#voltaic pile
Explore tagged Tumblr posts
Text
Sure they didn't have electricity as we know it today but batteries were being invented in the 1800s.

#battery#Voltaic Pile#Daniell Cell#Fuel Cell#Gaston Plante#Leclanche Carbon-Zinc Cell#J.A. Thiebaut#Carl Gassner#1881 the first commercially successful battery
46K notes
·
View notes
Text

More playing with science history. Here, I've hooked three voltaic cells in sequence; the liquid in the cells is salt water. I'm getting (as you can see) a little over 1.5 volts.
#frankenstein science#voltaic cell#voltaic pile#electricity#1800s electricity#history of science#not really sure how you use these electrify a whole entire giant man#Victor must have had racks of these things#yes I know the movie uses lightning but the book does NOT#the little voltaic piles actually produce more voltage
1 note
·
View note
Text
Love when batteries are referred to as voltaic piles. like yeah, you need some electricity? just go grab some from the pile
3 notes
·
View notes
Text
Alessandro Volta's Electric Eels
Okay so, it turns out that your cell phone battery is a basically a homunculus of an electric fish.


These are the same thing. Let me explain.
@fishteriously, a paleoichthyologist, told me that Alessandro Volta invented the electric battery after studying electric eels and rays. This sounded like a fun science factoid! I wanted to know more! I saw the claim repeated on any number of pop science articles from the last century or so, but none that quoted from primary sources.
The voltaic pile is one of the most important inventions, ever, of all time. Before Volta, electricity could be stored in Leyden jar capacitors, which would discharge in a single, brief burst. Volta's pile was the first method of producing a continuous electric current, which launched the modern era of electricity as we know it. His explanation for how it worked was incorrect, but it was still a massive breakthrough.
Batteries use the same principle to this day, just with different materials (e.g. cobalt oxide, graphite, and lithium salts rather than silver, zinc, and brine).
But is it a fish?
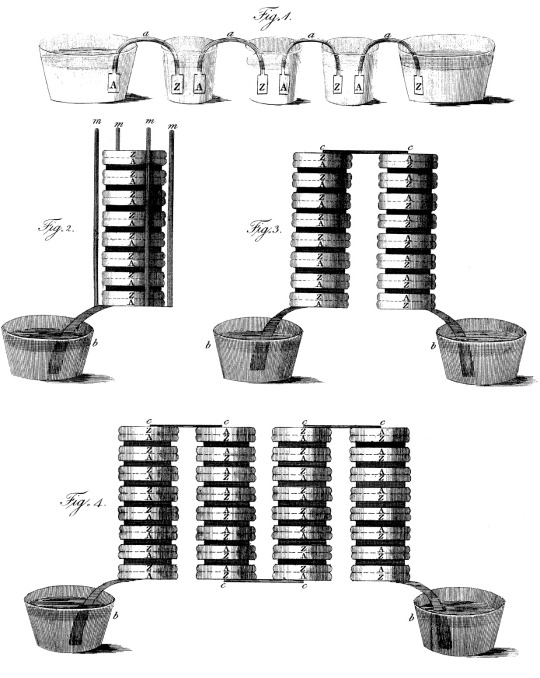
This is Volta's first schematic of a battery, or "voltaic pile" – at the time, "battery" referred to a bunch of Leyden jars linked in series, the term wouldn't come to refer to piles until later. "Z" and "A" stand for zinc and silver ("argentum"), with brine-soaked paper disks between. It does look a bit like an eel?
But is it truly?

Surely, if Volta modeled the pile after electric fishes, I’d be able to find a citation! Wikipedia is usually a good place to start when hunting primary sources, but no luck. No mention of fish at all. I trust fishteriously more than wikipedia, however, so I went digging. Looks like Volta first reported his discovery in a Letter to the Royal Society in 1800.

Found the letter!

Aw beans, it’s in French. I haven’t studied French since high school.

BUT WAIT. WHAT WAS THAT.
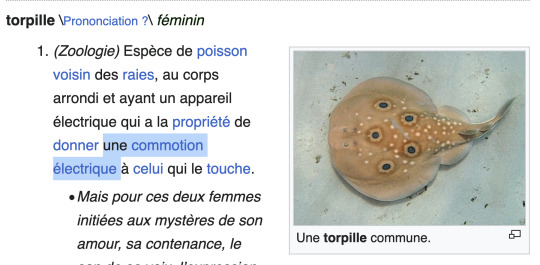

Une commotion électrique? A trembling eel???
Okay so now I NEEDED to read the letter in English. I found an English-language summary published by the Royal Society, but it looks like the only English translation of the full letter was in the appendix of an out-of-print book called “Alessandro Volta and the Electric Battery.”

So I bought a used copy. Let's see what Volta has to say about this:

"To this apparatus ... I have constructed it, in its form to the natural electric organ of the torpedo or electric eel, &c, than to the Leyden flask and electric batteries [battery = linked Leyden flasks], I would wish to give the name of artificial electric organ."
Yes! The voltaic pile was explicitly modeled after electric fishes – torpedo rays and electric eels. Fishteriously was 100% correct. Volta never even calls it a "pile," it is always "artificial electric organ." A significant portion of the letter is devoted to electric eels and torpedo rays, in fact.
But also, the rest of the letter is bonkers.

He wrote pages on painful experiments with the artificial electric organ – touching it, poking it into his eyes and ears, making other people touch it, generally just shocking the ever loving hell out of himself over and over. He routinely shocks himself so hard that he has to take breaks. And of course, he licks it.
But that's not the best part:

He says that the artificial electric organ can be turned sideways and submerged in liquid...
"...by which means these cylinders would have a pretty good resemblance to the electric eel ... they might be joined together by pliable metallic wires or screw springs, and then covered with a skin terminated by a head and tail properly formed, &c."
There you have it. One of the most important scientific discoveries of all time, and it includes a crafts project for building an authentic electric eel puppet.
In summary, next time you charge your phone, take a moment to thank the soul of the electric fish inside of it.
2K notes
·
View notes
Text
i understand how batteries USED TO work in the sense of like i have built / maintained / used voltaic piles & crowns of cups but i have legiterally no idea what is or isn't different between those and like a lithium ion battery so whenever my old batteries in things are shitting themselves and dying i am just picturing tgat my laptop needs to get its electrolytes topped off. like watering a piano you know how it goes
15 notes
·
View notes
Text
thinking about how if i was a scientist in the early modern period and i was trying to understand the whole deal with batteries and electric current and chemicals and stuff i would be so fucking confused. like electricity is confusing now when they can teach you about in spherical cow world where you have a nice neat power supply that lets you vary the voltage and stuff. imagine trying to understand the rules underlying electricity without an ammeter, without a voltmeter, without even electric lights! you just have batteries, and wires, and like, what, static electricity? magnets? its insane to me that people figured out electricity, and so fast. the voltaic pile was invented in 1800, maxwells equations were written up in 1862! galvani's frogs were in 1780! shocking frog legs to nice neat beautiful equations in less than 100 years! 100 years ago we were in early quantum! 80 years ago we had nuclear bombs! (well. 78. whatever)
99 notes
·
View notes
Text
this sounds unintentionally funny. fend off attackers by gaining Eel Powers
"He introduces her to his sister Zara, a sculptor, with whom she enjoys a close friendship, and to Prince Ivan, a rakish figure who pursues an unrequited attraction to Zara. She witnesses Ivan attempt to assault Zara, who repels him with electric power similar to that of an electric eel. From this, the protagonist gleans that the arts practised by Heliobas enable the strengthening of a human organ similar to a voltaic pile, granting health and longevity as well as other powers."
4 notes
·
View notes
Text
Whenever I get around to writing The Chains We Break (sequel to The Chains That Join Us). I will force upon the narrative a young boy who desires above all else to be a wizard's apprentice and banish him to the background immediately. He will be the eighth son of a sixteenth son of a thirty-secondth son (via artificial insemination). He bears no magical abilities. His name will be Octavian Pile.
But he will be pretty decent at chemistry and make the first voltaic pile battery in the story's world.
#nonsense#this is really just because the words “septimus heap” are stuck in my head#no other reason.#this happens sometimes for no reason when two to three words are trapped repeating in my mind for days on end
6 notes
·
View notes
Text
Come fuck this (gesturing to weird pile)
1K notes
·
View notes
Text
Powering the Future: A Casual Guide to Battery Technology
In simple words, the contemporary world as we know it would not be thinkable without batteries. From life-supporting devices like pacemakers to cellphones, batteries energize the several compact electronic devices all around you.
They have also discovered working in the past few years in electric vehicles and are touted as a "silver bullet" for the future of renewable power storage systems. They offer an easy and portable source of electrical power, permitting us to stay connected, and work efficiently, and can make a more supportable future possible when recharged with renewable power.
The battery technology market is witnessing development and is projected to reach USD 176.92 billion by 2030.
The Basics of Batteries:
Let's gain some basic knowledge regarding batteries. Batteries are power storage devices that change chemical power into electrical power. The most popular kind you encounter every day is the lithium-ion battery, popular for its high-power density and rechargeability. It is the powerhouse behind your devices and electric vehicles.
A Brief History Lesson:
Batteries are not a recent discovery. Alessandro Volta, an Italian physicist, made the first chemical battery in the early 19th century. It was known as the Voltaic Pile, made from alternating discs of copper and zinc and copper detached by cardboard saturated in salt water. Fast forward to today, and we've come a long way.

The Evolution of Battery Technology:
In the past few years, battery tech has changed significantly. We have witnessed improvements in materials, such as shifting from lead-acid to lithium-ion, which carried lighter and more effective batteries. Nowadays, academics are discovering solid-state batteries and other pioneering tech to make batteries secure, more powerful, and eco-friendly.
The Green Revolution:
As the world grapples with ecological worries, batteries play an important role in the shift towards renewable power. Battery storage permits us to use solar and wind power, loading it for when the sun is not shining or the wind is not blowing. This makes the path for a greener, more sustainable future.
Electric Vehicles (EVs):
One of the most thrilling workings of battery tech is in EVs. EVs are becoming progressively popular, thanks to enhancements in battery capacity and charging infrastructure. They are not just ecological; they are also extremely fun to drive!
In the coming few years, it is estimated that the increasing acceptance of EVs among customers will quicken the utilization of lithium-ion batteries, as they are an eco-friendlier substitute to orthodox fuels.
China is known as the world's topmost manufacturer of EVs. This is due to the demand for electric vehicles being more compared to other nations, as a result of increasing government steps in order to encourage the utilization of EVs.
Battery tech is an exciting field with a bright future. From your mobile phone to the worldwide shift toward clean power, batteries are at the heart of it all. As tech endures to progress, expect even more thrilling growths in the field of batteries. So, next time you plug in your device or hop into an electric vehicle, remember the extraordinary journey of these little powerhouses!
Source: P&S Intelligence
#Battery Technology Market Share#Battery Technology Market Size#Battery Technology Market Growth#Battery Technology Market Applications#Battery Technology Market Trends
1 note
·
View note
Text
The Evolution of Batteries: From the Voltaic Pile to Lithium-Ion Batteries

Batteries are an integral part of modern life, powering everything from smartphones to electric cars and home energy systems. However, the journey of batteries to their present form has been a long one, filled with remarkable innovation. As one of the leading Lithium-ion Battery Manufacturers, Yukinova is proud to be part of this exciting evolution. In this blog, we explore the fascinating history of batteries, from the early Voltaic Pile to today’s cutting-edge lithium-ion technology.
The Birth of the Battery: The Voltaic Pile
The invention of the battery dates back to 1800 when Italian physicist Alessandro Volta created the first chemical battery, known as the Voltaic Pile. This simple device was made from alternating discs of zinc and copper, separated by cardboard soaked in saltwater. While it was crude by modern standards, the Voltaic Pile was groundbreaking because it provided a continuous and steady source of electricity for the first time in human history.
This invention paved the way for the development of various electrochemical cells that formed the foundation of the battery technologies we rely on today. It also opened the door to experiments with electricity that would shape the future of science and technology.
The Rise of Lead-Acid Batteries
In 1859, French physicist Gaston Planté invented the lead-acid battery, a major leap forward in battery technology. Lead-acid batteries were rechargeable, making them highly practical for applications where a continuous supply of electricity was required. They became particularly useful in the automotive industry, providing the power needed to start internal combustion engines.
Lead-acid batteries are still widely used today, especially in cars and backup power systems. However, they are heavy and have a relatively short lifespan compared to modern batteries, leading to the need for new and more efficient energy storage solutions.
The Advent of Alkaline Batteries
In 1899, Swedish scientist Waldemar Jungner introduced the nickel-cadmium (NiCd) battery, followed by Thomas Edison’s nickel-iron battery in 1901. These batteries were more durable than lead-acid batteries and had a higher energy density, which made them suitable for more demanding applications, such as in early telecommunication systems and portable power tools.
Later, alkaline batteries emerged as a superior choice for consumer electronics due to their longer shelf life, lighter weight, and more efficient energy storage compared to other primary batteries like zinc-carbon.
The Revolution: Lithium-Ion Batteries
The most significant development in recent decades has been the invention of the lithium-ion battery. First commercialized by Sony in the early 1990s, lithium-ion batteries represented a quantum leap in energy storage. These batteries are lighter, have a much higher energy density, and are capable of many more charge-discharge cycles compared to lead-acid or nickel-based batteries.
Lithium-ion batteries have become the go-to choice for powering modern devices such as smartphones, laptops, electric vehicles, and renewable energy storage systems. Their ability to store large amounts of energy in a compact form, along with their longer lifespan, makes them ideal for a wide range of applications. Today, Lithium-ion Battery Manufacturers like Yukinova are pushing the boundaries of what these batteries can achieve, helping to power the future with clean and efficient energy.
The Future of Battery Technology
As we look to the future, batteries will continue to play an even larger role in our lives, especially with the shift towards renewable energy and electric mobility. Advances in solid-state battery technology, graphene batteries, and even bio-batteries are on the horizon, promising even more efficient, powerful, and environmentally friendly energy storage solutions.
At Yukinova, a renowned Lithium-ion Battery Manufacturer, we are committed to staying at the forefront of these advancements, developing high-performance lithium-ion batteries that meet the growing demand for efficient energy storage. Whether it’s for consumer electronics, electric vehicles, or renewable energy systems, our lithium-ion batteries are designed to deliver the best performance while supporting a sustainable future.
The evolution of battery technology, from the Voltaic Pile to today’s advanced lithium-ion batteries, has been a journey of innovation and discovery. As a leading Lithium-ion Battery Manufacturer, Yukinova continues to push the boundaries of battery technology to provide efficient, reliable, and eco-friendly energy storage solutions. Whether you’re looking for batteries to power your devices, vehicles, or home energy systems, our lithium-ion batteries are designed to meet your needs with superior performance and long-lasting power.
Embrace the future of energy with Yukinova—your trusted partner in high-performance lithium-ion battery technology.
Original Source: https://medium.com/@yukinovabattery/the-evolution-of-batteries-from-the-voltaic-pile-to-lithium-ion-batteries-5fd8f3e3b41e
0 notes
Video
youtube
How To Generate Free Electricity - Science Experiment With Vinegar And R...
Today we have a very simple and fun science experiment to try at home. We all have seen videos or science experiments of lighting our LED lights from lemons or potatoes. Today, in our science experiment we would use vinegar and 5 Rs coin to generate electricity and light our LED bulb.
In this science experiment, we explore the principles of electrochemistry and generate free electricity to light an LED bulb using coins and small cardboard pieces soaked in vinegar. The materials needed include several coins (preferably copper and zinc), cardboard pieces, vinegar, an LED light bulb, and wires.
First, prepare the cardboard pieces by cutting them into circles slightly smaller than the coins. Soak these cardboard pieces in vinegar for about 10 minutes. The vinegar acts as an electrolyte, facilitating the flow of electric current.
Next, assemble the voltaic pile. Begin by placing a copper coin (or any coin with a significant copper content) on a flat surface. Place a vinegar-soaked cardboard piece on top of the copper coin, followed by a zinc coin (or any coin with significant zinc content). Repeat this stacking process until you have a pile consisting of several such layers, always ensuring that the final layer is a zinc coin.
Once the voltaic pile is constructed, attach one wire to the topmost zinc coin and another wire to the bottommost copper coin. Connect these wires to the LED bulb. The chemical reaction between the vinegar-soaked cardboard and the coins creates an electric current, which flows through the wires and powers the LED bulb.
This science experiment for kids demonstrates how simple materials can be used to create a basic battery, illustrating the principles of chemical energy conversion to electrical energy, similar to the workings of a traditional battery.
0 notes
Text
History of Batteries, What Is a Battery, Recycling of Batteries

Introduction
Batteries power our world, from the smallest hearing aid to the largest electric vehicles. They are integral to modern life, making our gadgets portable and our green technologies possible. But what exactly are batteries, how did they come about, and how do we handle them responsibly? Let's dive into the fascinating history of batteries, explore what they are, and discuss the crucial topic of battery recycling.
What Is a Battery?
A battery is a device designed to store chemical energy and convert it into electrical energy through a chemical process. Typically, it comprises one or more electrochemical cells, each containing two electrodes - an anode and a cathode - separated by an electrolyte.
When in use, during discharge, chemical reactions take place at these electrodes, generating electrons that flow through an external circuit, thus creating electrical current. Rechargeable batteries, such as lithium-ion batteries, can reverse these chemical reactions when an external electrical current is applied, allowing the battery to be recharged and reused multiple times.
Batteries find applications in various fields, from powering electronic gadgets like smartphones and laptops to serving as energy storage units for renewable energy systems.
Composition
The battery consists of lead and lead dioxide plates submerged in concentrated sulfuric acid. During operation, reversible reactions occur where sulfate combines to form lead sulfate, accompanied by the addition of an electron. Discharge of the battery results in the accumulation of PBso4 and water in the acid, yielding a characteristic voltage of approximately 2 volts. By combining six cells, one can achieve the typical 12-volt output of a lead-acid battery. In comparison to zinc-carbon batteries, recharging lead-acid batteries is easier due to the fully reversible reactions. Zinc-carbon batteries lack the mechanism for returning hydrogen to the electrolyte, making recharging difficult.
what are types of batteries
primary batteries (disposable batteries), which are designed to be used once and discarded.
secondary batteries (rechargeable batteries ), which are designed to be recharged and used multiple times.
Early History of Batteries
One of the earliest known batteries is the Baghdad Battery, dating back to around 200 BC. This ancient artifact consists of a clay jar filled with a vinegar solution, containing an iron rod surrounded by a copper cylinder. Although its exact purpose is still debated, it is believed to have been used for electroplating or some form of electrical storage.
The Birth of the Modern Battery
In 1800, Alessandro Volta invented the voltaic pile, considered the first true battery. This invention consisted of alternating discs of zinc and copper, separated by pieces of cardboard soaked in saltwater. Volta's battery produced a steady current and laid the groundwork for future advancements in electrochemistry.
Development Through the 19th Century
John Daniell improved upon Volta's design in 1836 by creating the Daniell cell, which used copper and zinc in a more efficient configuration, reducing corrosion and increasing the battery's lifespan. In 1859, Gaston Planté invented the lead-acid battery, which became the first rechargeable battery. This type of battery is still widely used today, particularly in automotive applications.
20th Century Innovations
The 20th century saw significant advancements in battery technology. In 1899, Waldemar Jungner developed the nickel-cadmium (NiCd) battery, which offered better energy density and rechargeability compared to earlier designs. Later, in the 1950s, Lewis Urry invented the alkaline battery, which provided a longer shelf life and better performance for consumer electronics.
What is a lithium-ion battery?
Lithium-ion batteries are the most widely used rechargeable battery technology today, powering everyday devices such as mobile phones and electric vehicles. These batteries are made up of one or more lithium-ion cells and include a protective circuit board. They are called batteries once the cell or cells are placed inside a device with this protective circuit board.
What are the components of a lithium-ion cell?
Electrodes: The positively and negatively charged ends of a cell. Attached to the current collectors
Anode: The negative electrode
Cathode: The positive electrode
Electrolyte: A liquid or gel that conducts electricity
Current collectors: Conductive foils at each electrode of the battery that are connected to the terminals of the cell. The cell terminals transmit the electric current between the battery, the device and the energy source that powers the battery
Separator: A porous polymeric film that separates the electrodes while enabling the exchange of lithium ions from one side to the other
Applications of Batteries
Batteries are ubiquitous in our daily lives:
Consumer Electronics: Smartphones, laptops, and wearable devices rely heavily on rechargeable batteries.
Electric Vehicles: EVs use advanced battery packs to store and deliver the energy needed for transportation.
Renewable Energy Storage: Batteries store energy generated from renewable sources like solar and wind, providing a steady power supply even when the sun isn't shining or the wind isn't blowing.
Future of Battery Technology
The future of battery technology looks promising, with ongoing research focused on increasing energy density, reducing costs, and improving safety. Solid-state batteries, which use solid electrolytes instead of liquid ones, are a significant area of development. These batteries promise higher energy densities, longer lifespans, and enhanced safety features, potentially transforming everything from consumer electronics to electric vehicles.
Why do we care about batteries?
Batteries are essential in our modern world, powering a wide range of devices from smartphones to electric vehicles, offering convenience and mobility. They enable us to remain connected, access information, and conduct business wherever we are. Furthermore, as we shift towards renewable energy sources, batteries become vital for storing this intermittent energy, ensuring its reliable utilization. This not only reduces our dependence on fossil fuels but also aids in mitigating climate change. Beyond convenience, batteries are pivotal in advancing technology, fostering sustainability, and enhancing resilience, prompting extensive research and development globally.
Recycling of Batteries
Recycling batteries is crucial for mitigating their environmental impact. It conserves resources, reduces pollution, and prevents hazardous materials from entering the environment. Battery recycling involves several steps:
Collection: Batteries are collected from consumers and businesses.
Sorting: They are sorted by type and chemistry.
Processing: Batteries are dismantled, and valuable materials like metals are recovered.
Refinement: Recovered materials are purified for reuse in new batteries.
Conclusion
In conclusion, the history of batteries traces a remarkable journey of innovation and evolution, from ancient civilizations' rudimentary cells to today's sophisticated powerhouses driving our modern world. Understanding what a battery is, its composition, and its crucial role in powering our daily lives underscores the importance of responsible disposal and recycling. As we strive for a more sustainable future, initiatives like Big Country Recycling play a pivotal role, By partnering with Big Country Recycling, we not only contribute to environmental conservation but also ensure that valuable resources are recovered and reintegrated into the production cycle, fostering a circular economy for generations to come. Join us in championing a greener tomorrow with Big Country Recycling. Contact them today to learn more about their Recycling Services or to get a quote for your materials. Or call +1 325-949-5865.
0 notes
Text
A New Battery Technology is "Powering the Future"
Batteries Mcgraths Hill have become an indispensable part of our daily lives, from the small alkaline cells that power our remote controls to the lithium-ion behemoths driving electric vehicles. The evolution of battery technology is a testament to human innovation and our ever-growing need for portable and sustainable energy sources. In this 800-word essay, we will delve into the fascinating world of batteries, exploring their history, current advancements, and the promising future they hold in addressing critical global challenges.

The Historical Perspective
The history of batteries dates back to the late 18th century when Italian scientist Alessandro Volta developed the first true battery known as the "Voltaic Pile." This primitive contraption was built from alternating layers of zinc and copper separated by cardboard soaked in saltwater. The Voltaic Pile marked the birth of electrochemical energy storage and set the stage for further advancements.
Over the years, various types of batteries were developed, each with its unique properties. Lead-acid batteries, invented by Gaston Planté in 1859, became the standard for automotive use, while nickel-cadmium batteries revolutionized portable electronics in the mid-20th century. However, these early battery technologies had limitations, such as low energy density, toxicity, and relatively short lifespans, which prompted a relentless pursuit of better alternatives.
The Rise of Lithium-ion Batteries
The breakthrough came with the invention of lithium-ion batteries in the 1970s and their commercialization in the 1990s. These batteries offered a revolutionary combination of high energy density, longer cycle life, and reduced environmental impact compared to their predecessors. Lithium-ion batteries quickly became the backbone of the modern electronic age, powering laptops, smartphones, and a myriad of other devices. Today, lithium-ion batteries continue to dominate the market, finding applications in electric vehicles (EVs) and renewable energy storage systems. Their versatility and performance have played a crucial role in reducing greenhouse gas emissions and accelerating the adoption of clean energy technologies.
Innovation and Advancements
As the demand for better battery solutions grows, significant research and innovation have been directed toward improving the performance and sustainability of batteries. Here are some noteworthy advancements:
Recycling and Sustainability: Recognizing the environmental impact of battery production and disposal, many companies are developing sustainable practices. Recycling programs are emerging to recover valuable materials from old batteries, reducing the need for raw materials and minimizing waste.
Fast Charging: Research is ongoing to develop ultra-fast charging solutions that could reduce the time required to charge electric vehicles. Companies like Tesla and Porsche are pioneering high-speed charging technology.
Energy Storage: Batteries are pivotal in enabling the integration of renewable energy sources, such as wind and solar, into the grid. Utility-scale battery systems are being deployed to store excess energy and provide it when needed.
The Future of Batteries
The future of batteries holds great promise and a multitude of possibilities. These devices are integral to addressing some of the most pressing global challenges.
Electric Vehicles: As countries worldwide push for electric vehicle adoption to reduce emissions and combat climate change, batteries play a central role. Future advancements in battery technology, such as solid-state batteries and higher energy density, will extend EV range and reduce charging times.
Energy Access: In regions with limited access to reliable electricity, batteries can provide a lifeline. Portable and affordable battery solutions are vital in improving the quality of life for millions of people worldwide.
Conclusion
Batteries have come a long way since the days of Volta's Voltaic Pile, evolving from humble beginnings to becoming a linchpin of modern technology. Their importance in addressing current global challenges cannot be overstated, from enabling the electrification of transportation to supporting the transition to renewable energy sources. The ongoing research and development in battery technology promise a bright future with more efficient, sustainable, and powerful energy storage solutions. As we continue to innovate in this field, batteries will undoubtedly play a crucial role in shaping the world we live in, and the world of tomorrow.
Disclaimer: This is generic information and posts; content about the services can be changed from time to time as per your requirements and contract. To get the latest and updated information, contact us today or visit our website.
#car battery windsor#car batteries#car battery shop near me#Car Batteries Hawkesbury#Battery Mcgraths Hill#Battery Windsor
0 notes
Text
Consumer Electronics Batteries Market Expected to Reach $17.5 Billion, Globally, by 2032 at 10.1% CAGR: Allied Market Research
The Consumer Electronics Batteries market share is expected to witness considerable growth in coming years, owing to the rise in adoption of EVs and technological advancements
Wilmington, Delaware
According to a new report published by Allied Market Research, titled, “Consumer Electronics Batteries Market by Type and Application: Global Opportunity Analysis and Industry Forecast, 2023-2032" The consumer electronics batteries market was valued at $13.9 billion in 2022, and is estimated to reach $61.3 billion by 2032, growing at a CAGR of 16.2% from 2023 to 2032.
Download Research Report Sample & TOC: https://www.alliedmarketresearch.com/request-sample/208340 (We look forward to moving quickly to provide the Report Analysis needed for your Business Success) •116 – Tables •84 – Charts •272 – Pages
Consumer electronic batteries quietly serve as the backbone of the modern world. These compact, portable energy storage technologies have revolutionized the lifestyle. They provide ease and adaptability that are taken for granted by allowing the maintenance of the seamless operation of cellphones, computers, tablets, and many other technological devices. The history of batteries used in consumer electronics is intricately entwined with the history of battery technology as a whole. The first real battery, known as the Voltaic Pile, was developed by Italian scientist Alessandro Volta in the latter half of the 18th century. A constant flow of energy was produced by this early battery, which was made of alternating layers of zinc and copper separated by saltwater-soaked cardboard. Volta's discovery paved the way for later advancements in battery technology even though they are far different from the batteries we use today.
Prime determinants of growth:
Competitive analysis and profiles of the major global consumer electronics batteries market players that have been provided in the report include Samsung SDI Co., Ltd., LG Chem, Panasonic Corporation, Toshiba Corporation, Duracell, Sony Corporation, Apple, Inc., Hitachi, Ltd., Johnson Controls International Plc, and Energizer.
One of the significant factors that impact growth of the consumer electronics batteries market includes rise in adoption of EVs. Moreover, technological advancements is expected to drive the market growth. However, limited energy density and environmental concerns might hamper growth of the market. On the contrary, miniaturization and form-factor innovation offer potential growth opportunities for the consumer electronics batteries market.
Report Coverage and Details:
Aspects
Details
Market Size By 2032
USD 61.3 billion
Growth Rate
CAGR of 16.2%
Forecast period
2022 - 2032
Report Pages
272
By Type
Alkaline Batteries
Lithium-ion Batteries
Graphene Batteries
Nickel-Cadmium Batteries
Zinc-Carbon Batteries
Lead-Acid Batteries
Others
By Application
Smartphones
Laptops and Tablets
Digital Cameras
Wearable Devices
Medical Devices
Others
COVID-19 Scenario:
The COVID-19 pandemic caused a huge upheaval in the consumer electronics battery industry. There has been a growth in demand for consumer gadgets, such as laptops, tablets, smartphones, and gaming consoles, as a result of widespread remote working and more time spent at home. This increase in demand puts pressure on the supply system, causing sporadic shortages and delays.
The consumer electronics batteries market serves an instrumental part in the wider battery industry in addressing the ever-evolving requirements of the consumer electronics industry. The primary purpose of this market is the development and distribution of batteries developed particularly for an array of portable and mobile devices, including smartphones, laptops, tablets, digital cameras, gaming consoles, and smartwatches. The industry has its sights on delivering compact, lightweight, and rechargeable power alternatives in order to offer consumers the portability and accessibility that they seek from the items they purchase.
Inquiry Before Buying: https://www.alliedmarketresearch.com/purchase-enquiry/208340
Leading Market Players:
Hitachi, Ltd.,
Energizer Holdings, Inc.,
Sony Corporation,
LG Chem, Apple, Inc.,
Toshiba Corporation,
Samsung SDI Co., Ltd,
Johnson Controls International plc,
Duracell,
Panasonic Corporation
The report provides a detailed analysis of these key players of the global consumer electronic batteries industry. These players have adopted different strategies such as product development and product launch to increase their market share and maintain dominant shares in different regions. The report is valuable in highlighting business performance, operating segments, product portfolio, and strategic moves of market players to showcase the competitive scenario.
Key Benefits for Stakeholders:
This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the consumer electronics batteries market analysis from 2022 to 2032 to identify the prevailingcConsumer electronics batteries market opportunity.
The market research is offered along with information related to key drivers, restraints, and opportunities.
Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
In-depth analysis of the consumer
By Region:
North America (U.S., Canada, and Mexico)
Europe (U.K., Germany, France, Italy, Spain, Russia, Netherlands, Belgium, Poland, and Rest of Europe)
Asia-Pacific (China, Japan, India, South Korea, Australia, Malaysia, Thailand, Philippines, Indonesia, and Rest of Asia-Pacific)
LAMEA (Latin America, Middle East and Africa)
Trending Reports in Semiconductor and Electronics Industry (Book Now with Up to 20% Discount + COVID-19 Scenario):
Antenna Market size is projected to reach $40.1 billion by 2032, growing at a CAGR of 9.1% from 2023 to 2032.
Image Sensor Market size is projected to reach $87.5 billion by 2032, growing at a CAGR of 12.9% from 2023 to 2032.
Superconductors Market share is projected to reach $17.4 billion by 2032, growing at a CAGR of 10% from 2023 to 2032.
Power Cable Market size is projected to reach $277.8 billion by 2031, growing at a CAGR of 6.4% from 2022 to 2031
Embedded Systems Market size is projected to reach $163.2 billion by 2031, growing at a CAGR of 6.5% from 2022 to 2031.
About Us:
Allied Market Research (AMR) is a full-service market research and business-consulting wing of Allied Analytics LLP based in Wilmington, Delaware. Allied Market Research provides global enterprises as well as medium and small businesses with unmatched quality of "Market Research Reports Insights" and "Business Intelligence Solutions." AMR has a targeted view to provide business insights and consulting to assist its clients to make strategic business decisions and achieve sustainable growth in their respective market domain.
We are in professional corporate relations with various companies and this helps us in digging out market data that helps us generate accurate research data tables and confirms utmost accuracy in our market forecasting. Allied Market Research CEO Pawan Kumar is instrumental in inspiring and encouraging everyone associated with the company to maintain high quality of data and help clients in every way possible to achieve success. Each and every data presented in the reports published by us is extracted through primary interviews with top officials from leading companies of domain concerned. Our secondary data procurement methodology includes deep online and offline research and discussion with knowledgeable professionals and analysts in the industry.
Contact:
David Correa
1209 Orange Street, Corporation Trust Center, Wilmington, New Castle, Delaware 19801 USA.
Int'l: +1-503-894-6022 Toll Free: +1-800-792-5285
UK: +44-845-528-1300
India (Pune): +91-20-66346060 Fax: +1-800-792-5285 [email protected]
1 note
·
View note
Text
Alessandro Giuseppe Antonio Anastasio Volta: *invents the voltaic pile*
Everyone, apparently: "You know what that reminds me of? A bunch of cannons."
0 notes