#Cell Culture Media Market
Explore tagged Tumblr posts
Text
The Cell Culture Media Market in 2023 is US$ 4.58 billion, and is expected to reach US$ 11.7 billion by 2031 at a CAGR of 12.50%.
0 notes
Text
Viral Vaccine Cell Culture Media Market Will Grow At Highest Pace Owing To Rising Prevalence Of Viral Infections
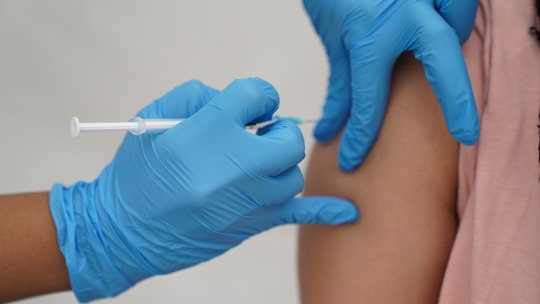
Viral vaccine cell culture media are essential growth mediums required for the propagation of viruses, used in the manufacturing of viral vaccines. It provides necessary nutrients to sustaining and propagation of cells outside of living organism. The composition of viral vaccine cell culture media varies depending on specific requirements and types of viruses being cultivated. It contains necessary salts, amino acids, proteins, vitamins and other organic compounds essential for growth of cells. The advantages associated with viral vaccine cell culture media include standardized and defined composition, ease of scale-up for large-scale manufacturing, and supporting growth of anchorage-dependent and suspension-adapted cells. The need for viral vaccine cell culture media is growing owing to rising incidences of viral infections and technological advancements in vaccine development.
The Viral Vaccine Cell Culture Media Market is estimated to be valued at US$ 1.8 Bn in 2024 and is expected to exhibit a CAGR of 5.8 % over the forecast period 2024-2031.
Key Takeaways
Key players operating in the viral vaccine cell culture media are Thermo Fisher Scientific, Merck, Sartorius,Creative Biolabs, Xell. Thermo Fisher Scientific dominates the market with wide range of viral vaccine cell culture media products.
Rising prevalence of viral infections such as influenza, COVID-19, hepatitis, and others is driving the demand for viral vaccines exponentially. As viral vaccines are manufactured using cell culture technologies, their growing demand is directly fueling the viral vaccine cell culture media market.
Advancements in cell culture technologies, serum-free and chemically defined media are helping overcome issues related to undefined compositions and lot-to-lot inconsistencies. These technologies are supporting more reproducible and scalable manufacturing of viral vaccines.
Market Trends
Serum-free and chemically defined media - These media eliminates risks of contamination from animal-derived components and assists consistent performance during vaccine production. Major players are focusing on development of serum-free formulations.
Single-use bioreactors and technologies - Single-use technologies are helping achieve flexible, consistent and scalable production compared to conventional stainless-steel bioreactors. This trend is positively impacting the viral vaccine cell culture media market.
Market Opportunities
Rising viral vaccine production in developing economies due to increasing disease burden is creating opportunities for viral vaccine cell culture media manufacturers to expand in emerging markets. Adoption of continuous manufacturing technologies using perfusion platforms can further improve productivity and efficiency of viral vaccine production. This presents lucrative opportunities for media manufacturers.
Impact Of COVID-19 On Viral Vaccine Cell Culture Media Market Growth
The outbreak of COVID-19 pandemic has severely impacted the growth of viral vaccine cell culture media market globally. The increased demand for vaccine development caused disturbance in the supply chain and manufacturing process of cell culture media. The lockdowns imposed by governments across various countries led to temporary closure of production facilities and disrupted import-export activities. This affected the availability of raw materials and components required for manufacturing cell culture media. Furthermore, restrictions on travel and transportation made it difficult for companies to conduct clinical trials and testing of vaccines under development.
However, with gradual lifting of lockdowns and resumption of business operations, the market is expected to regain lost momentum in post-COVID times. There is surge in R&D funding from governments and private organizations towards development of vaccines against coronavirus. This has boosted the demand for cell culture media from biopharmaceutical companies. Various start-ups and established players have entered into strategic collaborations with research institutes working on COVID-19 vaccines. They are focusing on expanding their production capacities to meet the growing requirements. Moreover, shift towards single-use technologies and automated solutions is expected to enhance production efficiency. Advancements in cell culture protocols will further drive the market growth in coming years.
Geographically, North America holds the major share of viral vaccine cell culture media market in terms of value, led by substantial research funding and presence of leading biopharma companies. Asia Pacific is emerging as the fastest growing regional market, supported by increasing government initiatives, improving healthcare infrastructure and growth of biosimilars industry in China and India. Recently, several Chinese manufacturers have started offering antibody and cell-based therapeutics against coronavirus. This is likely to boost the uptake of viral cell culture media in Asia Pacific post pandemic.
Impact Of COVID-19 On Viral Vaccine Cell Culture Media Market Growth In India
The COVID-19 outbreak hit India during the initial months of 2020. The country went into a nationwide lockdown enforcing travel restrictions and closure of non-essential services. This impacted the biopharmaceutical industry and temporarily disrupted the supply of cell culture media. Local production was halted as workforce mobility was constrained. Import of critical raw materials from other countries also reduced due to global supply chain disarray.
As a result, several vaccine developers faced challenges in terms of insufficient stock of cell culture media for ongoing R&D activities. Their pre-clinical research and clinical trials got delayed. However, as the lockdown rules were relaxed phase-wise, the Indian government initiated measures to resume operations following strict safety norms. It provided regulatory clearances and financial incentives for ramping up indigenous manufacturing of vaccine components including cell culture media.
India currently holds around 8-10% share of global biologics production. Post pandemic, efforts are being directed towards building self-reliance in vaccine manufacturing. Local players are augmenting their production capacities of viral cell culture media in collaboration with research institutes. Additionally, foreign companies are also evaluating India as an alternative manufacturing hub due to lower costs and large talent pool availability. Thus, India is anticipated to be one of the fastest growing regional markets for viral vaccine cell culture media in coming years.
Get more insights on this topic: https://www.trendingwebwire.com/viral-vaccine-cell-culture-media-market-set-to-grow-due-to-advancements-in-cell-culture-techniques/
Author Bio
Vaagisha brings over three years of expertise as a content editor in the market research domain. Originally a creative writer, she discovered her passion for editing, combining her flair for writing with a meticulous eye for detail. Her ability to craft and refine compelling content makes her an invaluable asset in delivering polished and engaging write-ups. (LinkedIn: https://www.linkedin.com/in/vaagisha-singh-8080b91)
What Are The Key Data Covered In This Viral Vaccine Cell Culture Media Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Viral Vaccine Cell Culture Media Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Viral Vaccine Cell Culture Media Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Viral Vaccine Cell Culture Media Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Viral Vaccine Cell Culture Media Market vendors
FAQ’s
Q.1 What are the main factors influencing the Viral Vaccine Cell Culture Media Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Viral Vaccine Cell Culture Media Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Viral Vaccine Cell Culture Media Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
#Viral Vaccine Cell Culture Media Market Trend#Viral Vaccine Cell Culture Media Market Size#Viral Vaccine Cell Culture Media Market Information#Viral Vaccine Cell Culture Media Market Analysis#Viral Vaccine Cell Culture Media Market Demand
0 notes
Text
Gene Therapy Cell Culture Media: The Backbone of Advanced Medicine
Gene therapy cell culture media are essential for the success of gene therapies, providing the critical environment needed for cell growth and maintenance. As gene therapy continues to advance, the demand for high-quality cell culture media is on the rise. Stay informed about the latest trends and developments in this rapidly growing market.
For More Details Click Here

1 note
·
View note
Text
Bridging the Gap: How the Global Cell Culture Media Market is Expanding Access to Care
The global cell culture media market is expected to have steady growth and, by 2034, be valued at an estimated US$6,699.93 million. From a market value of US$3,513.8 million in 2024, this amounts to a Compound Annual Growth Rate (CAGR) of 6.66% during the forecast period.
In the life sciences sector, the cell culture market is expanding quickly and is essential to many types of research, medication development, and biotechnology applications. The practice of cultivating and preserving cells outside of their natural habitat in a controlled environment is known as cell culture. These cultured cells are an invaluable resource for scientists and researchers because they offer important insights into disease causes, cell behavior, and treatment responses.
Preview Next-Level Insights Sample: https://www.futuremarketinsights.com/reports/sample/rep-gb-14366
The desire for personalized medication and the rising demand for biopharmaceutical goods are the main factors driving the growth of the cell culture industry. Pharmaceutical companies are extensively investing in cell culture technology to create new medications and enhance existing ones, as the prevalence of chronic diseases rises. The need for sophisticated cell culture methods has also increased as a result of the growth of tissue engineering and regenerative medicine.
The market is also expanding because to creative solutions and technological breakthroughs. To better replicate in vivo settings, scientists are always creating better culture medium, bioreactors, and 3D cell culture systems. These developments enhance cell viability, productivity, and overall efficiency, meeting the growing requirements of the biotechnology and pharmaceutical sectors.
Competitive Landscape:
Important companies like Avantor, Inc., Thermo Fisher Scientific, Inc., Caisson Laboratories Inc., Becton Dickinson and Company, Lonza Group AG, Bio-Rad Laboratories, Inc., General Electric Company, etc. are present in the worldwide cell culture media market.
Many businesses are making significant investments in R&D to develop novel and specialized formulations of cell culture medium. The aforementioned tasks involve creating specified and serum-free media, refining current formulations, and integrating cutting-edge technology to fulfill the dynamic requirements of biopharmaceutical and life sciences research.
Recent Developments
On July 12, 2023, Merck announced the expansion of its Lenexa, Kansas facility, making it the company’s largest dry powder cell culture media facility and Center of Excellence in North America.
In October 2023, London-based startup Multus Biotechnology focused on cutting the cost of cell culture media for cultivated meat by leveraging AI to optimize formulation efficiency. The startup addresses challenges in ingredient quality, scalability, and bioprocess productivity, aiming to enhance the sustainability and cost-effectiveness of cell culture media.
On July 27, 2023, MilliporeSigma invested $25 million to expand its cell culture media production facility in Lenexa, Kansas. The expansion included 98,000 ft2 of lab space, reinforcing the company’s commitment to meeting the increasing demand for cell culture media. Lenexa now serves as one of the three global centers of excellence for dry powder cell culture media manufacturing.
Key Companies in the Market:
Avantor, Inc.
Thermo Fisher Scientific, Inc.
Caisson Laboratories Inc.
Becton Dickinson and Company
Lonza Group AG
Bio-Rad Laboratories, Inc.
General Electric Company
Cell Culture Technologies LLC
Corning Incorporated
Fujifilm Holdings Corporation
Hi Media Laboratories Pvt. Ltd.
Merck & Co., Inc.
Market Segmentation:
By Type:
Serum-free Media
CHO Media
HEK 293 Media
BHK Media
VERO Cell Media
Insect Cell Media
Serum-free Stem Cell Media
CAR T-cell Media
Other Serum-free Media
Classical Media & Salts
Stem Cell Culture Media
Specialty Media
Chemically defined Media
Other Cell Culture Media
By Application:
Biopharmaceutical Production
Monoclonal antibodies
Cancer Research
Vaccines production
Other therapeutic proteins
Diagnostics
Drug Screening & Development
Tissue Engineering & Regenerative Medicine
Cell and gene therapy
Other tissue engineering & regenerative medicine applications
Other Application
By End User:
Pharmaceutical & Biotechnology Companies
Hospitals & Diagnostic Laboratories
Research & Academic Institutes
Others
By Region:
North America
Latin America
Europe
Asia Pacific
Middle East and Africa
0 notes
Text
CHO Cell Culture Media Market 2030 Has Huge Growth In Industry

CHO (Chinese Hamster Ovary) cell culture media are specifically formulated nutrient solutions used to support the growth, maintenance, and production of CHO cells in laboratory and industrial settings. CHO cells are commonly used in biopharmaceutical production for the expression of therapeutic proteins. Here is some information about CHO cell culture media:
For Download Sample Report Click Here: https://www.marketinforeports.com/Market-Reports/Request-Sample/520660
Basal Media: Basal media form the foundation of CHO cell culture media and provide essential nutrients and growth factors necessary for cell growth. They typically contain a balanced mixture of amino acids, vitamins, inorganic salts, glucose or other energy sources, and buffering agents to maintain pH stability. Basal media are usually supplemented with serum or serum substitutes, such as fetal bovine serum (FBS) or chemically defined serum-free supplements, to provide additional nutrients and growth-promoting factors.
Serum and Serum-Free Media: Traditionally, CHO cell culture media have utilized serum, such as FBS, to enhance cell growth and productivity. However, serum can introduce batch-to-batch variability and potential contamination risks. To overcome these challenges, serum-free media formulations have been developed. These media contain defined components that can support CHO cell growth and protein production without the use of animal-derived serum. Serum-free media often incorporate recombinant growth factors, hormones, and specific nutrients to mimic the growth-promoting properties of serum.
Feed Media: CHO cell cultures often require supplementation with feed media during the production phase to enhance cell viability, productivity, and protein quality. Feed media provide additional nutrients, such as amino acids, vitamins, and energy sources, to sustain the cells during the production of recombinant proteins. Feed media are typically added periodically or continuously to the culture to maintain optimal conditions for protein synthesis and prevent nutrient depletion.
Specialized Media: Depending on the specific requirements of CHO cell lines or the desired outcomes of the production process, specialized media formulations may be used. These specialized media can include chemically defined media, animal-component-free media, or media optimized for specific CHO cell lines or expression systems. These formulations are designed to provide precise control over the culture conditions and maximize protein yield and quality.
Buffer Systems: CHO cell culture media often incorporate buffering systems to maintain a stable pH within the optimal range for cell growth. Common buffer systems used include bicarbonate, phosphate, or HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid). The choice of buffer system depends on the specific experimental setup and requirements.
It’s important to note that the composition and formulations of CHO cell culture media can vary among different manufacturers and laboratories. Optimization of media components and culture conditions is critical for achieving high cell viability, productivity, and protein quality in CHO cell cultures. Specific media formulations are typically tailored to the specific needs of the cell line and the intended application, and they are often developed through a combination of empirical optimization and scientific expertise.
0 notes
Text
Biopharmaceutical Processing Equipment and Consumables Market - Global Opportunity Analysis and Industry Forecast (2022-2029)
Meticulous Research®– leading global market research company published a research report titled “Biopharmaceutical Processing Equipment and Consumables Market by Product Type {Filtration, Chromatography [Columns, Equipment], Disposable Bioreactors, Cell Culture Media, Shakers, Services), Application (Vaccine, mAb, R&D), and End User- Forecast to 2029.”
According to this latest publication from Meticulous Research, the global biopharmaceutical processing equipment and consumables market is expected to grow at a CAGR of 10.3% to reach $70.84 billion by 2029. Initiatives supporting the adoption of biopharmaceuticals, capacity expansions of biopharmaceutical manufacturing plants, and growing use of single-use technologies in commercial bioproduction are the factors driving the market growth.
Biopharmaceutical Processing Equipment and Consumables Market: Future Outlook
The global biopharmaceutical processing equipment and consumables market study presents, historical market data in terms of values (2020 and 2021), estimated current data (2022), and forecasts it for 2029 –by Product Type (Filtration Systems, Chromatography Systems {Resins, Columns, & Equipment}, Bioreactors {Reusable Bioreactors, Disposable/Single-use Bioreactors}, Cell Culture Products {Cell Culture Media [Cell Culture Media, by Physical Form–Dry Powder Media, Liquid Media], [Cell Culture Media, by Type–Off-the-Shelf Media, Custom Media], [Cell Culture Media Market, by Source-Chemically Defined Media, Natural Media], Reagents and Supplements, Cell and Cell Lines, Serum}, Mixing Systems, Bioprocessing Containers, Sterilizers, Centrifuges, Incubators, Shakers, Biosafety Cabinets, Other Consumables and Accessories, Services), Application (Commercial Bioproduction {Vaccine Manufacturing, mAb Production, Recombinant Protein Production, Cell and Gene Therapy Production}, Research and Development), End User (Biopharmaceutical/ Biotechnology Companies, Contract Development and Manufacturing Organizations and Contract Research Organizations (CDMOs/CROs), & Academics and Research Institutes), and Geography. The study also evaluates industry competitors and analyzes their market share at global and regional levels.

Download Free Sample PDF Copy: https://www.meticulousresearch.com/download-sample-report/cp_id=4200
Scope of the Report:
Global Biopharmaceutical Processing Equipment and Consumables Market, by Product Type
Resins
Columns
Equipment
Reusable Bioreactors
Disposable/Single-use Bioreactors
Dry Powder Media
Liquid Media
Off-the-Shelf Media
Custom Media
Chemically Defined Media
Natural Media
(Note: Other equipment and consumables include membrane adsorbers, cell disruption reagents, pipettes, syringes, vials, closures, tubing, connectors, and sensors)
Global Biopharmaceutical Processing Equipment and Consumables Market, by Application
Vaccine Manufacturing
mAb Production
Recombinant Protein Production
Cell and Gene Therapy Production
Global Biopharmaceutical Processing Equipment and Consumables Market, by End User
Biopharmaceutical/ Biotechnology Companies
Contract Development and Manufacturing Organizations and Contract Research Organizations (CDMOs/CROs)
Academics and Research Institutes
Biopharmaceutical Processing Equipment and Consumables Market, by Geography
U.S.
Canada
Germany
U.K.
France
Italy
Spain
Switzerland
Rest of Europe (RoE)
China
Japan
India
Rest of APAC (RoAPAC)
Speak to Our Analyst: https://www.meticulousresearch.com/speak-to-analyst/cp_id=4200 Based on product type, the filtration systems segment is estimated to account for the largest share of the market in 2022. The technological developments in filtration technologies and accelerated developments of single-use filtration systems to meet the growing need for single-use bioprocessing systems are contributing to the largest market share.
Based on application, the market is segmented into commercial bioproduction and R&D. The commercial bioproduction segment is estimated to account for the largest share of the market in 2022, owing to the growing number of biopharmaceuticals in the clinical development and nearing patent expiry of biologics.
Based on end user, the biopharmaceutical/biotechnology companies segment is estimated to account for the largest share of the market in 2022. The largest revenue share is attributed to the growing demand for biopharmaceutical equipment for commercial bioproduction in biopharmaceutical/biotechnology companies.
Geographic Review:
This research report analyzes major geographies and provides comprehensive analysis for North America (U.S. and Canada), Europe (Germany, France, U.K., Italy, Spain, Switzerland, and Rest of Europe), Asia-Pacific (China, Japan, India, and RoAPAC), Latin America, and Middle East & Africa.
North America is estimated to command the largest share of the biopharmaceutical processing equipment and consumables market in 2022, followed by Europe and Asia-Pacific. The U.S. is estimated to be the largest shareholding market in North America in 2022. An increase in the biotechnology R&D expenditure, the emergence of infectious diseases, supportive initiatives for increasing adoption of biosimilars, and large number of biopharmaceutical companies are some of the major factors driving the demand for biopharmaceutical processing equipment and consumables in the country.
BUY NOW: https://www.meticulousresearch.com/Checkout/94239620 Key Players
The key players operating in the global biopharmaceutical processing equipment and consumables market are 3M Company (U.S.), Thermo Fisher Scientific, Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Danaher Corporation (U.S.), Agilent Technologies, Inc (U.S.), Repligen Corporation (U.S.), Sartorius AG (Germany), Merck KGaA (Germany), Eppendorf AG (Germany), and Solaris Biotechnology Srl (Italy).
Key questions answered in the report-
Which are the high-growth market segments in terms of product type, application, end user, and regions/countries?
What was the historical market for biopharmaceutical processing equipment and consumables across the globe?
What are the market forecasts and estimates for the period 2022–2029?
What are the major drivers, restraints, challenges, opportunities, and trends in the global market of biopharmaceutical processing equipment and consumables?
Who are the major players in the global biopharmaceutical processing equipment and consumables market?
How is the competitive landscape, and who are the market leaders in the global biopharmaceutical processing equipment and consumables market?
What are the recent developments in the biopharmaceutical processing equipment and consumables market?
What are the different strategies adopted by the major players in the biopharmaceutical processing equipment and consumables market?
What are the geographical trends and high growth regions/countries?
top 10 companies operating in Biopharmaceutical Processing Equipment And Consumables Market- https://meticulousblog.org/top-10-companies-in-biopharmaceutical-processing-equipment-and-consumables-market/
Contact Us: Meticulous Research® Email- [email protected] Contact Sales- +1-646-781-8004 Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research Connect with us on Twitter- https://twitter.com/MeticulousR123
#Biopharmaceutical Equipment#Biopharmaceutical#Biopharmaceutical Processing#lifescience#Biopharmaceutical Processing Equipment#Biopharmaceutical Processing Equipment and Consumables Market#Biopharmaceutical Processing Equipment and Consumables#healthcare#drug discovery#drug development#medical devices#Filtration#Chromatography#Disposable Bioreactors#Cell Culture Media#Shakers#Vaccine#mAb#health#medical device#medical equipment
0 notes
Text
HOUSE MEANINGS IN ASTROLOGY



[READ] People often question why there’s so many meanings for each planet/house and the reason is so that you can learn more than just one thing about yourself through each placement. Otherwise astrology would be very vague and boring. These are all meanings that I’ve learned from my astrology classes at Kepler College

1ST HOUSE: identity/self, outward personality traits, outlook on life/approach to life, appearance, physical body, beauty, confidence, beginnings, how you initiate/ambition, your mannerisms, your outward behavior, physical fights, your presence, individuality, and passion
2ND HOUSE: money/finances (how we spend it, store it, and manage it), work, short term jobs, your work ethic, material possessions, self worth, values, emotional security, stability, financial security, how you meet financial obligations, your singing voice, giving/receiving, and resources (both material and non material)
3RD HOUSE: communication, your speaking voice/the way you talk, your mind, the way you think/your thinking skills, your perceptions, your opinions, your conscious mind, neighbors, siblings, interests, gossip, ideas/information, mathematics, literature, transportation (only ground not flying/air), local media, social media, cell phones, phone calls, visits, social activity, publishing, early education (before college), short trips, and short journeys
4TH HOUSE: homes/houses, family/family roots, your parents (particularly the mother/motherly figure), your inner child, emotions, foundations, your childhood, heredity, tradition, self-care, places of residence, real estate, properties, femininity, and conditions in early life
5TH HOUSE: children, childlike spirit, talent, creativity, drama, risk-taking, spotlight, romance (shows short term relationships, flings, hookups, and if long term relationships then only puppy love), hobbies, pleasures, objects of affection, vacations, games, speculation, fertility, concerts, festivals, and joy
6TH HOUSE: daily routine/day to day life/daily tasks, your health/fitness/the work you do on your body, your duties, self improvement, consistency, step-siblings, your hygiene, innocence, systems, service to others, co-workers, analytical nature, diets, animals, and your pets
7TH HOUSE: long term relationships, marriage, concern for others, attraction/attractiveness, charm, conflicts, partnerships, business partners, contracts, love affairs, open enemies, close associates, lower courts, negotiations, peers, agents, equality, harmony, and sharing
8TH HOUSE: major transformation, sex, death, longevity, changes, joint/shared finances, investments, stock market, your partners resources, taxes, inheritance, reproduction, seduction, intimacy (in general not only sexual), rebirth, merging, taboos, resurrection, loans, assets, secrets, mystery, businesses, spiritual transformation, magic (especially black magic), psychology, surgery/operations, trauma, periods, and the occult
9TH HOUSE: wisdom, law/laws, beliefs, religion, philosophy, higher education (college/university), viewpoints, languages, foreign environments, in-laws (your relatives through marriage), ethics, long journeys, travel, ideologies, higher courts, media, television, interviews, cross-cultural relations, grandparents, and learning
10TH HOUSE: your legacy, your career, your public image, your status, your reputation, fame, long-term goals, worldly attainment, sense of mission, responsibilities, recognition, authority, father/fatherly figure, experts, bosses, achievements, and professional aspirations
11TH HOUSE: friends, friend groups, gains, money made from career, desires, step/half parents, step/half children, uniqueness, inventions, technology, film, social awareness, influence, manifestations, hopes and wishes for the future, ideals, humanitarianism, associates (not just close ones), groups (in general), politics, social networking, where you make your debut into society, companions, allies, science, socialization/social interaction, clubs, organizations, and parties
12TH HOUSE: healing, the hidden, karma, karmic debts, old age, sleep, mental health, solitude/isolation, dreams (the ones you have when you sleep), hidden enemies, hidden causes, illusions, secret bed pleasures, spirituality, fears, losses, endings, escapism, impersonations, closure, need for withdrawal/privacy, afterlife, limiting beliefs, subsconcious memory, subconscious mind, hypnotism, self-undoing, hidden desires, the past, delay, and restrictions

MASTERLIST
MORE BEGINNER ASTROLOGY
PLANET MEANINGS

© 𝐚𝐬𝐭𝐫𝐨𝐬𝐤𝐲 𝟐𝟎𝟐𝟑 𝐚𝐥𝐥 𝐫𝐢𝐠𝐡𝐭𝐬 𝐫𝐞𝐬𝐞𝐫𝐯𝐞𝐝
#house meanings#astrology houses#houses in astrology#astro community#astrology#zodiac#astro placements#astrology tumblr#astro chart#birth chart#1st house#2nd house#3rd house#4th house#5th house#6th house#7th house#8th house#9th house#10th house#11th house#12th house
964 notes
·
View notes
Text

Picking Up Where the State Falls Through
None of this is new. The Black Panthers focused much of their work around meeting the needs of the Black community that the capitalist state and market had failed to fulfill. Projects like the Free Breakfast Program and ambulance services give credence to the extensive history of this type of mutual aid. It was the Panthers who exposed the extensive sickle cell anemia epidemic in the Black community by carrying out the work that the state should have done.
The concept of “revolutionary intercommunalism,” theorized by Black Panther leader Huey P. Newton, helped develop a strategy for structured community service programs also known as “survival programs.” These programs were meant to address the lack of helpful institutions and services in Black communities serving the needs of the people. The current situation demands proper respect given to its purpose. Intercommunalism focuses on and prioritizes Black self-determination outside of the state’s failures to adequately look after the needs of the Black community. The survival of underserved people is understood to be a part of the necessary politics of transformative change. Aside from the glitz of revolution that fuels popular depiction in the media, politics and culture, our current pre-revolutionary situation requires the everyday survival of those of us who would do the revolting in the first place. Intercommunalism pays respects to revolution as a process, and not merely an overnight reaction.
Across communities Black and all colors, we see a persistent need to address whatever shortcomings white supremacy delves out to us. It is not necessarily new for communities in the US dealing with white supremacy to support each other and build resistance from within. Starting our own services and building up each other is an everyday revolutionary politics of survival. However, what can and often does happen is that maintaining our own institutions within the bounds of capitalism becomes the objective when ending capitalism should be a necessary outcome. More than simply reacting to capitalism in anarchistic ways, we should be proactively working to overcome it by making our very models of resistance anti-capitalist. Depending on the likes of sympathetic capitalists and liberal elites is counterproductive in this respect. Instead of building ways to consistently respond to disaster, we must be proactive in ending the crisis of capitalism rather than solely attempting to counter it one day at a time.
A proactive pre-revolutionary situation will raise the consciousness of people to realize that they are already carrying out the radical politics they are often told to despise. Ahistorical liberal reimaginings of the past make tragedy into a necessary stepping stone for an empire that is learning at the expense of the oppressed. Real resistance positions people to build movements that undo the violence that oppression inflicts. We are not in need of excuses; we are in need of a better world. If we want that better world, we have to align our politics with a radical imagination, with sustainable everyday resistance and innovative strategy.
The task of making the planet a better place is a great task, but it is the only choice we have — lest we allow capitalism to destroy the carrying capacity of the one we currently inhabit. We can no longer afford to let crisis keep us entangled in this current state of disarray. Instead, we should charge our suffering to a system that must pay with its unacceptable existence.
Over 150 people worldwide have been murdered this year while defending the environment. This piece is in loving memory of those who have died and will die doing so. Thank you for all that you did for us.
#climate crisis#green anarchism#autonomous zones#autonomy#anarchism#revolution#ecology#climate change#resistance#community building#practical anarchy#practical anarchism#anarchist society#practical#daily posts#communism#anti capitalist#anti capitalism#late stage capitalism#organization#grassroots#grass roots#anarchists#libraries#leftism#social issues#economy#economics#anarchy works#environmentalism
9 notes
·
View notes
Text

I have so many nerdy thoughts about the Apple headset, particularly when it comes to interfaces and media, so read at your own risk:
I really think that Apple is a design company first, a technology company second. The fact it can do both well is impressive, but let’s be real: most of what was shown with the Reality Pro is stuff that other companies have done, piecemeal and less effectively, for the last 15+ years. Still, I bet even their competitors are relieved, and even excited, to have Apple in the VR headset market (and yes, it is a VR headset). Relieved because Apple didn’t show any new tech paradigm that puts them at a massive disadvantage; and excited because if someone is going to convince “normies” to put on a headset, it is going to be Apple. It may be through the Vision Pro that people get convinced of the value (such as it is) of spatial/volumetric/immersive interfaces, simply to go purchase a HTC Vive for a 3rd of the price. One can tell that Apple spent a lot of time and money showing what it would take to deliver some of the promises that VR manufacturers have been making for 15 years. Some users will happily take those promises as fulfilled with the Vision Pro, while others will agree to compromises and get other headsets.
But the real question is that of the value of spatial interfaces (what they really mean when they say “Spatial Computing”). It is not something we can answer in the abstract, as it involves a sort of media literacy accrued throughout generations, and spicy debates regarding immersive media. The generational issue is centered on a gamble these companies are making: That people who are naturalized to virtual worlds will demand novel user interfaces, expecting a 3rd dimension to simply “be there”. Why can’t I rotate my spreadsheet in Excel, revealing the transversal data space between the row and column? Can we put the formula in these new Z-Rows, instead of having to double-click on a single cell, like a caveman? What patterns will I discover once I can have graphs done based on rows, columns and Z-Rows, floating like holograms I can walk through? If these ideas sound bizarre to you, it may be because you have not been playing 3D games since childhood. Companies hope that new generations of users will ask these sorts of questions, however, as they need these spatial interfaces to become popular for their growth.
But even more foundational here is the issue of immersion. The concept of manipulation through media is as old as Plato, but it remains fresh and pressing in the face of social media and AI deep fakes. Most prescriptions on how to avoid manipulation put responsibility on individuals, who are supposed to “see through'' the BS (audience), or resist the monetary or libidinal temptations to create anti-social behavior (cultural producers). This is a deeply moralistic view, as it completely misses the role that the affordances of any given medium play in being a person. The fact is all and each subject is, at moments, manipulated and manipulator. Which of those roles we play is determined as much by individual “fixed” world views (morals), as by the relational space drawn by our communication technology (including language itself). This is why perfectly kind people can turn aggressive online, or why well-adjusted individuals consume objectionable content every day. The reptilian brain is always there, ready to be pleased or forgiven, and will slip into any medium it can regardless of how much puritanical restraint the medium is designed with.
To further complicate things, it is really hard to find the perfect split between audience and cultural producers as separate entities. No only because of the “prosumer” concept (which I find uninteresting), but because it is clear that even the most cool and collected cultural producer is, in themselves, a medium through which the program of immersive technology realizes “itself”. In other words: Apple is the way in which immersive media happens, turning the company into just an effective operator of an entity with its own agency and goals. What does “immersive media” want? That is the imminently political question for all of us in design, as we continue to carry its will. I am thinking about this myself, obviously, but trying to assert agency over it is REALLY HARD (specially as individuals).
Last thing: I find it fitting that Apple may be the one to finally push a bunch of people into immersive media, since it is the company that most effectively de-fanged minimalism as a political strategy. To me, Apple Minimalism “looks like” Brechtian alienation without the political radicalism, and the dematerialization of art without the materialist critique. We finally saw what all of those clean and smooth surfaces were for! It is not so that you reflect on your lived experience as a subject under capitalism or the police state. They are there so that you can watch the sexy cat-people in Avatar: The Way of Water, without anything (or anyone) bothering you.
37 notes
·
View notes
Text
https://webyourself.eu/blogs/426204/Cell-Culture-Media-Market-Size-Analysis-and-Forecast-2031
The Cell Culture Media Market in 2023 is US$ 4.58 billion, and is expected to reach US$ 11.7 billion by 2031 at a CAGR of 12.50%.
0 notes
Text
Cell Culture Media Market worth $13.0 billion by 2028
The global cell culture media market in terms of revenue was estimated to be worth $6.2 billion in 2023 and is poised to reach $13.0 billion by 2028, growing at a CAGR of 16.0% from 2023 to 2028.

Download an Illustrative overview:
Browse in-depth TOC on "Cell Culture Media Market"
359 - Tables
42 - Figures
315 – Pages
In 2022, the serum-free media segment accounted for the largest share of the type segment in the cell culture media market.
On the basis of type, the cell culture media market is divided into serum-free media, classical media & salts, stem cell culture media, specialty media, chemically defined media, and other cell culture media. In 2022, the serum-free media segment holds for the bigest share of the market. Factors responsible for the growth of this segment includes various advantages or benefits of serum-free media over other types of cell culture media.
In 2022, the biopharmaceutical production segment accounted for the largest share of the application segment in the cell culture media market.
On the basis of application, the cell culture media market is categorized into biopharmaceutical production, diagnostics, drug discovery & development, tissue engineering & regenerative medicine, and other applications. The biopharmaceutical production segment is estimated to grow at the highest growth rate during the forecast period. Factors responsible for the growth include, the growing production of cell culture-based vaccines, and expansion of pharmaceutical industry. The biopharmaceutical production applications of cell culture media include the manufacturing of biologic-based products such as vaccines, monoclonal antibodies, and other therapeutic proteins (such as anticoagulants, enzymes, blood factors, hormones, interferons, growth factors, interleukins, engineered proteins, and thrombolytics, among others).
In 2022, the Asia Pacific region is the fastest-growing region of the cell culture media market.
On the basis of the region, the global cell culture media market has been segmented into North America, Europe, the Asia Pacific, Latin America, and the Middle East and Africa. During the forecast period, the Asia Pacific market is estimated to register the highest CAGR during the forecast period. Increased focus of key market players on geographical expansion in emerging markets, favorable government policies and support for cell-based vaccines in the region and less manufacturing cost are some of the major factors anticipated to have positive impact on the market growth of Asia Pacific region.
Request Sample Pages:
Key Market Players:
Key players in the cell culture media market include Thermo Fisher Scientific, Inc. (US), Merck KGaA (Germany), Danaher Corporation (US), Sartorius AG (Germany), Corning Incorporated (US), FUJIFILM Irvine Scientific, Inc. (Japan), Lonza Group AG (Switzerland), Becton, Dickinson and Company (US), Miltenyi Biotec (Germany), and among others.
Recent Developments:
In June 2023, Thermo fisher launched Tumoroid Culture Medium to accelerate development of novel cancer therapies.
In April 2023, FUJIFILM Irvine Scientific launched BalanCD CHO Media Platform Portfolio for Bioprocessing.
In March 2022, FUJIFILM Irvine Scientific acquired Shenandoah Biotechnology. The deal would help the company to provide customers with a single point of access for their life science research, discovery, and cell and gene therapy needs.
In January 2022, Cytiva and Nucleus Biologics LLC collaborated to enhance custom cell media development for cell and gene therapies.
Cell Culture Media Market Advantages:
Versatility: Cell culture media provide a versatile platform for the growth and maintenance of various types of cells, including primary cells, stem cells, and cell lines derived from different tissues and species. This versatility allows researchers to study and manipulate diverse cell populations, facilitating a wide range of applications in basic research, drug discovery, and regenerative medicine.
Reproducibility: High-quality cell culture media formulations are standardized and extensively tested, ensuring consistent and reproducible results across different experiments and laboratories. This reliability is essential for generating meaningful and reliable data, which is critical for the advancement of scientific knowledge.
Customizability: The cell culture media market offers customizable formulations to cater to specific cell types and experimental requirements. Researchers can optimize media components to suit the unique needs of their cells, enhancing cell growth, proliferation, and differentiation, ultimately leading to more accurate and reliable experimental outcomes.
Scalability: Cell culture media have been developed to support large-scale production of cells, making them integral to biopharmaceutical manufacturing. With the increasing demand for cell-based therapies and biologics, scalable media solutions play a vital role in ensuring the efficient and cost-effective production of therapeutic cells and products.
Safety and Purity: High-quality cell culture media are manufactured under stringent quality control standards to ensure the absence of contaminants that may compromise cell viability or introduce experimental artifacts. These safety measures are essential to maintain the integrity of research findings and the safety of biopharmaceutical products.
Reduced Dependency on Animal Models: Advanced cell culture media formulations have contributed to reducing reliance on animal models in research, as they allow scientists to conduct more relevant experiments using human or cell-based models. This ethical advantage aligns with the growing focus on reducing animal testing while still advancing scientific progress.
Technological Advancements: The cell culture media market continually evolves, incorporating technological advancements, such as serum-free or xeno-free formulations, to improve cell culture systems' efficiency and reproducibility. These innovations empower researchers to work with more physiologically relevant and defined conditions, yielding results that better translate to clinical applications.
Accelerated Drug Discovery: Robust cell culture media accelerate drug discovery processes by enabling high-throughput screening of potential drug candidates on various cell models. This expedites the identification of promising compounds, shortens development timelines, and reduces drug development costs.
Overall, the cell culture media market's advantages have revolutionized modern biomedical research, playing a pivotal role in advancing scientific knowledge, drug development, and regenerative medicine, while contributing to the growth of the biotechnology and pharmaceutical industries.
#Cell Culture Media Market#Cell Culture Media Market Trends#Cell Culture Media Market Report#Cell Culture Media Industry
0 notes
Text

The viral vaccine cell culture media market possesses significant growth opportunities with the increasing prevalence of viral diseases and growing adoption of cell culture-based vaccine production processes. Cell culture media provide nutrients to sustain cell growth and maintain viability outside their natural surroundings. It finds widespread application in the cultivation of cells required for viral vaccine manufacturing.
#Viral Vaccine Cell Culture Media Market Trend#Viral Vaccine Cell Culture Media Market Size#Viral Vaccine Cell Culture Media Market Information#Viral Vaccine Cell Culture Media Market Analysis#Viral Vaccine Cell Culture Media Market Demand
0 notes
Text
Bennett University: A Leading Destination for Quality Higher Education
When it comes to choosing the right university, students are not just looking for academic excellence but also for institutions that provide holistic development. Bennett University, located in Greater Noida, Uttar Pradesh, is emerging as a prime destination for higher education in India. Established by the Times of India Group in 2016, Bennett University is dedicated to empowering students with cutting-edge skills and global perspectives.

Why Bennett University Stands Out
1. World-Class Curriculum
Bennett University offers a range of undergraduate, postgraduate, and doctoral programs designed in collaboration with leading global institutions. The curriculum is tailored to meet the demands of a rapidly evolving job market, especially in areas like engineering, management, law, and media.
The University emphasizes experiential learning, ensuring that students are equipped with practical skills to excel in real-world scenarios. The faculty includes accomplished academicians and industry professionals, providing students with invaluable insights into their fields.
2. Industry Partnerships and Internships
Bennett University has strong collaborations with leading companies, offering students unparalleled exposure to industry trends. These partnerships open up a wide array of opportunities, including internships and live projects, where students can apply their theoretical knowledge in a practical setting.
The University’s ties with global giants like Microsoft, Amazon Web Services (AWS), and IBM enable students to participate in exclusive training sessions, hackathons, and innovation challenges.
3. State-of-the-Art Infrastructure
The Bennett University campus is designed to foster an environment conducive to learning and innovation. Spread over 68 acres, the campus boasts modern classrooms, laboratories, and research centers. The University is equipped with the latest technological tools to provide students with a world-class education experience.
Additionally, the university offers excellent hostel facilities, libraries, recreational spaces, and sports amenities to ensure that students have a well-rounded campus life.
4. Global Exposure and International Collaborations
What truly sets Bennett University apart is its global outlook. The institution has partnered with internationally renowned universities, including Georgia Tech, Johnson Cornell, and Babson College, allowing students to gain global exposure through exchange programs, joint research initiatives, and international conferences.
This international collaboration enables students to understand global academic and professional standards, making them highly competitive in the global job market.
5. Entrepreneurship and Innovation Ecosystem
Bennett University is committed to nurturing the entrepreneurial spirit among students. The Center for Innovation and Entrepreneurship (CIE) is a hub where students can work on innovative ideas and develop them into market-ready products. The CIE provides mentorship, incubation, and funding opportunities to budding entrepreneurs.
With the growing startup culture in India, Bennett’s focus on entrepreneurship ensures that students are ready to contribute to and lead new ventures.
6. Placements and Career Support
Bennett University has a dedicated placement cell that works tirelessly to ensure that students secure positions in top companies. The university has an impressive placement record, with graduates being hired by leading multinational corporations such as Google, Deloitte, Microsoft, and Tata Consultancy Services.
The placement team also offers career counseling, soft skills training, and interview preparation to help students present themselves confidently to prospective employers.
Courses Offered at Bennett University
Bennett University offers a diverse range of courses across multiple disciplines:
Engineering: B.Tech (in various specializations including Computer Science, Electronics, and Biotechnology)
Management: BBA, MBA
Law: BA LLB (Hons.), BBA LLB (Hons.)
Media and Liberal Arts: BA (Journalism and Mass Communication), BA Liberal Arts
Doctoral Programs: Ph.D. in various fields
Each program is designed to provide in-depth knowledge while fostering critical thinking and problem-solving skills.
Campus Life at Bennett University
Campus life at Bennett is vibrant, with a wide range of cultural, social, and academic activities. The university organizes frequent guest lectures, workshops, and seminars to enrich students’ learning experiences. Moreover, Bennett has numerous student clubs and societies that cater to a variety of interests, from performing arts to robotics and coding.
Sports enthusiasts also have ample opportunities to engage in activities such as cricket, basketball, football, and swimming. With its blend of academics and extracurricular activities, Bennett University ensures that students develop both personally and professionally.
Why Choose Bennett University?
Choosing Bennett University is about more than just earning a degree. It’s about becoming part of a community that is committed to excellence, innovation, and leadership. With its focus on holistic development, Bennett University ensures that students are prepared to take on challenges, drive change, and lead in their respective fields.
For students looking for a university that offers world-class education, industry exposure, and global opportunities, Bennett University is the perfect choice.
#Bennett University#Quality Higher Education#Global Exposure#education#educationnews#universities#colleges#admissions#mba#higher education#students#imi university#university
2 notes
·
View notes
Text
Blogging in the era of TikTok and Instagram ///!!
In today's digital environment, it is important to consider if blogging is still relevant in the era of TikToks and Instagram. We may take into account a number of factors, such as communities, socialisation, digital data, and organisations, to investigate this subject.
Communities:
Blogging: Historically, specialised groups and subject matter experts have used blogging as a platform. Bloggers frequently provide material that appeals to specialised interests and subject matter. These groups of people may be incredibly helpful and engaging. TikTok and Instagram: These two platforms also contain communities, although their behaviours are usually rather different. Platforms for sharing short movies and images build communities around popular culture trends, influencers, and aesthetic ideas. Instead of extended conversations, engagement frequently consists of likes, comments, and shares.
Socialisation:
Blogging: Blogging enables in-depth topic investigation and fosters deliberative dialogue through comments. It encourages long-form material that encourages in-depth discussion and analysis. TikTok and Instagram are two sites that emphasise rapid and attractive content more than others. Through likes, emoticons, and brief remarks, they promote social interaction. The exchanges are frequently more informal and centred on visuals.
Digital Data:
Blogging: A considerable volume of text is produced by blogs. This information may be used for SEO (Search Engine Optimisation), making it an important source of organic traffic over the long run. Bloggers may get in-depth information about user behaviour and content performance from analytics tools. TikTok and Instagram are two social media sites that produce a lot of graphic and video content. Although they provide analytics tools, the data may be more difficult to understand and the content's shelf life is frequently shorter owing to the frequent revisions.
Organisations:
Blogging: For content marketing, thought leadership, and SEO, many organisations continue to see value in blogging. A technique to build authority and offer in-depth information is through blogs. Organisations frequently utilise TikTok and Instagram for visual marketing, influencer collaborations, and brand recognition. Short-form material might be useful for swiftly reaching larger audiences.
In conclusion, the objectives and tastes of content producers and users will determine if blogging is still relevant in the TikTok and Instagram era.

#blogging#social media#MDA2009#Dr_Bertha_Supremacy#blogger#instagram#tiktok#spotify#soundcloud#media#ronaldo#taylor swift#quora#socialmedia
7 notes
·
View notes