#zwitterion
Explore tagged Tumblr posts
Text
At this point, the concentration of the zwitterion equals that of the positively charged ion, and the pH of 2.35 equals the pKa value of the carboxyl group (pKa1):
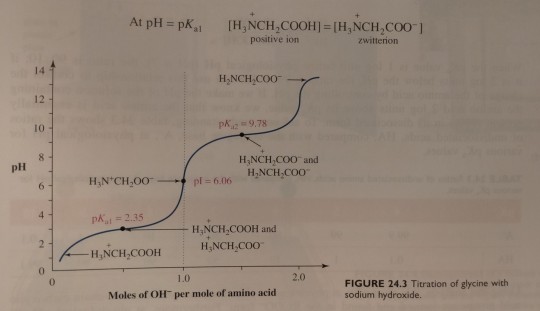
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#concentration#zwitterion#ions#carboxyl#titration#glycine#sodium hydroxide#chemical reactions
0 notes
Text
The general structural formula of an α-amino acid is shown in figure 24.1.

Although figure 24.1 seems to be a typical way of writing structural formulae for amino acids, it is not accurate because it shows an acid (-COOH) and a base (-NH2) within the same molecule. These acidic and basic groups react with each other to form an internal salt (a dipolar ion) (figure 24.1b), which is given the special name zwitterion.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#alpha#amino acid#structural formula#inaccurate#acid#base#salt#dipolar#ion#zwitterion
0 notes
Photo

1 note
·
View note
Text
Solar and wind are quickly transforming the energy landscape -- but if we are to realize the full potential of these intermittent, renewable energy sources, we'll need safe, affordable batteries capable of storing it. As part of an effort to overcome the long-term energy-storage challenge, University of Wisconsin-Madison engineers have invented a water-soluble chemical additive that improves the performance of a type of electrochemical storage called a bromide aqueous flow battery. "Bromide-based aqueous flow batteries are a promising solution, but there are many messy electrochemical problems with them. That's why there's no real successful bromide-based products today," says Patrick Sullivan who graduated from UW-Madison with a PhD in chemistry in 2023. "Yet, our one additive can solve so many different problems."
Read more.
#Materials Science#Science#Energy#Energy storage#Batteries#Electrochemistry#Flow batteries#Bromine#Zwitterions#University of Wisconsin
32 notes
·
View notes
Text
zwitterion is definitely one of the best words in the universe
#it’s just fun to say and very useful#and not traditional greek/latin chembabble either#your brain and blood are full of zwitterions!
3 notes
·
View notes
Text

TSRNOSS, p 709.
#clouds#polarization of light#bacterial infection#pneumonia#rotational energy of a molecule#release of potassium ions by neurones#hyperpolarization of a neurone#cryptobiosis#size of dinosaurs#ellipticity of Earth's orbit#zwitterions#alkaline soil#blood pH in infection#methionine#protein synthesis
0 notes
Note
I have a bio exam in about six hours (in the afternoon, this is an appropriate hour for me to be awake) and I don't wanna study so I'm gonna recite everything I know about proteins to you.
Proteins can have CHONS, Carbon Hydrogen Oxygen Nitrogen Sulfur. Made up of amino acids. Amino acids have NH2-R-C-H-COOH. NH2 is the amino group and COOH is the uhh carboxyl group? Amino acids exist as zwitterions, with the NH2 becoming NH3- and COOH becoming COO-. Because electronegativity and stuff. There are 20 amino acids (in my syllabus).
Proteins have primary structure (polypeptide chain, the specific sequence of amino acids) listed from N terminus to C terminus. Maintained by peptide bonds. Which is a condensation reaction. The COOH and the NH2 form a bond that looks like CONH. Then secondary structure, which is alpha helixes and beta sheets. Both are maintained by hydrogen bonds. Beta sheets can be parallel or antiparallel, though most of the ones in your blog or antiparallel (but also very short). Alpha helixes are more common and recognisable.
Next is tertiary structure. Four types of bonds in this one: Hydrogen bonds, ionic bonds, disulfide bonds, and hydrophobic interactions. In increasing order of strength, hydrophobic<hydrogen<ionic<disulfide. Disulfide bonds are covalent bonds and very heat stable. Formed by disulfide bridges. Very strong. Sulfur is found on Cysteine so the more cysteine is in a molecule the more heat stable it is likely to be. The single letter code for Cysteine is C. If I say CCCCCCCCCCCC, will disulfide bridges form in my protein? Hm. Ionic bonds is when uhh. Charged particles. Shit I forgot I need to revise this part. Hydrogen bonds are easier, I can identify them by identifying differences in electronegativity- in chem I remember these as FON and H, but in amino acids there's only O and N, no Fluorine. Very common, in most secondary structures. In polar particles or R groups. Ionic bonds are for charged particles, hydrogen bonds are for polar particles. Lastly in hydrophobic interactions, not to be confused with homophobic interactions. Which is when hydrophobic side chains- most commonly stuff with lots of carbon, or sometimes sulfur- gather in the middle of the protein, away from the aqueous exterior.
Next is Quaternary structure, which is when there are MULTIPLE polypeptide chains. Only when multiple polypeptide chains, if not it's tertiary. Not all proteins have these. Bonds are mostly the same as tertiary, I think?
CCCCCCCCCCCCCCCC.
There are globular, fibrous, and membrane proteins. But mostly I have to focus on the globular and fibrous part. My case study for globular proteins is haemoglobin. Haemoglobin has four polypeptide chains in it's quaternary structure, two alpha two beta. It is unclear if the names of the chains have anything to do with whether there are alpha helixes or beta sheets in them. It also has a prosthetic group, a haem group in this case, of iron- I think an Fe2+ molecule? Yeah. Which oxygen binds to. And once oxygen is bound to it it causes a change in confirmation in all four parts allowing it to bind to oxygen more easily. I need to know about sickle cell disease, which is when one of the amino acids- glutamic acid I think- is replaced with a hydrophobic one. Serine? Either way when oxygen is bound to it it's fine but when there's no oxygen there's a hydrophobic patch sticking out, which it doesn't like so the haemoglobin will change confirmation to hide, causing the haemoglobin to clump together in a way that causes the red blood cell to appear "sickle shaped". Also I was wrong it's not serine it's valine.
For fibrous, I have collagen. Which is mostly a repeating sequence of three amino acids- shit I forgot which ones I need to get my notes- glycine-X-Y. Glycine has a very small R group which allows it to fit in the small tight part in the alpha helix. X-Y is usually proline and hydroxyproline. Alpha helix, hydrogen bonds formed. Proline and hydroxyproline have relatively inflexible and bulky R groups which gives the structure rigidity. That's the secondary structure. But collagen is unique in that it has a secondary and quaternary structure, because in tropocollagen it's like three of these alpha helixes wound together. Then each tropocollagen cross links to neighbouring tropocollagen molecules. This is covalent bonds involving lysine residues. Lysine??? Where tf did Lysine come from??? I don't see it anywhere else on the page. This is worrying. Uhh this increases tensile strength and the staggered or overlapping arrangement of tropocollagen minimises points of weakness along the length of the fibrils. It's like, collagenchain-tropocollagen-fibrils-fibres. The staggering gives the bundle of fibrils a banded appearance. There was... A part where they talk about how the cell produces it on the outside... No, wait, that's cellulose. Carbohydrate. Completely different.
Then we move on to enzymes which is a whole different chapter and this is already a very long ask which I imagine will take very long to load. Thank you for letting me ramble in your inbox. CCCCCCCCC. I'm going to ramble about DNA&genomics to hellsitegenetics in a bit but first I need to finish looking through mitosis and meiosis. Ugh. I hope this protein has a high success rate and that my exam has an even higher success rate. Thank you for your time.
this sounds great and i bet you did awesome on your exam! i find explaining things to someone else helpful when studying, so i hope that doing this helped you as well. without even looking at it i can guarantee that your exam was much more successful than whatever proteins i'll get out of this.
i actually had to split this into two proteins and run them completely separately because it was simply too long. i am both excited and absolutely terrified to see what we get from all those cysteines
letter sequence in this ask matching protein-coding amino acids:
protein 1:
IhaveaieaminatsihrsintheafternnthisisanapprpriatehrfrmeteawakeandIdntwannastdysImgnnareciteeverythingIknwatprteinstyPrteinscanhaveCHNSCarnHydrgenygenNitrgenSlfrMadepfaminacidsAminacidshaveNHRCHCHNHistheamingrpandCHisthehhcarylgrpAminacidseistaswitterinswiththeNHecmingNHandCHecmingCecaseelectrnegativityandstffThereareaminacidsinmysyllasPrteinshaveprimarystrctreplypeptidechainthespecificseqencefaminacidslistedfrmNterminstCterminsMaintainedypeptidendsWhichisacndensatinreactinTheCHandtheNHfrmandthatlkslikeCNHThensecndarystrctrewhichisalphaheliesandetasheetstharemaintainedyhydrgenndsetasheetscaneparallelrantiparallelthghmstfthenesinyrlgrantiparalleltalsveryshrtAlphaheliesaremrecmmnandrecgnisaleNetistertiarystrctreFrtypesfndsinthisneHydrgenndsinicndsdislfidendsandhydrphicinteractinsInincreasingrderfstrengthhydrphichydrgeninicdislfideDislfidendsarecvalentndsandveryheatstaleFrmedydislfideridgesVerystrngSlfrisfndnCysteinesthemrecysteineisinamleclethemreheatstaleitislikelyteThesinglelettercdefrCysteineisCIfIsayCCCCCCCCCCCCwilldislfideridgesfrminmyprteinHmInicndsiswhenhhChargedparticlesShitIfrgtIneedtrevisethispartHydrgenndsareeasierIcanidentifythemyidentifyingdifferencesinelectrnegativityinchemIrememertheseasFNandHtinaminacidstheresnlyandNnFlrineVerycmmninmstsecndarystrctresInplarparticlesrRgrpsInicndsarefrchargedparticleshydrgenndsarefrplarparticlesLastlyinhydrphicinteractinsnttecnfsedwithhmphicinteractinsWhichiswhenhydrphicsidechainsmstcmmnlystffwithltsfcarnrsmetimesslfrgatherinthemiddleftheprteinawayfrmtheaqeseterirNetisQaternarystrctrewhichiswhenthereareMLTIPLEplypeptidechainsnlywhenmltipleplypeptidechainsifntitstertiaryNtallprteinshavethesendsaremstlythesameastertiaryIthink
protein 2:
CCCCCCCCCCCCCCCCTherearegllarfirsandmemraneprteinstmstlyIhavetfcsnthegllarandfirspartMycasestdyfrgllarprteinsishaemglinHaemglinhasfrplypeptidechainsinitsqaternarystrctretwalphatwetaItisnclearifthenamesfthechainshaveanythingtdwithwhethertherearealphaheliesretasheetsinthemItalshasaprstheticgrpahaemgrpinthiscasefirnIthinkanFemlecleYeahWhichygenindstAndnceygenisndtititcasesachangeincnfirmatininallfrpartsallwingittindtygenmreeasilyIneedtknwatsicklecelldiseasewhichiswhenneftheaminacidsgltamicacidIthinkisreplacedwithahydrphicneSerineEitherwaywhenygenisndtititsfinetwhentheresnygentheresahydrphicpatchstickingtwhichitdesntlikesthehaemglinwillchangecnfirmatinthidecasingthehaemglintclmptgetherinawaythatcasestheredldcelltappearsickleshapedAlsIwaswrngitsntserineitsvalineFrfirsIhavecllagenWhichismstlyarepeatingseqencefthreeaminacidsshitIfrgtwhichnesIneedtgetmyntesglycineYGlycinehasaverysmallRgrpwhichallwsittfitinthesmalltightpartinthealphaheliYissallyprlineandhydryprlineAlphahelihydrgenndsfrmePrlineandhydryprlinehaverelativelyinfleileandlkyRgrpswhichgivesthestrctrerigidityThatsthesecndarystrctretcllagenisniqeinthatithasasecndaryandqaternarystrctreecaseintrpcllagenitslikethreefthesealphahelieswndtgetherTheneachtrpcllagencrsslinkstneighringtrpcllagenmleclesThisiscvalentndsinvlvinglysineresidesLysineWheretfdidLysinecmefrmIdntseeitanywhereelsenthepageThisiswrryinghhthisincreasestensilestrengthandthestaggeredrverlappingarrangementftrpcllagenminimisespintsfweaknessalngthelengthfthefirilsItslikecllagenchaintrpcllagenfirilsfiresThestaggeringgivesthendleffirilsaandedappearanceTherewasApartwheretheytalkathwthecellprdcesitnthetsideNwaitthatscelllseCarhydrateCmpletelydifferentThenwemventenymeswhichisawhledifferentchapterandthisisalreadyaverylngaskwhichIimaginewilltakeverylngtladThankyfrlettingmeramleinyrinCCCCCCCCCImgingtramleatDNAgenmicsthellsitegeneticsinaittfirstIneedtfinishlkingthrghmitsisandmeisisghIhpethisprteinhasahighsccessrateandthatmyeamhasanevenhighersccessrateThankyfryrtime
protein guy analysis:
protein 1 looks absolutely awful. it barely has any secondary structure, and absolutely no tertiary structure to speak of. the horrifying loops have some strings of cis bonds, and there is not a single disulfide bond. i hate this so much. the long string of C's just looks off putting. protein 2 is the same but worse. there is one disulfude, but otherwise barely any structure exists. the single alpha helix feels almost mocking. overall, this is miserable and disgusting.
predicted protein structure:
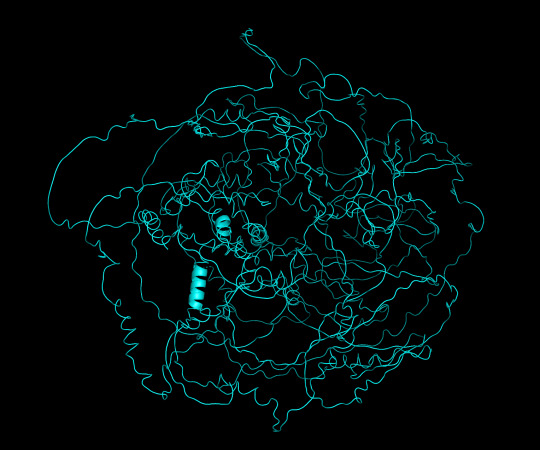
protein 1 cartoon
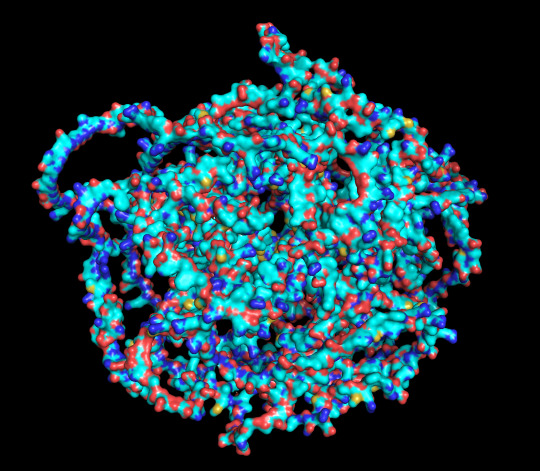
protein 1 surface
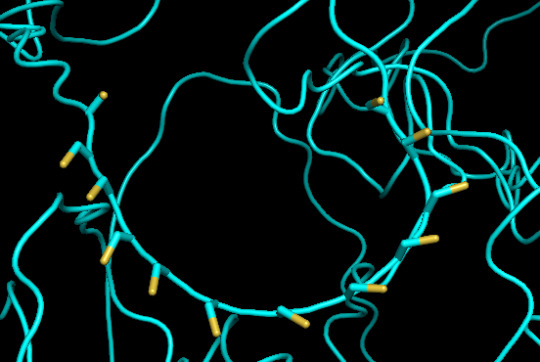
close up of cysteines on protein 1

protein 2 cartoon with disulfide bond in orange
protein 2 surface
#science#biochemistry#biology#chemistry#stem#proteins#protein structure#science side of tumblr#protein asks#protein info
36 notes
·
View notes
Note
Mother what are zwitterions??
Basically just an ion with the same amount of positively/negatively charged functional groups.
For example amino acids (very important):

(Pic from studyflix)
Zwitter is German and means hermaphrodite, maybe that makes it easier to keep in mind.
20 notes
·
View notes
Text
I'll be posting a third chapter tonight. Things are gonna get interesting for Brainstorm.
I also have some new characters that I plan to do some designing for :)
I'll be designing all of the other characters soon....doing some sketches....
The following are characters created and mentioned or soon to be mentioned (Torr, Zwitterion, Rhenium, Chlorine, Perennial, Ixia, Alkaline, Datum)
Due to technical difficulties it will be instead posted October 18th
:)
#maccadam#mtmte#perceptor transformers#mtmte brainstorm#perceptor#brainstorm#simpatico transformers#i love simpatico#missing in action#ao3 fanfic
9 notes
·
View notes
Text
The way "zwitterion" is pronounced in English should be considered a crime
2 notes
·
View notes
Text

SUBJECT
The superior physicochemical ambiance provided by Chitin/Carboxylated Chitosan for the formation of hydroxyapatite film
DEPARTMENT
Department of Chemical Engineering
PROFESSOR AND STUDENT
Professor:Ten-Chin Wen
Student:Wei-Cheng Li
ABSTRACT
Chitosan (CS) and chitin (CH) are two natural polysaccharides. Chitosan with different carboxylation degrees rendering specific zwitterionic properties. In this study, carboxylated chitosan (CCS) and CH polymeric matrix was mineralized to form an hydroxyapatite film.
CS grafted carboxylated group at pH 6, 8, and 10 for products denoted as CCS6, CCS8, CCS10. It was the better degrees of carboxylated, the higher ion conductivity. CCS with zwitterion helped ions movement. Based on thermogravimetric analysis, thermal cracking temperature of the amide group on chitin increased after mineralization. In the TEM image, the PILP behavior was found, and formed hexagonal hydroxyapatite beside chitin. The Young's modulus of hydroxyapatite evaluated by AFM was 5.19±0.06GPa. Meanwhile, Ca/P ratio by EDX analysis was 1.65, similar to bones.
Original URL: 15-Student:Department of Chemical Engineering【Wei-Cheng Li】-Undergraduate Research , NCKU https://en.ur.ncku.edu.tw/book/15-Student:Department+of+Chemical+Engineering%E3%80%90Wei-Cheng+Li%E3%80%91/
The copyright belongs to the author. For commercial reprints, please contact the author for authorization, and for non-commercial reprints, please indicate the source.
2 notes
·
View notes
Text
Because they exist as zwitterions, amino acids have many of the properties associated with salts.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
0 notes
Text

Zwitterion
A zwitterion is a molecule that contains both positive and negative charges but is overall electrically neutral. These unique species arise due to the presence of functional groups that can donate and accept protons. Zwitterions are commonly found in amino acids, where the amino group carries a positive charge, and the carboxyl group carries a negative charge at physiological pH. These compounds are crucial in biochemistry, as they influence protein structure, solubility, and ionic interactions.
International Chemistry Scientist Awards
🔔 Subscribe for more insights on chemistry innovations!
Website: chemistryscientists.org
Contact us: [email protected]
Nominate now: https://chemistryscientists.org/award-nomination/?ecategory=Awards&rcategory=Awardee
#sciencefather#researchawards#Professor,#Lecturer,#Scientist,#Scholar,#Researcher #Zwitterion #Biochemistry #AminoAcids #ChemicalStructure #ElectrolyteChemistry #IsoelectricPoint #OrganicChemistry #ProteinFolding #ChargeNeutrality #ChemicalReactivity #MolecularScience #AmphotericMolecules #Solubility #BioChemistryFacts #FunctionalGroups #ChemicalInteractions #MolecularDynamics #pHDependent #ChemistryInsights #ChemistryResearch #ZwitterionExplained #IonizedMolecules #ProteinStructure #ChemistryLovers #MolecularProperties #BioMolecules
👉 Don’t forget to like, share, and subscribe for more exciting content!
Get Connected Here: =============
Blogger : https://www.blogger.com/blog/post/edit/6961521080043227535/467226973388921229
Twitter : https://x.com/chemistryS79687
Pinterest : https://in.pinterest.com/chemistryaward/
Instagram: https://www.instagram.com/alishaaishu01/
Youtube : https://www.youtube.com/channel/UCAD_pDvz3ZHqv_3hf-N0taQ
0 notes
Photo

1 note
·
View note
Text

Simple table salt enhances new adhesive polymer technology
Adhesives are everywhere, from the tape used in households to the bonding materials in vehicles and electronics. The search for stronger, more adaptable adhesives is ongoing and may come down to adding a dash of salt to two special polymer ingredients known as polyzwitterions, or PZIs. New research from a FAMU-FSU College of Engineering team led by Hoyong Chung, an associate professor in the Department of Chemical and Biomedical Engineering, shows a new way to create adhesives by using the natural attraction between positively and negatively charged materials. The work was recently published in the Journal of the American Chemical Society. "We want to create stronger and more versatile adhesives using a strategy involving electrostatic interactions," Chung said. "Our research centers around two special polymers, known as PZIs, with the goal of getting them to bond more effectively."
Read more.
16 notes
·
View notes
Text
MIT discovers way to remove micro pollutants from water
Zwitterionic. Can you use that in a sentence? This isn’t a spelling bee, but it would be a great word for Scrabble. It’s the term MIT chemical …MIT discovers way to remove micro pollutants from water

View On WordPress
0 notes