#CYP3A4 inhibitors
Explore tagged Tumblr posts
Text

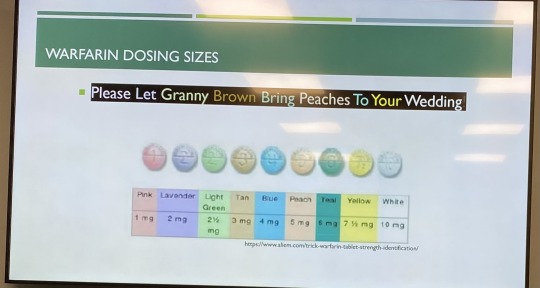


Anticoagulation Lecture 6/21/23
Factor Xa inhibitors = rivaroxaban, apixaban (Eliquis)
Direct thrombin inhibitor = dabigatran
Vitamin K inhibitor = warfarin
Apixaban has the lowest bleeding risk compared to the other Direct Oral Anticoagulants (DOACs); avoid in pts with BMI greater than or equal to 40 or weight more than 120 kg. Avoid dual inhibitors or inducers of CYP3A4 and P-gp.
VTE/PE tx = 10mg bid x7 days, then 5 mg bid
AFib = 5 mg bid (reduce to 2.5 mg bid if Cr greater than or equal to 1.5 or weight less than 60 kg or age greater than 80
Rivaroxaban (Xarelto) = once daily dosing compared to Eliquis
VTE/PE: 15 mg bid x21 days; then 20 mg qd
AFib: 20 mg qd with food to increase absorption
PAD/CAD: 2.5 mg bid (+antiplatelets if at increased risk)
Avoid with CYP3A4 inhibitors or inducers and P-gp
Edoxaban (Savaysa) – not used in pts with CrCl greater than 95
VTE/PE = parenteral anticoagulation for 5-10 days, then PO
VTE/PE/AFib = 60 mg qd
Does not have reversal agent like other DOACs do (which are reversed with Kcentra or andexanet)
Dabigatran (Pradaxa)
AFib: 150 mg bid; consider 110 mg bid if bleeding risk
VTE/PE: 5 days IV, then 150 mg bid
VTE ppx after THA/TKA: initial 110 mg once, then 220 mg qd x10-35 days
5-10 day bridge required for PE/VTE
Caution in pts greater than 75 years due to increased bleeding risk
Has own reversal agent (praxbind; idarucizumab)
C/I in pts with prosthetic heart valve
Store in original bottle and discard if unused after 4 months
Eliquis and Xarelto can be crushed and given via feeding tubes.
Warfarin (Coumadin)
Dosing is pt specific. Goal INR is 2-3. (2.5-3.5 if mechanical mitral valve).
Slow onset; slow time to steady state means dose taken today may not be reflected in INR for several days (2-4 days).
So many drug-drug interactions!
Metabolized by CYP pathways
Vitamin K antagonist
Dosing: recommend taking at night because INR is taken during the day, makes it easier to adjust dose.
Warfarin’s therapeutic steady state is based on half-lives of clotting factors. At least 5 days of consecutive warfarin needed for pt to be fully anticoagulated (this is why you use heparin until they get to this point).
Usually start with 5 mg qd. If bleeding risk, start with 2.5 mg qd. For obese pts or otherwise healthy/young can start with 7.5 mg qd. You go to maintenance protocol on 7th day.
Pts on VTE/PE tx are not anticoagulated the first 5 days, so use heparin IV or LMWH outpt. Once anticoagulated x24 hours, d/c heparin.
Start with 5 mg warfarin for first 3 days, then follow up with warfarin clinic on day 4. Typically, pt goes for weekly INRs. Then when at goal INR for 2 weeks, can spread out how often they f/u on their INR levels. Eventually they can go q6-8 weeks to check INR level.
VTE/PE – always bridge
AFib – risk assess to see whether heparin bridging is needed (assess bleeding risk [HASBLED]; CHA2DS2VASc for thrombotic risk).
Every 10 years, the dosing needed will decrease by 10%, so as pts age, the dose of warfarin needed will decrease.
DOACs = no INR monitoring, no dietary interactions, lower rates of bleeding, limited availability of reversal agent; contraindicated in pts with mechanical heart valves
AFib – AC (anticoagulate) indefinitely
1st VTE or PE that is provoked – AC for 3 months
1st episode of VTE/PE in setting of cancer – 3 months of AC (LMWH is better than DOAC or warfarin--new research shows DOAC may actually be more effective, so can use DOAC now; LMWH not preferred)
1st episode of VTE/PE unprovoked (no idea what caused it) – AC more than or equal to 3 months
2nd VTE – AC indefinitely
Heparin reversal – protamine (max dose is 50 mg), to reverse enoxaparin, give 1 mg protamine for each 1 mg of Lovenox
Warfarin reversal – vit K; 4 factor prothrombin compex (KCentra); FFP (if KCentra not available); KCentra is for life-threatening bleeding or if pt needs surgery for life-threatening condition
Apixaban/Rivaroxaban reversal – Kcentra (life threatening bleed or need emergency surgery; has thrombotic risk); FFP (Fresh Frozen Plasma); Andexanet Alfa. KCentra can cause clotting in 5% of pts; don’t give if you don’t need to.
Dabigatran reversal – idarucizumab (Praxbind); dosed as 5 g IV given 2.5 mg no more than 15 minutes apart)
Heparin lasts 2 hours
LMWH is preferred in pts who are pregnant (avoid warfarin). ESRD on dialysis – warfarin or Eliquis (avoid Xarelto). Obese pts – DOACs or warfarin. Cancer – use LMWH; recent study shows Eliquis is equal to or superior to LMWH in cancer pts who need anticoagulation.
Pregnancy = lovenox better, doesn’t cross placenta; UFH (unfractionated heparin) is an alternative in pts with poor renal function.
Stop heparin gtt and give Eliquis now or give 2 hours after stopping heparin gtt. Give loading dose Eliquis even if the pt was on heparin gtt.
4 notes
·
View notes
Note
fwiw my physiotherapist told me that people with eds apparently metabolise things faster than other people, so your fast metabolisation of medications/caffeine/etc could be in part linked to that?
yes people with EDS (and other forms of chronic pain) are highly likely to have the abnormal liver enzyme thing
we don't really know *why* but it's just. a thing.
#it's why loads of us are resistant to local anaesthetic also#something like 80% of chronic pain sufferers have at least one abnormality on the P450 cytochrome#around 30% have more than one#the most common (for us) is CYP2D6 which is the one responsible for - among other things - anaesthetic and most psychiatric medication#caffeine is CYP3A4#my 2D6 and 3A4 are for sure extremely overachieving#i have my suspicions about 2C9#incidentally CBD is a potent inhibitor of 2D6 - meaning it will slow the metabolism and 'make things work better'#which may go some way to explaining the popularity of CBD among chronic pain folks#bupropion (wellbutrin) does the same thing which is why i'm prescribed it lmao#taking wellbutrin to make my liver talk to amphetamine
69 notes
·
View notes
Text
very tragic that you can't eat tomato soup on latuda. evil, really.
#toamtoes are cyp3a4 inhibitors so they make my medicine stronger#which makes me overdose when i eat tomato soup and take my medicine :(
0 notes
Text
It's not even the vitamin C, grapefruit just acts as a CYP3A4 inhibitor, which fucks with how your body metabolizes certain medications. Something something talk to your doctor or pharmacist about potential interactions

[ID: a tag reading, “#don’t eat citrus if you have any mental health problems #the vitamin C is so bad for you” end ID]
losing my fucking mind over how people will come on here and say just the easiest to disprove absolutely inane lies. for no reason at all
#the more prior authorizations i do the more powerful i become#and by that i mean my brain is a constant string of pharmaceutical classes and contraindications
100K notes
·
View notes
Text
AI Deep Learning Accelerates Drug Development
Deep learning is a subset of artificial intelligence (AI) that mimics the neural networks of the human brain to learn from large amounts of data, enabling machines to solve complex problems. Deep learning technology has made significant progress in the biomedical field. Researchers have developed a series of application based on deep learning for disease diagnosis, protein design, and medical image recognition. The pharmaceutical industry is also beginning to recognize the importance of deep learning technology, hoping to leverage it to accelerate drug development and reduce costs.
1. Application of Deep Learning in Drug Development
Previous studies have demonstrated that deep learning technology offers significant advantages in several key areas of drug development, including optimization of chemical synthesis routes, ADME-Tox prediction, target identification and validation and generation of novel molecules.
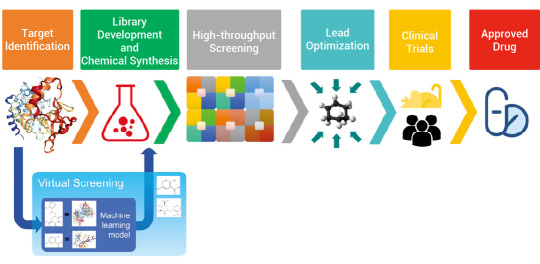
Figure 1. A broad overview of drug development and the place of virtual screening in this process[1].
1.1 Virtual Screening: Protein-Ligand Affinity
Deep learning can learn and identify potential binding patterns by comparing known protein-small molecule binding instances. During the training process, the deep learning models continuously optimize their parameters to enhance the accuracy and reliability of their predictions.
Yelena Guttman et al. developed a CYP3A4 inhibitor prediction model based on DeepChem framework. They created a KNIME workflow for data curation and employed the DeepChem module in Maestro to build a categorical classifier. This classifier was then used to virtually screen approximately 68,900 compounds from the FooDB database, leading to the successful identification of two new CYP3A4 inhibitors[2].
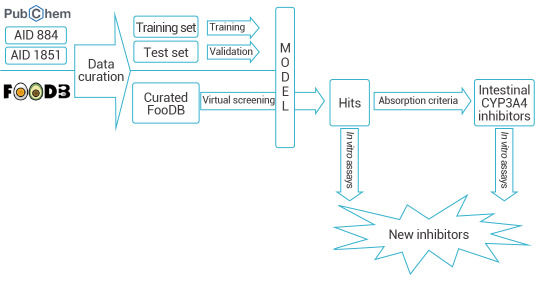
Figure 2. Prediction of CYP3A4 Inhibitors Based on DeepChem[2].
A workflow in KNIME analytics platform 4.0.314 was created to prepare and analyze the virtual screening.
1.2 ADME-Tox Prediction
Poor pharmacokinetic properties as well as toxicity issues are considered the main reasons for terminating the development process for drug candidates. Thus, there is an increasing need for robust screening methods to provide early information on absorption, distribution, metabolism, excretion, and toxicity (ADME-Tox) properties of compounds. Many studies have shown by leveraging these extensive ADME datasets, deep learning models can automatically identify and extract complex relationships between compound features and their corresponding ADMET properties. These trained models can then be used to predict the ADME properties of new compounds, thereby accelerating the process of drug discovery and development.
Liu et al. utilized directed message passing neural networks (D-MPNN, Chemprop) to predict the Nrf2 dietary-derived agonists and safety of compounds in the FooDB database. They successfully identified Nicotiflorin, a drug that exhibits both agonistic activity of Nrf2 and safety, which was validated in vitro and in vivo[3].
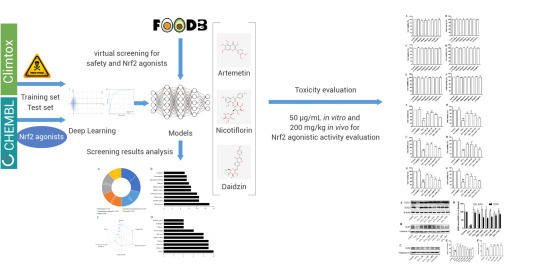
Figure 3. Using Deep-Learning Model D-MPNN to Assess Drug Safety[3].
1.3 Optimize Chemical Synthesis Routes
In recent years, it has been seen that artificial intelligence (AI) starts to bring revolutionary changes to chemical synthesis. However, the lack of suitable ways of representing chemical reactions and the scarceness of reaction data has limited the wider application of AI to reaction prediction. Deep learning is increasingly being applied to chemical synthesis, enabling the automatic identification and extraction of features and patterns from large datasets. This capability enhances the prediction of the efficiency and selectivity of new synthesis routes, significantly accelerating drug development and production.
Li et al. introduced a novel reaction representation, GraphRXN, for reaction prediction. G
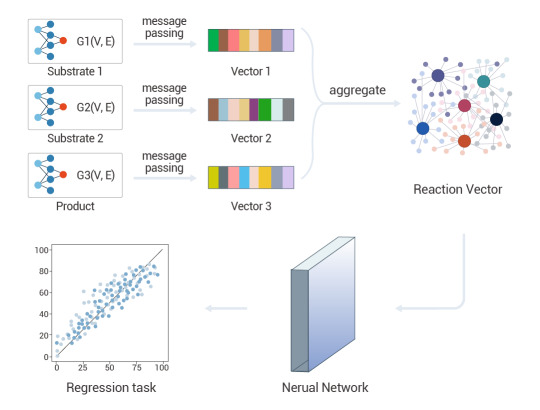
Figure 4. A deep-learning graph framework, GraphRXN, was proposed to be capable of learning reaction features and predicting reactivity[4].
2. Drug Screening Based on Deep Learning
The application of deep learning in the field of virtual screening primarily involves using neural networks to predict the activity or properties of compounds, thereby identifying potential candidate drugs or materials in a virtual environment. Commonly used deep learning models include Convolutional Neural Networks (CNN), Graph Neural Networks (GNN), Recurrent Neural Networks (RNN), Generative Adversarial Networks (GAN) and Transformer models.
CNNs excel at identifying patterns and features in structured data, such as chemical structures represented as images or graphs. Recent studies have demonstrated their effectiveness in predicting drug-drug interactions and assessing molecular properties by analyzing chemical substructures and other relevant features.
GNNs are designed to work directly with graph-structured data, making them particularly suitable for representing molecular structures where atoms are nodes and bonds are edges. They have shown remarkable performance in drug discovery by capturing the complex relationships between molecules and their properties.
RNNs are designed to handle sequential data, making them particularly effective for tasks where context from previous inputs is essential.
GANs consist of two neural networks—a generator and a discriminator—that work against each other to create new data instances.
Transformers have gained popularity for their ability to handle sequential data and capture long-range dependencies, making them suitable for tasks like natural language processing and time-series analysis.
In summary, deep learning is revolutionizing drug development by enhancing efficiency, accuracy, and cost-effectiveness across multiple stages of the process. As technology continues to evolve, its integration into pharmaceutical research is likely to deepen, paving the way for innovative therapeutic solutions.
Product Recommendation
Virtual Screening
MedChemExpress (MCE) provides high quality virtual screening service that enables researchers to identify most promising candidates. Based on the laws of quantum and molecular physics, our virtual screening services can achieve highly accurate results. Our optimized virtual screening protocol can reduce the size of chemical library to be screened experimentally, increase the likelihood to find innovative hits in a faster and less expensive manner, and mitigate the risk of failure in the lead optimization process.
50K Diversity Library
MCE 50K Diversity Library consists of 50,000 lead-like compounds with multiple characteristics such as calculated good solubility (-3.2 < logP < 5), oral bioavailability (RotB <= 10), drug transportability (PSA < 120). These compounds were selected by dissimilarity search with an average Tanimoto Coefficient of 0.52. There are 36,857 unique scaffolds and each scaffold 1 to 7 compounds. What’s more, compounds with the same scaffold have as many functional groups as possible, which make abundant chemical spaces.
MegaUni 10M Virtual Diversity Library
With MCE's 40,662 BBs, covering around 273 reaction types, more than 40 million molecules were generated. Compounds which comply with Ro5 criteria were selected. Inappropriate chemical structures, such as PAINS motifs and synthetically difficult accessible, were removed. Based on Morgan Fingerprint, molecular clustering analysis was carried out, and molecules close to each clustering center were extracted to form this drug-like and synthesizable diversity library. These selected molecules have 805,822 unique Bemis-Murcko Scaffolds (BMS) with diversified chemical space. This library is highly recommended for AI-based lead discovery, ultra-large virtual screening and novel lead discovery.
MegaUni 50K Virtual Diversity Library
MegaUni 50K Virtual Diversity Library consists of 50,000 novel, synthetically accessible, lead-like compounds. With MCE's 40,662 Building Blocks, covering around 273 reaction types, more than 40 million molecules were generated. Based on Morgan Fingerprint and Tanimoto Coefficient, molecular clustering analysis was carried out, and molecules closest to each clustering center were extracted to form a drug-like and synthesizable diversity library. The selected 50,000 drug-like molecules have 46,744 unique Bemis-Murcko Scaffolds (BMS), each containing only 1-3 compounds. This diverse library is highly recommended for virtual screening and novel lead discovery.
References
[1] Rifaioglu AS, et al.Brief Bioinform. 2019 Sep ;20(5):1878-1912.
[2] Guttman Y, et al.J Agric Food Chem. 2022 Mar ;70(8):2752-2761.
[3] Liu S, et al.J Agric Food Chem. 2023 May ;71(21):8038-8049.
[4] Li B, et al.J Cheminform. 2023 Aug;15(1):72.
[5] Segler MHS, et al. Planning chemical syntheses with deep neural networks and symbolic AI. Nature. 2018 Mar ;555(7698):604-610.
#biochemistry#chemistry#inhibitor#business#marketing#kit#developers & startups#AI Deep Learning Accelerates Drug Development
0 notes
Text
Vilitra 10 and Liver Health: Is It Safe for Men with Liver Conditions

Erectile dysfunction (ED) is a common condition that affects many men, especially as they age. Fortunately, medications like Vilitra 10, which contains the active ingredient Vardenafil, offer an effective solution for many men struggling with Performance issues . However, for men with liver conditions, there may be concerns about the safety of taking this medication. This article will explore the relationship between Vilitra 10 and liver health, examining whether it is safe for men with liver conditions to use it, and the considerations they must take into account.
What is Vilitra 10?
Vilitra 10 is a medication commonly prescribed to treat erectile dysfunction (ED) in men. It contains Vardenafil, a phosphodiesterase type 5 (PDE5) inhibitor that works by increasing blood flow to the penis, helping men achieve and maintain an erection when sexually aroused. The standard dosage for Vilitra 10 is 10 mg, typically taken about an hour before sexual activity.
While effective for many men, it is crucial to understand the potential risks and side effects of any medication, particularly for those with underlying health conditions like liver disease.
Liver Health and its Role in Medication Metabolism
The liver plays a vital role in metabolizing drugs and clearing them from the body. When a person has liver disease, this function can be impaired, meaning that medications might not be processed as efficiently. For men with liver conditions such as cirrhosis, hepatitis, or fatty liver disease, drugs like Vilitra 10 can accumulate in the body, potentially leading to adverse effects.
Therefore, understanding how a medication is metabolized and how liver function affects drug processing is essential when considering its safety. Vilitra 10, like many medications, is primarily broken down in the liver, which is why it is particularly important for men with liver conditions to exercise caution when using it.
Vilitra 10’s Interaction with the Liver
Vilitra 10 is metabolized by the liver enzymes, specifically the CYP3A4 enzyme. In healthy individuals, the liver processes the drug efficiently. However, for men with liver disease, this process can be slowed down. As a result, the drug can stay in the system for a longer period, potentially increasing the risk of side effects or complications.
For men with mild to moderate liver dysfunction, Vilitra 10 mg may still be prescribed, but adjustments to the dosage may be necessary. In cases of severe liver disease (such as cirrhosis), the use of Vilitra 10 is typically contraindicated or should be avoided altogether due to the risk of accumulation and toxicity. Therefore, it’s important for men with liver conditions to discuss their health status with their doctor before using this medication.
Liver Conditions and Erectile Dysfunction
Liver health is closely linked to sexual health, as liver disease can contribute to ED. Chronic liver disease can lead to hormonal imbalances, decreased testosterone levels, and vascular dysfunction, all of which can exacerbate erectile problems. Therefore, it is essential to address both liver disease and ED in a comprehensive treatment plan.
Although Vilitra 10 can be effective in treating ED in the general population, its use in men with liver conditions must be approached with caution. The liver plays a crucial role in the overall function of the body, and taking medication without proper management of liver disease may result in ineffective treatment or harmful side effects.
Safety Considerations for Men with Liver Conditions
For men with liver conditions, the use of Vilitra 10 requires careful consideration. While some men with mild liver impairment may be able to use the medication safely under medical supervision, others with more severe liver dysfunction may be at a higher risk of complications. Here are a few key safety considerations:
Lower Starting Doses: Men with liver conditions may need to start with a lower dose of Vilitra 10. A healthcare provider might adjust the dose based on liver function to reduce the risk of side effects.
Frequent Monitoring: Regular liver function tests and follow-up visits with a healthcare provider are essential for men using Vilitra 10 with liver conditions. Monitoring liver function can help ensure the medication is being processed properly and that no harmful effects are occurring.
Avoiding Certain Medications: Certain medications that interact with Vilitra 10 can increase the risk of side effects. These include medications that affect liver enzymes or other drugs metabolized by the liver. It's essential to inform your doctor about all other medications you're taking.
What to Do Before Taking Vilitra 10
Before using Vilitra 10, men with liver conditions should have an honest discussion with their healthcare provider. The doctor may recommend tests to assess liver function, such as liver enzyme tests or imaging studies, to determine whether it is safe to use the medication. Based on the results, the healthcare provider may adjust the dose or suggest alternative treatments.
Potential Side Effects and Complications
Like all medications, Vilitra 10 can cause side effects. Common side effects include headaches, flushing, and indigestion. For men with liver conditions, however, the potential for more serious side effects may be higher. These include dizziness, vision changes, and, in rare cases, priapism (a prolonged erection).
In men with liver disease, Vilitra 10 may also exacerbate liver-related issues, leading to more severe complications. If any unusual symptoms occur, such as yellowing of the skin or eyes (jaundice), abdominal pain, or swelling, immediate medical attention is required.
Alternative Treatments for ED in Men with Liver Disease
For men with liver conditions who are concerned about the safety of Vilitra 10, there are alternative treatments available. Lifestyle changes, such as improving diet, exercising, and managing stress, can help alleviate ED symptoms in some cases.
Additionally, other medications and therapies may be more suitable for men with liver disease. For instance, lower-dose alternatives or medications that are less reliant on liver metabolism may be prescribed. Non-medication treatments, such as penile injections or vacuum pumps, may also be recommended.
Conclusion
Vilitra 10 can be an effective treatment for erectile dysfunction, but its use in men with liver conditions requires careful consideration. Liver disease can affect how the body metabolizes the drug, potentially increasing the risk of side effects or complications. Therefore, men with liver conditions should consult with a healthcare provider before using Vilitra 10. By monitoring liver function and adjusting dosages as needed, many men with mild liver disease can safely use this medication. However, for those with more severe liver impairment, alternative treatments should be explored.
0 notes
Text
What is the classification of Barigen 4 mg (Baricitinib)?
Baricitinib is classified as a Janus kinase (JAK) inhibitor. It is an oral medication primarily used for the treatment of inflammatory conditions, particularly autoimmune diseases. The drug specifically inhibits JAK1 and JAK2, which are part of the JAK-STAT signaling pathway involved in immune responses and inflammation.
The classification of Baricitinib 4 mg can be categorized as follows:
Pharmacological Class: Janus kinase (JAK) inhibitor
Therapeutic Class: Disease-modifying anti-rheumatic drug (DMARD)
Route of Administration: Oral
Indications: Rheumatoid arthritis (RA), COVID-19-related complications, atopic dermatitis, alopecia areata, and other inflammatory conditions
Regulatory Classification: Prescription-only medication
Description of Baricitinib 4 mg
1. General Overview
Barigen 4 mg (Baricitinib) is a small molecule inhibitor of JAK1 and JAK2 enzymes, which play an essential role in the immune response by transmitting cytokine signals to the cell nucleus. By inhibiting these enzymes, Baricitinib helps reduce inflammation and autoimmune activity, making it effective in treating chronic inflammatory conditions.

2. Mechanism of Action
Baricitinib works by selectively inhibiting JAK1 and JAK2, preventing the phosphorylation and activation of STAT (Signal Transducers and Activators of Transcription) proteins. These STAT proteins play a crucial role in cytokine signaling, which is responsible for the immune response and inflammation.
By blocking JAK1/JAK2-mediated pathways, Baricitinib reduces cytokine-driven inflammation, which is particularly beneficial for patients with autoimmune diseases such as rheumatoid arthritis. In the case of COVID-19, it helps reduce hyperinflammation and cytokine storm, improving patient outcomes.
3. Indications and Uses
Baricitinib 4 mg is approved for several conditions, including:
Rheumatoid Arthritis (RA): Used in adult patients with moderate to severe RA who have not responded adequately to other disease-modifying anti-rheumatic drugs (DMARDs). It helps reduce joint pain, swelling, and long-term disease progression.
Severe Alopecia Areata: Approved for patients with extensive hair loss caused by autoimmune conditions affecting hair follicles.
Atopic Dermatitis: Used in some patients with moderate to severe atopic dermatitis when other treatments have failed.
COVID-19 Treatment: In hospitalized patients with COVID-19, Baricitinib has been used to manage severe inflammation, often in combination with corticosteroids, reducing mortality and the need for ventilation.
4. Dosage and Administration
The standard dose for rheumatoid arthritis and alopecia areata is 4 mg once daily.
Patients with kidney impairment may require a lower dose, typically 2 mg per day.
In COVID-19 treatment, the recommended duration is generally 14 days or until discharge, depending on the patient’s condition.
5. Contraindications and Warnings
Baricitinib should be used with caution in certain populations:
Patients with severe infections: Since it suppresses the immune system, there is a risk of serious infections like tuberculosis or bacterial infections.
Patients with a history of thrombosis: Increased risk of blood clots, including deep vein thrombosis (DVT) and pulmonary embolism (PE).
Hepatic and renal impairment: Dosage adjustments may be necessary.
Pregnant and breastfeeding women: Safety has not been well established in these populations.
6. Side Effects
Some common side effects of Baricitinib 4 mg include:
Upper respiratory tract infections
Nausea
Headaches
Elevated liver enzymes
Increased risk of infections
Severe side effects include blood clot formation, serious infections, and possible malignancies with long-term use.
7. Drug Interactions
Baricitinib may interact with other immunosuppressants, increasing the risk of infections. It should also be used cautiously with strong CYP3A4 inhibitors or inducers, which may alter its metabolism.
Conclusion
Baricitinib 4 mg is a potent JAK inhibitor used to treat inflammatory and autoimmune diseases, including rheumatoid arthritis, alopecia areata, and severe COVID-19 complications. Its mechanism of action involves blocking the JAK-STAT pathway, reducing immune system overactivity. While effective, it requires careful monitoring due to the risk of infections, thrombosis, and other side effects. Patients must use Baricitinib under the supervision of a healthcare provider to ensure safe and effective treatment.
1 note
·
View note
Text
How Safe is Krrista Force for Your Liver?
If you're considering using Krrista Force tablets, you might wonder, "Is it safe for my liver?" Let's break it down in simple terms to help you understand.
What is Krrista Force?
Krrista Force combines sildenafil and dapoxetine, two active ingredients used to treat erectile dysfunction (i.e., impotence) and premature ejaculation. With a strength of 150 mg of sildenafil and 60 mg of dapoxetine, this powerful medication aims to provide a solution to cure ED and improve overall intimacy.
But what about its effects on your liver? That's an important question, especially for long-term health.
How Does Krrista Force Affect the Liver?
The liver plays a crucial role in metabolizing medications, including Krrista Force. Here's how the active ingredients interact with the liver:
Sildenafil
Sildenafil, known for its role in improving blood flow, has been shown to positively impact liver health. Researchers at the University of Pittsburgh discovered that sildenafil increases the liver's production of a protein called cyclic GMP, which can help reduce inflammation and protect the liver from damage due to sepsis (immune system life-threatening, dangerous reaction to an infection).
This protection is especially significant in cases like sepsis, a condition that can cause severe liver damage. By reducing harmful signals in liver cells, sildenafil might actually offer some protective benefits.
In another study, sildenafil was found to be effective in recovery of liver function tests that decreased due to obstructive jaundice.
Dapoxetine
Dapoxetine, a selective serotonin reuptake inhibitor (SSRI), works to delay ejaculation by modulating certain neural pathways. Its metabolism occurs primarily in the liver, involving enzymes like CYP2D6 and CYP3A4.
The good news? Dapoxetine is rapidly cleared from the body, which minimizes the risk of long-term liver stress. This makes it a relatively safe option for most users, as it avoids accumulation in the liver.
The Big Question: Is Krrista Force Safe for Your Liver?
In general, Krrista Force tablets are considered safe for liver health when taken as prescribed. However, a few factors should be kept in mind:
Existing Liver Conditions: If you have pre-existing liver issues, it's always a good idea to consult your doctor before taking any medication.
Dosage: Stick to the recommended dosage. Overuse can strain your liver unnecessarily.
Alcohol: Avoid drinking alcohol while using Krrista Force. Alcohol can increase the liver's workload and reduce the effectiveness of the medication.
Additional Benefits for Liver Health
Surprisingly, medications like sildenafil (found in Krrista Force) might even support liver health. Studies suggest that sildenafil could be repurposed to reduce inflammation in conditions like sepsis. By increasing cyclic GMP levels, it may help protect against liver damage during systemic inflammation.
While more research is needed, these findings are promising. It's rare to find a medication that addresses how to treat ED and offers potential protective benefits for other organs, like the liver.
How to Use Krrista Force Safely?
If you're purchasing Krrista Force from a trusted and reliable Krrista Force wholesaler online, follow these guidelines to ensure safe use:
Consult Your Doctor: Always talk to a healthcare professional before starting Krrista Force, especially if you have liver or kidney concerns.
Take as Directed: Follow the prescribed dosage. Taking more doesn't mean faster results and could harm your liver.
Monitor Your Body: If you notice any unusual symptoms, such as nausea, jaundice, or abdominal pain, contact your doctor immediately.
Stay Hydrated: Drinking plenty of water helps your liver process medications efficiently.
Krrista Force and Overall Health
While its primary purpose is to cure ED, Krrista Force provides more than just improved intimacy. Its active ingredients, when used responsibly, have a favorable safety profile. By following your doctor's advice and using the medication as prescribed, you can enjoy its benefits while keeping your liver healthy.
Conclusion
Is Krrista Force safe for your liver? Yes, for most men, it's a safe and effective option. With careful use, this medication not only addresses erectile dysfunction impotence but may even offer some protective benefits for liver health.
If you're looking for a solution to treating ED, Krrista Force might be the answer. Just remember to prioritize your overall health by using the medication responsibly and seeking medical advice when needed.
Your liver is a vital organ. Thankfully, with the right precautions, you can take care of it while effectively addressing your health concerns.
1 note
·
View note
Text
Resihance

Resihance
Regorafenib is a multi-kinase inhibitor used primarily for the treatment of cancer. It is particularly effective in targeting angiogenesis (the formation of new blood vessels), which plays a crucial role in cancer growth and metastasis. Below is a detailed overview of Regorafenib:
Mechanism of Action:
Regorafenib inhibits multiple protein kinases involved in tumor angiogenesis, oncogenesis, and the tumor microenvironment. Specifically, it targets:
VEGFR (Vascular Endothelial Growth Factor Receptor) – involved in blood vessel formation.
PDGFR (Platelet-Derived Growth Factor Receptor) – involved in the growth and survival of cells.
RAF kinases (including BRAF) – involved in cell proliferation and survival.
By blocking these pathways, Regorafenib reduces tumor growth and the spread of cancer.
Indications:
Regorafenib is used in the treatment of several cancers, including:
Colorectal Cancer: It is used in metastatic colorectal cancer (mCRC) that has progressed after standard therapy.
Gastrointestinal Stromal Tumors (GIST): It is prescribed for GIST after imatinib and sunitinib treatment have failed.
Hepatocellular Carcinoma (HCC): For patients with advanced liver cancer who have been previously treated with sorafenib.
Common Side Effects:
Fatigue
Hand-foot skin reaction (redness, swelling, pain in palms and soles)
Diarrhea
Hypertension (high blood pressure)
Nausea and vomiting
Abdominal pain
Decreased appetite
Weight loss
Serious Side Effects:
Liver toxicity: Regorafenib can lead to severe liver damage, including elevated liver enzymes, jaundice, and, in rare cases, liver failure.
Bleeding: Regorafenib can increase the risk of severe bleeding, especially in patients with cancer that has spread to the liver.
Cardiovascular complications: It can lead to high blood pressure and may increase the risk of heart attack or stroke.
Gastrointestinal perforation: A rare but potentially life-threatening complication.
Monitoring and Precautions:
Liver function should be monitored regularly because of the risk of liver toxicity.
Blood pressure should be checked frequently to detect any early signs of hypertension.
Skin reactions should be monitored closely, as they can affect the patient's quality of life.
Kidney function should also be assessed periodically, especially in patients at risk of kidney damage.
Pharmacokinetics:
Absorption: Regorafenib is well absorbed after oral administration but should be taken with a low-fat meal to ensure proper absorption.
Metabolism: The drug is metabolized in the liver primarily through CYP3A4, and its active metabolites also play a role in its efficacy.
Excretion: Regorafenib and its metabolites are excreted primarily through feces, with a small portion eliminated through urine.
0 notes
Text
Attend the speech of "Dr. Juliana Bicca" about “Estrogen Metabolism And Women´S Health: Implications For Disease And Therapeutic Interventions” at the 14GASTROUCG
Estrogen, a primary female sex hormone, plays a critical role in women's health. Beyond its well-known effects on reproductive tissues, estrogen influences various physiological systems, including the cardiovascular, skeletal, and central nervous systems. Understanding estrogen metabolism is crucial for appreciating its implications for women's health and developing effective therapeutic interventions. This blog delves into the intricate world of estrogen metabolism, its impact on health, and potential therapeutic approaches.

The Basics of Estrogen Metabolism
Estrogen metabolism involves the conversion of estrogens, such as estradiol, into various metabolites. This process primarily occurs in the liver through a series of enzymatic reactions. The key players in estrogen metabolism include:
Aromatase: This enzyme converts androgens (male hormones) into estrogens, making it pivotal in regulating estrogen levels.
CYP450 Enzymes: Cytochrome P450 enzymes, particularly CYP1A1, CYP1B1, and CYP3A4, are responsible for hydroxylating estrogens. These reactions produce various metabolites, including 2-hydroxyestrone and 16α-hydroxyestrone, with differing biological activities.
COMT (Catechol-O-Methyltransferase): This enzyme methylates hydroxylated estrogen metabolites, rendering them less active and more easily excreted.
Implications of Estrogen Metabolism on Women's Health
Breast Cancer: The balance between different estrogen metabolites can influence breast cancer risk. For example, 16α-hydroxyestrone has been associated with a higher risk, while 2-hydroxyestrone is considered less estrogenic and may have protective effects. Understanding these pathways can help in assessing risk and developing preventive strategies.
Bone Health: Estrogen plays a crucial role in maintaining bone density. During menopause, the decline in estrogen levels can lead to increased bone resorption, contributing to osteoporosis. Estrogen replacement therapies can help mitigate this risk, but understanding individual metabolic pathways is vital for personalizing treatment.
Cardiovascular Health: Estrogen has protective effects on the cardiovascular system, including promoting vasodilation and reducing cholesterol levels. However, its metabolism can produce metabolites with different effects, influencing cardiovascular risk. For instance, some metabolites may increase oxidative stress, while others have antioxidant properties.
Neuroprotection: Estrogen's role in the central nervous system includes neuroprotective effects, potentially lowering the risk of neurodegenerative diseases like Alzheimer's. However, the benefits may vary depending on the balance of estrogen metabolites.
Therapeutic Interventions and Considerations
Hormone Replacement Therapy (HRT): HRT is commonly used to alleviate menopausal symptoms and prevent osteoporosis. However, the type of estrogen and the metabolic pathways it engages are crucial in determining the therapy's safety and efficacy. For instance, bioidentical hormones may offer a more favorable risk profile compared to synthetic hormones.
Selective Estrogen Receptor Modulators (SERMs): SERMs are compounds that selectively activate or block estrogen receptors in different tissues. They can be used to mimic the beneficial effects of estrogen in some tissues while avoiding adverse effects in others, such as breast tissue.
Aromatase Inhibitors: These drugs inhibit the aromatase enzyme, reducing estrogen production. They are often used in treating estrogen receptor-positive breast cancer, as they lower estrogen levels and limit tumor growth.
Nutritional and Lifestyle Interventions: Diet and lifestyle can significantly impact estrogen metabolism. For example, cruciferous vegetables contain compounds that can promote the formation of beneficial estrogen metabolites. Regular exercise and maintaining a healthy weight also positively influence hormone balance.
Conclusion
Estrogen metabolism is a complex but crucial aspect of women's health, influencing a wide range of physiological processes and disease risks. As our understanding of these metabolic pathways grows, it opens new avenues for personalized medicine and targeted therapies. By considering individual differences in estrogen metabolism, healthcare providers can offer more tailored and effective treatments, ultimately improving women's health outcomes. Whether through pharmacological interventions, lifestyle changes, or a combination of both, the goal remains to harness the benefits of estrogen while minimizing potential risks.

Important Information:
Conference Name: 14th World Gastroenterology, IBD & Hepatology Conference Short Name: 14GHUCG2024 Dates: December 17-19, 2024 Venue: Dubai, UAE Email: [email protected] Visit: https://gastroenterology.universeconferences.com/ Call for Papers: https://gastroenterology.universeconferences.com/submit-abstract/ Register here: https://gastroenterology.universeconferences.com/registration/ Exhibitor/Sponsor: https://gastroenterology.universeconferences.com/exhibit-sponsor-opportunities/ Call Us: +1 (207) 707-7298 WhatsApp Us: +442033222718
0 notes
Text
medical talk and health updates under the cut
1st: i think i posted about this a week or so ago, but i think i had viral meningitis (which goes away by itself) for a couple weeks in october but now that's better :)
2nd: i've noticed a pattern of me vomiting up my antipsychotic (lurasidone aka latuda) whenever i eat tomato soup or other tomato-dominant dishes. this has been happening for over a year at this point, and i finally put 2 and 2 together.
so i did some digging, and i found something that no doctor seems to know and/or tell people?? apparently tomato juice is a cyp3a4 inhibitor (the same thing that makes grapefruit juice dangerous for many people on different medications) (i can explain it more if people want, but tl;dr it makes my medication at least twice as strong, and i already take the second highest dose possible for latuda)
so basically, when i eat tomato soup and then take my latuda (which has to be taken with a full meal), i'm basically being overdosed by at least twofold if not more, hence, i feel really sick and inevitably vomit. but much like many lactose intolerant people, i won't let this revelation stop me from enjoying food, so i've decided to not take my medication when i have tomato soup.
so yay!! mystery solved!!! :)
but 3rd: my stupid baka body is hurting me again. my hips hurt?? kinda bad?? idk why, and i'm not in the mood to investigate rn, so i'll see if it passes.
1 note
·
View note
Text
Unraveling the Interactions: Aspirin's Influence on Drug Metabolism and Therapy
Aspirin, a ubiquitous medication renowned for its pain-relieving and anti-inflammatory properties, has a long-standing history of medical use. Beyond its primary indications, recent research has shed light on its potential impact on the metabolism and efficacy of various drugs. Understanding these interactions is crucial for optimizing therapeutic outcomes and mitigating potential risks associated with polypharmacy.
Interactions with Drug Metabolism:
Aspirin’s Influence on Drug, also known as acetylsalicylic acid, exerts its effects through inhibition of cyclooxygenase enzymes, particularly COX-1 and COX-2. While primarily recognized for its antiplatelet and analgesic actions, aspirin's influence extends to drug metabolism pathways mediated by cytochrome P450 enzymes (CYPs). Studies have demonstrated that aspirin can modulate the activity of certain CYP isoforms, potentially altering the pharmacokinetics of co-administered drugs. Specifically, aspirin has been implicated in inhibiting CYP2C9, CYP2C19, and CYP3A4 enzymes, which are involved in the metabolism of a wide array of medications, including anticoagulants, antiplatelet agents, statins, and selective serotonin reuptake inhibitors (SSRIs).
Clinical Implications:
The interactions between aspirin and other drugs carry significant clinical implications. For instance, concurrent use of aspirin with anticoagulants like warfarin or direct oral anticoagulants (DOACs) may potentiate the risk of bleeding due to impaired metabolism of these agents. Similarly, co-administration of Aspirin’s Influence on Drug with certain antidepressants, such as fluoxetine or sertraline, may lead to elevated drug concentrations and an increased risk of adverse effects. Furthermore, the impact of aspirin on antiplatelet agents like clopidogrel underscores the importance of tailored therapy regimens to balance efficacy and safety in patients with cardiovascular conditions.
Future Directions:
While current evidence elucidates some of the interactions between aspirin and other drugs, further research is warranted to comprehensively characterize these phenomena. Future studies should explore the molecular mechanisms underlying aspirin-mediated alterations in drug metabolism and assess the clinical relevance of these interactions across diverse patient populations. Additionally, advances in pharmacogenomics may facilitate personalized approaches to drug therapy, considering individual variations in enzyme activity and drug response.
Conclusion:
In conclusion, aspirin's influence on drug metabolism presents a multifaceted phenomenon with implications for therapeutic efficacy and safety. Clinicians should remain vigilant when prescribing aspirin in conjunction with other medications, considering potential interactions that may impact treatment outcomes. Continued research efforts are essential to refine our understanding of these interactions and optimize pharmacotherapeutic strategies in clinical practice.
0 notes
Text
What You Need to Know About Vilitra 40 and Its Interaction with Antifungal Drugs

Vilitra 40 is a widely used medication for treating erectile dysfunction (ED), a condition that affects millions of men worldwide. Its active ingredient, vardenafil, is a phosphodiesterase type 5 (PDE5) inhibitor that helps increase blood flow to the penis, enabling men to achieve and maintain an erection during sexual activity. While Vilitra 40 can be highly effective, there are certain precautions that users need to be aware of, especially when it comes to interactions with other medications. One such concern is the interaction between Vilitra 40 and antifungal drugs, which can potentially cause harmful effects. This article will explore this interaction and provide guidance on how to use Vilitra 40 safely if you are taking antifungal medications.
Understanding Vilitra 40 and How It Works
Vilitra 40 contains the active ingredient vardenafil, which is part of a class of drugs known as PDE5 inhibitors. PDE5 is an enzyme that breaks down cGMP, a molecule that helps relax smooth muscles and dilate blood vessels, leading to increased blood flow. In men with erectile dysfunction, PDE5 inhibitors like vardenafil block this enzyme, allowing blood vessels in the penis to remain open during sexual arousal. This improved blood flow helps men achieve and maintain an erection.
While Vilitra 40 is generally safe for most users, it can cause side effects, including headaches, dizziness, nasal congestion, and flushing. These effects are typically mild and go away after a few hours. However, combining Vilitra 40 with certain medications, especially antifungal drugs, can cause more severe complications. This is why it's important to be aware of potential interactions before taking Vilitra 40.
What Are Antifungal Drugs and Their Common Uses?
Antifungal drugs are medications used to treat infections caused by fungi. These drugs are commonly prescribed for conditions like athlete's foot, ringworm, and candidiasis, as well as systemic fungal infections. Some of the most commonly prescribed antifungal drugs include:
Ketoconazole
Itraconazole
Fluconazole
These antifungal medications work by targeting the cell walls or enzymes of fungi, inhibiting their ability to grow and reproduce. They are effective in treating a wide range of fungal infections, but they can also have unintended effects on other medications, including those used for erectile dysfunction, like Vilitra 40.
The Potential Interaction Between Vilitra 40 and Antifungal Drugs
The key concern when taking Vilitra 40 with antifungal drugs lies in how these medications are metabolized in the body. Antifungal drugs, particularly azole antifungals like ketoconazole and itraconazole, can inhibit the activity of certain enzymes in the liver, specifically the CYP3A4 enzyme. This enzyme is responsible for breaking down many drugs, including vardenafil (the active ingredient in Vilitra 40).
When antifungal drugs inhibit CYP3A4, they slow down the metabolism of vardenafil, causing it to remain in the bloodstream for longer periods at higher concentrations. This means that the levels of vardenafil in your system can become dangerously elevated, increasing the risk of side effects associated with Vilitra 40, including low blood pressure, dizziness, and prolonged erections (priapism).
Why the Interaction Is Dangerous
The interaction between Vilitra 40 and antifungal drugs can be dangerous due to the potential for serious side effects. If vardenafil accumulates in the bloodstream, it can cause:
Low Blood Pressure: Elevated levels of vardenafil can lead to an excessive drop in blood pressure, causing dizziness, lightheadedness, and fainting. For individuals with heart problems, this can be particularly risky.
Prolonged Erection (Priapism): One of the most concerning side effects of high vardenafil levels is priapism, a prolonged and painful erection that lasts for more than four hours. If left untreated, priapism can cause permanent damage to the penis.
Other Side Effects: Users may also experience headaches, blurred vision, and chest pain due to high levels of vardenafil.
In extreme cases, the combination of Vilitra 40 and antifungal drugs can lead to serious heart complications, including arrhythmia or heart attack, particularly in individuals with pre-existing heart conditions.
Signs of an Interaction: What to Look Out For
If you are taking Vilitra 40 mg and an antifungal drug, it's essential to monitor for any unusual symptoms. Some signs that an interaction may be occurring include:
Dizziness or Lightheadedness: If you experience dizziness or feel faint, this could be a sign that your blood pressure has dropped too much.
Headaches or Blurred Vision: Elevated vardenafil levels can cause headaches or affect your vision.
Prolonged Erection (Priapism): If you experience an erection that lasts longer than four hours and is painful, this is a medical emergency that requires immediate attention.
Chest Pain or Heart Palpitations: If you experience any chest discomfort or heart-related symptoms, seek medical help immediately.
If you experience any of these symptoms, it's important to seek medical advice right away to avoid further complications.
How to Safely Use Vilitra 40 with Antifungal Drugs
To avoid the dangerous interaction between Vilitra 40 and antifungal drugs, consider the following steps:
Consult a Doctor: Before using Vilitra 40 with antifungal medications, talk to your healthcare provider. They may suggest adjusting the dosage of either the antifungal drug or Vilitra 40.
Alternative Treatments for ED: If you're required to take antifungal drugs, your doctor might recommend alternative ED treatments that are less likely to interact with antifungal medications.
Timing and Dosage Adjustments: In some cases, your doctor may advise adjusting the timing of when you take Vilitra 40 and the antifungal medication, or they may suggest a lower dose of Vilitra 40 to minimize risks.
Alternatives to Vilitra 40 and Antifungal Drug Combinations
If you need both antifungal treatment and erectile dysfunction medication, there are alternatives to Vilitra 40 that may have less risk of interaction. Some options include:
Other PDE5 Inhibitors: Other medications like sildenafil (Viagra) or tadalafil (Cialis) may be safer choices, but this depends on your specific health situation. Always consult your doctor before switching medications.
Non-Pharmaceutical Approaches: For some men, lifestyle changes such as exercise, stress reduction, and improved diet can help alleviate erectile dysfunction. Vacuum erection devices or penile injections are other non-drug options for ED.
Conclusion
Vilitra 40 is an effective treatment for erectile dysfunction, but when combined with antifungal drugs, it can cause serious interactions that increase the risk of dangerous side effects. By understanding how these drugs interact and consulting with your healthcare provider, you can safely manage both your ED and fungal infection treatments. Always seek medical advice before combining Vilitra 40 with any other medications to ensure your safety and well-being.
0 notes
Text
Everolimus (Dosing-1)
In this article, we will discuss Everolimus (Dosing-1). So, let’s get started. Recommended Dose in SEGA with TSC The recommended starting dose is 4.5 mg/m2, once daily. The recommended starting dose for patients with severe hepatic impairment (Child-Pugh class C) or requiring moderate CYP3A4/PgP inhibitors is 2.5 mg/m2, once daily. The recommended starting dose for patients requiring a…
View On WordPress
0 notes
Text
How effective is Finerenone?
New medicine for treating diabetic nephropathy – LUCIFINE finerenone tablets
LUCIFINE Finerenone Tablets Is a non-steroidal mineralocorticoid receptor antagonist (MRA) indicated to reduce the risk of sustained eGFR decline end stage kidney disease, cardiovascular death non-fatal myocardial infarction, and hospitalization for hear failure in adult patients with chronic kidney disease(CKD) associated with type 2 diabetes T2D)
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of a drug and may not reflect the rates observed in practice.
The safety of LUCIFINE was evaluated in 2 randomized, double-blind, placebo-controlled, multicenter pivotal phase 3 studies, FIDELIO-DKD and FIGARO-DKDin which a total of 6510 patients were treated with 10 or 20 mg once daily over a mean duration of 2.2 and 2.9 years, respectively.
Overall, serious adverse events occurred in 32% of patients receiving LUCIFINE and in 34% of patients receiving placebo in the FIDELIO-DKD study: the findings were similar in the FIGARODKD study. Permanent discontinuations due to adverse events also occurred in a similar proportion of patients in the two studies (6-7% of patients receiving LUCIFINE and in 5-6% of patients receiving placebo).
The most frequently reported (≥10%)adverse reaction in both studies was hyperkalemia [see Warnings and Precautions(5.1)]. Hospitalization due to Hyperkalemia for the LUCIFINE was 0.9% vs 0.2% in the placebo group across both studies. Hyperkalemia led to permanent discontinuation of treatment in 1.7% receiving LUCIFINE versus 0.6% of patients receiving placebo across both studies.
LUCIFINE is a CYP3A4 substrate. Concomitant use with a strong CYP3A4 inhibitor increases Finerenone exposure, which may increase the risk of LUCIFINE adverse reactions. Concomitant Use of LUCIFINE with strong CYP3A4 inhibitors is contraindicated see Contraindications (4) Avoid concomitant intake of grapefruit or grapefruit juice.
Moderate and Weak CYP3A4 Inhibitors LUCIFINE is a CYP3A4 substrate. Concomitant use with a moderate or weak CYP3A4 inhibitor increases Finerenone exposure, which may increase the risk of LUCIFINE adverse reactions. Monitor serum potassium during drug initiation or dosage adjustment of either LUCIFINE or the moderate or weak CYP3A4 inhibitor, and adjust LUCIFINE dosage as appropriate [(see Dosing and Administration (2.3) and Drug interaction (7 .2)]. Strong and Moderate CYP3A4 inducers.
LUCIFINE is a CYP3A4 substrate. Concomitant use of LUCIFINE with a strong or moderate CYP3A4 inducer decreases Finerenone exposure. which may reduce the efficacy of LUCIFINE. Avoid concomitant use of LUCIFINE with strong or moderate CYP3A4 inducers.

#Diabetic Kidney Disease#Finerenone#diabetic nephropathy#Diabetic#long-term diabetes#Kidney Disease#DKD#CKD#blood sugar#protein in the urine#kidney failure#eGFR#Blood Sugar Control#Obesity#Hypertension
0 notes
Text
TIBSOVO: Acute Myeloid Leukemia.
FDA-approved drug
Tibsovo, an FDA-approved IDH1 inhibitor approved in 2018, treats adult patients with susceptible IDH1 mutations found by an FDA-approved test.
Acute myeloid leukaemia (AML) is a type of blood malignancy.
Patients over 75 with comorbidities that preclude intensive induction chemotherapy should not be treated for recurrent or refractory AML.
The disease was discovered in patients previously receiving either locally advanced or metastatic cholangiocarcinoma treatment.
Although Tibsovo is a low-cost medication, patients should know the following drug interactions before taking it. Speak with your doctor if you require any additional information.
Tibsovo: Suppliers in India
Tibsovo pills are available for purchase. Tibsovo should be used once a day until the condition worsens or there is severe toxicity.
Tibsovo can be taken with or without food. Tibsovo should not be taken with a high-fat meal since the Tibsovo concentration will rise. Tibsovo pills should not be divided or separated. Tibsovo capsules should be taken orally at the same time every day. If you miss a Tibsovo dose because of vomiting, wait until the next scheduled dose.
With a legitimate prescription and from a trustworthy provider such as Indian Pharma Networks, this is available. Any generic and innovative supplier with the relevant authorization, a valid and recognized prescription, and adherence to the criteria may provide the medication. You may buy tibsovo online from Indian Pharma Networks at amazingly low prices because we are one of the most reliable and reputable generic pharmaceutical providers.

Tibsovo: Precautions and Warnings
With benefits, there are few Warnings and Precautions associated with Tibsovo 250 mg, which are given as follows-
The minerals and electrocardiograms should be used to check for QTc interval prolongation. If QTc interval prolongation occurs, reduce or pause the dose, resume the dose, or discontinue Tibsovo completely.
In those who may have Guillain-Barre syndrome (GBS), look for new motor and sensory impairments and associated signs and symptoms. If you have Guillain-Barré syndrome, you should never play Tibsovo again.
Tibsovo: Contradictions
If you take a strong or moderate CYP3A4 inhibitor, reduce your Tibsovo dosage. Examine the patients for signs of a prolonged QTc interval.
If you are a CYP3A4 inducer, avoid taking Tibsovo (7.1, 12.3) simultaneously.
When utilizing CYP3A4 substrates that are sensitive, avoid taking Tibsovo (7.2, 12.3) at the same time.
Tibsovo should not be taken with any medications that cause QTc prolongation. Patients should be closely monitored for an increased risk of QTc interval prolongation if coadministration is unavoidable.
If you experience symptoms of differentiation syndrome, your doctor may prescribe a corticosteroid or a drug known as hydroxyurea while keeping an eye on you in the hospital.
This highly effective drug is widely available and may be acquired from any reliable seller. Tibsovo is very easy to incorporate into treatment plans in India due to its affordable price.
Tibsovo: After-Negative Effects
The most common adverse effects in AML patients (20%) were fatigue, arthralgia, leukocytosis, diarrhoea, edoema, nausea, dyspnea, mucositis, and abnormal ECG readings. QT prolongation symptoms include rash, cough, decreased appetite, myalgia, constipation, and pyrexia.
The most common laboratory abnormalities (20%) in AML patients were haemoglobin drop, calcium drop, sodium drop, magnesium drop, uric acid drop, potassium drop, alkaline phosphatase drop, aspartate aminotransferase drop, phosphate drop, and creatinine drop.
The most prevalent side symptoms in cholangiocarcinoma patients (15%) were fatigue, nausea, stomach discomfort, diarrhoea, coughing, decreased appetite, ascites, vomiting, anaemia, and rash.
The most common laboratory abnormalities in 10% of cholangiocarcinoma patients were low haemoglobin, increased aspartate aminotransferase, and high bilirubin.
To learn about side effects and how much Tibsovo costs, go to https://www.indianpharmanetwork.in/contact-us/. Indian Pharma Network is a rapidly expanding pharmaceutical consultancy and service firm that provides authentic pharmaceuticals through a dependable network.
Tibsovo: Distributors in India
The small drug ivosidenib blocks the action of the mutant isocitrate dehydrogenase 1 (IDH1) enzyme. R132H and R132C mutations are the most frequent in AML patients.
When compared to wild-type IDH1, ivosidenib significantly reduced the quantity required to inhibit a range of IDH1 R132 mutants in vitro. Ivosidenib lowered 2HG levels and enhanced myeloid growth in mice xenograft models of AML with an IDH1 mutation in vitro and in vivo by inhibiting the mutant IDH1 enzyme. Blood samples from people with AML and mutant IDH1 showed a decrease in ex-vivo 2-HG levels, blast counts, and the fraction of mature myeloid cells after ivosidenib treatment.
This efficient drug is accessible for purchase from any reliable seller. Because tibsovo price in India is so low and readily available, adding it to therapy is so straightforward.
Tibsovo: Availability in India
Tibsovo has a molecular weight of 583.0 g/mol and the chemical formula C28H22ClF3N6O3. Ivosidenib is exceedingly insoluble in water and has a pH range of 1.2 to 7.4.
Tibsovo film-coated pills containing ivosidenib are marketed as Tibsovo. Among the inactive ingredients are colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulphate. The coating of the tablet contains FD&C blue #2, hypromellose, lactose monohydrate, titanium dioxide, and triacetin.
This efficient drug is accessible for purchase from any reliable seller and is available in India. Because tibsovo price in India is so low and readily available, adding it to therapy is so straightforward. For more information.
0 notes