it with a one seat majority, so no excuses. Forget about the next election and focus on doing as much good as you can, while you still can. 👏🏿👏🏿👏🏿👏🏿👏🏿👏🏿👏🏿
👉🏿 https://threadreaderapp.com/thread/1660846689450688514.html
#politics#minnesota#social justice#culture wars#this is what democracy looks like#republicans are evil
25K notes
·
View notes
Text
Drug Manufacturing Licenses | Dueranconsultancy
Getting a drug manufacturing license is not an easy process to handle. You have to register yourself and submit your documents to the Central Drugs Standard Control Organization (CDSCO). If you need support to get your license, you must connect with me. I am a professional regulatory service provider and know the process of getting a license for drug manufacturing in India.
#drug licence#drug manufacturing license#drug licence online apply#drug establishment registration#import drug license#drug import license#online drug license#licence for fixed dose combination
0 notes