#right ventricular hypertrophy
Explore tagged Tumblr posts
Text
Right Ventricular Hypertrophy
Does anyone know anything about RVH?
My recent emergency ECG indicated I have signs of RVH, and I was wondering if anyone was aware of what it was, possible effects, treatments, etc?
RVH might be the answer to what I’ve been experiencing that we previously suspected was POTS. We’ll have to see what comes of my cardiologist appointment!
Thanks in advance lovelies!! 🫶🫶
#RVH#right ventricular hypertrophy#heart health#medical issues#heart defects#science#medicine#nursing#doctors#question
0 notes
Text
She've trained for more than a year and given the seen results, she rapidly fell in love with the weights. Changes in her appearance are so good she couldn't stop thinking about having more and faster. Having studied some biology in the past, she decided to rely on anabolic steroids to maxime muscle growth in the shortest possible period of time.
She's now in front of the mirror, at the gym, looking at herself. She looks so strong and healthy. For some unknown reasons (maybe black magic) she was able to avoid the androgenic effects of anabolic steroids but some other effects can't be avoided at all. Inside of her other changes are occuring:
The heart is a muscle and during high effort training it will react like any other muscle to the use of steroids. Androgenic steroids alters both electrical and structural features of the myocardium. Supraphysiological doses of those steroids induces toxicity of the cardiovascular system, including changes in the lipid profile, elevations in blood pressure, myocyte [working heart cells] hypertrophy, disarray and apoptosis [controlled cell death] and a procoagulant state. Thereby, contributing to disorders such coronary artery disease, hypertension, cardiomyopathy and thromboembolic disorders. Hypertension is an important reported phenomenon in anabolic steroids users, described to be a consequence of increased sympathetic drive and endothelial [inner layer of blood vessels] dysfunction. Those steroids are involved in promoting the growth of cardiac tissue, resulting in significant adverse adaptations such as an increase in wall thickness, and left ventricular cavity size. Diastolic [heart relaxation after a beat] function appears to be affected, whereby a reduction in early and late diastolic filling velocity ratios is expected. Interestingly, immunohistochemical analyses revealed greater expression of proinflammatory mediators, showing ongoing silent myocardial injury in steroids users. Cardiomegaly [giant heart], myocardial fibrosis and necrosis have been also seen in post-mortem studies on people who used anabolic steroids. Other observed complications are: ventricular rigidity, increase in right ventricular strain, left atrial dysfunction, myocardial infarction and heart failure. Importantly, hypertrophy, fibrosis and necrosis are all substrates for arrhythmias that are further made worse by exercise. Testosterone, in particular, has been associated with rhythmic disturbances, possibly through the potentiation of potassium channels involved in ventricular repolarisation.
Anabolic androgenic steroids have the potential of increasing the risk of sudden cardiac death through four potential mechanisms: the thrombosis model, the atherogenic model, the direct myocardial injury model, and model of vasospasm.
In few words to her heart the simple act of keep beating has become a struggle, a struggle that can become unbearable any moment.
(Further details about this topic are provided in the article: "The Cardiac Effects of Performance-Enhancing Medications: Caffeine vs. Anabolic Androgenic Steroids" by Sivalokanathan et al. 2021 from which this post is mainly taken)
62 notes
·
View notes
Text
Christine’s Malpractice Case
Every year, there are thousands of medical malpractice cases reported in the United States. Ranging from surgical or procedural errors, to misdiagnosis, to anesthesia errors, and many other possible factors not listed. We all have a certain level of trust in medical professionals because of their many years of training and education. However, these professionals are people too, and are prone to making mistakes from time to time. Unfortunately when medical professionals make a mistake, it can have major consequences for their patient- leading to further injury, disability, or even death. Sadly, one such case took place at our hospital recently.
The patient was Christine Rossi. She was 47 years old and stood at only 5 feet tall, but her big personality made up for her lack of height. She had a pleasantly plump figure, beautiful brown eyes, shoulder length brown hair, was olive skinned since she was of Italian descent, and always had a fresh mani+pedi. She looked good for her age since she never had kids, and she was never married- but definitely married to her career as a medical malpractice attorney.
Christine’s case began when she was brought into our emergency department one evening straight from her office. She was wheeled into trauma 1 sitting up on the gurney, stripped down to just her bra and underwear. She was wearing an oxygen mask, had EKG electrodes all over her chest, and had IVs going in both arms. “hi, I’m Dr Lindsay. Can you tell me what’s wrong?” Dr Lindsay, the ER attending from that evening asked Christine in a calm, inviting tone. Christine was gasping for air and had one hand on her chest. Her eyes were absolutely bugging out at times, and she was visibly uncomfortable. “my chest…” Christine utters to Lindsay. “your chest hurts? How long has it been hurting you?” Dr Lindsay asks in response. “since yesterday… but it got worse- a lot worse just now…” Christine tells Dr Lindsay.
On the heart monitors, Dr Lindsay saw that Christine was tachycardic, hypotensive, and had an abnormal EKG. The EKG showed unifocal PVCs with ST elevation. The doctor ordered some blood tests: a CBC, BMP, tox screen, and a stat cardiac enzyme test. An echocardiogram and chest x ray were also ordered while the blood was being drawn for the labs.
While the blood samples were sent off to the lab, the chest x ray was performed first. The only thing that was abnormal was some swelling and irritation in both lungs. This can be caused in part by Christine’s rapid, labored breathing, but it can also be associated with blood clots in the lungs, heart attacks, or fluid buildup in the lungs (for example, from pneumonia, covid, and sometimes severe bronchitis). The chest x ray definitely provided some good information, but it didn’t give Dr Lindsay the whole picture, so an echocardiogram was ordered. The echo showed right ventricular hypertrophy. Basically, the right side of her heart was enlarged and working much harder than it should. With the stat cardiac enzyme lab still pending, a dose of nitro was given for chest pain, and cardiology was called for consultation.
The two members of our cardio team to arrive were Dr Rachel, one of our cardiothoracic surgeons, and her cardio resident Dr Sarah. “hey guys, I appreciate you coming down. I think she’s having an acute STEMI and needs the cath lab, just waiting on the cardiac enzyme test to come back to confirm. What do you think?” Dr Lindsay says to the 2 cardio doctors. Dr Sarah looks at Dr Rachel, waiting for her to do the talking. “don’t look at me! What do you think of Dr Lindsay’s assessment?” Dr Rachel told Sarah, trying to get her resident to take some initiative. “I um… I agree.” The resident replies hesitantly. “why do you agree? Go on!” Dr Rachel tells Sarah. “well… um… the EKG shows ST elevation. And uh…. The patient has angina pectoris and shortness of breath.” The resident replies, nervously, and without confidence.
Nurse Nancy walks into the room with a few pieces of paper. “labs are back.” She says, handing the papers to Dr Lindsay. “Cardiac enzymes are high. This is definitely a STEMI.” Dr Lindsay says thinking out loud. “ok, let’s get her to the cath lab. We need to start a central line and get a stent in her.” Dr Rachel called out to the rest of the ER team. “what… what’s going on?” a nervous Christine asked, still breathing heavily. “you’re having a heart attack and we have to put a stent in, ok?’” Dr Lindsay tells the nervous lawyer. “a heart attack?!” Christine asks in response, surprised at what she’s heard. “am I going to die?!” Christine continued. “you’re in great hands! We’ve seen plenty of heart attacks like this. We’re going to place a stent, keep you here for a day or two, and you should be good to go.” Dr Lindsay replies with relative confidence, oblivious to the fact of what was to come. “Can you call my mom? I’m scared…” Christine asks Lindsay, still short of breath, visibly in pain from the crushing pressure she felt in her chest. “of course! We’ll have one of the nurses reach out to her, ok?” Lindsay replies, reassuring.
Over the following few minutes, Christine is taken up to the cardiac catheterization lab. She’s laid flat on the table and her bra is removed, allowing her large, D cup breasts to spill out. “alright Christine, our resident Dr Sarah will place the line and the stent. We’ll be getting started shortly.” Dr Rachel tells the nervous attorney. “the resident? I don’t want her to practice on me…” Christine protests, having a gut feeling against having the resident perform the catheterization and stent placement. “don’t worry ma’am, me and Dr Lindsay have done these plenty of times. Sarah will have plenty of adult supervision!” Dr Rachel tells Christine, attempting to add a little comedic relief to the urgent situation. Christine still had a bad feeling about it, but ultimately agreed to let Sarah perform the procedure.
The upper right portion of Christine’s chest was splashed with betadine to sterilize the area. The resident identifies the superior midpoint of the clavicle, and moves down a few centimeters. This is the location of the subclavian vein, so it’s important that the correct location be identified in the early stages of the procedure. Next, a local anesthetic is injected into Christine’s chest to numb the skin and some of the underlying tissues. She winced in pain, feeling a pinch and a burn from the injection. It normally takes 45-60 seconds for the local anesthetic to numb the area effectively, so in the meantime, an ultrasound was set up. This is to further confirm the location of the subclavian vein, and to follow the catheter’s path once placed. Next, a hollow needle was advanced through the skin. Christine could feel the pressure of the needle being inserted, but no pain. The resident Sarah advanced the needle slowly into the beautiful attorney’s chest, looking at the ultrasound monitor. Eventually, the needle was in the correct depth and blood was aspirated. The needle was held in place for a moment while the blunt guide wire was maneuvered through the needle and into the subclavian vein. While inserting the guide wire, Sarah pulled it out and inserted it again quickly, unnoticed by Rachel or Lindsay. However, everything seemed fine at the time. But in that moment, unbeknownst to everyone, Sarah introduced an air bubble into the central line, which would now become a ticking time bomb.
Eventually, the guide wire and catheter were sent to the correct location, and the occluded coronary artery was identified. A small stent was navigated into the central line and carefully and methodically navigated to the correct location. Once the stent was in place, it was placed and opened, restoring blood flow to the previously blocked artery. After confirming the placement of the stent via ultrasound and x ray, the guide wire was removed and a port was left in the initial site to leave the central line open for the duration of Christine’s hospital stay.
After the procedure was completed, Christine was brought back to an exam room in the ER to wait until a bed opened up in the recovery area. “how’re you feeling?” Dr Lindsay asked. “I definitely notice a difference. Thank you…” Christine replied, no longer breathing heavily, and seemed a lot more calm than earlier. “look who’s here!” nurse Nancy says excited, bringing Christine’s 70 year old mother Marie into the room. Marie hurries over to the bed as fast as her 70 year old body can, and gives her daughter a hug and a kiss. “How are you doing sweetie? They said you had a heart attack!” the concerned mother asks. “I’m doing a lot better mom! Thanks for coming.” She replies, with a smile on her face. “we’ll leave you two alone. It’s been quite a day, right?” Dr Lindsay said, exiting the room with nurse Nancy.
Approximately 2 hours go by. “something’s wrong! Come in, quick!” 70 year old Marie shouts to the ER team while scurrying out of the exam room, visibly worried. Dr Lindsay, nurse Heather, and nurse Nancy head into the room. The heart monitors are chirping loudly, showing that Christine is severely hypotensive and tachycardic. Christine’s eyes are shut, but she’s groaning. “christine? What’s wrong?” Dr Lindsay asks, doing a gentle sternal rub, to which Christine doesn’t respond. “she passed out and won’t wake up! What happened?!” Marie asks in a panicked tone. “We’re gonna get to the bottom of this, ok?” Dr Lindsay replied. Heather shined a pen light into Christine’s eyes and both pupils were fixed and dilated. “Pupils blown Linds” Heather tells Lindsay, shaking her head. “lets get her intubated! Get cardio back down here NOW!” Lindsay shouts, wondering what the hell just happened. “christine? Can you squeeze my hand?” Lindsay asks, receiving no response. Marie was holding her daughter’s other hand and talking to her while chaos ensued. “get me a 7.0 ET tube!” Lindsay shouted.
The ET tube was being navigated carefully into the woman’s airway by Lindsay. “no pulse, starting compressions!” Heather called out. “damn it!” Lindsay said frustrated, finishing her rapid sequence intubation. Heather delivered deep, violent chest compressions on Christine while her 70 year old mother continued to hold her hand and stroke her hair. “she’s in PEA. Push epi and atropine. And where the hell’s cardio?!” Dr Lindsay shouted again, frustrated. While Lindsay ambu bagged and lead the code, Heather continued delivering CPR. Christine’s chest caved in, and her belly jiggled outwards. Her breasts shook and trembled from the residual force of the compressions being received.
Dr Rachel and Sarah enter the room and are shocked, seeing their seemingly stable patient having her chest pumped violently. “what happened?!” Rachel asked, stunned. “I figured you two might try to figure that out for us. Any ideas?” Lindsay replied sternly. “what do you mean? She was fine a little while ago!” Rachel replied. “sarah even did a good job on her first stent placement and central line.” Rachel continued. “wait! This was the first time she ever operated on someone?!” Marie shouted, overhearing what was said. “ma’am… believe me, she is absolutely qualified. And every procedure has its risks.” Rachel replied, jumping to Sarah’s immediate defense. “did she kill my baby girl?!” Marie asked, becoming teary eyed. “Ma’am, why don’t we bring you to a private waiting room while the doctors work.” Nurse Nancy suggested, trying to gently direct the 70 year old woman out of the room. “no no no, I’m not going anywhere! That’s my daughter!” Marie shouted, tears running down her face, still holding her daughter’s hand as her chest was being absolutely pummeled.
The heartbreaking scene was interrupted by Dr Lindsay announcing that v-fib was on the monitors. “alright, charge the paddles to 200.” Lindsay called out. Nancy gently made Marie back away from the table because of the impending shock. The paddles were pressed up against Marie’s bare chest, the ambu bag was temporarily detached, and the shock was delivered. Marie’s body flopped on the table while a KA-THUNK was heard in the room. “still no change, charge to 250.” Lindsay called out, shaking her head a bit. After a cycle of compressions, the next shock was delivered. The electricity ran through the 47 year old’s limp, lifeless body, causing her to twitch sharply in response. “no pulse, let’s hit her again at 300.” Lindsay responded, looking at the monitors. “please… save my baby! That’s my little girl!” Marie begged the team while living every parent’s worst nightmare. “paddles charged.” Heather called out. The defibs were placed back onto Christine’s chest, and shock #3 was promptly delivered. Christine’s feet kicked up above the table and slammed back down half a second later, showing off the deep, soft, silky, prominent wrinkles throughout the soles of her size 7 feet. “still nothing doc.” Heather said, having 2 fingers placed on Christine’s neck for a carotid pulse. The paddles were recharged, and in a moment’s notice, Christine was shocked at 360j. Her body reacted more violently to the stronger shock, with her eyes opening up halfway, staring blankly up above. “PEA, resuming compressions.” Dr Lindsay said, taking over CPR for Heather.
More meds were pushed while CPR went on. However, it took another 6 minutes to produce another shockable rhythm. Nonetheless, when v-fib appeared on the monitors again, the paddles were recharged to 360 joules, and Christine was shocked again. Marie’s lifeless body twitched abruptly in reaction to the shock while her eyes remained open, staring blankly at the ceiling above. After another cycle of chest compressions, the next shock was delivered, causing Christine’s toes to curl, once again showing off the deep, soft wrinkles in the soles of her feet. But unfortunately at that point, the code started to become more redundant: CPR, shock, meds, repeat.
It was now 24 minutes into the code and Christine was still in v-fib. Her complexion was a ghastly pale color, her skin was ice cold to the touch, and there was a huge bruise on the center of her chest from all the CPR she’d received. At that point it was Dr Rachel doing CPR while Lindsay still ran the code. Lindsay looked around the room, eventually making eye contact with Rachel. Lindsay shook her head at Rachel, knowing Christine wasn’t coming back. Dr Rachel backed off, and nurse Heather detached the ambu bag. “what’s going on? Why are you stopping?” Marie asked the team, still holding her daughter’s hand. “I’m so sorry ma’am…” Dr Lindsay said, before Marie interrupted, “no no no! Shock her again! Keep pounding her chest! There’s gotta be SOMETHING you can do, right?!” Dr Lindsay paused for a moment, then said “I’m so sorry ma’am. We did everything we could. Your daughter’s heart won’t restart, and her brain has been deprived of oxygen for so long.” Marie started to cry at the point, practically crumbling to the floor. “time of death, 8:45pm.” Dr Lindsay said, peeling her gloves off. “no no no!” Marie wept. Nurse Nancy scurried over to try and console the woman while Heather began basic postmortem care.
The monitors were switched off, the EKG electrodes were disconnected, and the ambu bag was detached. A toe tag was filled out and placed on the big toe of Christine’s left foot, dangling in front of her beautiful, wrinkly soles. Her body was covered up, but Heather lowered the blanket down to Christine’s shoulders so Marie could have as much time as she needed to grieve her daughter’s tragic passing.
Since the exact cause of Christine’s death was unknown, an autopsy was ordered. The results of said autopsy concluded that Christine died from an air embolism that traveled to her brain. Essentially, air was introduced in the central line by Sarah, and it eventually traveled to the brain and got stuck in the smaller, more delicate vessels there. With these findings in mind, Marie was able to sue the hospital for Malpractice and received a hefty settlement payment. It was an absolute tragedy that Marie witnessed the death of her own daughter, and it was also a bit ironic that a medical malpractice attorney died from medical malpractice.
72 notes
·
View notes
Text


is anything the way it used to be
[ID: flat color digital art of 2 anthro dogs from the chest up, morgan and sasha. sasha is a tall, thin longhaired orange and white belgian sheepdog/siberian husky with an atrophied and weak face, forehead wrinkles, freckles, a snow nose, messy bangs, and orange eyes, and morgan is a short, fat black and white siberian husky with splotches over his eyes and freckles near his nose. his bangs are combed to the side, he has stubble, and his left ear is torn.
1: both have their foreheads pressed together, and their eyes closed. sasha is wearing a pale brown sweater under a grey brown coat, and morgan is wearing a grey purple collared shirt under a black coat with a black and grey striped tie under the lapels. the background is orange.
2: morgan is hugging sasha with his face pressed into their shoulder and his hands clenched in their coat, eyes squeezed shut. he has a silver shield shaped pin with a red lowercase cursive D whose tail swings into a heart in between his fingers. sasha looks apprehensive. above them, a larger version of the same pin morgan holds instead depicts an anatomical heart with right ventricular hypertrophy. sasha is wearing a red brown coat with a lot of loose lint, and morgan is wearing a black collared shirt under a lighter black coat. the background is light grey purple. end ID]
32 notes
·
View notes
Text
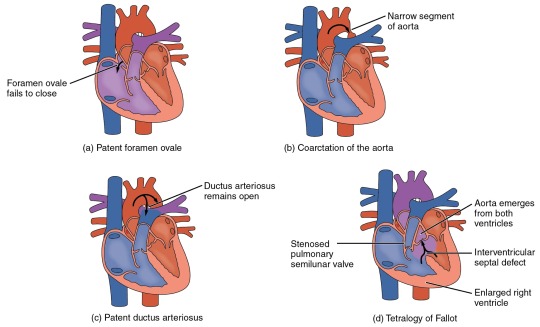
Heart Defects
Heart Defects
One very common form of interatrial septum pathology is patent foramen ovale, which occurs when the septum primum does not close at birth, and the fossa ovalis is unable to fuse. The word patent is from the Latin root patens for “open.” It may be benign or asymptomatic, perhaps never being diagnosed, or in extreme cases, it may require surgical repair to close the opening permanently. As much as 20–25 percent of the general population may have a patent foramen ovale, but fortunately, most have the benign, asymptomatic version. Patent foramen ovale is normally detected by auscultation of a heart murmur (an abnormal heart sound) and confirmed by imaging with an echocardiogram. Despite its prevalence in the general population, the causes of patent ovale are unknown, and there are no known risk factors. In nonlife-threatening cases, it is better to monitor the condition than to risk heart surgery to repair and seal the opening.
Coarctation of the aorta is a congenital abnormal narrowing of the aorta that is normally located at the insertion of the ligamentum arteriosum, the remnant of the fetal shunt called the ductus arteriosus. If severe, this condition drastically restricts blood flow through the primary systemic artery, which is life threatening. In some individuals, the condition may be fairly benign and not detected until later in life. Detectable symptoms in an infant include difficulty breathing, poor appetite, trouble feeding, or failure to thrive. In older individuals, symptoms include dizziness, fainting, shortness of breath, chest pain, fatigue, headache, and nosebleeds. Treatment involves surgery to resect (remove) the affected region or angioplasty to open the abnormally narrow passageway. Studies have shown that the earlier the surgery is performed, the better the chance of survival.
A patent ductus arteriosus is a congenital condition in which the ductus arteriosus fails to close. The condition may range from severe to benign. Failure of the ductus arteriosus to close results in blood flowing from the higher pressure aorta into the lower pressure pulmonary trunk. This additional fluid moving toward the lungs increases pulmonary pressure and makes respiration difficult. Symptoms include shortness of breath (dyspnea), tachycardia, enlarged heart, a widened pulse pressure, and poor weight gain in infants. Treatments include surgical closure (ligation), manual closure using platinum coils or specialized mesh inserted via the femoral artery or vein, or nonsteroidal anti-inflammatory drugs to block the synthesis of prostaglandin E2, which maintains the vessel in an open position. If untreated, the condition can result in congestive heart failure.
Septal defects are not uncommon in individuals and may be congenital or caused by various disease processes. Tetralogy of Fallot is a congenital condition that may also occur from exposure to unknown environmental factors; it occurs when there is an opening in the interventricular septum caused by blockage of the pulmonary trunk, normally at the pulmonary semilunar valve. This allows blood that is relatively low in oxygen from the right ventricle to flow into the left ventricle and mix with the blood that is relatively high in oxygen. Symptoms include a distinct heart murmur, low blood oxygen percent saturation, dyspnea or difficulty in breathing, polycythemia, broadening (clubbing) of the fingers and toes, and in children, difficulty in feeding or failure to grow and develop. It is the most common cause of cyanosis following birth. The term “tetralogy” is derived from the four components of the condition, although only three may be present in an individual patient: pulmonary infundibular stenosis (rigidity of the pulmonary valve), overriding aorta (the aorta is shifted above both ventricles), ventricular septal defect (opening), and right ventricular hypertrophy (enlargement of the right ventricle). Other heart defects may also accompany this condition, which is typically confirmed by echocardiography imaging. Tetralogy of Fallot occurs in approximately 400 out of one million live births. Normal treatment involves extensive surgical repair, including the use of stents to redirect blood flow and replacement of valves and patches to repair the septal defect, but the condition has a relatively high mortality. Survival rates are currently 75 percent during the first year of life; 60 percent by 4 years of age; 30 percent by 10 years; and 5 percent by 40 years.
In the case of severe septal defects, including both tetralogy of Fallot and patent foramen ovale, failure of the heart to develop properly can lead to a condition commonly known as a “blue baby.” Regardless of normal skin pigmentation, individuals with this condition have an insufficient supply of oxygenated blood, which leads to cyanosis, a blue or purple coloration of the skin, especially when active.
Septal defects are commonly first detected through auscultation, listening to the chest using a stethoscope. In this case, instead of hearing normal heart sounds attributed to the flow of blood and closing of heart valves, unusual heart sounds may be detected. This is often followed by medical imaging to confirm or rule out a diagnosis. In many cases, treatment may not be needed.
#atomic heart#science#biology#college#education#school#student#medicine#doctors#health#healthcare#nursing#physiology#pathology
3 notes
·
View notes
Text
Understanding the R Wave ECG: Insights into Cardiac Health

The R wave is a key component of the QRS complex on an ECG, signifying the depolarization of the heart's ventricles, which precedes their contraction. It is the first positive deflection following the P wave, typically the most prominent part of the QRS complex. The R wave's amplitude and duration (usually 0.06 to 0.10 seconds) help determine the heart's electrical axis, which is crucial for diagnosing various conditions.
High R waves can indicate ventricular hypertrophy or conduction abnormalities, with specific patterns suggesting right or left ventricular hypertrophy. Low R waves may point to a previous myocardial infarction or conditions like COPD, pericardial effusion, or cardiomyopathy. Normal R wave progression shows increasing amplitude from V1 to V6, while poor progression may indicate an anterior myocardial infarction or left bundle branch block.
Interpreting R wave abnormalities requires considering the patient's clinical history, symptoms, and other ECG findings. Serial ECGs are valuable for tracking changes over time. By analyzing the R wave, healthcare providers can gain critical insights into the heart's electrical activity and structural health, aiding in the diagnosis and management of various cardiac conditions.
0 notes
Text
Poor R wave Progression
Author:- Mr. Ritesh Sharma
Poor R Wave Progression is a well-known abnormality within the domain of an electrocardiogram as you must already know an ECG test is performed to check the electrical activity of the heart over a period of time. Furthermore, the heart abnormalities in an ECG test are determined in waveforms. So, R Wave is one of these waves that can potentially show abnormalities on an ECG test. However, poor R wave progression is associated with abnormalities in the heart’s conduction system.
Now, before we proceed to explain the poor R wave progression, let us try to understand the conduction system of the heart. This system is responsible for orchestrating the circulation system in the body. It consists of the natural pacemaker of the heart, i.e., the sinoatrial node, and different other parts such as the atrioventricular node, the bundle of his, and Purkinje fibers. This system lets the heart contract and relax through the chambers of the heart and supply blood to all vital parts of the body.
Therefore, the abnormalities related to this conduction system of the heart are showcased on an abnormal ECG through poor R wave progression. In this blog, we will cover the intricacies of PRWP, exploring its causes, diagnostic significance, and treatment options.
Understanding Poor R Wave Progression (PRWP)
The R wave in an ECG represents the electrical depolarization of the ventricles, specifically the left ventricle. Normally, as the ECG leads move from the right side of the chest to the left, the R wave should gradually increase in amplitude. However, in cases of poor R wave progression, this progression is blunted or absent. PRWP can indicate several underlying cardiac issues, ranging from benign anatomical variations to more serious conditions like myocardial infarction or left ventricular hypertrophy.
Causes of Poor R Wave Progression
Anatomical Variations: In some individuals, the positioning of the heart within the chest cavity can lead to atypical ECG patterns, including PRWP. While these variations are often benign, they can cause confusion during interpretation, necessitating further evaluation to rule out pathology.
Myocardial Infarction (Heart Attack): One of the most concerning causes of PRWP is myocardial infarction, commonly known as a heart attack. When a portion of the heart muscle is deprived of oxygen due to a blocked coronary artery, it can result in abnormal electrical activity, including changes in the ECG pattern such as PRWP.
Left Ventricular Hypertrophy (LVH): LVH is a condition characterized by an enlargement and thickening of the muscle wall of the left ventricle. This can occur due to hypertension, aortic stenosis, or other underlying cardiac conditions. In LVH, the ECG may show signs of abnormal electrical conduction, including poor R wave progression.
Cardiomyopathy: Various forms of cardiomyopathy, including hypertrophic cardiomyopathy and dilated cardiomyopathy, can lead to changes in the ECG pattern, including PRWP. These conditions involve abnormalities in the structure and function of the heart muscle.
Pericardial Effusion: Pericardial effusion refers to the accumulation of fluid around the heart. This can compress the heart and interfere with its electrical conduction system, resulting in abnormal ECG findings, including poor R wave progression.
Diagnosis of Poor R Wave Progression
Diagnosing PRWP begins with obtaining an ECG. The ECG waveform is analyzed to determine the presence and severity of poor R wave progression. However, it’s essential to consider the clinical context alongside the ECG findings. A thorough medical history, physical examination, and additional tests such as echocardiography, stress testing, or cardiac MRI may be necessary to identify the underlying cause of PRWP.
Treatment and Management
The management of PRWP depends on its underlying cause:
Anatomical Variations: If PRWP is attributed to benign anatomical variations, no specific treatment may be required. However, periodic monitoring and follow-up ECGs may be recommended to ensure stability.
Myocardial Infarction: In cases where PRWP is indicative of myocardial infarction, prompt intervention is crucial. Treatment may involve medications to relieve symptoms, reperfusion therapy (such as thrombolytics or percutaneous coronary intervention) to restore blood flow to the affected area, and lifestyle modifications to reduce the risk of future events.
Left Ventricular Hypertrophy: Management of LVH focuses on addressing the underlying cause, such as controlling hypertension or treating aortic stenosis. Medications to reduce blood pressure or surgical interventions may be necessary in some cases.
Cardiomyopathy: Treatment of cardiomyopathy aims to manage symptoms, slow disease progression, and reduce the risk of complications. This may involve medications such as beta-blockers, ACE inhibitors, or diuretics, as well as lifestyle modifications and, in some cases, surgical interventions such as implantable cardioverter-defibrillator (ICD) placement or heart transplantation.
Pericardial Effusion: Management of pericardial effusion depends on its severity and underlying cause. Small effusions may resolve on their own, while larger effusions or those causing symptoms may require drainage procedures such as pericardiocentesis or surgical intervention.
The Poor R Wave Progression can be classified as a tricky condition on an electrocardiogram. Furthermore, the underlying causes of this condition are also hard to deal with. However with the correct treatment approach considering the underlying condition and its severity, healthcare professionals can cure this condition after being detected on an electrocardiogram.
Prompt recognition and appropriate management of PRWP are essential for optimizing patient outcomes and preventing further cardiac complications. As with any cardiac abnormality such as cardiac arrhythmias or heart dysfunctions, a multidisciplinary approach involving cardiologists, primary care physicians, and other healthcare providers is key to providing comprehensive care to patients with poor R wave progression.
0 notes
Text
31. Who inspires you?
Everybody and many things inspire me. My problem at this age is my nearly impossible power to do anything about that inspiration.
In the Spring when I was 12 years old, I had my appendix removed which in those days meant ether as an anesthetic and a week in hospital. I can still experience nausea at the very thought of ether. However the experience of being cared for by nurses was likely an experience that impacted the rest of my life. I was always allowed to remove wood splinters from my Dad’s hands when I was very little and not very adept. I liked hearing my Dad call me his nurse but after my appendectomy the mold was cast. My destiny was determined.
At my 40th high school class reunion we all agreed without exception, that our favorite teacher was Miss Gillis. She was a matronly woman of maybe 50 years who always had her hair done neatly. She was an inspiration to all of us. And she was certain we could all love Shakespeare and despite all evidence to the contrary she was sure we could all go on to higher education. She was right in most cases.
When my granddaughter, Anne was in third grade she wrote a Greek play which in itself was quite remarkable. She called me one evening to ask if I could make her costumes as her play was going to be performed for her school. The play was two days hence. Luckily, both men and women of Greece at that time wore chitons. These were short robes tied at the waist with rope. That was easy enough. Anne was the narrator and had a crown and her mother’s jewelry. The play was a success and made the Newburyport newspaper’s front page. How could I not be inspired.
I lived on my farm in Warner for 22 years. It was a beautiful place tucked in a valley in the Mink Hills. It also had a swimming pool and my grandchildren spent time there with me during their summer vacation. On one such occasion they came pool ready. They were wearing their swimsuits and
carrying colorful pieces of foam called noodles. I asked if they really held them above water or how many noodles would be needed to keep me afloat. Merton suggested I might need 17 noodles. Anne immediately came to my defense chiding Merton for implying I was heavier than I ought to be. I assured both that there was no harm done and it would be our secret. I could tell them I stuck to my diet by announcing I lost a noodle or two. My grandchildren have all inspired me to be a better person as each of them certainly are such.
My friend, David inspires me. He has a hereditary eye condition that never seemed to limit him. He graduated from Harvard and went on to Medical School. He is now chief of staff in Neuro Oncology in a very large teaching hospital. Years before when he was taking a gap year deciding to go into law or medicine, we worked together. One weekend we went up to Cranmore, NH to watch the US tennis open. We saw Michael Chang play. I don’t know how much of the match David actually saw but it was a well spent and inspiring day.
It is my brother, Kip who inspires me to this day. He has been gone for more than 15 years now but remains vivid in my memory. We share the dubious distinction of having hereditary, congenital anomalies. He had Epstein’s disease and was actually missing a large portion of his right atrium. i have a ventricular hypertrophy that inhibits the closing of the mitral valve. My problem was not diagnosed until I was in my 70’s. Kip’s was evident at birth and my parents were cautioned not to send him to school fearing it would be too taxing. He graduated from high school and went on to have a successful career with the Imperial Bank. I am now experiencing the shortness of breath that Kip knew from birth. I can now relate to the admission Kip made to me two months before he died. He without rancor, but as a matter of fact, said he did not wish to just be here taking up space and using up Oxygen. I now know of what he spoke. He will inspire me to the end.
0 notes
Text
AUTOPSY REPORT
Name of Deceased: Noah Jenkins Age: 21 Sex: Male Height: 6’0” Weight: 165 lbs Hair: Red Date of Death: [DATE] Time of Death: 2:34 PM Location of Death: University Gym Cause of Death: Massive Acute Myocardial Infarction (Heart Attack) Genetic Disorder Identified: Familial Hypertrophic Cardiomyopathy (FHC)
EXTERNAL EXAMINATION
The body is that of a well-developed, well-nourished 21-year-old Caucasian male with a lean and fit physique. The decedent is 6 feet tall and weighs 165 pounds. His red hair is matted with perspiration. The skin appears pale with light freckling, particularly on the face and shoulders.
Trauma Related to Resuscitation: Significant damage is evident from aggressive resuscitation efforts. The chest shows extensive bruising and ecchymosis across the sternum and left anterior thorax. Palpation reveals multiple fractures, with three ribs on the left side (ribs 3, 4, and 5) and two ribs on the right side (ribs 4 and 5) clearly fractured, consistent with intense CPR chest compressions.
The sternum itself is fractured at its midline, a common injury during forceful chest compressions. The surrounding tissue exhibits deep hemorrhaging, with extensive subcutaneous bruising extending laterally.
Despite these injuries, the decedent's airway and thoracic cavity remain intact, with no signs of tracheal trauma from intubation attempts.
INTERNAL EXAMINATION
Cardiovascular System:
The heart is significantly enlarged, weighing 550 grams (normal adult male heart weight is 300-350 grams), showing pronounced hypertrophy due to familial hypertrophic cardiomyopathy (FHC). This genetic condition led to abnormally thickened heart muscle walls, impairing the heart's ability to pump blood effectively.
Left Ventricle: The left ventricular wall is notably thickened, measuring 2.5 cm (normal thickness is 1.0-1.5 cm). The left ventricular cavity is constricted as a result, indicating severe hypertrophic cardiomyopathy. There are large regions of fibrosis within the septum and posterior wall of the left ventricle, suggesting long-standing damage from chronic strain on the heart.
Right Ventricle: The right ventricular wall, while less affected, also shows signs of mild hypertrophy with a thickness of 1.0 cm (normal is 0.5 cm).
Coronary Arteries: There is no significant coronary artery blockage or atherosclerosis, ruling out coronary artery disease as the cause of the heart attack.
Myocardium: A large myocardial infarction is present on the posterior wall of the left ventricle, covering approximately 40% of the myocardial tissue. The infarcted area is dark, necrotic, and extends deeply into the heart muscle, consistent with a massive acute myocardial event. Scarring and fibrosis around the infarcted region indicate previous ischemic episodes, likely leading up to the fatal heart attack.
Lungs:
Both lungs are congested and edematous, weighing 600 grams each (normal weight is 450 grams). Frothy fluid is present in the bronchi, consistent with pulmonary edema secondary to acute heart failure. The presence of pink, frothy material is noted around the nose and mouth, indicating fluid leakage during resuscitation efforts.
Chest Wall:
Extensive hemorrhaging is noted in the intercostal muscles and subcutaneous tissues adjacent to the rib fractures. The left and right ribs (3, 4, 5) on both sides are fractured, with associated contusions and bleeding in the surrounding muscle tissue. The mid-sternum is fractured, and the surrounding connective tissues show significant hemorrhagic infiltration.
The pleural spaces are free of fluid or air, ruling out pneumothorax as a contributing factor.
Abdomen and Other Organs:
The liver is mildly enlarged, indicating passive congestion secondary to heart failure. The spleen, kidneys, and gastrointestinal tract appear grossly normal on inspection. The stomach contains partially digested food, consistent with the decedent’s last known meal 3 hours prior to death.
CONCLUSION
Noah Jenkins died as a result of a massive acute myocardial infarction precipitated by familial hypertrophic cardiomyopathy (FHC), a rare genetic disorder causing excessive thickening of the heart muscle. The significant cardiac hypertrophy, particularly in the left ventricle, compromised blood flow and caused a fatal heart attack. Extensive resuscitation efforts, including forceful chest compressions, resulted in multiple rib fractures, a fractured sternum, and severe bruising of the chest wall. Despite these efforts, the damage to the heart was too severe to overcome.
Manner of Death: Natural Cause of Death: Massive Acute Myocardial Infarction due to Familial Hypertrophic Cardiomyopathy

67 notes
·
View notes
Text
Pseudocoarctation of the aorta by Phong Teck Lee in Journal of Clinical and Medical Images, Case Reports

Introduction
Pseudocoarctation of the aorta was first described in 1951 and remains a relatively rare congenital anomaly [1]. We present a case of pseudocoarctation of the aorta which was detected on magnetic resonance imaging (MRI).
Case Report
A 19-year-old gentleman with suspected coarctation of aorta was referred for magnetic resonance imaging (MRI). Twelve-lead electrocardiogram showed right bundle branch block. On clinical examination, there was a soft ejection systolic murmur in the left parasternal edge. He was asymptomatic with excellent exercise capacity. MRI was performed with Siemens Aera 1.5-Tesla scanner. The entire aorta was reconstructed with three-dimensional rendered imaging from non-contrast aortogram. The aorta appears elongated an unusually “high” aortic arch up to the level of the clavicle. This results in a large distance between the aortic arch and the pulmonary artery bifurcation. The descending aorta is mildly kinked at the level of the ligamentum arteriosum with no significant stenosis (Figure/Video). Phase contrast assessment at the level of the kink demonstrated absence of significant stenosis with a maximal velocity of 1.5 m/s. There was no evidence of collateral artery formation. The left ventricular volumes and systolic function were normal and there was no evidence of myocardial hypertrophy. These findings are consistent with a diagnosis of pseudocoarctation of the aorta.
Discussion
Pseudocoarctation of the aorta consists of elongation and kinking of the aortic arch and narrowing of the aortic isthmus without significant obstruction. The exact etiology of pseudocoarctation of the aorta is unknown. Postulated embryologic cause include failure of the compression of the third through the seventh segments of the dorsal aortic roots and the fourth arch segment [2].
Features of pseudocoarctation of the aorta are best visualized using three-dimensional reconstruction of the aorta by computed tomography (CT) or magnetic resonance imaging (MRI). The elongation of the arch frequently produces an unusually high aortic arch in the mediastinum and increased distance between the origins of left common carotid artery and left subclavian artery [3]. The left subclavian artery also has a more caudal origin [3]. Other features include absence or only a mild degree of luminal stenosis, absence of collateral circulation and absence of left ventricular hypertrophy and ascending aortic dilatation [4]. These features are present in our case. Concomitant congenital heart lesions have been reported in association with pseudocoartation of the aorta [2]. It could also be associated with distal aneurysmal dilatation. While pseudocoarctation of the aorta is usually benign, cases of aneurysm formation and rupture have been reported [2, 3]. As such, surgical treatment is recommended for symptomatic cases, or those associated with aneurysm formation, and regular follow-up for asymptomatic patients.
This case highlights the imaging features of pseudocoarctation of the aorta. We demonstrated the utility of CMR, especially three-dimensional reconstruction imaging and phase contrast assessment in the diagnosis of pseudocoarctation of the aorta.
For more details : https://jcmimagescasereports.org/author-guidelines/
#Pseudocoarctation#magnetic resonance imaging#asymptomatic#aortic#ligamentum#myocardial hypertrophy#embryologic#computed tomography#Phong Teck Lee#JCMICR
0 notes
Text
Understanding Tetralogy Of Fallot Pathophysiology: A Comprehensive Overview

Tetralogy of Fallot (TOF) is a serious congenital heart defect characterized by four primary abnormalities: a ventricular septal defect (VSD), pulmonary stenosis, right ventricular hypertrophy, and an overriding aorta. These defects lead to a mix of oxygen-poor and oxygen-rich blood being pumped into the body, causing a bluish discoloration of the skin known as cyanosis.
In TOF, the VSD allows blood to flow between the left and right ventricles, mixing oxygen-rich and oxygen-poor blood. Pulmonary stenosis, which is a narrowing of the outflow tract from the right ventricle to the pulmonary artery, obstructs blood flow to the lungs. This causes the right ventricle to thicken and enlarge due to increased workload. The overriding aorta is positioned above the ventricular septal defect, enabling both oxygen-poor and oxygen-rich blood to enter the systemic circulation.
Symptoms of TOF include cyanosis, difficulty breathing, and "tet spells," which are sudden episodes of severe cyanosis, often triggered by activities that increase oxygen demand, such as crying or feeding. Surgical intervention, usually performed in early infancy, involves patching the VSD and relieving the obstruction caused by pulmonary stenosis, thereby improving blood flow to the lungs and overall oxygenation.
0 notes
Text
Giving Little Hearts a Second Chance: Surgical Treatment of Congenital Heart Disease
For many families, the news of a congenital heart defect (CHD) in their child can be overwhelming. These structural abnormalities in the heart, present from birth, can disrupt normal blood flow and pose significant health risks. However, advancements in medical technology and the expertise of skilled pediatric cardiologist like Dr. Nidhi Rawal offer hope and a chance for a normal life for these children.
Understanding CHDs:
CHDs encompass a wide range of conditions, varying in severity and complexity. Some common examples include:
Ventricular septal defect (VSD): A hole in the wall separating the heart's lower chambers.
Atrial septal defect (ASD): A hole in the wall separating the heart's upper chambers.
Tetralogy of Fallot: A combination of four heart defects, including VSD, pulmonary valve stenosis, right ventricular hypertrophy, and an overriding aorta.
Coarctation of the aorta: A narrowing of the aorta, the main artery carrying blood from the heart to the body.
Symptoms and Early Detection:
While symptoms can vary depending on the specific defect, some common signs in newborns and infants include:
Bluish tint to the skin, lips, or nail beds (cyanosis)
Rapid breathing or difficulty breathing
Poor feeding or failure to thrive
Excessive sweating during feeding
Tiring easily during activities
Early detection is crucial for optimal outcomes. Prenatal screening, echocardiograms (ultrasound of the heart), and pulse oximetry can help identify CHDs before or shortly after birth.
The Role of Surgery:
Surgery plays a vital role in treating many CHDs. Depending on the specific condition and its severity, different surgical approaches may be employed:
Open-heart surgery: This involves stopping the heart, using a heart-lung bypass machine to circulate blood, and accessing the heart through an incision in the chest. The surgeon then repairs the defect using various techniques, such as patching holes, widening narrowed valves, or replacing damaged valves.
Minimally invasive cardiac surgery: This approach utilizes smaller incisions and specialized instruments to perform certain procedures, offering faster recovery times and less scarring.
Catheter-based interventions: In some cases, catheters inserted through blood vessels can be used to repair or open narrowed valves or close small defects.
Dr. Nidhi Rawal's Expertise:
Dr. Nidhi Rawal is a highly skilled and experienced pediatric cardiologist dedicated to providing comprehensive care for children with CHDs. With extensive training and expertise in various surgical techniques, she is committed to offering the most appropriate and advanced treatment options for each individual case.
Benefits of Surgical Intervention:
Successful surgical intervention for CHDs can significantly improve a child's quality of life. Benefits include:
Improved blood flow and oxygenation
Reduced symptoms like shortness of breath and fatigue
Normal growth and development
Increased life expectancy
Living with a CHD:
Following surgery, regular follow-up care with a cardiologist is essential to monitor the child's health and ensure optimal long-term outcomes. Depending on the complexity of the CHD, some children may require additional interventions or medications throughout their lives.
Hope for the Future:
While CHDs can be challenging, advancements in surgical techniques and the dedication of skilled professionals like Dr. Nidhi Rawal offer immense hope for children with these conditions. Early detection, proper treatment, and ongoing care can empower these children to lead healthy and fulfilling lives.
Remember:
If you have any concerns about your child's heart health, consult a pediatrician or pediatric cardiologist promptly.
Early diagnosis and intervention are crucial for optimal outcomes.
Surgical advancements offer hope and a chance for a normal life for children with CHDs.
Dr. Nidhi Rawal is a dedicated and experienced pediatric cardiologist committed to providing the best possible care for your child.
By working together, we can ensure that every child with a CHD has the opportunity to thrive and reach their full potential.
1 note
·
View note
Text

From The Only EKG Book You'll Ever Need.
I think when I learned this in school it was the R wave in lead V5 or V6 (whichever one is taller) plus the S wave in V1 or V2 is greater than 35 mm.

3 notes
·
View notes
Text
Subchronic toxicity of the pulmonary hypertension model due to low-dose monocrotaline in rats
Subchronic toxicity of the pulmonary hypertension model due to low-dose monocrotaline in rats @JPPRes

Image: pixabay Article published in Journal of Pharmacy & Pharmacognosy Research 8(4): 308-315, 2020. Vicente Benavides-Cordoba1, Melissa Silva-Medina1, María Ximena Varela2,3, Mauricio Palacios Gómez1*
1Department of Pharmacology, Basic Sciences School, Universidad del Valle. Cali, Colombia.2Department of Pathology, Universidad del Valle. Cali, Colombia.3Department of Pathology,…
View On WordPress
0 notes
Photo

The Non-Believer - Horror at Camp Covaire AU
As the youngest child born to two doctors, logic and facts have always dominated Israel’s world. He was never one to believe in childhood fantasies such as Santa Claus, the Easter Bunny, the Tooth Fairy, or even the Boogeyman. And he definitely doesn’t believe in true love. For most of his life Israel was homeschooled due to left ventricular hypertrophy (LVH) and hypertrophic cardiomyopathy (HOCM). Having to always watch from the sidelines at age ten he had asked his parents if he could go to Camp Covaire like his brother before him.
After begging them for months on end they finally relented but still had their doubts. Ultimately, they had been right. After over-exerting himself while swimming Israel had landed himself in the hospital and underwent a heart transplant. Everyone at camp thought he had died and for awhile he became another ghost story.
In all honesty his chance of survival had been low. Many proclaimed it was a miracle the boy had lived. Others claimed it was God’s mercy that let Israel see birthday after birthday. Israel just claims it was dumb luck and science. God isn’t real. And if he was why would he save him of all people? He was nothing remarkable. He was a quiet wallflower. And despite being popular and attractive he had a reputation for turning down those who approached him romantically. Why? He simply doesn’t believe in love. Or anything else for that matter.
Now at age 21, Israel has returned from attending university abroad in Sweden to attend camp, this time as a camp counselor. Back at university he is head of the archery team and usually can be found at the archery range on site. But he keeps his distance far from the lake for obvious reasons. Many of the campers and his fellow staff members call him Izzy, much to his unexpressed annoyance. Personality-wise, he tends to keep more to himself than the more boisterous staff and tends to hide in the background. Although, it's clear he has a soft spot of the shy and homesick campers.
As far as monsters and things that go bump in the night... They’re just campfire stories. Or are they?
3 notes
·
View notes
Text
Heart of a Champion
Iva Haugen was an up and coming professional tennis player from Norway. She was a naturally beautiful 23 year old girl who was tall and thin, standing at 5'9 with platinum blonde hair, blue eyes, and fair skin. Iva was in the United States training for an upcoming professional tournament with her coach and a few other pros who were participating in the same event as her. Her training was going exceptionally well, and she really liked her chances in the upcoming tournament. Unfortunately, fate had other plans for the young tennis pro.
After several hours of training yesterday, Iva felt as if her heart was going to leap right out of her chest. She attributed this symptom to nerves about the tournament and excessive exercise that day. While showering off back at her hotel a little while later, the heart palpitations returned along with sudden, severe dizziness. After her shower, she felt a bit tired and decided to take a nap.
Approximately 30 minutes later, one of the other tennis pros who was friends with Iva entered the room; it was Lara Nielson, a Swiss tennis player. Lara wanted to see if Iva wanted to hang out that night and grab as bite to eat. However, Lara found her friend passed out on the bed and not responding to her voice. Lara shook Iva, but all she did was groan weakly. Iva's eyes were rolled back and her breaths were fast but shallow. Lara knew something was seriously wrong, so she called 911.
When paramedics arrived on scene minutes later, Iva was still drifting in and out of consciousness. The medics asked Lara a few questions in an attempt to assess the situation. The questions were routine such as: “any medical conditions?” “is she on any medications?” “did she use any illegal drugs?” “any injuries that you know of?” “any allergies?” Lara answered no to all these questions and insisted that Iva was previously healthy.
After that brief conversation with Lara, the medics began their initial assessment and set up equipment. One medic snipped off Iva's top, only sparing her dark blue bra to set up a 5 lead ECG with a portable heart monitor. Medic #2 set up 2 large bore IVs and hung a bag of normal saline, and then set Iva up on an o2 mask with high flow oxygen. On scene, Iva's vital signs were: BP 162/103, heart rate 126bpm, pulse oxygenation 98%. The ECG showed Unifocal PVCs with narrow Q waves. With these stats in mind, the medics knew this was a cardiovascular issue of some sort and decided to bring her into the ambulance and transport her to the emergency department.
During transport, the medics pushed vasodilators in order to regulate her blood pressure, but the young tennis star continued to deteriorate. Her heart rate was dangerously high, and she was now unconscious, barely breathing. For the purpose of airway management, the medics decided to intubate their attractive, foreign patient. Her head was tilted back and the laryngoscope was placed into her mouth. A 7.0 ET tube was then carefully navigated into her airway, and secured with a blue tube holder.
Around 2 minutes after intubation, the young athlete’s condition worsened, converting to pulseless v-tach. The medic snipped off Iva’s dark blue bra, exposing her B cup breasts. The defibrillator pads were then stuck onto her bare chest and charged to 150 joules. Iva jolted around on the gurney in response to the controlled dose of electricity. The heart monitors still displayed pulseless v-tach, so the medics decided to shock her again at 200 joules. The 2nd shock made the young woman's chest shoot up, arching her back a bit. Shock #2 converted the young woman to v-fib, so a full code blue was initiated.
Epinephrine and atropine were injected into Iva’s IV, and CPR was started while the defibs were being recharged. The young athlete received deep, harsh chest compressions from the medic. Her perky, B cup breasts bounced in sync with the compressions while her head lolled around a bit. Once the defibs were ready, the medic backed away and delivered a 250j shock. The shock caused her nude body to bounce around in response, but v-fib was still present on the heart monitors. Another shock was delivered, but it had no effect whatsoever.
CPR was still in progress upon arrival to our emergency department. At that point, Iva was shocked 4 times, given 2 doses of drugs, and had a total down time of 6 and a half minutes. Once we had her in the trauma bay, we transferred her onto the table and resumed the code. One of our ER nurses took over compressions. She delivered strong, rapid compressions that caused the patient’s belly to ripple and bounce outward. I then began ordering tests: I ordered stat trauma labs (CBC, BMP, tox screen), but I also threw in a cardiac enzyme test to rule out an MI, and a D-dimer to rule out a blood clot. The next order of business was to order an echocardiogram since the medics’ account of what happened suggested a heart problem. With CPR ongoing, an echocardiogram was performed. The echo showed mild left ventricular hypertrophy, and major thickening of the intraventricular septum. I immediately knew this was hypertrophic cardiomyopathy. “page cardio. If we get a pulse back, she’s gonna need surgery.” I called out. Hypertrophic cardiomyopathy is a condition where the muscular wall between the bottom 2 chambers of the heart is abnormally thick. This can cause major problems with bloodflow into and out of the heart. Not every patient with hypertrophic cardiomyopathy has a long history of symptoms, which is why it can cause a sudden cardiac arrest.
After all my diagnostic tests were completed, we decided to shock Iva again. The defib pads made a whining sound as they charged. Once the pads were ready, everyone backed away and the shock was delivered. Iva’s feet leapt up above the table and slammed back down, showing of the prominent, silky wrinkles in the soles of her size 10 feet. V-fib still ran across the heart monitors, so another shock was delivered after a cycle of chest compressions. The next shock caused the young lady to jolt violently on the bed as the electricity ran through her dying body. This shock sent Iva into PEA, so CPR was resumed at that point.
The next rounds of epinephrine and atropine were pushed, along with the first dose of bicarb. The drugs and several rounds of compressions failed to produce a shockable rhythm. Iva was given more drugs at the 12 minute mark of the code, but she still remained in PEA. Her chest was starting to get bruised from all the compressions she’s received. Her fair complexion quickly turned into a ghastly white, and she was beginning to become cool to the touch. The situation grew increasingly tense, but she still had pupil reactivity so the code ensued.
It took another 4 and a half minutes, but Iva was able to be converted back into v-fib. The defibrillator pads were charged and everyone backed away in anticipation of the shock. 300 joules of electricity were sent into Iva’s lifeless body, causing her to twitch violently on the table. With v-fib still displaying on the monitors, a cycle of compressions were performed and the next shock was delivered moments later, causing her battered body to react violently to the shock. This shock sent Iva back into PEA, so CPR was resumed at that point.
The ER team maxed the young athlete out on drugs and performed numerous cycles of chest compressions on her, but she failed to respond to the life saving efforts. After a 22 minute code, the ER team ceased their efforts and called time of death at 16:41.
The ambu bag was detached and the flatlined heart monitors were shut off. The defibrillator pads were peeled off her battered chest while another nurse disconnected the ECG electrodes. Lastly, Iva’s lifeless body was covered and a toe tag was placed.
We unfortunately broke the news to Lara, Iva’s coach, and a few other tennis pros who came to the hospital after hearing what happened.
Iva’s death was honored with a moment of silence on the 1st day of the tournament, and this experience encouraged Lara to become an advocate for providing more in-depth sports physicals to athletes in all sports.
81 notes
·
View notes