#rhombohedral
Explore tagged Tumblr posts
Text

1 note
·
View note
Text
admiring my mineral girlfriends rhombohedral cleavage
43 notes
·
View notes
Text

Not all calcite crystals perfect; synthesis methods can alter internal structure, affect chemical reactivity
When looking at calcite under a microscope, a scientist would immediately recognize the crystalline form of calcium carbonate by its rhombohedral appearance. That is, calcite is shaped like a distorted cube. One of Earth's most abundant minerals, calcite is a major component of limestone and marble. It is also the most stable of the three common, naturally occurring crystal forms of calcium carbonate; the other two forms are aragonite and vaterite. Studying calcite is important as it has broad relevance in several ways. When calcium carbonate is synthesized, it transforms carbon dioxide (CO2) into solid carbonate, the final reaction product needed for long-term carbon storage in the fight against climate change. Additionally, as researchers recently discovered, the presence of defects in calcite can be controlled by how it is synthesized and can alter many of its properties. For example, defects can affect calcite's ability to soak up harmful substances in the environment like heavy metals. They can also affect the mechanical strength of calcite—which has implications for the development of more durable materials—and the ability to improve catalysts used in industrial processes.
Read more.
#Materials Science#Science#Calcite#Minerals#Materials characterization#Calcium carbonate#Crystals#Crystallization
11 notes
·
View notes
Text
Mineral Cup Round 1: Quartz vs Mercury
Could this be the most controversial match-up of round one? The always a bridesmaid, never a MinCup champion icon quartz goes up against infamous, alluring "is it even a mineral" mercury.
https://www.mineralcup.org/2024/vote/r1m11
QUARTZ
The most common single mineral in continental crust, we live on quartz! It may be the most recognized mineral around - but can it make it to the championship?
Name: From German Quarz, ultimately from Proto-Slavic *tvrd-, "hard".
Bling: Rainbows, ombres, inclusions, cat's eyes - you could make a gem exhibit on quartz alone! It carves beautifully, but its natural spikes are the definition of crystal. (And have I mentioned... conchoidal fracture?!)
Uses: In addition to art, quartz crystals are crucial in electronics - it's the key ingredient for silicon wafers, and its piezoelectricity (makes a current under stress) lets watches, radios, and computers keep time. There would be no MinCup without quartz!
Team Lick: Sure! It tastes like nothing.

Parent Géry. Creative Commons Attribution-Share Alike 3.0 Unported license.

Fatimid ewer, 10th-11th century. Photo by Marie-Lan Nguyen. Public domain.
MERCURY
It's a liquid on Earth, but freezes into a rhombohedral crystal structure. Mineral enough to be in MinCup? Will its mirror shine capture your vote?
Name: From the planet Mercury, which was associated with the metal in medieval alchemy.
Bling: The shine! The beading! The more-liquid-than-water flow!
Uses: Makes liquid-mirror telescopes, electrodes, and is useful for extracting silver and producing chlorine. It had many more uses in the past - in addition to lighthouses and thermometers (and medicine), it's responsible for the discovery of superconductivity!
Team DO NOT Lick! Though liquid mercury isn't well absorbed, mercury vapor will still poison you.

Bionerd. Creative Commons Attribution 3.0 Unported license.

Dnn87. Creative Commons Attribution 3.0 Unported license.
#minerals#geology#quartz#mercury#mincup24#team do not lick#certainly the hardest round for me so far...#i would be devastated if quartz got kicked out of next year's competition though#i'll accept it leaving if it wins the cup!
2 notes
·
View notes
Text
Cool natural Pseudomorph. Perfect Calcite rhombohedral crystal into Siderite with sparkling quartz overgrowth. Bor Quarry, Dalnegorsk
GoldenHourMinerals.com
49 notes
·
View notes
Text
Kosovo Combo! Rhombohedral Mangano Calcite and Clear Quartz Cluster.
https://www.oldearthminerals.com/shop/p/q0ghsshyf5s09bhqgpic2tzw7q6pqd-7jsb2
#calcite #quartzcluster #crystalcluster #mineralspecimens #minerals #crystals #quartzcrystals #healingcrystals
12 notes
·
View notes
Text
Preliminary Poll - Opal Varieties




More information under the cut!
Opal! Opal is technically NOT a mineral because it lacks one of the defining traits of minerals - a specific, ordered internal structure. Opal is amorphous, so the atoms are arranged randomly, they don't have a pattern to them that would make opal a mineral!
Chemically, opal is hydrous silica. Something fun about the preliminary polls is that aside from tourmaline and jade, all the other minerals included are essentially variations of quartz, which is really just silica. The chemical formula for quartz is SiO2 and the chemical formula for opal is SiO2 with H2O.
Opals in general are known for their iridescence (the shiny colors that change when you rotate an opal in your hand). This iridescence happens BECAUSE opal is not a mineral; the random internal structure makes it so that light reflects in all kinds of crazy ways.
About the listed opals themselves, Australian opals are the most common, because most opal comes from Australia. Australian opal is probably what most people think of when they think of opal: shiny, with bright pastel colors.
Ethiopian opals, meanwhile, tend to have more fiery colors (think fire opals) and are also hydrophane which means they absorb water.
Harlequin opals are opals where the iridescence looks like geometric shapes. Unfortunatly, I don't know what is happening on the microscopic level that creates these rhombohedral shapes in harlequin opals. Most of the information I'm sharing in these posts is stuff I already know from the classes I had to take in undergrad, or introductory-level facts that I've been able to summarize from Wikipedia pages or Mindat.org pages. That being said, I'm always willing to learn from people who know more than me or to do more of my own research if people have questions and aren't sure where to start looking (hint: Wiki and mindat are great places to START your research, regardless of what high-and-mighty teachers might say).
Lastly, opalized fossils are fossils where the organic material from a previously living organism is replaced by silica (in this case, specifically opal). Most often, fossils form when minerals of some kind (silica, carbonates, pyrite, etc) fill in the empty spaces of a dead organism, like the inside of a snail or bivalve shell. The opalization of fossils is only one way this process, called permineralization, can occur, but it sure is pretty!
26 notes
·
View notes
Text



#nature#minerals#crystals#collection#gemstone#crystalshop#flowers#for sale#galena#crystalhealing#raw crystals#gift#home decor#collectibles
2 notes
·
View notes
Text

Optical Calcite
Also known as Iceland Spar or Iceland Crystal this is a transparent variety of Calcite often used in demonstrating the polarization of light. It occrs in large rhombohedral crystals.
The refractive index of this crystal is different for light with differing polarizations and as such unpolarized light entering the crystal will divide into two mutually perpendicular rays resulting in objects seen through the crystal appearing to have been doubled.
It has also been speculates that the 'sunstone' mentioned in medieval icelandic texts was actually Optical Calcite.
1 note
·
View note
Text
I found a coffee mug with little rhombohedral crystals in the bottom yesterday

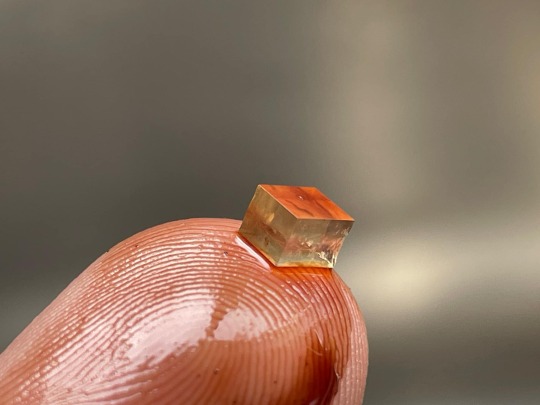
this guy's beef got old enough to have geology
110K notes
·
View notes
Text
Aggregate of rhombohedral Siderite crystals on matrix, from the "La gitana" mine, an old iron mine. The specimen shows brown and cream-colored crystals. A beautiful crystal aggregate of Siderite crystals.
0 notes
Text
Calcium Carbonate (CC) and Precipitated Calcium Carbonate (PCC) are chemically identical (both have the chemical formula CaCO₃), but they differ in their origin, purity, particle size, shape, and applications. Here’s a breakdown of their differences:

1. Origin:
Calcium Carbonate (CC): Often referred to as Ground Calcium Carbonate (GCC), this form is derived from natural sources like limestone, marble, or chalk. It is mechanically ground from these materials.
Precipitated Calcium Carbonate (PCC): This is a synthetically produced form of calcium carbonate. PCC is manufactured by chemical processes, typically by reacting calcium oxide (lime) with carbon dioxide.
2. Purity:
CC (GCC): The purity depends on the natural source and may contain impurities such as clay or other minerals.
PCC: Being synthesized, PCC has higher purity as it is manufactured under controlled conditions, ensuring a more uniform composition.
3. Particle Size and Shape:
CC (GCC): The particles are irregular and coarse, with sizes depending on how finely the material is ground. The shapes are typically rhombohedral, irregular, or even amorphous.
PCC: PCC has a well-defined particle shape (often cubic, spherical, or scalenohedral), and its particle size is much finer and more controlled. PCC can be customized to have specific properties based on its application.
4. Production Process:
CC (GCC): Produced by grinding limestone or marble to different particle sizes.
PCC: Produced by a chemical process where calcium oxide is reacted with carbon dioxide in water, resulting in the precipitation of calcium carbonate.
5. Applications:
CC (GCC): Used in construction materials, paints, plastics, rubber, and as a filler in products like paper. It is cost-effective but less refined.
PCC: Used in high-end applications where particle size, shape, and purity are critical, such as in pharmaceuticals, food additives, high-quality papers, and as a whitening agent in products like toothpaste and cosmetics.
6. Cost:
CC (GCC): Generally cheaper due to its natural extraction process.
PCC: More expensive due to its synthetic production process and its customizable nature.
In summary, PCC offers finer control over particle size, shape, and purity, making it ideal for specialized applications, while GCC is a more cost-effective option used in larger-volume industrial applications.
#branding#business#commercial#precipitated calcium carbonate#accounting#calcium carbonate manufacturers in india#ecommerce#economy#entrepreneur#finance
0 notes
Text
The X-Ray Diffraction Pattern of the Ash Content on Ignition at 900 ˚C of a Copy Paper Sample and its Mechanical and Optical Properties_Crimson Publishers

Abstract
The ash on ignition at 900 ˚C of a A4 copy paper sample UPM New Future Multi was determined to be 15.4%w/w and its X-ray diffraction pattern showed the presence of only portlandite syn Ca(OH)2 peaks placed at 2θ values of 18o, 29o, 34o, 46o, 47o, 51o, 54o, 63o, 72o which corresponded to crystal planes of 111, 110, 112. The high tensile force value of 4.5KN/m of the dry A4 copy paper sample correlated well with its high pH value of 9.6 and high ash on ignition content of 15.4%w/w. Along with them the roughness Bendtsen of the paper was found in a scale which related well with its pick resistance measured to be 1.96m/s in the machine direction and 0.39m/s in the counter direction. The band gap energy Eg from the Diffuse Reflection Spectroscopy (DRS) study of the A4 copy paper sample was calculated to be 1.169715722005400000000000eV. The absorption coefficient α(ν) near the edges was calculated to be 4.27185997cm-1 and the band gap was obtained by intercepting the linear fitted line in the plot of (αhv)^2 versus hv. The X-Ray powder diffraction pattern of portlandite was indexed by trial and error, and some peaks were assigned their hkl values. Finally, the Miller indices of the reflections were determined as well as the separation of planes. The separation of the 111 plane in the rhombohedral lattice was calculated to be 0.2984038nm. The separation of the 110 plane in the rhombohedral lattice was calculated to be 0.423379nm and of the 112 plane was 0.2205806429nm.
Read more about this article: https://crimsonpublishers.com/acsr/fulltext/ACSR.000566.php
For more articles in our journal: https://crimsonpublishers.com/acsr/
#annals of chemical science research#chemistry open access journals#crimson publishers#peer reviewed journal of acsr#chemical sciences journal#crimson publishers google scholar#acsr#chemistry journals#open access journals#peer review journals
0 notes
Text

Periodic Table Championship: Round 1, Day 2, Antimony vs. Terbium
Match 2 of day 2 for round 1 is element 51, antimony, versus element 65, terbium.
Antimony is a metalloid known since ancient times, with artifacts of antimony and its compounds found dating back to 3000BC. Crystallizing in the rhombohedral crystal system, it has a layered structure with weak bonding between the layers, making it relatively soft and brittle. Antimony is commonly alloyed with lead and tin for solders, bullets, and bearings. The origin of the element's name is uncertain.
Terbium is a rare earth, lanthanide element, crystallizing in with a hexagonal crystal structure. It is malleable, ductile, and soft, and while relatively stable in air, reacts readily with water. Terbium is used in green phosphors, dopants in solid-state devices, and as a component of the alloy Terfenol-D. It is one of four elements named after the town of Ytterby in Sweden.
4 notes
·
View notes
Text
Phonon-mediated unconventional superconductivity in rhombohedral stacked multilayer graphene
http://dlvr.it/TB8gHQ
0 notes
Text
Kosovo Combo! Rhombohedral Mangano Calcite and Clear Quartz Cluster $65 plus shipping
https://www.oldearthminerals.com/shop/p/q0ghsshyf5s09bhqgpic2tzw7q6pqd-7jsb2
#kosovominerals #quartzcluster #manganocalcite #crystalhealing #crystalcommunity #crystals #fineminerals #minerals #quartz
4 notes
·
View notes