#Biosimilar market Trends
Explore tagged Tumblr posts
Text
#India Biosimilar Market Report#India Biosimilar Market Trends#India Biosimilar Market Growth#India Biosimilar Market Share
0 notes
Text
Biosimilar Market Size, Share, Key Growth Drivers, Trends, Challenges and Competitive Landscape
Data Bridge Market research has recently issued comprehensive industry research on Global Biosimilar Market which includes growth analysis, regional marketing, challenges, opportunities, and drivers analysed in the report.
Besides, Biosimilar market report studies market growth opportunities and restraining factors. The geographical division of this market analysis report offers data that gives an idea of the revenue of the companies and sales figures of the market growth. The market report also contains the drivers and restraints for the Biosimilar market that are obtained with the help of SWOT analysis, and also shows all the recent developments, product launches, joint ventures, mergers and acquisitions by the several key players and brands with their systemic company profiles, that are driving the market.
Access Full 350 Pages PDF Report @
Data Bridge Market Research analyses that the global biosimilar market which was USD 37,270.20 million in 2022, is expected to reach USD 343,520.90 million by 2030, and is expected to undergo a CAGR of 32.00% during the forecast period 2023-2030. “Oncology” dominates the product segment of the global biosimilar market owing to the owing to the increase in number of research studies, increasing research and development activities by key players focused on oncology and advancements in healthcare technologies. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Key Questions Answered with this Study
1) What makes Biosimilar Market feasible for long term investment?
2) Know value chain areas where players can create value?
3) Teritorry that may see steep rise in CAGR & Y-O-Y growth?
4) What geographic region would have better demand for product/services?
5) What opportunity emerging territory would offer to established and new entrants in Biosimilar Market?
6) Risk side analysis connected with service providers?
7) How influencing factors driving the demand of Biosimilarin next few years?
8) What is the impact analysis of various factors in the Global Biosimilar Market growth?
9) What strategies of big players help them acquire share in mature market?
10) How Technology and Customer-Centric Innovation is bringing big Change in Biosimilar Market?
Some of the major players operating in the global biosimilar market are:
Novartis AG (Switzerland)
Orion Pharma AB (Sweden)
Pfizer Inc. (U.S.)
Samsung Bioepis. (South Korea)
Coherus BioSciences, Inc. (U.S.)
Amgen Inc. (U.S.)
Eli Lilly and Company (U.S.)
Takeda Pharmaceutical Company Limited. (Japan)
Bristol-Myers Squibb Company (U.S.)
Merck KGaA (Germany)
Teva Pharmaceutical Industries Ltd. (U.S.)
Biocon. (India)
Bayer AG (Germany)
AbbVie Inc. (U.S.)
Allergan (Ireland)
Dr. Reddy’s Laboratories Ltd. (India)
Boehringer Ingelheim International GmbH. (Germany)
Biogen (U.S.)
Browse Trending Reports:
Prenatal Testing And New Born Screening Market
Rapid Oral Fluid Screening Device Market
North America Eclinical Solutions Market
Medical Tuning Fork Market
North America Cbct Dental Imaging Market
Myopia Treatment Market
Induced Pluripotent Stem Cells Market
Pneumococcal Vaccine Market
Dupuytrens Disease Market
Mitogen Activated Protein Kinase Mapk Inhibitors Therapeutics Market
Neonatal Seizures Drugs Market
Chikungunya Treatment Market
Sickle Cell Disease Treatment Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: [email protected]
#Biosimilar Market Size#Key Growth Drivers#Challenges and Competitive Landscape#market report#market share#market size#market trends#market research#market analysis#marketresearch#markettrends#Biosimilar Market
0 notes
Text
Navigating the Biosimilars Market: Key Players and Strategies

The biosimilars market represents a significant opportunity for pharmaceutical companies to offer more affordable alternatives to costly biologic drugs. As the demand for biosimilars continues to rise, understanding the key players and strategies driving this market becomes essential for stakeholders aiming to navigate and succeed in this competitive landscape.
According to the study by Next Move Strategy Consulting, the global Biosimilars Market size is predicted to reach USD 263.57 billion with a CAGR of 34.0% by 2030. This forecast underscores the immense growth potential within the biosimilars sector, prompting both established pharmaceutical giants and emerging players to strategically position themselves in this burgeoning market.
Request a FREE sample, here: https://www.nextmsc.com/biosimilars-market/request-sample
Current Landscape of the Biosimilars Market
The biosimilars market has witnessed rapid growth in recent years, driven by factors such as patent expirations of biologic drugs, increasing healthcare costs, and growing demand for affordable treatment options. Biosimilars, which are highly similar but not identical to reference biologics, offer a cost-effective alternative for patients while maintaining similar efficacy and safety profiles.
Key Players in the Biosimilars Market
Amgen Inc.: Amgen Inc. is a leading biotechnology company known for its innovative biologic and biosimilar products. With a strong emphasis on research and development, Amgen has successfully launched several biosimilar therapies across therapeutic areas such as oncology, immunology, and hematology. The company's biosimilars portfolio includes products such as filgrastim, bevacizumab, and adalimumab, catering to the diverse needs of patients worldwide.
F. Hoffmann-La Roche Ltd.: F. Hoffmann-La Roche Ltd., commonly known as Roche, is a global pharmaceutical company with a significant presence in the biosimilars market. Leveraging its expertise in biotechnology, Roche has developed biosimilar alternatives to blockbuster biologic drugs, including rituximab and trastuzumab. The company's commitment to quality and innovation has positioned it as a key player in driving biosimilar adoption across geographies.
Sandoz International GmbH: Sandoz International GmbH, a subsidiary of Novartis, is a pioneer in the biosimilars market. With a focus on expanding patient access to affordable biologic therapies, Sandoz has developed a diverse portfolio of biosimilar products, including filgrastim, etanercept, and rituximab. Through strategic partnerships and collaborations, Sandoz continues to drive biosimilar innovation and market penetration worldwide.
Dr. Reddy’s Laboratories Ltd.: Dr. Reddy’s Laboratories Ltd. is a leading Indian pharmaceutical company with a growing presence in the biosimilars market. The company's biosimilar portfolio includes products such as filgrastim, pegfilgrastim, and trastuzumab, offering cost-effective treatment options for patients with cancer and autoimmune diseases. Dr. Reddy’s commitment to quality and affordability has enabled it to establish a strong foothold in the biosimilars market globally.
Teva Pharmaceutical Industries Ltd.: Teva Pharmaceutical Industries Ltd. is a multinational pharmaceutical company that has emerged as a key player in the biosimilars market. With a focus on therapeutic areas such as oncology, neurology, and autoimmune diseases, Teva has developed biosimilar alternatives to biologic drugs such as filgrastim and rituximab. The company's robust biosimilars pipeline and strategic partnerships position it for continued growth and market expansion.
Pfizer Inc.: Pfizer Inc. is a global pharmaceutical company known for its innovative biologic and biosimilar products. With a diverse biosimilars portfolio spanning therapeutic areas such as oncology, inflammation, and immunology, Pfizer aims to improve patient access to high-quality, affordable biologic therapies. Through strategic acquisitions and collaborations, Pfizer continues to drive biosimilar innovation and market growth worldwide.
Samsung Bioepis: Samsung Bioepis is a biopharmaceutical company focused on developing biosimilar therapies for various diseases. Leveraging its expertise in biotechnology and manufacturing, Samsung Bioepis has successfully launched biosimilar alternatives to biologic drugs including infliximab, etanercept, and trastuzimab. The company's commitment to quality, affordability, and innovation has established it as a key player in the biosimilars market.
Inquire before buying, here: https://www.nextmsc.com/biosimilars-market/inquire-before-buying
Biocon: Biocon is a leading biotechnology company based in India, specializing in biosimilar and generic pharmaceuticals. With a strong focus on research and development, Biocon has developed biosimilar products targeting therapeutic areas such as oncology, diabetes, and immunology. The company's biosimilars portfolio includes products such as trastuzumab, pegfilgrastim, and insulin glargine, catering to the needs of patients globally.
Stada Arzneimittel AG: Stada Arzneimittel AG is a European pharmaceutical company that has ventured into the biosimilars market in recent years. With a focus on developing high-quality, affordable biosimilar alternatives, Stada aims to address unmet medical needs and improve patient access to essential biologic therapies. The company's biosimilars portfolio includes products including filgrastim and epoetin alfa, offering cost-effective treatment options for patients with cancer and chronic diseases.
Mylan N.V.: Mylan N.V. is a global pharmaceutical company known for its diverse portfolio of generic and specialty pharmaceuticals. With a strategic focus on biosimilar development, Mylan has launched biosimilar products targeting therapeutic areas such as oncology, immunology, and diabetes. The company's biosimilars portfolio includes products including trastuzumab, adalimumab, and insulin glargine, providing patients with affordable alternatives to costly biologic drugs.
Strategies in the Biosimilars Market
Research and Development: Key players in the biosimilars market invest heavily in research and development to develop high-quality biosimilar products that meet regulatory standards and address unmet medical needs. By leveraging innovative technologies and scientific expertise, companies can accelerate the development and commercialization of biosimilar therapies.
Strategic Partnerships: Collaborations and partnerships play a crucial role in driving biosimilar adoption and market penetration. By partnering with contract manufacturing organizations, academic institutions, and regulatory agencies, companies can enhance their biosimilar development capabilities, access new markets, and navigate complex regulatory pathways more effectively.
Regulatory Compliance: Regulatory compliance is paramount in the biosimilars market, given the complex nature of biologic drugs and the stringent requirements set forth by regulatory authorities. Key players in the biosimilars market adhere to rigorous regulatory standards to ensure the safety, efficacy, and quality of their biosimilar products, thereby gaining the trust and confidence of healthcare providers and patients.
Market Access Strategies: Access to markets is critical for the success of biosimilar products. Key players in the biosimilars market implement strategic market access strategies to overcome barriers such as pricing and reimbursement challenges, formulary restrictions, and competition from originator biologics. By collaborating with payers, healthcare providers, and patient advocacy groups, companies can enhance market access for their biosimilar products and drive uptake among prescribers and patients.
Educational Initiatives: Education and awareness play a crucial role in fostering acceptance and uptake of biosimilar therapies among healthcare providers, patients, and other stakeholders. Key players in the biosimilars market invest in educational initiatives to provide accurate information about biosimilars, address misconceptions, and promote the benefits of biosimilar treatment options. By engaging in scientific exchange activities, continuing medical education programs, and patient advocacy efforts, companies can empower stakeholders to make informed decisions about biosimilar therapies.
Global Expansion: The biosimilars market offers significant growth opportunities beyond traditional markets, with emerging economies presenting untapped potential for biosimilar adoption. Key players in the biosimilars market prioritize global expansion strategies to capitalize on the growing demand for affordable biologic therapies in regions such as Asia-Pacific, Latin America, and the Middle East. By establishing local partnerships, investing in market-specific research and development, and navigating regulatory complexities, companies can position themselves for success in diverse geographic markets.
Conclusion
The biosimilars market presents a dynamic and rapidly evolving landscape, driven by the increasing demand for affordable biologic therapies and the emergence of innovative biosimilar products. Key players in the biosimilars market play a critical role in shaping the future of healthcare by developing high-quality, cost-effective biosimilar alternatives and implementing strategic initiatives to drive market growth and adoption.
By understanding the key players and strategies driving the biosimilars market, stakeholders can navigate this complex landscape effectively and contribute to improving patient access to essential biologic treatments worldwide.
#biosimilars market#biotechnology#healthcare#market trends#market research#industry analysis#innovation
0 notes
Text
Biosimilars Unleashed: The Future of Healthcare in the US
Buy Now
What is the Size of US Biosimilar Industry?
US Biosimilar Market is expected to grow at a CAGR of ~ % between 2017-2022 and is expected to reach ~USD Bn by 2028. Biosimilars enhance patient access to essential treatments, especially in therapies with high demand, like oncology, by providing more affordable options. Additionally, Growing evidence of biosimilars' comparable efficacy and safety fosters trust among healthcare professionals, driving adoption.

Click here to Download a sample Report
Biosimilars offer cost savings compared to originator biologics, addressing the need for affordable healthcare solutions in the face of rising medical costs. Favorable regulatory frameworks, like the BPCIA, streamline biosimilar approval processes, encouraging manufacturers to invest in development.
Furthermore, The expiration of patents for numerous reference biologics creates opportunities for biosimilar entry, leading to increased competition and market expansion. Pharmaceutical companies are investing in biosimilar R&D and production, expanding the pipeline and market availability. Supportive healthcare policies and reimbursement models incentivize biosimilar adoption, creating a favorable environment for market growth.
US Biosimilar Market by drug class
The US Biosimilar market is segmented by Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-inflammatory agents and Others. Based on drug class, Monoclonal Antibodies segment dominates the bio similar market in 2022.
Monoclonal antibodies have diverse applications across various therapeutic areas. From cancer treatment to autoimmune diseases, biosimilar Mabs addressed a wide range of medical needs, leading to a broad and growing market. Biosimilars, with their potential for cost savings while maintaining comparable efficacy and safety, gained significant attention as viable alternatives.
US Biosimilar Market by application
In US Biosimilar market, they are segmented by application into Oncology, Blood disorders, Chronic diseases and autoimmune conditions and Others. On the basis of application, Oncology segment was the dominant in 2022.
The increasing prevalence of cancer and the high cost of traditional biologics used in oncology treatment have created a strong incentive for the adoption of biosimilars. Biosimilars offer the potential to provide similar therapeutic outcomes at a lower cost, making them an attractive option for both healthcare providers and patients.
Additionally, the rigorous clinical trials and regulatory processes that biosimilars undergo to gain approval provide reassurance to healthcare professionals and patients regarding their safety and efficacy. This has led to increased acceptance and adoption of biosimilars in oncology.
US Biosimilar by Region
The US Biosimilar market is segmented by Region into North, East, West and South. In 2022, the dominance region is North region in US Biosimilar market.
The North region benefits from a concentration of healthcare providers and academic institutions that are at the forefront of adopting and integrating biosimilars into their treatment protocols. These institutions are more likely to have the expertise to evaluate and incorporate biosimilars effectively, driving their adoption among healthcare professionals and patients.
Click here to Download a Custom Report
Competition Scenario in US Biosimilar Market
The US biosimilar market has witnessed an evolving competitive landscape, with several key players competing for market share. Prominent pharmaceutical companies such as Amgen, Pfizer, Sandoz (Novartis), and Boehringer Ingelheim have been actively involved in developing and marketing biosimilar products. These established players have utilized their expertise in biologics and significant resources to navigate the regulatory landscape and compete effectively.
The competition in the US biosimilar market is characterized by a balance between established pharmaceutical giants and emerging biotech companies. While the major players possess the advantage of resources and experience, smaller biotech firms are also contributing to the market with innovative approaches and niche biosimilar offerings.
What is the Expected Future Outlook for the Overall US Biosimilar Market?
The US Biosimilar market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Billion by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. The US biosimilar market is likely to experience significant growth in the coming years, driven by several factors. Biosimilars are biologic drugs that are highly similar to already approved reference biologics. They offer potential cost savings, increased competition, and improved patient access to crucial treatments.
Firstly, the regulatory environment is becoming more favorable for biosimilars. The Biologics Price Competition and Innovation Act (BPCIA) established a pathway for biosimilar approval in the US, allowing for a smoother regulatory process. As more biosimilars receive approval, competition in the market is expected to intensify.
Secondly, patents for several blockbuster biologics are expiring or have already expired. This creates opportunities for biosimilar manufacturers to enter the market with more affordable alternatives, offering healthcare systems and patients a choice in treatment options.
Thirdly, as healthcare costs continue to rise, biosimilars present an attractive solution for reducing expenses. Their potential to offer cost savings without compromising therapeutic efficacy could lead to increased adoption by healthcare providers, insurers, and patients alike.
Physician and patient education are crucial, as misconceptions about biosimilars' safety and effectiveness might hinder their adoption. Additionally, legal and market access barriers, including patent litigation and complex distribution systems, could slow down the growth of the biosimilar market.
The biosimilar market witness consolidation as larger pharmaceutical companies acquire or partner with smaller biotech firms to bolster their biosimilar portfolios. This will lead to more resources being devoted to biosimilar development and marketing. Changes in healthcare policies, such as reimbursement models and value-based care initiatives, can influence the biosimilar market's growth. Favourable policies that incentivize biosimilar adoption drives their market growth.
#US Biosimilar Market#US Biosimilar Industry#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market#US homologous biologics market#US oncology biosimilar market#US immunology biosimilar sector#US insulin biosimilar industry#US Generics Biologics market challenges#US leading Biosimilar drug providers#US leading Biosimilar drug manufacturers
0 notes
Text
#Biosimilars Market#Biosimilars Market size#Biosimilars Market share#Biosimilars Market trends#Biosimilars Market analysis
0 notes
Text
Biosimilars Market Size 2022 Industry Recent Developments and Technology, Size, Trends, Growth, and Forecast Research Report 2030| By R&I

The report is titled as ‘Biosimilars Market: Opportunity Analysis and Future Assessment 2020-2028’. An overview of conceptual frameworks, analytical approaches of the biosimilars market is the main objective of the report, which further consists the market opportunity and insights of the data involved in the making of the respective market. Biosimilars market is expected to grow with a significant rate in the near future.
The global biosimilars market in 2020 is estimated for more than US$ 11,036.2 Mn and expected to reach a value of US$ 1,09,501.8 Mn by 2028 with a significant CAGR of 33.5%.
Request a Sample Copy of this Report @: https://reportsandinsights.com/sample-request/1243
Biosimilars Market Dynamics
Patent expiration in the biological drug industry is the major factor driving the growth of global biosimilars market. Moreover, the introduction of clear regulatory pathways for the introduction of biosimilars to the market by many countries is further encouraging biosimilar development. For instance, recently India and Canada introduced regulations for approval of biosimilar drugs in respective countries. However, unlike generic chemical entities, producing identical copies of innovator cell-based drugs is impossible and takes more time, which is the biggest challenge for the growth of global biosimilars market. Biologics are produced by cells in culture or whole organisms, which are inherently more variable than chemical synthesis methods.
Biosimilars Market Regional Overview
On the basis of region, the global biosimilars market is segmented into six regions namely North America, Latin America, Asia Pacific, Europe, Middle East, and Africa. North America biosimilars market is expected to be the most dominating market throughout the forecast period due to due to the expiry of patents for blockbuster biological drugs. The presence of key players in Europe makes it second-largest market for biosimilars. Asia Pacific biosimilars market is expected to witness delayed growth due to relatively long patent term for biological drugs
MMC Overview
The non-identical approach of Reports and Insights stands with conceptual methods backed up with the data analysis. The novel market understanding approach makes up the standard of the assessment results that give a better opportunity for the customers to put their effort.
A research report on the biosimilars market by Reports and Insights is an in-depth and extensive study of the market based on the necessary data crunching and statistical analysis. It provides a brief view of the dynamics flowing through the market, which includes the factors that support market and the factors that are acting as impedance for the growth of the market. Furthermore, the report includes the various trends and opportunities in the respective market in different regions for a better understanding of readers that helps to analyze the potential of the market.
Wish to Know More About the Study? Click here to get a Report Description: https://reportsandinsights.com/report/global-biosimilars-market
Biosimilars Market Key Players
Some of the key participating players in global Biosimilars market are:
 Pfizer
 Sandoz International
 Teva Pharmaceuticals
 Amgen
 Biocon
 Dr. Reddy’s Laboratories
 Celltrion
 Samsung Biologics
 Synthon Pharmaceuticals, Inc.
 LG Life Sciences
 Biocon
 Hospira
 Merck Serono (Merck Group)
 Biogen Idec Inc.
 Genentech (Roche Group)
Biosimilars Market Segmentation
The global Biosimilars market is segmented on the basis of product type, manufacturing, diseases, and region.
By Product Type
 Recombinant Non-Glycosylated Proteins
 Recombinant Human Growth Hormone (RHGH)
 Granulocyte Colony-Stimulating Factor (Filgrastim)
 Insulin
 Interferons
 Interferon-Beta
 Interferon-Alpha
 Recombinant Glycosylated Proteins
 Erythropoietin (EPO)
 Monoclonal Antibodies (MABS)
 Infliximab
 Rituximab
 Adalimumab
 Other Monoclonal Antibodies
 Follitropin
 Recombinant Peptides
 Glucagon
 Calcitonin
By Type of Manufacturing
 In-House Manufacturing
 Contract Manufacturing
By Disease
 Oncology
 Chronic Diseases
 Autoimmune Diseases
 Blood Disorders
 Growth Hormone Deficiency
 Infectious Diseases
 Other Diseases
By Region
 North America
 Latin America
 Europe
 Asia Pacific
 Middle East
 Africa
To view Top Players, Segmentation and other Statistics of Biosimilars Industry, Get Sample Report @: https://reportsandinsights.com/sample-request/1243
About Reports and Insights:
Reports and Insights is one of the leading market research companies which offers syndicate and consulting research around the globe. At Reports and Insights, we adhere to the client needs and regularly ponder to bring out more valuable and real outcomes for our customers. We are equipped with strategically enhanced group of researchers and analysts that redefines and stabilizes the business polarity in different categorical dimensions of the market.
Contact Us:
Neil Jonathan
1820 Avenue M, Brooklyn
NY 11230, United States
+1-(718) 312-8686
Find Us on LinkedIn: www.linkedin.com/company/report-and-insights/
View Latest Market Updates At: https://marketsresearchanalytics.com
#Biosimilars Market Research#Biosimilars Market Report#Biosimilars Market Share 2021#Biosimilars Market Size 2022#Biosimilars Market Trends#Biosimilars Market Key Players#Global Biosimilars Market Analysis#Biosimilars Industry News#Biosimilars Industry Analysis#Biosimilars Market Forecast#Biosimilars Market CAGR#USA Biosimilars Market#Japan Biosimilars Market#Biosimilars Market Demand#Argentina Biosimilars Market#Australia Biosimilars Market#Belgium Biosimilars Market#Brazil Biosimilars Market#Canada Biosimilars Market#Chile Biosimilars Market#China Biosimilars Market#Columbia Biosimilars Market#Egypt Biosimilars Market#France Biosimilars Market#Germany Biosimilars Market#Global Biosimilars Market#India Biosimilars Market#Indonesia Biosimilars Market#Biosimilars Applications#Biosimilars Industry
0 notes
Text
The Evolving Intraocular Lens Market: Trends, Drivers, and Growth Opportunities
Introduction: The global intraocular lens (IOL) market is poised for significant growth, spurred by factors such as the aging population and advances in cataract surgery technologies. Estimated to grow from USD 4.2 billion in 2023 to USD 6.0 billion by 2029, this market is shaped by innovations from industry leaders like Alcon, Johnson & Johnson Vision Care, and Carl Zeiss Meditec. This blog provides a comprehensive overview of key trends, market drivers, and growth opportunities that make the IOL market a focal point for healthcare advancements.

Download PDF Brochure
1. Market Dynamics in the Intraocular Lens Market:
Rising Demand for Cataract Surgery IOLs: Cataracts affect millions globally, especially in older adults. Monofocal IOLs, which currently hold the largest share in the IOL market, are widely used for cataract surgeries due to their cost-effectiveness and performance in restoring basic vision. The demand for monofocal and premium IOLs, including multifocal and extended depth-of-focus (EDOF) lenses, continues to grow as patients seek solutions that improve near and distant vision simultaneously.
Technological Advances in IOL Design: Innovations in hydrophobic and hydrophilic IOLs, as well as extended-wear options, enhance patient outcomes. Hydrophobic acrylic IOLs, in particular, dominate due to their durability, water resistance, and reduced risk of post-surgical complications. These features make them a preferred choice, contributing to the lens’s popularity in high-volume procedures such as cataract surgery.
2. Market Drivers Fueling Growth:
Aging Population as a Catalyst: The growing elderly demographic is a significant driver in the eye care market. Aging increases the prevalence of cataracts and presbyopia, leading to a higher demand for both cataract surgery IOLs and presbyopia-correcting lenses. With a notable proportion of the population aged over 65 in North America and Europe, the demand for advanced IOL technologies is expected to surge.
Increase in Cataract Procedures: As a leading cause of vision impairment, cataracts account for a large volume of global surgeries. In regions with high healthcare access, such as North America, the availability of insurance coverage further supports the uptake of IOLs. With the growing adoption of minimally invasive cataract procedures worldwide, particularly in emerging economies, the market for cataract lens implants is expanding.
3. Opportunities in Emerging Markets:
Expansion into Emerging Economies: Markets in Asia-Pacific, Latin America, and the Middle East & Africa offer high-growth potential for IOLs. Rising disposable incomes and greater healthcare awareness are fueling demand for vision correction and cataract surgeries. Companies are increasingly investing in these regions, seeking to tap into new consumer bases with unmet needs in eye care.
Innovations in Lens Implants: Technological advancements present opportunities for biosimilar IOL development, AI-driven diagnostics, and teleophthalmology, which allow remote diagnosis and monitoring. These innovations are especially beneficial in emerging markets, where access to ophthalmologists and specialized eye care centers remains limited.
4. Key IOL Segments:
Monofocal IOLs: Cost-effective and widely used, monofocal IOLs capture a major portion of the IOL market due to their reliability in cataract surgeries. They are particularly popular in high-demand markets with aging populations and are likely to remain dominant given their proven effectiveness in vision restoration.
Premium IOLs: For patients desiring improved quality of life post-surgery, premium IOLs—including multifocal, EDOF, and accommodating lenses—offer enhanced capabilities, addressing both near and far vision needs. Although premium IOLs require out-of-pocket expenses, demand is rising, especially among patients in developed regions who can afford customized solutions.
Phakic IOLs: Phakic IOLs, implanted without removing the eye’s natural lens, cater to younger patients with high myopia or hyperopia. While they have a smaller market share, these lenses are gaining attention due to their non-invasive correction of refractive errors, offering a valuable alternative for patients seeking vision correction without laser surgery.
5. Regional Market Highlights:
North America and Europe: Dominant regions in the intraocular lens market, North America and Europe benefit from robust healthcare infrastructure, increased awareness of eye health, and greater disposable incomes. Insurance coverage and government-supported healthcare initiatives further support IOL uptake. In these regions, the adoption of premium IOLs is notably high, with patients willing to invest in improved visual outcomes.
Asia-Pacific and Latin America: Rapid economic growth, rising disposable incomes, and improved healthcare access in countries like China, India, and Brazil are transforming these regions into lucrative markets. As awareness of advanced IOL technologies increases, companies are likely to experience significant demand growth for both monofocal and premium IOLs.
6. Challenges Facing the Intraocular Lens Industry:
High Cost of Premium Lenses: While standard IOLs are generally covered by insurance, premium lenses often require out-of-pocket payments, limiting their accessibility. The price sensitivity of patients, especially in low- and middle-income regions, presents a barrier to widespread adoption of advanced IOL options.
Shortage of Skilled Ophthalmologists: A growing demand for cataract surgeries faces a counterbalance in the form of a shortage of ophthalmologists. This is particularly challenging in developing countries, where fewer eye care professionals are available to meet the rising patient needs. Addressing this gap requires increased funding in medical training and support for telemedicine to expand the reach of eye care.
7. Future Trends and Opportunities in the IOL Market:
AI and Digital Health Integration: Artificial intelligence is becoming integral to the development and customization of IOLs, with AI algorithms helping identify optimal lenses based on patient-specific factors. Additionally, AI-powered platforms can enhance surgical precision and support virtual consultations, enabling remote areas to access specialized eye care.
Personalized Vision Correction Solutions: The trend toward personalized medicine is also influencing the IOL market. Advances in IOL technology enable customization of lenses to cater to specific patient profiles, such as correcting astigmatism or enhancing low-light vision. This approach will likely drive demand for premium IOLs in developed regions, where patients seek optimal visual quality.
Conclusion:
The intraocular lens market is on a promising growth trajectory, driven by an aging global population, increased cataract surgeries, and significant advancements in IOL technologies. Emerging markets and AI integration represent exciting avenues for growth, as companies focus on innovative solutions to meet diverse patient needs. For stakeholders in the intraocular lens market, keeping pace with these trends is essential to capture market opportunities and contribute to a future where vision care is accessible, effective, and tailored to each patient.
0 notes
Text
The Growth of Contract Manufacturing Companies in India: Trends and Future Outlook
India has become a major player in the global pharmaceutical industry, with contract manufacturing companies playing a key role in this growth. Contract manufacturers, or third-party manufacturing pharma companies in India, produce a wide range of medicines for companies looking to outsource production. By partnering with India’s experienced manufacturing sector, companies can meet global demand and lower costs without sacrificing quality. This blog will explore the factors driving this sector’s growth, key trends, and the future outlook for contract manufacturing companies in India.

What Are Contract Manufacturing Companies in India?
Contract manufacturing companies partner with pharmaceutical firms to produce their products, typically at a lower cost and with higher efficiency. This partnership model is especially beneficial for global pharma companies, which can focus on distribution and R&D while leaving production to specialized firms in India. Many of these companies, which serve as the Top Pharma Export Companies in India, are highly certified and able to manufacture drugs for international markets under strict regulatory guidelines.
Trends Driving the Growth of Contract Manufacturing in India
Several factors and trends have contributed to the growth of contract manufacturing in India:
1. High Global Demand for Generics and APIs
India’s contract manufacturers are highly skilled at producing generic drugs and Active Pharmaceutical Ingredients (APIs) cost-effectively. With the global demand for generics growing—especially in emerging markets—India’s contract manufacturers play a vital role in meeting this demand, while top pharma export companies in India continue to expand their reach.
2. Diverse Service Offerings
Many third-party manufacturing pharma companies in India now offer a broad range of services beyond production, including research, packaging, and distribution. This versatility allows international pharma companies to find complete solutions under one roof, reducing logistics and operational costs.
3. Cost Efficiency and Government Support
India’s relatively low costs of labor, raw materials, and infrastructure make it a preferred destination for contract manufacturing. In addition, government policies offer support for contract manufacturing through simplified regulations, investment incentives, and tax breaks. This supportive environment encourages both domestic and international companies to expand production through Indian contract manufacturers.
4. Focus on Specialized Drug Manufacturing
The demand for complex drugs, including biosimilars and high-potency formulations, is rising globally. Indian contract manufacturers are investing in the technology and expertise needed to produce these specialized drugs, making them valuable partners for companies in regulated markets like the US and Europe.
Benefits of Contract Manufacturing in India
The growth of contract manufacturing companies in India brings various benefits to the global pharmaceutical sector, including:
Quality Compliance: Many Indian contract manufacturers meet global standards with certifications from bodies like the US FDA and EU GMP, allowing them to supply to strict international markets.
High Capacity Production: Indian manufacturers can handle large-scale production, providing a reliable supply chain for companies in need of bulk medicines.
Innovation in Drug Development: With increased investment in research, contract manufacturers in India are innovating in areas like generic formulations and biosimilars.
Future Outlook for Contract Manufacturing in India
The future of contract manufacturing companies in India looks bright, with several factors expected to drive continued growth:

1. Increased Global Collaborations
With rising healthcare costs worldwide, global pharmaceutical companies are likely to continue forming partnerships with Indian manufacturers for cost-effective production. These collaborations will help India’s contract manufacturing sector reach new markets and strengthen its global presence.
2. Investment in Biologics and Biosimilars
Indian contract manufacturers are increasingly focusing on biologics and biosimilars, which have high demand in international markets. By developing specialized capabilities in these areas, India’s manufacturers can provide advanced solutions for pharmaceutical companies seeking high-quality biologic drugs.
3. Adoption of New Technologies
Many contract manufacturers are embracing automation, AI, and data analytics to improve efficiency and reduce errors in production. By integrating these technologies, companies can enhance the speed and accuracy of their processes, making Indian contract manufacturers even more competitive on the global stage.
4. Environmental Responsibility
With sustainability becoming a global priority, India’s contract manufacturers are beginning to adopt green practices, such as waste reduction and energy-efficient processes. This commitment to environmental responsibility aligns with international standards, attracting companies that prioritize sustainable practices.
Challenges in Contract Manufacturing
Despite a promising future, contract manufacturers in India face certain challenges:
Regulatory Compliance: Keeping up with the complex regulations of global markets requires constant investment in quality and compliance.
Intellectual Property (IP) Concerns: Handling proprietary formulations and drug patents can be complex in third-party manufacturing relationships, as companies must take extra care to protect sensitive IP.
Intense Competition: With many players in the contract manufacturing market, companies face pressure to offer high quality at competitive prices.
Conclusion
India’s contract manufacturing sector is essential in meeting the world’s demand for affordable and high-quality medicine. Supported by favorable policies, cost advantages, and high production capacity, contract manufacturing companies in India are set to maintain their role as reliable partners for the global pharmaceutical industry. As these companies continue to grow, investing in innovation, sustainability, and specialized drug development, they will further solidify India’s position as a global leader. This outlook also holds promising opportunities for third-party manufacturing pharma companies in India to reach new markets and expand their impact on global healthcare, backed by support from Top Pharma Export Companies in India that contribute to India’s success as a leading exporter of pharmaceuticals.
#contract manufacturing companies in India#third party manufacturing pharma companies in India#Top Pharma Export Companies in India
0 notes
Text
Clonazepam Market : Technology Advancements, Industry Insights, Trends And Forecast 2033
The clonazepam global market report 2024 from The Business Research Company provides comprehensive market statistics, including global market size, regional shares, competitor market share, detailed segments, trends, and opportunities. This report offers an in-depth analysis of current and future industry scenarios, delivering a complete perspective for thriving in the industrial automation software market.
Clonazepam Market, 2024 report by The Business Research Company offers comprehensive insights into the current state of the market and highlights future growth opportunities.

Market Size - The clonazepam market size has grown strongly in recent years. It will grow from $1.37 billion in 2023 to $1.48 billion in 2024 at a compound annual growth rate (CAGR) of 7.9%. The growth in the historic period can be attributed to increasing prevalence of anxiety disorders, rising cases of panic disorders, growing awareness about mental health, increased prescription rates, rising acceptance of benzodiazepines.
The clonazepam market size is expected to see strong growth in the next few years. It will grow to $2.01 billion in 2028 at a compound annual growth rate (CAGR) of 8%. The growth in the forecast period can be attributed to growing prevalence of epilepsy, rising geriatric population, increasing adoption of telemedicine, rising prevalence of insomnia, growing awareness of mental health disorders. Major trends in the forecast period include expansion of digital health solutions, development of novel formulations, shift toward sustainable and ethical sourcing, advancement in biosimilar and biogeneric production.
Order your report now for swift delivery @ https://www.thebusinessresearchcompany.com/report/clonazepam-global-market-report
The Business Research Company's reports encompass a wide range of information, including:
1. Market Size (Historic and Forecast): Analysis of the market's historical performance and projections for future growth.
2. Drivers: Examination of the key factors propelling market growth.
3. Trends: Identification of emerging trends and patterns shaping the market landscape.
4. Key Segments: Breakdown of the market into its primary segments and their respective performance.
5. Focus Regions and Geographies: Insight into the most critical regions and geographical areas influencing the market.
6. Macro Economic Factors: Assessment of broader economic elements impacting the market.
Market Drivers - The increasing prevalence of anxiety and panic disorders is expected to propel the growth of the clonazepam market going forward. Anxiety and panic disorders are mental health conditions characterized by persistent and excessive worry or fear that can lead to physical symptoms and disrupt daily functioning. The increasing prevalence of anxiety and panic disorders is largely due to rising stress levels, societal pressures, and greater awareness and diagnosis. Clonazepam is used in the treatment of anxiety and panic disorders to help alleviate symptoms by calming the nervous system and reducing excessive neural activity. For instance, in May 2024, according to the American Psychiatric Association, a US-based non-profit organization, in 2024, 43% of adults report feeling more anxious compared to the previous year, a rise from 37% in 2023 and 32% in 2022. Therefore, the increasing prevalence of anxiety and panic disorders will drive the growth of the clonazepam market.
Market Trends - Major companies operating in the clonazepam market are focusing on strategic product expansion approaches, such as active pharmaceutical ingredients (API) facilities, to diversify their offerings, improve market presence, and cater to a broader range of therapeutic needs while meeting growing global demand for high-quality clonazepam formulations. An active pharmaceutical ingredients (API) facility for clonazepam is responsible for producing the raw material used in the formulation of clonazepam-based medications, ensuring compliance with regulatory standards such as GMP for quality and safety. For instance, in July 2024, Rusan Pharma Private Limited, an India-based pharmaceutical company, received Good Manufacturing Practice (GMP) approval from the United States Food and Drug Administration (USFDA) for its Active Pharmaceutical Ingredient (API) facility. This milestone paves the way for the company to enter the US API market, bolstered by an active US Drug Master File (DMF) for specialized APIs such as apomorphine, buprenorphine, naloxone, clonazepam, diazepam, and others.
The clonazepam market covered in this report is segmented –
1) By Type: Tablet, Injection 2) By Application: Adult, Child 3) By End User: Hospitals, Homecare Settings, Specialty Clinics
Get an inside scoop of the clonazepam market, Request now for Sample Report @ https://www.thebusinessresearchcompany.com/sample.aspx?id=19004&type=smp
Regional Insights - North America was the largest region in the clonazepam market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the clonazepam market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Key Companies - Major companies operating in the clonazepam market are Novartis AG, Abbott Healthcare Pvt Ltd., Boehringer Ingelheim, Teva Pharmaceutical Industries Ltd., Aurobindo Pharma Ltd., Dr Reddy’s Laboratories Ltd., Intas Pharmaceuticals, Cipla Ltd., Lupin Limited, Orion Corporation, CHEPLAPHARM Arzneimittel GmbH, Alkem Laboratories Ltd., Torrent Pharmaceuticals, Alembic Pharmaceuticals Ltd., Neuraxpharm, Pharmascience Inc., Medopharm Ltd., Global Calcium, Sun Pharmaceutical Industries Ltd., Prinston Pharmaceutical Inc., MITS Healthcare Private Limited, Emenox Healthcare, DR BEST Pharmaceuticals, Intra Life Pvt Ltd., Neuracle Lifesciences
Table of Contents 1. Executive Summary 2. Clonazepam Market Report Structure 3. Clonazepam Market Trends And Strategies 4. Clonazepam Market – Macro Economic Scenario 5. Clonazepam Market Size And Growth ….. 27. Clonazepam Market Competitor Landscape And Company Profiles 28. Key Mergers And Acquisitions 29. Future Outlook and Potential Analysis 30. Appendix
Contact Us: The Business Research Company Europe: +44 207 1930 708 Asia: +91 88972 63534 Americas: +1 315 623 0293 Email: [email protected]
Follow Us On: LinkedIn: https://in.linkedin.com/company/the-business-research-company Twitter: https://twitter.com/tbrc_info Facebook: https://www.facebook.com/TheBusinessResearchCompany YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ Blog: https://blog.tbrc.info/ Healthcare Blog: https://healthcareresearchreports.com/ Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
Immunology Market 2024 Future Scope Analysis with Size, Trend, Opportunities, Revenue, Future Scope

The Immunology Market is witnessing unprecedented growth, fueled by the rising prevalence of chronic diseases, innovative advancements in therapeutic approaches, and increased investment in research and development. According to a recent study by SNS Insider, titled Immunology Market Revenue, the global immunology market is projected to experience robust expansion over the next several years. This growth is largely attributed to the increasing focus on personalized medicine, innovative immunotherapies, and a global rise in autoimmune and infectious diseases.
As healthcare systems worldwide continue to prioritize preventive and therapeutic care, the immunology sector is evolving with groundbreaking solutions designed to enhance treatment efficacy and patient outcomes. Key market drivers include advancements in biologics and biosimilars, the integration of artificial intelligence in immunology research, and the expansion of healthcare infrastructure in emerging economies. These trends indicate a significant shift toward a proactive, preventive approach to healthcare, which further elevates the importance of immunology in addressing complex medical conditions.
The report by SNS Insider provides a comprehensive analysis of the immunology market, covering detailed market segmentation, regional insights, and key trends that are shaping the industry. It offers in-depth data and projections on market size, revenue growth, and product demand, which provide valuable insights for stakeholders, investors, and healthcare professionals aiming to make informed decisions within this dynamic sector.
Get Free Sample Report
Key Insights and Trends in the Immunology Market
Rising Demand for Advanced Therapeutics: Immunotherapy, including treatments such as monoclonal antibodies, cytokines, and cell-based therapies, has become a central focus in immunology due to its ability to target specific immune responses. With a surge in chronic disease cases worldwide, these advanced therapies are now essential in treatment plans, improving both the efficiency and specificity of healthcare solutions. Furthermore, the need for personalized medicine is pushing the boundaries of immunology research, leading to more tailored and patient-specific therapies.
Growth in Biologics and Biosimilars: As the demand for effective, long-term treatments for chronic and autoimmune diseases grows, biologics and biosimilars are becoming pivotal in immunology. Biologics, with their precision-targeted approach, have become increasingly popular for treating conditions like rheumatoid arthritis, lupus, and multiple sclerosis. Additionally, the emergence of biosimilars is enabling cost-effective options for healthcare providers and patients, facilitating access to life-saving treatments in regions where affordability and accessibility are primary concerns.
Technological Advancements in Research and Development: Artificial intelligence, machine learning, and big data analytics are revolutionizing immunology research. These technologies allow researchers to process complex biological data, uncover insights into immune responses, and accelerate drug discovery and development processes. This technological evolution is enabling more efficient and precise development of immunotherapies, which could result in faster time-to-market for new drugs and, ultimately, improved patient outcomes.
Regional Insights and Future Projections
The Immunology Market report also highlights significant growth opportunities across various regions, with North America and Europe currently dominating the market due to established healthcare infrastructure, strong research ecosystems, and high levels of investment in advanced therapies. However, Asia-Pacific is projected to witness remarkable growth in the coming years. The expansion of healthcare infrastructure, coupled with rising awareness of autoimmune and chronic diseases, is expected to fuel market demand across emerging economies in the region.
Additionally, government initiatives to strengthen healthcare systems and the increased prevalence of lifestyle-related diseases are driving growth in the Asia-Pacific immunology market. With significant R&D investments and growing collaboration among leading pharmaceutical and biotech companies, the immunology sector is poised for substantial advancements, paving the way for innovative therapeutic options that will positively impact global healthcare outcomes.
Competitive Landscape and Key Players
In the competitive landscape, major players in the immunology market are focusing on strategic partnerships, mergers, and acquisitions to expand their portfolios and geographical reach. Companies are also investing heavily in R&D to introduce novel therapies and improve existing products. With a strong emphasis on quality, efficacy, and patient safety, these companies are driving market growth and enhancing their market position.
The report identifies several key players, each of which contributes to shaping the immunology landscape with innovative products and solutions. Their efforts underscore the importance of immunology as a cornerstone of modern medicine, addressing critical health challenges and providing hope for millions of patients worldwide.
About Us
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Contact Us: Akash Anand – Head of Business Development & Strategy [email protected] Phone: +1-415-230-0044 (US) | +91-7798602273 (IND)
#Immunology Market#Immunology Market Size#Immunology Market Share#Immunology Market Growth#Market Research
0 notes
Text
Biosimilar Market Size, Share, Key Growth Drivers, Trends, Challenges and Competitive Landscape
Data Bridge Market research has recently issued comprehensive industry research on Global Biosimilar Market which includes growth analysis, regional marketing, challenges, opportunities, and drivers analysed in the report.
Besides, Biosimilar market report studies market growth opportunities and restraining factors. The geographical division of this market analysis report offers data that gives an idea of the revenue of the companies and sales figures of the market growth. The market report also contains the drivers and restraints for the Biosimilar market that are obtained with the help of SWOT analysis, and also shows all the recent developments, product launches, joint ventures, mergers and acquisitions by the several key players and brands with their systemic company profiles, that are driving the market.
Data Bridge Market Research analyses that the global biosimilar market which was USD 37,270.20 million in 2022, is expected to reach USD 343,520.90 million by 2030, and is expected to undergo a CAGR of 32.00% during the forecast period 2023-2030. “Oncology” dominates the product segment of the global biosimilar market owing to the owing to the increase in number of research studies, increasing research and development activities by key players focused on oncology and advancements in healthcare technologies. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Access Full 350 Pages PDF Report @
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Key Questions Answered with this Study
1) What makes Biosimilar Market feasible for long term investment?
2) Know value chain areas where players can create value?
3) Teritorry that may see steep rise in CAGR & Y-O-Y growth?
4) What geographic region would have better demand for product/services?
5) What opportunity emerging territory would offer to established and new entrants in Biosimilar Market?
6) Risk side analysis connected with service providers?
7) How influencing factors driving the demand of Biosimilarin next few years?
8) What is the impact analysis of various factors in the Global Biosimilar Market growth?
9) What strategies of big players help them acquire share in mature market?
10) How Technology and Customer-Centric Innovation is bringing big Change in Biosimilar Market?
Some of the major players operating in the global biosimilar market are:
Novartis AG (Switzerland)
Orion Pharma AB (Sweden)
Pfizer Inc. (U.S.)
Samsung Bioepis. (South Korea)
Coherus BioSciences, Inc. (U.S.)
Amgen Inc. (U.S.)
Eli Lilly and Company (U.S.)
Takeda Pharmaceutical Company Limited. (Japan)
Bristol-Myers Squibb Company (U.S.)
Merck KGaA (Germany)
Teva Pharmaceutical Industries Ltd. (U.S.)
Biocon. (India)
Bayer AG (Germany)
AbbVie Inc. (U.S.)
Allergan (Ireland)
Dr. Reddy’s Laboratories Ltd. (India)
Boehringer Ingelheim International GmbH. (Germany)
Biogen (U.S.)
Browse Trending Reports:
Vitamins Market
Triage B Type Natriuretic Peptide Bnp Testing Market
Healthcare Finance Solutions Market
Biosimilar Market
Surgical Instrument Tracking Systems Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: [email protected]
#Biosimilar Market Size#Share#Key Growth Drivers#Trends#Challenges and Competitive Landscape#market size#market report#market trends#market share#marketresearch#market research#markettrends#market analysis
0 notes
Text
Arthritis Therapeutics Market: Future Trends Shaping Patient Care

The future of the arthritis therapeutics market is poised for remarkable transformation, driven by technological advancements and a deeper understanding of the disease's complexities. As research progresses, the market is likely to witness a shift towards more personalized and targeted treatment approaches that cater to the unique needs of individuals suffering from arthritis.
One of the most promising trends is the development of biologic therapies, which have revolutionized the treatment landscape. These therapies target specific pathways involved in the inflammatory process, offering more effective relief for patients. Ongoing research into new biologics and biosimilars is expected to enhance treatment options, making them more accessible and affordable.
Additionally, the rise of precision medicine is set to play a crucial role in the market's evolution. By utilizing genetic and molecular profiling, healthcare providers can tailor treatments to the specific characteristics of each patient’s condition. This personalized approach not only improves patient outcomes but also fosters a deeper connection between patients and healthcare providers.
The integration of digital health technologies, such as telemedicine and mobile health applications, is likely to enhance patient management and engagement. These tools can facilitate better monitoring of symptoms, adherence to treatment plans, and communication with healthcare professionals. As patients become more engaged in their care, the demand for innovative therapies that align with their preferences will increase.
The aging population also presents significant opportunities for growth within the arthritis therapeutics market. As the number of elderly individuals rises, so too does the incidence of arthritis, driving the need for effective treatment solutions. Companies that can swiftly adapt to the changing demographics and address the specific needs of older patients will find themselves well-positioned for success.
In summary, the future of the arthritis therapeutics market is bright, characterized by innovation and patient-centric approaches. With continued research and development, the industry is set to deliver more effective and personalized therapies, ultimately improving the quality of life for those affected by arthritis.
For a sample report click on:
0 notes
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
1.Pfizer
Click to read more about Pfizer
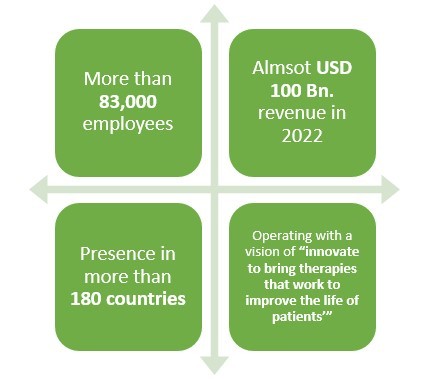
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
2.Amgen
Click here to Download a Sample Report
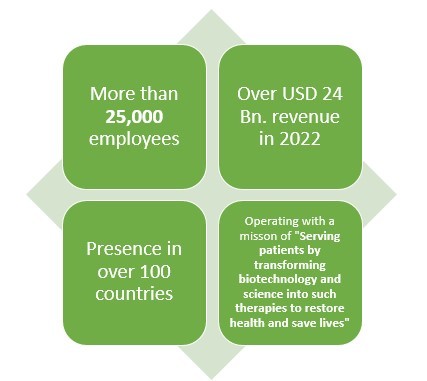
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
4.Coherus Biosciences:
Click here to Ask for a Custom Report
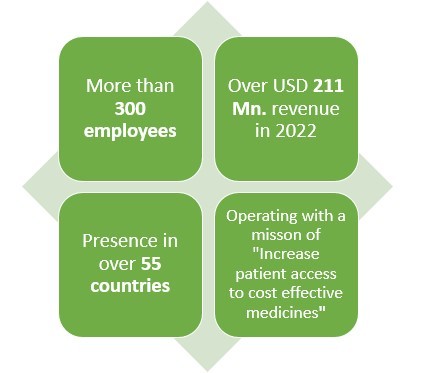
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
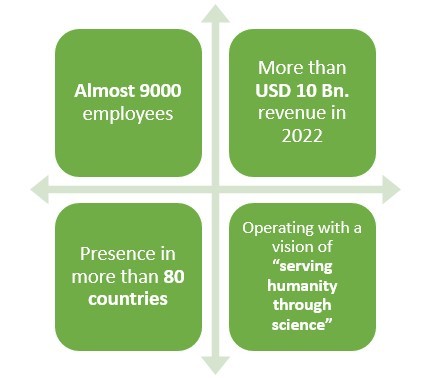
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Link
0 notes
Text
The Active Pharmaceutical Ingredients (API) Industry: Driving Growth and Innovation in Pharmaceuticals
The Active Pharmaceutical Ingredients (API) Market Size is projected to be valued at USD 216.5 billion in 2024, with expectations to grow to USD 306.90 billion by 2029, reflecting a CAGR of 7.22% over the forecast period (2024-2029).

Market Overview and Growth Factors
The global API market is experiencing robust expansion, driven by the rising prevalence of chronic and lifestyle-related diseases, advancements in biopharmaceuticals, and growing generic drug production. The demand for high-quality, affordable drugs is fueling the need for APIs, especially as healthcare accessibility improves worldwide. Additionally, the surge in demand for innovative drugs and targeted therapies, particularly for diseases like cancer, diabetes, and cardiovascular disorders, is propelling the API sector forward.
Key Trends in the API Market
Growing Demand for Specialty APIs Specialty APIs, such as high-potency active pharmaceutical ingredients (HPAPIs) and biologics, are seeing heightened demand due to their effectiveness in treating complex diseases. HPAPIs, particularly, are gaining traction in oncology treatments due to their targeted approach, which minimizes adverse effects and improves patient outcomes.
Focus on Quality and Compliance Regulatory standards for API manufacturing are becoming increasingly stringent, with a focus on Good Manufacturing Practices (GMP). This emphasis on compliance ensures product quality and safety, essential for patient health. Global regulatory bodies, such as the FDA and EMA, are imposing rigorous oversight, and compliance is now a competitive differentiator in the API industry.
Rise in Biotech and Biopharmaceuticals Biotechnology-based APIs are gaining ground in treating previously unmanageable conditions. Biologics, biosimilars, and vaccines are increasingly contributing to API demand. As biopharmaceutical R&D investments increase, the demand for biologic APIs is expected to climb, fueling industry growth.
API Manufacturing Outsourcing Many pharmaceutical companies are outsourcing API production to focus on core competencies. This trend reduces operational costs and taps into specialized expertise in regions like Asia-Pacific, where API manufacturing is more cost-effective and has well-established supply chains.
Innovation in Drug Manufacturing Processes Advanced technologies such as continuous manufacturing, green chemistry, and AI-driven optimization are reshaping the API landscape. These innovations enhance production efficiency, reduce costs, and minimize environmental impact, making API manufacturing more sustainable.
Conclusion
The Active Pharmaceutical Ingredients (API) industry is set for sustained growth, supported by rising healthcare needs, regulatory advancements, and innovations in drug manufacturing. As APIs remain integral to drug formulation and therapeutic efficacy, this market is crucial to advancements in global healthcare. Strategic outsourcing, enhanced quality standards, and technological progress in API production are likely to remain key drivers in the sector's development. In a dynamic pharmaceutical landscape, the API industry is well-positioned to support the demand for high-quality, effective medications that enhance patient outcomes worldwide.
For a detailed overview and more insights, you can refer to the full market research report by Mordor Intelligence https://www.mordorintelligence.com/industry-reports/global-active-pharmaceutical-ingredients-api-market
#Active Pharmaceutical Ingredients (API) Market#Active Pharmaceutical Ingredients (API) Market Size#Active Pharmaceutical Ingredients (API) Market Share#Active Pharmaceutical Ingredients (API) Market Trends#Active Pharmaceutical Ingredients (API) Market Analysis#Active Pharmaceutical Ingredients (API) Market Forecast
0 notes
Text
The Pegfilgrastim Biosimilars Market is transforming with trends in biologics development and manufacturing efficiency
The Pegfilgrastim Biosimilars Market is transforming with trends in biologics development and manufacturing efficiency The pegfilgrastim biosimilars market consists of biosimilar drugs developed to be equivalent to Neulasta, a long-acting form of the granulocyte colony-stimulating factor filgrastim. Pegfilgrastim biosimilars help patients undergoing chemotherapy by stimulating the bone marrow to produce more white blood cells, reducing the risk of infections. The drugs provide an affordable alternative to originator brand Neulasta due to their similar safety and efficacy profiles but lower costs. The Global Pegfilgrastim Biosimilars Market is estimated to be valued at US$ 703 million in 2024 and is expected to exhibit a CAGR of 8.9% over the forecast period 2024 to 2031. Get More Insights On Pegfilgrastim Biosimilars Market https://www.insightprobing.com/blog/pegfilgrastim-biosimilars-market/
0 notes