#Caenorhabditis elegans (C. elegans)
Explore tagged Tumblr posts
Text

Caenorhabditis elegans
Photo credit: Unknown. All earliest source links (circa 2008) are dead.
#caenorhabditis elegans#caenorhabditis#c elegans#fez#microbiology#microbes#biology#hat#hats#microbes in hats#microorganisms#bacteria#protozoa#microscopy
112 notes
·
View notes
Text
Phylum #12: Nematoda, the roundworms!

Imagine for one second if the entire world disappeared. Except for roundworms. Then, we would still see through them the ghostly outlines of forests, rivers, cities, and even people. Numbering in the hundred billion billions, nematodes are by far the most abundant phylum on Earth. Easily overlooked, but truly everywhere.
With their abundance comes a huge amount of different species. More than 20 thousand species are already known, with more than a million estimated to exist in total. Either parasitic or free-living, they are found in virtually every environment, from the poles to the tropics and from mountaintops to seafloor.
Most are superficially very similar, sharing the same round worm body plan with a soft, unsegmented cuticle that must be shed. This phenomenon, called cryptic diversity, is also known from other phyla like placozoans. A single morphological "species" can easily reveal itself to be a massive lineage of genetically distinct species.
Despite the staggering amount of nematode species, one in particular is crucial to scientific research. Caenorhabditis elegans is the prototypical model organism, having had its genome sequenced and the fate of every single one of its cells determined, including its connectome or "neuron map". From spaceflight to medical research, C. elegans has been at the forefront of numerous studies, making it possibly the most well-understood animal ever.

51 notes
·
View notes
Text
Powering the brain: How energy is distributed within single cells - Technology Org
New Post has been published on https://thedigitalinsider.com/powering-the-brain-how-energy-is-distributed-within-single-cells-technology-org/
Powering the brain: How energy is distributed within single cells - Technology Org
Every system in a living organism depends upon a finite energy supply to function. No organ is more energy-intensive in humans than the brain, which consumes about 20% of the body’s metabolic energy.
Using a novel biosensor known as HYlight — a fluorescent imaging technology — researchers could monitor and map metabolic activity within individual neurons of C. elegans (a type of nematode worm) over time and under different conditions.
But how is energy distributed in the nervous system to assure its functioning? In a new study, Yale scientists unravel part of the mystery. Using a novel biosensor, they were able to map the energy metabolism in single cells of a living organism, the nematode worm C. elegans.
The technology, which Yale scientists developed, allowed researchers to map a “landscape” of energy distribution across cells — and even within individual neurons.
Their findings are published in Proceedings of the National Academy of Sciences journal.
Scientists have long been interested in questions related to the body’s energy metabolism, from how the energy is produced biochemically to how it is distributed throughout the organism — including the brain. In past studies, neuroimaging technologies such as functional MRI have revealed that energy distribution in the brain changes to accommodate the brain’s different activity states, which underpin thought and cognition. But these technologies lack the cellular resolution needed to understand how energy metabolism is distributed within single cells in the nervous system.
“It’s known that energy production is not distributed equally throughout the brain, but where exactly is it happening? And how does its distribution affect function of the nervous system?” said Aaron Wolfe, a postdoctoral associate in neuroscience at Yale School of Medicine and lead author of the study. “Those were the questions that drove this work.”
To answer them, the Yale team, led by Wolfe and Daniel Colón-Ramos, the Dorys McConnell Duberg Professor of Neuroscience and Cell Biology and co-corresponding author, used a biosensor called HYlight to study metabolic activity within individual neurons in C. elegans under different conditions.
The biosensor was originally developed by a research group led by Richard Goodman, a former faculty member at the Vollum Institute and now an associate research scientist in the Colón-Ramos lab.
The researchers found that energy is differentially distributed across specific cells, and maps onto the identity of individual neurons. This unequal distribution, they said, forms “energy landscapes” that might shape how information flows through neurons and likely influences behavior.
“Energy is the force that animates life. In the context of the nervous system, it animates thought and behavior,” said Colón-Ramos. “Energy is produced via specific metabolic reactions, that we can now track in living animals.
“Visualizing this energy metabolism allows us to understand how its distribution constrains nervous system function in health, disease, and aging.”
Added Wolfe: “By understanding energy production at a cellular level, we can help identify exactly how deficits may arise that can impair neuronal function.”
For example, he said, the researchers found that energy is not only distributed across different cells, but also within compartments of the cells.
“In neurons, our findings indicate that this distribution happen near synapses, which are structures that neurons use to communicate with one another,” Wolfe said. “Maps exist for synaptic connections, and now we can make new maps for energy distribution as animals perform behaviors.”
Source: Yale University
You can offer your link to a page which is relevant to the topic of this post.
#aging#Animals#Behavior#Biology#biosensors#Biotechnology news#Brain#Caenorhabditis elegans (C. elegans)#cell#cell biology#Cells#cognition#Disease#energy#energy production#Faculty#Featured life sciences news#Forms#Health#how#humans#identity#Imaging#it#Landscape#LED#life#Link#map#Medicine
0 notes
Text
OFMD characters if they were worms
(and whether or not I would still love them)
Izzy: Pseudobiceros hancockanus

Also known as "Hancock's Flatworm," it has a distinct black coloring and is part of a genus known for engaging in "penis fencing" for reproduction, which I like to imagine is something Izzy does too. Obviously I would still love him; I don't think there's anything that could make me stop loving him.
Stede: Sabellastarte spectabilis

Also known as the "feather duster worm." It's popular in squariums because of its flamboyant plume of tentacles, which are almost as ridiculous as Stede's hair. If anything I think I'd love him more as a worm.
Ed: Eunice aphroditois

Also known as the "sand striker" or "bobbit worm." This thing is a horrifying monster that feeds on fish unfortunate enough to come near it with its sharp mandibles. The rainbow iridescence is pretty though. Sorry Ed but I've read too many horror stories about these; I would not love you if you were a worm.
Calico Jack: Trichuris trichiura

Also known as the "whipworm," which is the only reason I chose it. Unlike Calico Jack, I wouldn't want one of these in my large intestine because they're the cause of trichuriasis, a parasitic infection. I'm not that into parasites so I'm unfortunately going to have to pass on loving Jack as a worm.
Roach: Hirudo medicinalis

One of a few species of "medicinal leeches." Leeches are still used for medical purposes to this day because of the beneficial secretions in their saliva, and they're also cool as fuck. They're like vampires except they're worms, so obviously I'd still love Roach as a worm.
Frenchie: Lagis koreni

Frenchie is a "trumpet worm" because that was the only worm I could find with a name related to music. Also the tubes they build for themselves to live in are super cool and I wanted to include them somewhere. I would for sure love him if we was a worm.
Wee John: Megascolides australis

Also known as the "giant Gippsland earthworm." Because he's big, get it? 10/10, would still love him as a worm.
Lucius: Spirobranchus giganteus

Commonly known as the "christmas tree worm." The two spiral things on either side of its body function both as gills and to capture food, and they're also gay as fuck. Love that for him, and I'd absolutely love him as a worm.
Jim: Bipalium kewense

Jim is a "hammerhead flatworm," mostly because it kind of looks like it's wearing a hat but also because it produces a deadly paralyzing neurotoxin. Obviously I love that, and I'd love them if they were a worm.
Oluwande: Maritigrella crozierae

Commonly known as the "tiger flatworm." I chose this for Oluwande because they apparently often live together in pairs and Jim/Oluwande is the best couple in the show. You already know I'd still love him if he was a worm.
Buttons: Plagiostomum vittatum

Not much is known about this mysterious species of marine flatworm aside from the fact that it's native to the Atlantic ocean (by which I mean there isn't a Wikipedia article for it and I can't be bothered to do more research). I do like a mystery so yeah, I'd love him if he were a worm.
Fang: Hermodice carunculata

The "bearded fireworm," like Fang, looks soft but is also deadly. It's namesake white bristles are capable of penetrating skin and injecting a powerful neurotoxin. I would love him if he was a worm but I'd keep my distance.
Ivan: Arthurdendyus triangulatus

Known as the "New Zealand flatworm," it fades into the background a bit but it's still cool. Apparently they roll up when they rest which would be really cool if I could find a picture of it. Anyways yeah I'd love him as a worm.
The Swede: Caenorhabditis elegans

C. elegans is a species of nematode notable for being the first multicellular organism to have its entire genome sequenced, because it's so simple. I'd definitely love him as a worm.
Black Pete: Lumbricus terrestris

The common earthworm. It's a bit plain, but it plays a vitally important role in its ecosystem. Of course I'd love him as a worm.
Mary: Riftia pachyptila

Also known as the "giant tubeworm," it's capable of surviving in the extremely hot, toxic environments of deep-sea vents, which is almost as impressive as Mary being able to survive living in a house with Stede. Obviously I'd still love her as a worm, she's an icon.
#i spent so long researching worms for this post you'd better not let it flop#i feel like i shouldn't have to say this but don't read this if you don't want to see a bunch of pictures of worms#our flag means death#ofmd#izzy hands#stede bonnet#edward teach#calico jack#roach ofmd#frenchie ofmd#wee john feeney#lucius spriggs#jim jimenez#oluwande boodhari#nathaniel buttons#fang ofmd#ivan ofmd#the swede#black pete#mary bonnet#shitpost#long post
165 notes
·
View notes
Text
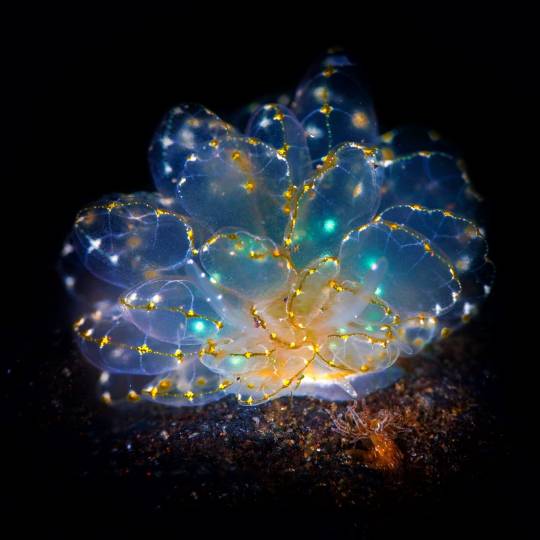
I spent several minutes staring at this in confusion because I know C. elegans, I have worked with C. elegans, I am familiar with the entire developmental process of C. elegans, and these are not C. elegans
And then I remembered the difference between Cyerce elegans and Caenorhabditis elegans, and felt silly.
'fairies dont exist' WRONG❗❗cyerce elegans



88K notes
·
View notes
Text
A cell protector collaborates with a killer
New Post has been published on https://sunalei.org/news/a-cell-protector-collaborates-with-a-killer/
A cell protector collaborates with a killer

From early development to old age, cell death is a part of life. Without enough of a critical type of cell death known as apoptosis, animals wind up with too many cells, which can set the stage for cancer or autoimmune disease. But careful control is essential, because when apoptosis eliminates the wrong cells, the effects can be just as dire, helping to drive many kinds of neurodegenerative disease.
By studying the microscopic roundworm Caenorhabditis elegans — which was honored with its fourth Nobel Prize last month — scientists at MIT’s McGovern Institute for Brain Research have begun to unravel a longstanding mystery about the factors that control apoptosis: how a protein capable of preventing programmed cell death can also promote it. Their study, led by Robert Horvitz, the David H. Koch Professor of Biology at MIT, and reported Oct. 9 in the journal Science Advances, sheds light on the process of cell death in both health and disease.
“These findings, by graduate student Nolan Tucker and former graduate student, now MIT faculty colleague, Peter Reddien, have revealed that a protein interaction long thought to block apoptosis in C. elegans likely instead has the opposite effect,” says Horvitz, who is also an investigator at the Howard Hughes Medical Institute and the McGovern Institute. Horvitz shared the 2002 Nobel Prize in Physiology or Medicine for discovering and characterizing the genes controlling cell death in C. elegans.
Mechanisms of cell death
Horvitz, Tucker, Reddien, and colleagues have provided foundational insights in the field of apoptosis by using C. elegans to analyze the mechanisms that drive apoptosis, as well as the mechanisms that determine how cells ensure apoptosis happens when and where it should. Unlike humans and other mammals, which depend on dozens of proteins to control apoptosis, these worms use just a few. And when things go awry, it’s easy to tell: When there’s not enough apoptosis, researchers can see that there are too many cells inside the worms’ translucent bodies. And when there’s too much, the worms lack certain biological functions or, in more extreme cases, can’t reproduce or die during embryonic development.
Work in the Horvitz lab defined the roles of many of the genes and proteins that control apoptosis in worms. These regulators proved to have counterparts in human cells, and for that reason studies of worms have helped reveal how human cells govern cell death and pointed toward potential targets for treating disease.
A protein’s dual role
Three of C. elegans’ primary regulators of apoptosis actively promote cell death, whereas just one, CED-9, reins in the apoptosis-promoting proteins to keep cells alive. As early as the 1990s, however, Horvitz and colleagues recognized that CED-9 was not exclusively a protector of cells. Their experiments indicated that the protector protein also plays a role in promoting cell death. But while researchers thought they knew how CED-9 protected against apoptosis, its pro-apoptotic role was more puzzling.
CED-9’s dual role means that mutations in the gene that encode it can impact apoptosis in multiple ways. Most ced-9 mutations interfere with the protein’s ability to protect against cell death and result in excess cell death. Conversely, mutations that abnormally activate ced-9 cause too little cell death, just like mutations that inactivate any of the three killer genes.
An atypical ced-9 mutation, identified by Reddien when he was a PhD student in Horvitz’s lab, hinted at how CED-9 promotes cell death. That mutation altered the part of the CED-9 protein that interacts with the protein CED-4, which is proapoptotic. Since the mutation specifically leads to a reduction in apoptosis, this suggested that CED-9 might need to interact with CED-4 to promote cell death.
The idea was particularly intriguing because researchers had long thought that CED-9’s interaction with CED-4 had exactly the opposite effect: In the canonical model, CED-9 anchors CED-4 to cells’ mitochondria, sequestering the CED-4 killer protein and preventing it from associating with and activating another key killer, the CED-3 protein — thereby preventing apoptosis.
To test the hypothesis that CED-9’s interactions with the killer CED-4 protein enhance apoptosis, the team needed more evidence. So graduate student Nolan Tucker used CRISPR gene editing tools to create more worms with mutations in CED-9, each one targeting a different spot in the CED-4-binding region. Then he examined the worms. “What I saw with this particular class of mutations was extra cells and viability,” he says — clear signs that the altered CED-9 was still protecting against cell death, but could no longer promote it. “Those observations strongly supported the hypothesis that the ability to bind CED-4 is needed for the pro-apoptotic function of CED-9,” Tucker explains. Their observations also suggested that, contrary to earlier thinking, CED-9 doesn’t need to bind with CED-4 to protect against apoptosis.
When he looked inside the cells of the mutant worms, Tucker found additional evidence that these mutations prevented CED-9’s ability to interact with CED-4. When both CED-9 and CED-4 are intact, CED-4 appears associated with cells’ mitochondria. But in the presence of these mutations, CED-4 was instead at the edge of the cell nucleus. CED-9’s ability to bind CED-4 to mitochondria appeared to be necessary to promote apoptosis, not to protect against it.
Looking ahead
While the team’s findings begin to explain a long-unanswered question about one of the primary regulators of apoptosis, they raise new ones, as well. “I think that this main pathway of apoptosis has been seen by a lot of people as more-or-less settled science. Our findings should change that view,” Tucker says.
The researchers see important parallels between their findings from this study of worms and what’s known about cell death pathways in mammals. The mammalian counterpart to CED-9 is a protein called BCL-2, mutations in which can lead to cancer. BCL-2, like CED-9, can both promote and protect against apoptosis. As with CED-9, the pro-apoptotic function of BCL-2 has been mysterious. In mammals, too, mitochondria play a key role in activating apoptosis. The Horvitz lab’s discovery opens opportunities to better understand how apoptosis is regulated not only in worms but also in humans, and how dysregulation of apoptosis in humans can lead to such disorders as cancer, autoimmune disease, and neurodegeneration.
0 notes
Text
The Raf/LIN-45 C-terminal distal tail segment negatively regulates signaling in Caenorhabditis elegans
http://dlvr.it/T9l2S2
0 notes
Text
Research shows how RNA 'junk' controls our genes
Researchers at Arizona State University have made a significant advance in understanding how genes are controlled in living organisms. The new study, published in the journal Nucleic Acids Research, focuses on critical snippets of RNA in the tiny, transparent roundworm Caenorhabditis elegans (C. elegans). The study provides a detailed map of the 3’UTR regions of RNA in C. elegans. 3’UTRs…
0 notes
Text
Découverte d’un nouveau gène capable de lutter contre le vieillissement
See on Scoop.it - EntomoNews
"Des chercheurs chinois ont identifié un gène chez la mouche du vinaigre qui pourrait ralentir le vieillissement."
Tuniscope
Publié le 07-06-2024
"En étudiant l'ADN de 1 283 morceaux de ces insectes, ils ont découvert que le gène CG11837 influençait leur durée de vie. Lorsque ce gène était plus actif, les mouches vivaient jusqu'à 59 % plus longtemps que la normale.
Une correspondance de 93 % a été trouvée entre ce gène et un gène humain appelé DIMT1. Lorsque les cellules humaines contenant ce gène ont été exposées à des radiations similaires au vieillissement, elles ont vieilli 65% moins vite que les cellules non modifiées.
Les gènes, qu'ils soient humains ou insectes, agissent sur la forme et la structure des mitochondries, les centrales énergétiques des cellules. Ces mitochondries produisent de l'ATP, l'énergie nécessaire au bon fonctionnement cellulaire. Quand cette énergie manque, le processus de vieillissement s'amorce.
Les chercheurs espèrent que cette découverte encouragera la recherche de méthodes pour activer ce gène et ainsi potentiellement ralentir le vieillissement."
------
NDÉ
L'étude
Identification of a longevity gene through evolutionary rate covariation of insect mito-nuclear genomes - Nature Aging, 04.06.2024 https://pubmed.ncbi.nlm.nih.gov/38834883/
M Tao, J Chen, C Cui, Y Xu, J Xu, Z Shi, J Yun, J Zhang… - Nature Aging, 2024 - nature.com
… Additionally, we discovered CG11837, a longevity gene putatively considered an ortholog of the human gene DIMT1, contributing to lifespans from worms to insects and protecting …
Abstract
Oxidative phosphorylation, essential for energy metabolism and linked to the regulation of longevity, involves mitochondrial and nuclear genes. The functions of these genes and their evolutionary rate covariation (ERC) have been extensively studied, but little is known about whether other nuclear genes not targeted to mitochondria evolutionarily and functionally interact with mitochondrial genes. Here we systematically examined the ERC of mitochondrial and nuclear benchmarking universal single-copy ortholog (BUSCO) genes from 472 insects, identifying 75 non-mitochondria-targeted nuclear genes. We found that the uncharacterized gene CG11837-a putative ortholog of human DIMT1-regulates insect lifespan, as its knockdown reduces median lifespan in five diverse insect species and Caenorhabditis elegans, whereas its overexpression extends median lifespans in fruit flies and C. elegans and enhances oxidative phosphorylation gene activity. Additionally, DIMT1 overexpression protects human cells from cellular senescence. Together, these data provide insights into the ERC of mito-nuclear genes and suggest that CG11837 may regulate longevity across animals.
------
[Image] via Xingxing Shen sur X, 04.06.2024
"Happy to share our recent work in @NatureAging We systematically investigated the evolutionary rate covariation of mito-nuclear genomes in 472 insects and identified a new nuclear gene that may regulate longevity across animals. A 🧵 https://t.co/4bGJmrZI1c https://t.co/lcWg9ykIRH"
https://x.com/shenxingxing1/status/1797927617720631748
0 notes
Text
The Caenorhabditis #RNA-seq Browser – a web-based application for on-demand analysis of publicly available Caenorhabditis spp. bulk #RNA-sequencing data
The Caenorhabditis #RNA-seq Browser is an open-source Shiny web app that enables on-demand visualization and quantification of bulk #RNA-sequencing data for five Caenorhabditis species: C. elegans, C. briggsae, C. brenneri, C. japonica, and C. remanei... https://www.rna-seqblog.com/the-caenorhabditis-rna-seq-browser-a-web-based-application-for-on-demand-analysis-of-publicly-available-caenorhabditis-spp-bulk-rna-sequencing-data/?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Quote
The recent discovery of liquid droplets within living cells was first made in the germ cells of a soil-dwelling worm, Caenorhabditis elegans (C. elegans). Within the worm's embryo, membraneless structures called P granules serve essential reproductive functions. When probed further, investigators found that the P granules lacked membranes and could drip, join together or dissolve away, having characteristics just like liquids. Further, these P granules could hold their integrity within the jelly-like cytoplasm, much like oil droplets in water. "There was a fundamental change in 2009 in thinking about cellular compartmentalization in terms of the emergence of droplet-like structures,"
Rich molecular language guides tiny liquid droplet formation in cells
0 notes
Text
Mirando las imágenes que tomé en el microscopio entendí por qué había hecho el experimento. Porque no me acordaba. Pero cuando lo entendí, muchas cosas cobraron sentido.
Creo que necesito recuperar mi mirada. Mi mirada curiosa, mi mirada inocente, esa mirada libre de prejuicios, esa mirada que me permite fascinarme con los fenómenos más elementales que hacen a la vida posible.
Porque hay algo de eso en la ciencia. Lo siento mucho, pero hay algo de "inútil" si la medida de lo útil es cuánta plata nos va a dar saber x cosa. No porque el conocimiento no sirva, si no porque no podemos saber de antemano lo que descubriremos, ergo no sabemos en qué medida nos permitirá entender mejor la vida y el universo, o si será aplicable al desarrollo de alguna tecnología. Hacer ciencia no es como fabricar autos, no se puede calcular un costo de producción y estimar un rendimiento monetario. Ojo, tampoco estoy diciendo que la ciencia no sirva para producir dinero, porque claro que impulsa industrias (la farmacéutica, la química, la agropecuaria,* -solo por nombrar algunas- ¿o qué se creen?). Lo que estoy intentando decir es que hacer ciencia sirve fundamentalmente para comprender el mundo en que vivimos, para vivir mejor, para elevar el espíritu -como dice Dolina.
Por cierto, una deuda que tenemos lxs científicxs es compartir eso que descubrimos, tener más y mejor llegada. Pero como sociedad toda entera, creo que nos debemos entender que producir (dinero) es un medio para vivir, y no al revés -como dice Kartun. (Miren cito las fuentes y todo, porque yo pienso esto, pero además lo dicen dos referentes del arte y la cultura nacional que peinan canas, miren si no sabrán algo). Y ojo de nuevo, eso no quiere decir tampoco que tengamos que conformarnos todxs ganando dos pesos con cincuenta eh, que eso tampoco alcanza para vivir bien. Y no me vengan con que vivir plena y dignamente no puede ser la aspiración de cada ser humano, porque debería ser un derecho.
*Nota al pie: los ejemplos que puse est��n relacionados sobre todo a las ciencias naturales, porque son los que se me ocurrieron por ser esa mi área de conocimiento. Entiendo que las ciencias sociales, dado que su campo de estudio son las humanidades (y su objetivo es entender dinámicas sociales, fenómenos culturales, estudiar la historia, la literatura, las artes, la filosofía, preguntar por el sentido de todo -de absolutamente todo, nada menos, ¿nunca se preguntaron por el sentido de algo? ¿de su vida? ¿de lo que hacen? ¿de lo que les llena el alma? ¿no les importa eso?) se aplican en el desarrollo de políticas públicas para garantizar el acceso a la educación, a la salud, a atender necesidades sociales (que previamente debieron ser estudiadas y entendidas), entre otras cosas; en suma para generar una sociedad más equitativa y garantizar derechos, miren si no van a ser importantes. Ah, y también entiendo que se aplican mucho en comunicación, marketing, estudios de mercado y demás cosas que todas las empresas hoy en día necesitan. Así que no sé, vayan a revisar sus parámetros de utilidad antes de venir a decir que la ciencia no sirve.
Les dejo los links de las entrevistas a estos referentes que me hicieron hacer las pases con mi profesión, mi situación y distintas contradicciones que me venían atravesando últimamente. Capaz les arroja algo de luz a algunx de ustedes también.
youtube
youtube
Para más contexto, dejo una de las imágenes de microscopía aquí:

Caenorhabditis elegans LIU1, detalle de la cola tomado con microscopio de epi-fluorescencia usando un filtro FITC, la barra de escala son 50 micras. Esta variedad de C. elegans expresa una proteína fluorescente en la superficie de unas organelas llamadas "lipid droplets" cuya función es almacenar lípidos. Dicho en criollo, los circulitos que se ven son los bordes de las organelas.
1 note
·
View note
Link
0 notes
Text
Nematode proteins shed light on infertility - Technology Org
New Post has been published on https://thedigitalinsider.com/nematode-proteins-shed-light-on-infertility-technology-org/
Nematode proteins shed light on infertility - Technology Org
We have two copies of each chromosome in every cell in our bodies except in our reproductive cells. Sperm and egg cells contain a single copy of each chromosome with a unique mix of genes from our parents, an evolutionary trick to give our offspring genetic variability. The sperm and egg are made during meiosis, the process by which cells with two chromosome copies reduce their chromosome numbers to one. For meiosis to work, the two chromosomes must align perfectly and exchange the correct amount of genetic information. Any deviation puts fertility at risk.
A cartoon of paired homologous chromosomes (blue) with the synaptonemal complex (SC, green) assembled between them. Below, from top to bottom, are cartoons depicting the localization of proteins in a normal SC, in the SC of the researcher-bred infertile nematodes, and in the SC of the nematodes with the suppressor-mutation. Image credit: Lisa Kursel and Maria Diaz de la Loza
Enter the synaptonemal complex (SC), a zipper-like protein structure that lines up and anchors the two parental chromosomes together, end-to-end, to facilitate successful genetic exchanges. Failure to regulate this exchange is a leading cause of age-related infertility in humans and could compromise fertility across the tree of life. Humans, fungi, plants, worms and anything that reproduces sexually uses the SC to make reproductive cells, known as gametes. Despite its importance, we don’t understand how proteins within the SC regulate chromosomal interactions because this multi-step process happens in internal organs and has been impossible to recreate in a lab.
In a new study, University of Utah biologists developed a method for illuminating the intricate interactions of the SC in the nematode C. elegans. The authors identified a trio of protein segments that guide chromosomal interactions, and pinpointed the location where they interact with each other. Their novel method uses a technique known as genetic suppressor screening, which can serve as a blueprint for research on large cellular assemblies that resist traditional structural analysis.
“This is a way to lock in on systems in cells that are too ‘loosie-goosey’ to use methods that rely on crystallization,” said Ofer Rog, associate professor of biology at the U and senior author of the study. “A lot of the interactions in cells are loosely bonded together. You can’t look at it under an electron microscope because nothing is stable enough—everything is constantly moving. Our approach allows you to study even the interactions that are relatively weak or transient.”
The study published in the journal Proceedings of the National Academy of Sciences (PNAS).
The birds and the bees… and the nematodes
Let’s dig into meiosis. Chromosomes are thread-like structures made of DNA that carry genetic information when cells divide and from generation to generation. Regular cells have a certain number of chromosomes; humans have 46 and C. elegans have 12. Chromosomes come in pairs called homologous chromosomes that carry the genes we inherited from each of our parents—one from our mom, one from our dad. When meiosis begins, homologous chromosomes arrange themselves into elongated structures organized along a backbone called the axis. The axes of homologous pairs are aligned lengthwise to each other while at the same time, the synaptonemal complex (SC) forms between the parallel axes. The homologous pairs have matching genes arranged in the same order, with small variations within the genes—these are the variations that make each individual unique.
“You can think of it like a zipper,” Rog explained. “The axes of the chromosomes are like the two sides of your shirt. The synaptonemal complex is kind of like the teeth of the zippers that lock onto each other and can pull and align the two sides of the shirt correctly.”
Scientist previously knew that the SC of C. elegans formed between homologs, but the U biologists are the first to pinpoint the exact position where the SC interacts with itself to facilitate genetic exchanges.
“When you exchange information between the chromosomes, you want to make sure that at the end you still have two complete chromosomes,” said Rog. “The way the cell does it is that the two chromosomes are perfectly aligned. So, when you exchange segments between them, you’re not losing any information in the process.
How to analyze loosie-goosey structures
The researchers bred 50,000 nematodes that had temperature-sensitive defects in the SC. At high temperatures, the worms were unable to form the SC protein zipper needed to join the chromosomes together. Without the zipper, the gene exchanges during meiosis either didn’t happen at all or didn’t occur at the right number. Lisa Kursel, postdoctoral researcher and lead author of the study ran the experiments.
“We grew the worms at the permissive, cooler temperature, then exposed them to a chemical that caused millions of mutations along their chromosomes and watched to see if any of the mutated worms could reproduce at the warmer temperature,” Kursel said. The chemically induced mutations that corrected the nematode’s infertility are known as suppressor mutations. “Then we’d know if the suppressor mutations restored their fertility.”
To identify the animals with mutations that made them fertile again, the researchers put the nematodes on agar plates filled with yummy bacteria. The agar plates that had fertile nematodes were soon empty as their progeny ate the food. The agar plates with sterile worms died off before they could clean their plate, allowing the bacteria to flourish.
Once they had fertile nematodes, they could test if the mutation “fixed” the protein zipper. They then screened every single base pair on the DNA—100 million base pairs—and identified which mutations restored the worms’ ability to reproduce. They found that all the helpful mutations occurred in short segments of three proteins, SYP-1, SYP-3, SYP-4. Moreover, the mutations carried distinct signatures of interaction. For example, while the original mutations changed the electric charge from positive to negative, the helpful mutations flipped the charge back.
“This was a strong indication that SYP-1, SYP-3 and SYP-4 interact with each other like magnets, with positive and negative regions attracted to each other,” said Rog. Such “sticky” interactions could also help tether the chromosomes together.
Jesus Aguayo Martinez, a senior biology major and co-author of the study, looked at the behavior of the suppressor mutation in nematodes without the original SC-disrupting mutation.
“We thought that since the original mutation alone produced a fertility defect, then the nematodes with the suppressor mutation alone would also have a fertility defect. That wasn’t the case,” said Aguayo Martinez. “Surprisingly, normal worms and worms with only the suppressor mutations produced similar numbers of progeny.”
Next steps
Uncovering the SC’s role in meiosis may help to better understand fertility in humans. The SC has a similar role across all eukaryotes, from nematodes to fungi to plants to humans. Previous research by the Rog Lab at the U showed that the structure itself looks the same and acts similarly to bring in parental chromosomes to facilitate exchanges. However, the actual sequences of the protein components are different between organisms. Such a pattern is unusual: Most cellular structures that carry essential, basic functions like cell division, genome duplication or metabolism are highly conserved, and could in fact be swapped between different organisms.
“A question that we think a lot about is what is special about the SC? Why can it do the same thing and look the same way, but consist of different building blocks?” Rog asked.
Source: University of Utah
You can offer your link to a page which is relevant to the topic of this post.
#000#Analysis#Animals#approach#Bacteria#bees#Behavior#Biology#Biotechnology news#birds#Blue#Building#Caenorhabditis elegans (C. elegans)#cell#Cells#cellular structures#chemical#chromosomes#DNA#electron#Explained#fertility#Food#form#Forms#fungi#genes#genetic#Genetic engineering news#genome
1 note
·
View note
Text
A single-cell transcriptomics atlas for the parasitic nematode Heligmosomoides bakeri: Extrapolating model organism information to non-model systems
Single cell atlases aim to collect the gene expression information for every cell type in an organism but can be challenging to perform in non-model organisms. To try to circumvent the problem of having no verified cell type markers in the parasitic nematode Heligmosomoides bakeri to use for an atlas, we attempted to use orthologs of verified markers from the closely related model organism Caenorhabditis elegans. This resulted in a useful comparison between the two worms for each of the cell types recovered in preliminary H. bakeri single cell RNA-sequencing. For H. bakeri males and females, robustly recovered cell types include the gametes, embryos, and male intestine, while hypodermis, neurons, muscles, and pharyngeal cells were under-represented cell types. The two worms appear to have a similar hypodermis, cuticle, eggshell, and spermatogenesis process. On the other hand, putative cell identities and cell cycle scores suggest the intestine and muscle cells in H. bakeri may still be cycling and dividing, unlike in C. elegans. Additionally, embryogenesis and early development appear to be quite different between the two worms, with only eight out of 94 confirmed paternal contributions to the embryo in C. elegans (with an ortholog) predicted to also be paternal contributions in H. bakeri. Overall, this new dataset allowed me to move beyond the presence or absence of orthologs to include their tissue specificity and expression level similarities and differences when comparing these two worms to better identify biological processes and traits in a parasitic nematode that are modelled well by C. elegans. http://dlvr.it/T3Q2Hv
0 notes
Text
Fully Funded PhD Modelling Rare Diseases in C. elegans University College Dublin We are seeking a PhD student to join us in beautiful #Dublin for a fully funded 4 year project modelling ciliopathies in C. elegans See the full job description on jobRxiv: https://jobrxiv.org/job/university-college-dublin-27778-fully-funded-phd-modelling-rare-diseases-in-c-elegans/?feed_id=59847 #ScienceJobs #hiring #research @KarenL_PhD #cilia #celegans Dublin #Ireland #PhDStudent
0 notes