Don't wanna be here? Send us removal request.
Text
Ophthalmic Drugs Contract Manufacturing: An Overview Of The Rapidly Evolving

Regulatory compliances play a pivotal role in ophthalmic drugs manufacturing due to stringent norms and quality standards set by regulatory bodies like US FDA, EMA, etc. Ophthalmic products manufacturing needs to adhere to Current Good Manufacturing Practices (CGMP) to ensure product safety, efficacy, and quality. Ophthalmic drugs contract manufacturers invest heavily in approvals, certifications, and manufacturing facilities upgradation to meet evolving regulatory guidelines. They focus on establishing robust quality management systems, validation protocols, change control systems, and document controls. Regular product quality reviews and internal audits also help contract manufacturers deliver regulatory compliance.
Leading Technology And Specialized Equipment
Ophthalmic drugs require highly sophisticated and precise manufacturing technologies and equipment due to small volumes and concentrations. Contract manufacturers leverage leading technologies like microprocessing, microfluidics, micro-molding, and precision coating to produce a diverse range of ophthalmic products. They invest in specialized, high-end equipment like micronizing mills, soft-gel encapsulation systems, and laser-marking machines. Automation and digitization using IoT, AI, and analytics also help boost production efficiency and quality. The technologies and equipment enable contract manufacturers to cater to customized packaging and dosing requirements of ophthalmic drugs.
Specialized Facilities And Cleanrooms
Ophthalmic products demand stringent environmental control and hygiene standards owing to small dosage forms and direct exposure to eyes. Contract manufacturers operate highly specialized facilities with ISO classified cleanrooms to minimize microbial and particulate contaminations. Features like laminar air flow, differential air pressures, high-efficiency particulate air (HEPA) filtration help maintain critical environmental conditions. Periodic qualification and calibration of facilities and utility systems also ensure process validation. Designated areas for raw material receipts, products manufacturing, quality testing, packaging, and warehousing follow zoning principles. These specialized facilities enable contract manufacturers to ensure sterility, stability, and preserved efficacy of Ophthalmic Drugs Contract Manufacturing and storage.
Capabilities In Sterile Fill/Finish
A major portion of ophthalmic drugs require sterile fill/finish due to direct administration into eyes. Contract manufacturers have dedicated sterile suites equipped with barrier isolators, aseptic processing equipment, and self-contained environmental control systems. Technologies like lyophilization and terminal sterilization aid microbial decontamination. Stringent personnel training on garbing and cross-transfer procedures helps avoid contamination risks. Regular media fills and endotoxin challenge simulations validate sterilization process efficacy. Quality control testing through rapid microbiological methods, particulate testing and endotoxin assays ensure sterility assurance of aseptically filled ophthalmic products. These capabilities enable outsourcing of sterile fill/finish operations for preservative-free ophthalmic drugs.
Specialized Analytical Testing
Ophthalmic drugs require meticulous analytical characterization and quality testing due to small amounts administered near eye region. Contract manufacturers invest in -leading analytical instruments like HPLC, GC, dissolution testing equipment, and particle size analyzers. Methods involve testing of identity, purity, content uniformity, particulate matters, pH, osmolarity, sterility, bacterial endotoxin, and preservative content. Stability indicating methods help real-time product monitoring on storage. Various ophthalmic dosage forms like ointments, gels, suspensions etc. also require formulations development and evaluation of rheological properties, spreadability and bioavailability. Contract testing laboratories employ highly trained analytical experts, validated methods and computerized data integrity systems. These specialized testing capabilities help ensure efficacy and safety of outsourced ophthalmic products.
Case Studies And Fill-Finish Agreement
A leading UK-based ophthalmic drug firm outsourced development and fill-finish operations of its novel anti-inflammatory eye drop to a US-based contract manufacturer. Impressed by proven sterile fill/finish capabilities, quality systems, and regulatory compliance track record, six-month technology transfer was completed on schedule. Further, a 1-year commercial supply agreement was signed with production scale-up clauses. Another Ophthalmic Drugs Contract Manufacturing giant outsourced manufacturing of its portable multi-dose ophthalmic dispensers to a reputed Japanese contract manufacturer specializing in medical-device moulding. Leveraging expertise in micro-molding and precision assembly, the firm ensured precise dosing and improved patients' compliance. These cases illustrate effective collaborations aiding faster access of critical ophthalmic therapies.
Get more insights on this topic: https://www.trendingwebwire.com/ophthalmic-drugs-contract-manufacturing-meeting-global-ophthalmic-medications-demands-through-specialized-services/
About Author:
Priya Pandey is a dynamic and passionate editor with over three years of expertise in content editing and proofreading. Holding a bachelor's degree in biotechnology, Priya has a knack for making the content engaging. Her diverse portfolio includes editing documents across different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. Priya's meticulous attention to detail and commitment to excellence make her an invaluable asset in the world of content creation and refinement. (LinkedIn - https://www.linkedin.com/in/priya-pandey-8417a8173/)
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
0 notes
Text

The global ophthalmic drugs has seen steady growth in recent years due to rising prevalence of eye diseases and an aging population. Key drivers behind this trend include growing incidence of chronic diseases like diabetes that can damage vision over time. Advancements in diagnosis and treatment have also improved health outcomes and boosted demand for specialized medications. However, drug development and manufacturing requires significant financial investments and dedicated facilities to adhere to stringent cGMP and regulatory compliance standards for products intended for ocular delivery. This is where contract service providers play a vital role in supporting drug companies through their expertise and scalable manufacturing capacity.
0 notes
Text
Non-Viral Transfection Reagents - A Safer Alternative For Gene Delivery
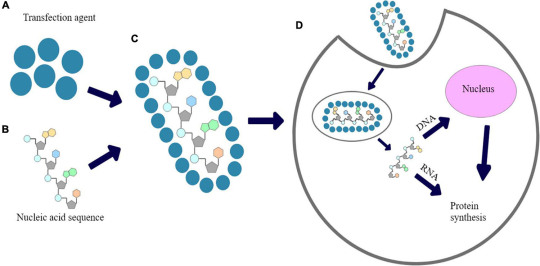
One of the earliest and simplest methods of non-viral transfection is through physical disruption of the cell membrane. Physical transfection methods such as electroporation apply an electric pulse to cells, causing the formation of temporary pores in the membrane through which nucleic acids can pass into the cell. Electroporation is a cost-effective technique that is widely used in research and industrial applications. However, it can be relatively toxic to cells and has low transfection efficiency compared to viral and other chemical methods. A related physical approach is particle bombardment or biolistics, which uses a gene "gun" to literally fire DNA-coated microscopic gold or tungsten particles into cells. While effective in some cell types, biolistics can damage cells and has limitations in scale-up for therapeutic use.
Cationic Lipid And Polymer-Based Transfection Agents
More advanced non-viral vectors take advantage of the natural ability of cationic lipids and polymers to condense and complex with negatively charged nucleic acids like DNA and RNA. When cationic molecules bind to nucleic acids, they form nano-sized particles called lipoplexes or polyplexes that are able to fuse with and enter cells. Some of the most popular cationic lipids used in research and therapies include DOTMA, DDAB, and DOTAP. Common cationic polymers used include polyethyleneimine (PEI) and poly-L-lysine. These cationic complexes protect nucleic acids from degradation while facilitating cellular uptake primarily through endocytosis. Cationic lipid- and polymer-based agents provide reasonable transfection efficiencies and scalability while displaying lower cytotoxicity compared to viral vectors. Continuous improvements aim to enhance transfection rates and reduce toxicity further.
Dendrimers And Other Nanoparticle Carriers
More engineered nanoparticles are also being explored as Non-Viral Transfection Reagents. Dendrimers are synthetic, nanoscale macromolecules with a highly branched treelike structure and numerous chemical functionalities on their surface. Their architecture makes them ideal for uniformly encapsulating drugs or genes. Positively charged dendrimers readily complex with nucleic acids through electrostatic interactions. Early generations showed some cytotoxic effects, but newer designs demonstrate efficient gene transfer capabilities comparable to viral vectors with significantly reduced toxicity. Gold nanoparticles, silica nanoparticles, carbon nanotubes and other inorganic nanomaterials are also being investigated as platforms for nucleic acid delivery. Surface functionalization allows conjugation of targeting ligands to facilitate cellular internalization. These novel carrier systems offer intriguing prospects as safer, targeted gene therapy vectors.
Cell-Penetrating Peptides (CPPs)
Cell-penetrating peptides represent another class of non-viral transfection agent. These are short, cationic peptide sequences often derived from naturally occurring proteins that are taken up efficiently by many cell types. A widely used CPP is TAT (trans-activating transcriptional activator) peptide from HIV-1. Others include penetratin and transportan. In combination with nucleic acids, CPPs are believed to traverse the plasma membrane and endosomal barriers, enabling direct cytoplasmic and nuclear delivery. CPP conjugation can significantly boost transfection compared to transfection reagents alone, while avoiding safety issues linked to viral or non-biodegradable carriers. CPPs face technical hurdles like aggregation and off-target effects that require addressing, but they offer a promising biocompatible approach. Further advances may yield CPP vectors effective enough for clinical gene therapy.
Combination Strategies And In Vivo Applications
Given the benefits and limitations of individual classes of Non-Viral Transfection Reagents, combination approaches hold promise to maximize desirable properties. For instance, cationic lipids or polymers can condense genes into nanoparticles for protection and increased cellular association, while CPPs or targeting ligands incorporated at the surface facilitate internalization and destination. Sequential layer-by-layer assembly enables tailoring of vector components for optimized transfection profiles in different cell types and disease contexts. Non-viral vectors also continue enhancing for in vivo gene delivery applications. These include functionalization with PEG to evade immune detection and cell-specific targeting with antibodies or other moieties.Successful non-viral gene therapy demonstrations in animal models have been reported for conditions like cancer, pulmonary disease, cardiovascular defects and CNS disorders. Well-designed combination systems may one day achieve viral-level gene transfer efficiencies needed for widespread clinical gene therapy with improved safety.
Get more insights on this topic: https://www.trendingwebwire.com/non-viral-transfection-reagents-alternative-methods-for-efficiently-introducing-nucleic-acids-into-cells/
Author Bio
Vaagisha brings over three years of expertise as a content editor in the market research domain. Originally a creative writer, she discovered her passion for editing, combining her flair for writing with a meticulous eye for detail. Her ability to craft and refine compelling content makes her an invaluable asset in delivering polished and engaging write-ups. (LinkedIn: https://www.linkedin.com/in/vaagisha-singh-8080b91)
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
1 note
·
View note
Text

Non-viral transfection techniques utilize physical or chemical methods to deliver nucleic acids like DNA, RNA, siRNA etc. into cells. Unlike viral vectors, non-viral methods don’t integrate delivered genetic material into the host cell’s chromosome, so they are safer but generally less efficient. They are widely used in research, biotechnology and potential gene therapy applications.
0 notes
Text
Non-Muscle Invasive Bladder Cancer: A Growing Healthcare Concern

Non-muscle invasive bladder cancer (NMIBC) is a common form of bladder cancer that has not yet spread beyond the inner layer of the bladder. NMIBC accounts for around 75% of new cases of bladder cancer diagnosed each year in the United States. The exact causes of NMIBC are still unknown, however certain risk factors such as smoking, exposure to chemicals in the workplace, and genetic factors may increase the likelihood of developing this type of bladder cancer.
Symptoms And Diagnosis Of NMIBC
The main symptoms of Non-Muscle Invasive Bladder Cancer include blood or blood clots in the urine, frequent urges to urinate, and painful urination. However, these symptoms can also be caused by other non-cancerous health conditions as well. A doctor will usually recommend cystoscopy and urine testing to help diagnose NMIBC. During cystoscopy, a thin camera is inserted through the urethra to examine the inner surface of the bladder. Abnormal tissues detected during cystoscopy are then removed for biopsy to check for cancerous cells under a microscope. Urine tests can also detect traces of blood or tumor cells in the urine which may indicate the presence of bladder cancer.
Stages And Grading Of Non-Muscle Invasive Bladder Cancer
NMIBC is divided into three stages - stage 0, stage Ta, and stage T1. Stage 0 refers to carcinoma in situ (CIS), where the cancer is found only in the inner layer of the bladder cells and has not grown into deeper layers. Stage Ta means the cancer has grown into the inner layer but not into deeper layers of the bladder. Stage T1 is when the cancer has grown into the connective tissue just beneath the inner lining of the bladder but has not spread outside the bladder.
In addition to staging, NMIBC tumors are also assigned a grade from G1 to G3 based on how aggressive they look under the microscope. Low-grade (G1) tumors usually grow slowly while high-grade (G3) tumors tend to grow and spread more quickly. Accurately staging and grading NMIBC helps doctors determine the appropriate treatment approach and predict the likelihood of recurrence.
Treatment Options For NMIBC
Treatment for NMIBC depends on the stage and grade of the cancer. For stage 0 or Ta G1 grade tumors, the standard first-line treatment is transurethral resection of the bladder tumor (TURBT). During this procedure, any visible tumors are surgically removed but the bladder lining and muscle layer remain intact. For higher risk stage Ta or T1 tumors, doctors may recommend additional treatment with intravesical therapy immediately after TURBT.
Intravesical therapy involves administering chemotherapy or immunotherapy medications directly into the bladder using a catheter. The most common intravesical medications are Bacillus Calmette-Guerin (BCG) immunotherapy and Mitomycin C chemotherapy. BCG treatment has the benefit of reducing recurrence rates more than chemotherapy alone in high-grade NMIBC. For recurrent or residual tumors after initial treatment, further TURBT or more aggressive therapies may be needed. In rare cases where the cancer recurs as muscle-invasive disease, radical cystectomy (complete bladder removal) may become necessary.
Rising Incidence And Healthcare Burden
Statistics show the incidence and prevalence rates of Non-Muscle Invasive Bladder Cancer have been rising over the past few decades. This increased occurrence corresponds to aging populations and growing risk factors like smoking in many Western countries. The National Cancer Institute estimates around 80,470 new cases of bladder cancer will be diagnosed in the United States in 2022 alone. Managing NMIBC poses a considerable economic burden on the healthcare system as well. The costs of long-term surveillance and recurrent treatment make bladder cancer one of the most expensive cancers to manage over a patient's lifetime. Experts project costs associated with NMIBC will continue escalating unless steps are taken to curb risk factors and improve outcomes through new treatment innovations.
Bladder Preservation As An Alternative To Cystectomy
For select high-risk NMIBC cases where cystectomy may otherwise be recommended, bladder preservation approaches are being explored as a potentially better alternative. Using maximal transurethral resection combined with multidrug intravesical chemo-immunotherapy and close monitoring, some studies show it is possible to preserve the bladder in over 70-80% of such patients, avoiding the morbidities of bladder removal surgery. However, patient selection criteria and long-term oncologic outcomes still need validation through larger randomized trials before bladder preservation can become a standard option. Ongoing research into new targeted drugs, immunotherapies, and molecular biomarkers may also help improve risk stratification and personalize bladder preservation approaches in the future.
Outlook
Non-Muscle Invasive Bladder Cancer accounts for the majority of new bladder cancer cases seen annually. Although less aggressive than muscle-invasive disease, NMIBC poses growing socio-economic and healthcare challenges due to high recurrence rates requiring life-time surveillance. Current management relies on transurethral resection combined with intravesical chemo-immunotherapies, with cystectomy reserved for refractory cases. Bladder preservation is being explored as an alternative to radical cystectomy in selected high-risk patients. Going forward, better prevention, accurate risk stratification aided by biomarkers, newer drugs, and personalized treatment protocols hold promise to reduce morbidity and costs related to NMIBC in the coming years.
Get more insights on this topic: https://www.trendingwebwire.com/non-muscle-invasive-bladder-cancer-symptoms-stages-and-treatment-options/
About Author:
Priya Pandey is a dynamic and passionate editor with over three years of expertise in content editing and proofreading. Holding a bachelor's degree in biotechnology, Priya has a knack for making the content engaging. Her diverse portfolio includes editing documents across different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. Priya's meticulous attention to detail and commitment to excellence make her an invaluable asset in the world of content creation and refinement. (LinkedIn - https://www.linkedin.com/in/priya-pandey-8417a8173/)
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
0 notes
Text

Non-Muscle Invasive Bladder Cancer (NMIBC) refers to the early stages of bladder cancer that have not yet spread into the deeper muscular layers of the bladder. In this article, we will discuss the symptoms, stages and treatment options for NMIBC
0 notes
Text
NAMPT Inhibitors: A Promising New Class Of Anti-Cancer Drugs

NAMPT (nicotinamide phosphoribosyltransferase), also known as visfatin or pre-B cell colony-enhancing factor (PBEF), is an enzyme involved in mammalian NAD+ biosynthesis. NAMPT catalyzes the rate-limiting step in the mammalian NAD+ salvage pathway by converting nicotinamide to nicotinamide mononucleotide (NMN). Nicotinamide phosphoribosyltransferase inhibitors are a new class of drugs that work by inhibiting this NAMPT enzyme, thereby blocking NAD+ biosynthesis in cancer cells.
Impact On Cancer Metabolism
Cancer cells exhibit increased metabolic needs compared to normal cells to support their rapid proliferation. They meet these high metabolic demands partly by upregulating the NAD+ salvage pathway mediated by NAMPT. Inhibiting NAMPT Inhibitors impairs this crucial source of NAD+ for cancer cells, significantly impacting their metabolism and survival. Depriving cancer cells of NAD+ is an attractive therapeutic strategy since NAD+ plays a central role in many cellular processes like glycolysis, mitochondrial function, and DNA repair - all of which are dysregulated in cancer.
Potential As Anti-Cancer Agents
Several preclinical studies have demonstrated potent anti-tumor activity of NAMPT inhibitors as single agents and in combination with other anti-cancer drugs across various cancer types. They cause metabolic catastrophe in cancer cells by depleting NAD+ levels, inhibiting glycolysis and disrupting mitochondrial function. This results in increased reactive oxygen species production, DNA damage, and eventual cancer cell death through mechanisms like necrosis or autophagy. The promising anti-tumor efficacy of Nicotinamide phosphoribosyltransferase inhibitors seen in animal models has translated to early clinical trials establishing their potential as anti-cancer therapeutics.
Leading Nicotinamide Phosphoribosyltransferase Inhibitors Drugs
FK866 (APO866) was the first Nicotinamide phosphoribosyltransferase inhibitors developed. It demonstrated robust anti-tumor activity in preclinical studies. However, it had formulation challenges and troublesome side effects in clinical trials. This led to the development of next-generation orally bioavailable Nicotinamide phosphoribosyltransferase inhibitors with improved pharmacokinetic and safety profiles.
Some Of The Leading NAMPT Inhibitors Drugs Currently In Clinical Trials Include:
- GMX1777 (EŌS-850) - Developed by Gemogenetics and currently in Phase 1/2 trials for solid tumors and lymphoma. It has shown an acceptable safety profile and signs of clinical responses.
- BMN673 - Developed by BioMedicine and currently in Phase 1 trials for solid tumors and lymphomas. It has a favorable pharmacokinetic profile and signals of clinical anti-tumor activity. - CHS-828 - Developed by Calithera Biosciences and currently in Phase 1 trials. It has shown preliminary anti-cancer activity and tolerability in patients with advanced solid tumors.
Clinical Trial Progress And Outcomes
While trials with early generation Nicotinamide phosphoribosyltransferase inhibitors FK866 and GMX0120 saw dose-limiting toxicities, next-gen candidates have exhibited better safety profiles in ongoing Phase 1 studies. They are generally well-tolerated with mostly low-grade adverse events reported. Dose escalation is ongoing to establish maximum tolerated and recommended Phase 2 doses.
Some patients with diverse cancer types including hepatocellular carcinoma, colorectal cancer, lymphoma, etc. have shown clinical responses and disease stabilization based on immune-related response criteria. Promising Phase 1 efficacy results have prompted expansion cohorts to further evaluate responses. Combination studies evaluating Nicotinamide phosphoribosyltransferase inhibitors along with immunotherapy or other metabolic modulating agents are also actively recruiting patients.
Based on accumulating clinical data, NAMPT inhibitors appear to be differentiating themselves from earlier candidates and demonstrating therapeutic potential. Developers are optimistic these agents could offer meaningful clinical benefits either as monotherapy or in combination regimens, pending outcomes from ongoing and planned late-stage trials.
Future Directions And Commercial Prospects
With novel mechanisms targeting cancer metabolism, Nicotinamide phosphoribosyltransferase inhibitors represent a promising new class of anti-cancer drugs. If ongoing and planned clinical studies establish their safety and efficacy profiles, major pharmaceutical companies may become interested in these assets given the large oncology opportunities. Developers are hoping to progress lead candidates into pivotal trials and partnerships over the next few years based on Phase 1 results.
While more research is still needed, NAMPT inhibitors could potentially help address unmet needs across several cancer types either as single agents or in combinations. Their commercial prospects will depend on demonstrating meaningful clinical benefits compared to existing standard-of-care regimens. Developers and research institutions worldwide continue advancing this new therapy class through collaborative clinical trials and projects aimed at fully elucidating the role of NAMPT inhibition in cancer treatment.
Get more insights on this topic: https://www.trendingwebwire.com/nampt-inhibitors-a-potential-therapy-for-various-cancers-and-other-diseases/
Author Bio:
Alice Mutum is a seasoned senior content editor at Coherent Market Insights, leveraging extensive expertise gained from her previous role as a content writer. With seven years in content development, Alice masterfully employs SEO best practices and cutting-edge digital marketing strategies to craft high-ranking, impactful content. As an editor, she meticulously ensures flawless grammar and punctuation, precise data accuracy, and perfect alignment with audience needs in every research report. Alice's dedication to excellence and her strategic approach to content make her an invaluable asset in the world of market insights. (LinkedIn: www.linkedin.com/in/alice-mutum-3b247b137 )
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
0 notes
Text

NAMPT inhibitors are a class of investigational drugs that work by inhibiting nicotinamide phosphoribosyltransferase (NAMPT), an enzyme that regulates NAD+ biosynthesis in the body. NAMPT catalyzes the rate-limiting step in one of the primary NAD+ salvage pathways in mammals. By blocking NAMPT, these drugs aim to decrease cellular NAD+ levels and disrupt various NAD+-dependent processes and pathways involved in diseases.
0 notes
Text
Sleep Tech Devices Market Set To Grow At Highest Pace Owing To Rising Preference For Wireless Devices

The Sleep Tech Devices Market comprises products used for tracking and monitoring various sleep parameters. Sleep tech devices help diagnose sleep disorders and improve sleep quality through continuous monitoring. Key sleep tech devices include sleep tracker watches, sleep apps, under mattress sensors, and wearables. Sleep trackers help track critical parameters like snoring levels, heart rate, sleep cycles, and oxygen levels. The growing demand for wireless products to monitor health in real-time is fueling the demand for sleep tech devices.
The Sleep Tech Devices Market is estimated to be valued at US$ 19.9 Bn in 2024 and is expected to exhibit a CAGR of 17% over the forecast period 2024-2031.
Rising awareness regarding the importance of good sleep and various sleep-related disorders is driving the demand for sleep tech devices among consumers. Sleep tech devices help diagnose sleep apnea and insomnia and recommend lifestyle changes and therapies to enhance sleep quality.
Key Takeaways
Key players operating in the Sleep Tech Devices Market are Koninklijke Philips N.V., Huawei Device Co Ltd., ResMed, Xiaomi, and Apple Inc. Philips offers a wide range of sleep and respiratory care solutions including ventilators, masks, and sleep tracking devices. Huawei launched its Band 6 sleep tracker in 2021 with 90+ workout modes and two-week battery life. With over 90 million happy customers, ResMed is a global leader in sleep apnea treatment solutions including sleep apnea masks and VPAP devices.
Rising health awareness is fueling the adoption of sleep tech devices globally. Data from sleep trackers indicating poor sleep quality is encouraging users to opt for CPAP machines and dental appliances. CPAP machine shipments grew over 7% during the pandemic as more people were diagnosed with sleep apnea symptoms owing to remote monitoring via oximeters and pulse oximeters.
Technological advancements are boosting the adoption of wearables and apps for tracking sleep. Integration of advanced technologies like infrared sensors, GPS, and heart rate sensors has enhanced the accuracy of sleep data from wearables. The availability of advanced data analytics tools is further helping identify correlations between sleep, activity, and health parameters.
Market Trends
The rising popularity of biointegrated electronics is a key trend in the Sleep Tech Devices Market. Devices implanted under the skin can seamlessly and continuously track critical health data without the need for wearables or apps. Another major trend is the growing recognition of circadian rhythms and development of lighting and ambient products to sync body clock and enhance sleep cycles. Companies are integrating lighting, audio, and fragrance elements into devices and apps to create customizable circadian lighting programs and schedules.
Market Opportunities
The rapid pace of digital transformation also opens avenues to leverage AI and big data analytics to gain deeper insights into sleep-health correlations. Longitudinal health data from a large population of sleep tech users can help develop highly customized sleep solutions. Another key area is pediatric sleep tech which is still in nascent stages. Pediatric-focused sleep solutions can help address rising concerns over children’s screen time, bedtimes, and sleep quality.
Impact Of COVID-19 On Sleep Tech Devices Market Growth
The COVID-19 pandemic has significantly impacted the growth of the Sleep Tech Devices Market globally. During the initial stages of the pandemic in 2020, many economies imposed strict lockdowns and restrictions on travel. As people were confined to their homes, lifestyle changes such as increased screen time, disrupted sleeping schedules and high stress levels seriously affected sleep quality and patterns. This drove higher demand for sleep tech devices as consumers sought solutions to monitor and improve their sleep. However, supply chain disruptions and factory shutdowns posed challenges for manufacturers. As the pandemic progressed through 2021, while restrictions eased in some regions, demand continued to rise. Manufacturers responded by ramping up production capacities with safety protocols and accelerating their digital transformation strategies to sustain business continuity. Going forward, popularity of remote work arrangements and awareness about benefits of monitoring health vitals are expected to keep propelling the Sleep Tech Devices Market even in the post-pandemic era.
Europe Currently Concentrates Highest Value In Sleep Tech Devices Market
Among geographical regions, Europe currently holds the leading share of the Sleep Tech Devices Market in terms of value. Countries such as Germany, UK, France have high consumer spending power and awareness regarding importance of quality sleep. Several leading players have their headquarters and manufacturing facilities located within Europe, allowing for easy availability of a wide variety of products. However, COVID-19 induced lockdowns disrupted regional sales initially. With restrictions now easing, demand recovery is strong. Still, high energy costs and changing economic conditions pose near-term challenges to market growth in Europe. The region will continue facing stiff competition from rapidly expanding Asian markets in the forecast period.
Asia Pacific Emerging as the Fastest Growing Region for Sleep Tech Devices
The Asia Pacific region has emerged as the fastest growing regional market for sleep tech devices globally. Countries like China, India and Indonesia have large populations experiencing rising lifestyle diseases due to changing working patterns and digitization of daily lives. This is generating significant demand for sleep monitoring solutions. Additionally, these developing economies also offer attractive market opportunities owing to growing disposable incomes, increasing health awareness and technology adoption. Leveraging advantageous policies, low manufacturing costs and presence of supply chain facilities, key players have commenced local production in Asia to tap into its high growth potential. If distribution channel expansion, brand awareness campaigns and economic revival efforts sustain post-pandemic, the Asia Pacific Sleep Tech Devices Market is slated for continued strong expansion through 2031.
Get more insights on this topic: https://www.trendingwebwire.com/sleep-tech-devices-market-is-estimated-to-witness-high-growth-owing-to-advancements-in-connected-devices/
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
What Are The Key Data Covered In This Sleep Tech Devices Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Sleep Tech Devices 's growth between 2024 and 2031.
:- Accurate calculation of the size of the Sleep Tech Devices and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Sleep Tech Devices Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Sleep Tech Devices vendors
FAQ’s
Q.1 What are the main factors influencing the Sleep Tech Devices ?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Sleep Tech Devices companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Sleep Tech Devices ’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
#Sleep Tech Devices Market Trend#Sleep Tech Devices Market Size#Sleep Tech Devices Market Information#Sleep Tech Devices Market Analysis#Sleep Tech Devices Market Demand
0 notes
Text

The Sleep Tech Devices Market has seen immense growth opportunities owing to the growing adoption of smart technologies used for monitoring and improving sleep quality. Sleep tech devices such as smartwatches, wearables, and sleep apps have gained popularity among users looking to track their sleep patterns and duration. Products like sleep trackers, smart beds, and waking lamps are being incorporated to optimize the sleep environment.
#Sleep Tech Devices Market Trend#Sleep Tech Devices Market Size#Sleep Tech Devices Market Information#Sleep Tech Devices Market Analysis#Sleep Tech Devices Market Demand
0 notes
Text
The Short-Acting Insulin Market To Grow At Highest Pace Owing To Increasing Prevalence Of Diabetes

The short-acting insulin market comprises human insulin, insulin lispro, insulin aspart, and insulin glulisine. Short-acting insulin helps patients with diabetes to manage their blood sugar level within a few hours after injection. It is commonly used before or during meals or when blood sugar level is high. The increasing prevalence of diabetes worldwide has significantly boosted the demand for short-acting insulin for effective management and treatment of the disease.
The Short-Acting Insulin Market is estimated to be valued at US$ 9.5 Bn in 2024 and is expected to exhibit a CAGR of 5.1% over the forecast period 2024-2031.
Key Takeaways
Key players operating in the short-acting insulin are Eli Lilly and Company, Novo Nordisk, Sanofi, Biocon, and Adocia.
Eli Lilly and Company is a global leader in diabetes care with prominent products such as Humalog and Admelog. Novo Nordisk is one of the leading pharmaceutical companies in diabetes care with short-acting insulin products such as NovoRapid and Fiasp.
The growing incidence of diabetes due to obesity, lack of physical activity, and unhealthy lifestyles has spurred the demand for short-acting insulin worldwide. According to the International Diabetes Federation, approximately 537 million adults were living with diabetes in 2021, and the number is expected to rise to 643 million by 2030 and 784 million by 2045.
Technological advancements in drug delivery systems have led to the development of novel short-acting insulin formulations for improved efficacy and patient compliance. For instance, Fiasp by Novo Nordisk is an ultra-fast-acting insulin aspart indicated for adults with diabetes.
Market Trends
Growing popularity of pre-filled insulin pens - Pre-filled insulin pens offer convenience of use and accurate dose delivery compared to vials and syringes. This is encouraging more patients and healthcare providers to shift from conventional vials to pre-filled insulin pens.
Growing demand for human insulin analogs - Second-generation human insulin analogs mimicking rapid-acting insulin have demonstrated faster absorption and onset of action. Hence, they are gaining traction over conventional human insulins.
Market Opportunities
Smart insulin delivery devices - Integration of connectivity features in insulin pumps and pens can help synchronize insulin delivery with daily activities and meals. This represents a lucrative avenue.
Emerging economies in Asia - Countries like China, India, Indonesia, and Vietnam are expected to fuel future demand growth owing to increasing diabetes prevalence and enhanced access to treatment options.
In conclusion, the Short-Acting Insulin Market is expected to grow notably in the forecast period owing to the rising prevalence of diabetes worldwide along with technological developments for convenient and more efficient insulin therapies.
Impact Of COVID-19 On Short-Acting Insulin Market Growth
The COVID-19 pandemic has significantly impacted the growth of the Short-Acting Insulin Market. During the initial months of the pandemic, several nations imposed strict lockdowns and social distancing measures to curb the spread of the virus. This led to disruptions across the healthcare sector and supply chain networks. Many diabetic patients faced challenges in regularly accessing insulin therapy and management services during this period. It became difficult for patients to physically visit hospitals or clinics for insulin injections or consultations with doctors. Telehealth and remote monitoring emerged as viable options to ensure continuity of care. However, not all patients had access to these virtual healthcare platforms.
With lockdowns being gradually lifted in many countries post mid-2020, the short-acting insulin market is recovering. Healthcare systems have implemented new protocols and guidelines to minimize infection risks while delivering services. Insulin manufacturers ramped up production to meet any surge in demand. Governments also focused on strengthening supply chains and ensuring uninterrupted availability of diabetes treatment and management products. The COVID-19 pandemic highlighted the need for innovative insulin delivery methods like pens, pumps and wearable devices. It also emphasized the importance of personalized therapy and glucose monitoring technology. In the coming years, the market is expected to grow backed by increasing diagnosis rates, growing awareness about diabetes self-management, and advances in insulin therapy products.
Geographical Regions With Highest Short-Acting Insulin Market Value
North America represented the largest short-acting insulin market in terms of value in 2024. This is attributed to the rising prevalence of diabetes, growing obesity rates, availability of advanced treatment options and high healthcare spending in the US and Canada. Europe held the second position driven by increasing government focus on noncommunicable diseases and presence of major market players. Asia Pacific is projected to be the fastest growing regional market between 2024-2031 backed by growing awareness, expanding patient reach of key players and rising healthcare investments in countries like China and India.
Fastest Growing Regional Market For Short-Acting Insulin
Asia Pacific is poised to be the fastest growing regional market for short-acting insulin during the forecast period from 2024 to 2031. This can be attributed to factors like rising diabetes prevalence, growing medical expenditures, increasing focus on prevention and management of chronic diseases, improving access to diagnosis and treatment services, and expansion initiatives by leading manufacturers. Furthermore, adoption of technologies like smartphones and smart insulin pens is supporting diabetes care in the region. Asia Pacific countries such as China, India and Indonesia offer lucrative growth opportunities for market players due to rising healthcare infrastructure, surge in medical tourism and rapidly increasing patient pool.
Get more insights on this topic: https://www.trendingwebwire.com/advanced-technologies-are-estimated-to-drive-growth-in-the-short-acting-insulin-market/
Author Bio:
Money Singh is a seasoned content writer with over four years of experience in the market research sector. Her expertise spans various industries, including food and beverages, biotechnology, chemical and materials, defense and aerospace, consumer goods, etc. (https://www.linkedin.com/in/money-singh-590844163 )
What Are The Key Data Covered In This Short-Acting Insulin Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Short-Acting Insulin Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Short-Acting Insulin Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Short-Acting Insulin Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Short-Acting Insulin Market vendors
FAQ’s
Q.1 What are the main factors influencing the Short-Acting Insulin Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Short-Acting Insulin Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Short-Acting Insulin Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
#Short-Acting Insulin Market Trend#Short-Acting Insulin Market Size#Short-Acting Insulin Market Information#Short-Acting Insulin Market Analysis#Short-Acting Insulin Market Demand
0 notes
Text

The short-acting insulin market has witnessed significant growth over the past few years owing to the rising prevalence of diabetes worldwide. Short-acting insulin helps manage blood glucose levels in diabetes patients immediately after a meal. The most commonly used short-acting insulin products include insulin lispro, insulin aspart, and insulin glulisine. These synthetic insulin analogs work faster as compared to regular human insulin and have a shorter duration of action.
#Short-Acting Insulin Market Trend#Short-Acting Insulin Market Size#Short-Acting Insulin Market Information#Short-Acting Insulin Market Analysis#Short-Acting Insulin Market Demand
0 notes
Text
Seasonal Allergic Rhinitis Market Will Grow At Highest Pace Owing To Increasing Demand For Immunotherapy Treatment
The seasonal allergic rhinitis market comprises drugs, immunotherapy, and antihistamines used in the treatment of seasonal allergies, also known as hay fever. Allergic rhinitis is caused by an allergic reaction to airborne allergens such as pollen from trees, grass, flowers, and weeds. Common symptoms of seasonal allergic rhinitis include sneezing, nasal congestion, runny nose, and itchy, watery eyes. The demand for immunotherapy treatment, also known as allergy shots, is increasing as it provides long-term relief from allergies with minimal side effects as compared to medication.
The Seasonal Allergic Rhinitis Market is estimated to be valued at US$ 10.8 Bn in 2024 and is expected to exhibit a CAGR of 2.9% over the forecast period 2024-2031.
Key Takeaways
Key players operating in the seasonal allergic rhinitis market are Regeneron Pharmaceuticals, Revolo Biotherapeutics, Allergy Therapeutics, Emergo Therapeutics, ALKAbello. Regeneron Pharmaceuticals dominates the market with its successful drug Dupixent which treats both seasonal allergic rhinitis and other allergic conditions.
The seasonal allergic rhinitis market is witnessing high demand due to the growing prevalence of allergic rhinitis worldwide. According to a study by the American Academy of Allergy Asthma & Immunology, more than 50 million Americans suffer from allergies every year with seasonal allergic rhinitis affecting 20% of the global population.
Technological advancements are being made in allergy immunotherapy which involves exposing patients to gradually increasing doses of allergen extracts to boost immunity. New advanced therapies like sublingual and subcutaneous immunotherapy are gaining popularity due to easy mode of administration and higher efficacy.
Market Trends
The increased adoption of combination therapies using corticosteroids with antihistamines for treating moderate to severe symptoms is a key trend in the seasonal allergic rhinitis market. This provides better relief than single therapy. Another trend is the growing preference for generic drugs due to their lower cost compared to branded drugs.
Market Opportunities
Rising pollution levels have made seasonal allergies more severe creating opportunities for seasonal allergic rhinitis drugs. Over-the-counter remedies and nasal sprays are expected to witness high demand owing to convenience for self-treatment of mild symptoms.
Impact Of COVID-19 On Seasonal Allergic Rhinitis Market Growth
The COVID-19 pandemic has impacted the seasonal allergic rhinitis market in ways. The supply chain disruptions led to shortages in antihistamine nasal spray supplies causing patients difficulties. The lockdowns and social distancing measures reduced outdoor activities which provided temporary relief to seasonal allergy patients. However, it also delayed in-person doctor consultations and allergy testing limiting appropriate diagnoses and treatment plans. Telehealth emerged as an important tool for doctors to remotely monitor patients and adjust medications.
The pandemic shifted priorities of drug makers away from new product development and clinical trials towards vaccines and antiviral drugs. This slowed new seasonal allergy drug approvals. Post pandemic, the growth is expected to rebound faster in developing countries as healthcare budgets recover and access to treatment improves. Meanwhile, developed regions may see a moderate growth due to preference for online consultations, home-based allergy management devices and expectation of new innovations that provide lasting relief.
To sustain the market potential, companies need strategies addressing supply chain resilience, funding of new therapies and leveraging digital platforms. Collaborations with telehealth providers will help expand access while monitoring pandemics' long term impact on seasonal patterns is important for production planning.
Geography: Europe
Europe accounts for the largest share of the seasonal allergic rhinitis market, both in terms of value and volume. This is attributed to high per capita healthcare spending, advanced medical infrastructure and greater awareness about allergy diagnosis and management. Countries such as Germany, United Kingdom and France have a major market presence due to large patient pools and strong reimbursement structures supporting quality care.
The market is also rapidly growing in Central and Eastern European nations as healthcare reforms attract international pharmaceutical investments. Rising environmental pollution and allergen exposures in developing cities are contributing to higher disease incidence. Overall, Europe will continue dominating the seasonal allergic rhinitis space backed by strong research environments discovering novel therapeutic targets.
Get more insights on this topic: https://www.trendingwebwire.com/seasonal-allergic-rhinitis-market-is-estimated-to-witness-high-growth-owing-to-emerging-immunotherapies/
Author Bio:
Alice Mutum is a seasoned senior content editor at Coherent Market Insights, leveraging extensive expertise gained from her previous role as a content writer. With seven years in content development, Alice masterfully employs SEO best practices and cutting-edge digital marketing strategies to craft high-ranking, impactful content. As an editor, she meticulously ensures flawless grammar and punctuation, precise data accuracy, and perfect alignment with audience needs in every research report. Alice's dedication to excellence and her strategic approach to content make her an invaluable asset in the world of market insights. (LinkedIn: www.linkedin.com/in/alice-mutum-3b247b137 )
What Are The Key Data Covered In This Seasonal Allergic Rhinitis Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Seasonal Allergic Rhinitis Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Seasonal Allergic Rhinitis Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Seasonal Allergic Rhinitis Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Seasonal Allergic Rhinitis Market vendors
FAQ’s
Q.1 What are the main factors influencing the Seasonal Allergic Rhinitis Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Seasonal Allergic Rhinitis Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Seasonal Allergic Rhinitis Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
#Seasonal Allergic Rhinitis Market Trend#Seasonal Allergic Rhinitis Market Size#Seasonal Allergic Rhinitis Market Information#Seasonal Allergic Rhinitis Market Analysis#Seasonal Allergic Rhinitis Market Demand
0 notes
Text

Seasonal allergic rhinitis, commonly known as hay fever, is an allergic inflammatory disease of the nasal airways caused by exposure to airborne allergens such as pollen and mold spores. Common symptoms include sneezing, nasal congestion, runny nose, and itchy eyes. Seasonal allergic rhinitis is usually treated through oral or nasal decongestants, antihistamines, immunotherapy, and conjunctival or nasal anti-allergic therapy. Immunotherapy introduces small and gradually increasing doses of allergens to build tolerance and offer long-term symptom relief.
#Seasonal Allergic Rhinitis Market Trend#Seasonal Allergic Rhinitis Market Size#Seasonal Allergic Rhinitis Market Information#Seasonal Allergic Rhinitis Market Analysis#Seasonal Allergic Rhinitis Market Demand
0 notes
Text
Polymyalgia Rheumatica Drugs Market Will Grow At Highest Pace Owing To Increasing Demand For Effective Treatments

Polymyalgia rheumatica (PMR) is a disorder characterized by muscle pain and stiffness, usually around the shoulder and hip areas. PMR drugs offer targeted therapies to reduce pain and inflammation. Corticosteroids such as prednisone are commonly prescribed as they suppress immune systems and reduce discomfort. Biologics like humanized monoclonal anti-IL6 receptor antibody drugs like tocilizumab are increasingly being used to treat PMR as well.
The Polymyalgia Rheumatica Drugs Market is estimated to be valued at US$ 266 Mn in 2024 and is expected to exhibit a CAGR of 13% over the forecast period 2024-2031.
Key Takeaways
Key players operating in the Polymyalgia Rheumatica Drugs are Sparrow Pharmaceuticals, Novartis Pharmaceuticals, Roche Chugai, Chugai Pharmaceutical, Genentech Inc.
Growing geriatric population is driving demand as PMR is more prevalent in older adults. Technological advancements are helping develop targeted biologic drugs with lesser side effects compared to traditional corticosteroids.
Market Trends
Increasing preference for biologics over corticosteroids: Due to lesser side effect profile, biologics like tocilizumab are becoming first line choices for PMR treatment. This trend will boost the biologics segment.
Rising R&D into pipeline drugs: Several companies are conducting clinical trials on new molecular entities for PMR. This will expand treatment options if approved.
Market Opportunities
Emerging markets in Asia Pacific: With developing healthcare infrastructure and raising healthcare spends, China, India offer high growth potential for PMR drugs.
Combination therapies: Combining biologics with corticosteroids can enhance efficacy while reducing steroid doses. This presents an opportunity area.
Impact Of COVID-19 On Polymyalgia Rheumatica Drugs Market Growth
The outbreak of COVID-19 pandemic has significantly impacted the growth of polymyalgia rheumatica drugs market globally. During the pandemic, the focus of healthcare professionals and resources shifted majorly towards managing COVID-19 patients. This led to postponement of various non-critical medical procedures and outpatient department visits. The postponement and cancellation of routine doctor consultations and elective surgeries resulted in reduced diagnosis and treatment rates of polymyalgia rheumatica. Moreover, lockdowns and social distancing measures imposed by various governments restricted the movement of people as well as disrupted the supply chain of drugs manufacturing. This caused shortage of essential drugs used for treatment of polymyalgia rheumatica in different regions. However, with eased lockdowns and restrictions in 2021, the market is recovering slowly as diagnosis and treatments have resumed under strict safety guidelines. The key manufacturers are focusing on developing new drugs and treatment options to cater to the growing needs. They are also working on strengthening the supply chain to avoid future disruptions.
European Region Dominates The Polymyalgia Rheumatica Drugs Market
The European region currently dominates the Polymyalgia Rheumatica Drugs Market in terms of value. This is majorly attributed to high prevalence of polymyalgia rheumatica in the region coupled with advanced healthcare infrastructure and high healthcare spending by governments as well as patients. Within Europe, countries such as Germany, United Kingdom, Italy, and France collectively account for over 50% of the regional market revenue due to their strong economies and large patient pool affected by the disease. The rising geriatric population which is highly susceptible to polymyalgia rheumatica further supports the market growth in the region. Moreover, active government support for research and manufacturing of innovative treatment options also contributes to Europe's leading position in the global market.
Asia Pacific Emerges As The Fastest Growing Region In The Global Market
The polymyalgia rheumatica drugs market in the Asia Pacific region is estimated to witness the fastest growth during the forecast period. This can be attributed to increasing healthcare expenditure, improving access to diagnosis and treatment, rapidly growing geriatric population, and rising incidence of polymyalgia rheumatica in Asia Pacific countries. Additionally, strategic partnerships between international manufacturers and local players are helping in technology transfer and increasing availability of advanced drugs. Countries like China, India, Japan and South Korea are expected to be the major revenue generators for the APAC market owing to their huge patient bases and emerging economies. The APAC polymyalgia rheumatica drugs market is projected to expand at a CAGR of over 15% until 2031.
Get more insights on this topic: https://www.trendingwebwire.com/polymyalgia-rheumatica-drugs-market-is-estimated-to-witness-high-growth-owing-to-advancements-in-targeted-therapeutics/
Author Bio
Vaagisha brings over three years of expertise as a content editor in the market research domain. Originally a creative writer, she discovered her passion for editing, combining her flair for writing with a meticulous eye for detail. Her ability to craft and refine compelling content makes her an invaluable asset in delivering polished and engaging write-ups. (LinkedIn: https://www.linkedin.com/in/vaagisha-singh-8080b91)
What Are The Key Data Covered In This Polymyalgia Rheumatica Drugs Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Polymyalgia Rheumatica Drugs Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Polymyalgia Rheumatica Drugs Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Polymyalgia Rheumatica Drugs Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Polymyalgia Rheumatica Drugs Market vendors
FAQ’s
Q.1 What are the main factors influencing the Polymyalgia Rheumatica Drugs Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Polymyalgia Rheumatica Drugs Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Polymyalgia Rheumatica Drugs Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area��s assessment of agreements, income, and value implicate?
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
#Polymyalgia Rheumatica Drugs Market Trend#Polymyalgia Rheumatica Drugs Market Size#Polymyalgia Rheumatica Drugs Market Information#Polymyalgia Rheumatica Drugs Market Analysis#Polymyalgia Rheumatica Drugs Market Demand
0 notes
Text

The polymyalgia rheumatica drugs market involves drugs that help relieve symptoms of polymyalgia rheumatica such as pain and stiffness in the shoulders, hip and neck muscles. Polymyalgia rheumatica (PMR) is a common inflammatory disorder that occurs in older adults and causes aching and tenderness in the shoulder, hip and neck muscles. Corticosteroids are currently the standard treatment to alleviate symptoms of PMR. However, newer targeted biologic therapeutics hold promise in safely and effectively treating PMR without major side effects associated with corticosteroids.
#Polymyalgia Rheumatica Drugs Market Trend#Polymyalgia Rheumatica Drugs Market Size#Polymyalgia Rheumatica Drugs Market Information#Polymyalgia Rheumatica Drugs Market Analysis#Polymyalgia Rheumatica Drugs Market Demand
0 notes
Text
Restless Legs Syndrome Treatment Market Will Grow At Highest Pace Owing To Rising Awareness And Diagnosis Of RLS Disorder

The restless legs syndrome treatment market has been witnessing significant growth owing to the rising prevalence of RLS disorder globally. Restless legs syndrome or Willis-Ekbom disease is a common sensorimotor disorder characterized by unpleasant sensations in the legs and an urge to move them to relieve those sensations. The symptoms of RLS usually occur late in the day resulting in difficulty in falling asleep. The mainstream treatment options for RLS include dopaminergic drugs such as pramipexole, ropinirole, and benzodiazepines. The increasing awareness about the symptoms and management of RLS disorder and improving diagnosis rates are the key factors propelling the demand for effective RLS treatment drugs and devices.
The Restless Legs Syndrome Treatment Market is estimated to be valued at US$ 2.5 Bn in 2024 and is expected to exhibit a CAGR of 5.7% over the forecast period 2024-2031.
Key Takeaways
Key players operating in the restless legs syndrome treatment market are GlaxoSmithKline, Teva Pharmaceuticals, Boehringer Ingelheim, Pfizer Inc., and UCB Pharma. GlaxoSmithKline accounted for the dominant market share in 2021 owing to its blockbuster drugs Mirapex and Requip being used for RLS treatment.
The key growing demand in the market can be attributed to the rising prevalence of RLS disorder mainly due to increasing risk factors like advanced age, chronic kidney disease, iron deficiency, and pregnancy. According to estimates, around 10% of adults are affected by RLS worldwide. This high prevalence of RLS and associated symptoms are driving more people to seek effective treatment options.
Technological advancements like the development of next-generation neuromodulation devices for deep brain stimulation therapy and wearable devices with built-in sensors to monitor symptoms are boosting the adoption of non-drug treatment choices for RLS. Innovation in drug delivery systems to achieve 24x7 symptom relief without major side effects is also fueling the growth of the restless legs syndrome treatment market.
Market Trends
Increased adoption of combination drug therapies - The trend of prescribing more than one RLS drug in combination is growing as it helps relieve symptoms better than monotherapy in severe cases. Dopamine agonists are often combined with alpha-2-delta ligands.
Rising popularity of neurostimulation therapies - Advancements in neurostimulation devices and techniques like spinal cord stimulation and transcutaneous electrical nerve stimulation are providingrelief to RLS patients with fewer side effects than drugs.
Focus on developing personalized treatment protocols - With more insights into disease underlying pathophysiology, treatment protocols are becoming tailored to individual patient needs based on symptom triggers, severity, and comorbidities to achieve optimal outcomes.
Market Opportunities
Development of oral extended-release formulations - There is scope for developing oral long-acting RLS medications that reduce dosing frequency and boost adherence to the prescribed treatment regimen.
Combination of drug and non-drug therapies - Integration of dopaminergic drugs with neuromodulation and physical therapy holds potential for synergistic effects in RLS management by targeting multiple disease aspects.
Impact Of Covid-19 On Restless Legs Syndrome Treatment Market Growth:
The COVID-19 pandemic has impacted the Restless Legs Syndrome Treatment market in several ways. During the initial lockdowns imposed by various governments globally, there was a disruption in manufacturing and supply chain activities. This led to delay in production as well as unavailability of key raw materials. However, as Restless Legs Syndrome is a chronic neurological condition, the demand for its treatment remained constant.
With the scare of infection, patients started preferring online consultation and home delivery of medicines over visiting hospitals and clinics for treatment. This boosted the telemedicine and e-pharmacy sectors. Pharmaceutical companies also shifted their focus to ensuring uninterrupted supply of drugs via online channels. However, priorities of healthcare systems changed drastically during the pandemic with more focus on COVID patients. Resources and funding were diverted for coronavirus treatment leading to delay in new drug development projects and clinical trials for Restless Legs Syndrome treatment.
As lockdowns are gradually lifting now, manufacturing and supply chains are getting back on track. The pharmaceutical industry is also focusing on expansion of their online presence and delivery networks to cater to the changed consumer behavior. Researchers are accelerating drug development processes to launch new and improved treatment options in the market. It is expected that with rising vaccination rates and adaptation to new normal, the Restless Legs Syndrome Treatment market will see steady growth over the forecast period.
Regions With Highest Consumption Of Restless Legs Syndrome Treatment:
North America accounts for the largest share of the Restless Legs Syndrome Treatment Market in terms of value. This is majorly attributed to the rising prevalence of the neurological condition in the region coupled with high diagnosis and treatment rates. According to estimates, around 12% of the adult population in the United States suffers from Restless Legs Syndrome. Availability of advanced healthcare infrastructure and favorable reimbursement policies further drive the market growth in North America.
Europe is also one of the key geographical regions concentratrating consumption of Restless Legs Syndrome drugs. Countries like Germany, United Kingdom, France have reported large patient pools undergoing medication therapy. Rising neurological disorders due to aging population and growing awareness aid the European market expansion.
Fastest Growing Region in Restless Legs Syndrome Treatment Market:
The Asia Pacific region is projected to witness the fastest growth in the Restless Legs Syndrome Treatment Market over the forecast period. This can be attributed to increasing healthcare expenditures of developing nations like India and China. Rapid economic development, rising living standards and growing medical tourism are improving access to diagnosis and treatment in the Asia Pacific region.
Moreover, key international players are expanding their presence in Asia Pacific by collaborating with local pharmaceutical manufacturers. evolving healthcare infrastructure and rising disease awareness campaigns by government organizations are further boosting the Restless Legs Syndrome patient pool. The growth momentum is expected to continue in the forthcoming years as well.
Get more insights on this topic: https://www.ukwebwire.com/restless-legs-syndrome-treatment-market-is-estimated-to-witness-high-growth-owing-to-advancements-in-novel-drug-development/
About Author:
Priya Pandey is a dynamic and passionate editor with over three years of expertise in content editing and proofreading. Holding a bachelor's degree in biotechnology, Priya has a knack for making the content engaging. Her diverse portfolio includes editing documents across different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. Priya's meticulous attention to detail and commitment to excellence make her an invaluable asset in the world of content creation and refinement. (LinkedIn - https://www.linkedin.com/in/priya-pandey-8417a8173/)
What Are The Key Data Covered In This Restless Legs Syndrome Treatment Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Restless Legs Syndrome Treatment Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Restless Legs Syndrome Treatment Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Restless Legs Syndrome Treatment Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Restless Legs Syndrome Treatment Market vendors
FAQ’s
Q.1 What are the main factors influencing the Restless Legs Syndrome Treatment Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Restless Legs Syndrome Treatment Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Restless Legs Syndrome Treatment Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
#Restless Legs Syndrome Treatment Market Trend#Restless Legs Syndrome Treatment Market Size#Restless Legs Syndrome Treatment Market Information#Restless Legs Syndrome Treatment Market Analysis#Restless Legs Syndrome Treatment Market Demand
0 notes