#osteogenic differentiation
Explore tagged Tumblr posts
Text
Abstract Numerous experimental data shows crucial involvement of mirs in skeletal development in embryos, osteogenic differentiation, and maturation. However, molecular mechanisms of mirs' action—in other words, their target signaling pathways and transcriptional factors that specific drives osteogenic differentiation—is far from being understood. With meta-analysis, the authors identified mirs significantly involved in hMSCs osteogenic differentiation. Statistical analysis revealed a significant trend of upregulation of let-7a, mir-21, mir-26a, mir-29b, mir-101, mir-143, and mir-218 during hMSCs differentiation into osteoblast. And the opposite trend was shown for mir-17, mir-31, mir-138, and mir-222: their content was significantly lower during osteogenic differentiation. Using bioinformatics approaches, the authors identified predictable genes-target for each mirs and analyzed signaling networks and biological process enriched by these genes. Bioinformatic assay shows that mirNAs specifically involved in hMSCs transition into osteogenic differentiation via microenvironment formation (i.e., let-7a, mir-17, mir-21, mir-29b, and mir-101), TGF-β/BMP-SMAD-dependent pathway (i.e., let-7a, mir-17, mir-21, mir-26a, and mir-101) and MAPK signaling pathway (i.e., let-7a, mir-21, mir-26a, mir-29b, mir-143, and mir17). Yap-dependent expression of osteogenic transcriptional factors are modulated by let-7a, mir-31mir-101, mir-138, and mir-222. We predicted that mir-17, mir-26a, mir-29b, mir-101, mir-138, and mir-222 are specifically involved in canonical Wnt sig-naling-dependent osteogenesis as well as in osteoblast maturation, together with let-7a, mir-29b, and mir-218, which modulate AMPK signaling. Additionally, identified mir-101 is likely involved into osteoblast homeostasis via Hedgehog signaling. The data presented here expands knowledge in the field of hMSCs’ fate and osteogenesis orchestration by mirs and points to proosteogenic and antiosteogenic mirs and their potential molecular pathways.
2 notes
·
View notes
Text
Antioxidants, Vol. 13, Pages 470: Tricarboxylic Acid Cycle Regulation of Metabolic Program, Redox System, and Epigenetic Remodeling for Bone Health and Disease
Imbalanced osteogenic cell-mediated bone gain and osteoclastic remodeling accelerates the development of osteoporosis, which is the leading risk factor of disability in the elderly. Harmonizing the metabolic actions of bone-making cells and bone resorbing cells to the mineralized matrix network is required to maintain bone mass homeostasis. The tricarboxylic acid (TCA) cycle in mitochondria is a crucial process for cellular energy production and redox homeostasis. The canonical actions of TCA cycle enzymes and intermediates are indispensable in oxidative phosphorylation and adenosine triphosphate (ATP) biosynthesis for osteogenic differentiation and osteoclast formation. Knockout mouse models identify these enzymes’ roles in bone mass and microarchitecture. In the noncanonical processes, the metabolites as a co-factor or a substrate involve epigenetic modification, including histone acetyltransferases, DNA demethylases, #RNA m6A demethylases, and histone demethylases, which affect genomic stability or chromatin accessibility for cell metabolism and bone formation and resorption. The genetic manipulation of these epigenetic regulators or TCA cycle intermediate supplementation compromises age, estrogen deficiency, or inflammation-induced bone mass loss and microstructure deterioration. This review sheds light on the metabolic functions of the TCA cycle in terms of bone integrity and highlights the crosstalk of the TCA cycle and redox and epigenetic pathways in skeletal tissue metabolism and the intermediates as treatment options for delaying osteoporosis. https://www.mdpi.com/2076-3921/13/4/470?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
Similar, but not the same: multi-omics comparison of human valve interstitial cells and osteoblast osteogenic differentiation expanded with an estimation of data-dependent and data-independent PASEF
Osteogenic differentiation is crucial in normal bone formation and pathological calcification, such as calcific aortic valve disease (CAVD). Understanding the proteomic and transcriptomic landscapes underlying this differentiation can unveil potential therapeutic targets for CAVD. In this study, we employed the timsTOF Pro platform to explore the proteomic profiles of valve interstitial cells (VICs) and osteoblasts during osteogenic differentiation, utilizing three data acquisition/analysis techniques: Data-Dependent Acquisition (DDA-PASEF) and Data-Independent Acquisition (DIA-PASEF) with a classic library based and machine learning-based "library-free" search (DIA-ML). RNA-seq complemented comparative proteome coverage analysis to provide a comprehensive biological reference. We reveal distinct proteomic and transcriptomic profiles between VICs and osteoblasts, highlighting specific biological processes in their osteogenic differentiation pathways. Furthermore, the study identified potential therapeutic targets for CAVD, including the differential expression of proteins such as MAOA and ERK1/2 pathway in VICs. From a technical perspective, the DIA-ML offers significant advantages and seems the method of choice for routine proteomics. http://dlvr.it/T55XBS
0 notes
Text
DBM Market Dynamics: Trends and Predictions for the Next Decade By 2023 to 2030

The global demineralized bone matrix (DBM) market is expected to have a US$ 702,29 million global market in 2023, rising to US$ 920,84 million by 2030 at a projected compound yearly growth rate of 5.6%. The growth of the market is driven by the increasing demand for bone graft substitutes in orthopedic and spine surgeries. DBM is a natural bone graft substitute that is derived from human or animal bone. It is processed to remove the inorganic minerals, leaving behind a collagen matrix that promotes bone growth.
Within the larger medical and orthopedic business, the demineralized bone matrix (DBM) market is a dynamic subsector. DBM is a bio-implantation material generated from allogeneic bone that has undergone processing to eliminate mineral content while preserving vital organic elements. The end product is a matrix that is abundant in proteins, collagen, and growth factors that support the regeneration and healing of broken bones.
Request A Sample Copy of This Research Report! https://absolutemarketresearch.com/Global-Demineralized-Bone-Matrix-(DBM)-Market/1236/request-sample
The market for demineralized bone matrix is anticipated to grow quickly due to developments in grafting and an increase in cosmetic surgery. In addition, the younger population and athletes' growing need for surgical treatments and soft tissue allografts will definitely propel this industry ahead. A comprehensive analysis of the market is given in the Global Demineralized Bone Matrix Market study. The study provides a thorough examination of the market's major segments, trends, drivers, constraints, competitive environment, and other elements that are significantly influencing it.
Definition of the Global Demineralized Bone Matrix Market:
An allograft bone that has had the inorganic mineral removed, leaving just the organic "collagen" matrix, is known as demineralized bone matrix (DBM). In 1965, Marshall Urist made the initial discovery that more biologically active bone morphogenetic proteins are revealed when bone minerals are removed. The development of progenitor cells into osteoprogenitor cells, which are in charge of creating bone and cartilage, is influenced by these growth factors. Because of the demineralization process, DBM has much lower mechanical qualities but is more physiologically active than demineralized bone transplants.
The success of a bone graft depends on its capacity to draw host cells to the graft site and control how they differentiate into osteoblasts, which produce new bone, to heal the deficiency. The osteoconductive, osteoinductive, and osteogenic qualities of the transplant will dictate this. Autograft bone taken from the iliac crest is currently regarded as the "gold standard" due to its superior osteogenic qualities. Its application is, however, restricted by donor site morbidity, lengthier surgical and recuperation periods, and a shortage of donor bone.
Allograft bone has the potential to replace autograft bone. It needs to be extensively treated and terminally sterilized before implantation in order to reduce the possibility of an immunological reaction or the spread of illness. During this process, the graft's osteogenic and osteoinductive qualities are eliminated, leaving just an osteoconductive scaffold. These scaffolds come in a range of preparations (such struts and morselized particles) for different orthopedic uses. Since the mineral is removed, DBM's biological qualities are superior to those of demineralized allograft bone, hence enhancing the graft's osteoinductivity.
Key Takeaways:
The global demineralized bone matrix (DBM) market is expected to have a US$ 702,29 million global market in 2023, rising to US$ 920,84 million by 2030 at a projected compound yearly growth rate of 5.6%.
The growth of the market is being driven by the increasing demand for bone graft substitutes in orthopedic and dental applications.
North America is the largest market for DBM, followed by Europe and Asia Pacific.
The increasing prevalence of bone-related disorders, such as osteoporosis and osteoarthritis, is also driving the growth of the market.
Regional Outlook:
North America is the largest market for DBM, owing to the high prevalence of bone-related disorders and the increasing adoption of minimally invasive surgical procedures.
Europe is the second-largest market for DBM, driven by the growing demand for regenerative medicine products.
Asia Pacific is the fastest-growing market for DBM, due to the rising disposable incomes and the increasing awareness of bone graft substitutes.
Key Players:
Stryker Corporation
Zimmer Biomet
RTI Surgical
Ossur
MiMedx
Amedica Corporation
Geistlich Biomaterials
Bioventus
Globus Medical
Depuy Synthes
Segmentation:
By type:
Purified DBM
Injectable DBM
Putty DBM
Strip DBM
By application:
Spine surgery
Orthopedic surgery
Dental surgery
By region:
North America
Europe
Asia Pacific
Latin America
Middle East and Africa
0 notes
Text
Osteogenic Differentiation Potential of Mesenchymal Stem Cells Using Single Cell Multiomic Analysis
Pubmed: http://dlvr.it/Sy4zxR
0 notes
Link
So researchers are studying how to clone teeth to replace teeth that need to be removed and as an intermediary step how to regenerate dental pulp and ligaments after root canal. The report above is newly published this month Sept 2021.
Two and a half years ago I regrew my periodontal ligament after a root canal, and I know how I did it. The answer is long term High Dose Omega-3 fat supplementation. Our bodies make the fancy growth factors in the report above from Omega-3 Fat.
I redacted personal information from the report below.

We will find out if I can do it again. I’m seeing this guy again for another root canal today. Wish me luck.
6 notes
·
View notes
Link
Human bone marrow-derived mesenchymal stem cells (hBMSCs) and their derivative enhanced green fluorescent protein (eGFP)-hBMSCs were employed to evaluate an innovative hybrid scaffold composed of granular hydroxylapatite and collagen hemostat (Coll/HA). The cellular morphology/cytoskeleton organization and cell viability were investigated by immunohistochemistry (IHC) and AlamarBlue metabolic assay, respectively. The expression of osteopontin and osteocalcin proteins was analyzed by IHC and ELISA, whereas osteogenic genes were investigated by quantitative PCR (Q-PCR). Cell morphology of eGFP-hBMSCs was indistinguishable from that of parental hBMSCs. The cytoskeleton architecture of hBMSCs grown on the scaffold appeared to be well organized, whereas its integrity remained uninfluenced by the scaffold during the time course. Metabolic activity measured in hBMSCs grown on a biomaterial was increased during the experiments, up to day 21 (p < 0.05). The biomaterial induced the matrix mineralization in hBMSCs. The scaffold favored the expression of osteogenic proteins, such as osteocalcin and osteopontin. In hBMSC cultures, the scaffold induced up-regulation in specific genes that are involved in ossification process (BMP2/3, SPP1, SMAD3, and SP7), whereas they showed an up-regulation of MMP9 and MMP10, which play a central role during the skeletal development. hBMSCs were induced to chondrogenic differentiation through up-regulation of COL2A1 gene. Our experiments suggest that ...
1 note
·
View note
Link
In the study, the research team reported that red-light absorbing carbon nitride (C₃N₄) sheets lead to remarkable proliferation and osteogenic differentiation by runt-related transcription factor 2 (Runx2) activation, a key transcription factor associated with osteoblast differentiation. (...)
Professor Kim and Professor Suh examined the C₃N₄sheets. They discovered that this material absorbs red light and then emits fluorescence, which can be used to speed up bone regeneration. Professor Kim's team synthesized carbon nitrogen derivatives from melamine compounds. Then, they analyzed the light-absorbing characteristics of C₃N₄sheets at a wavelength range of 455-635 nanometers (nm). As a result, the C₃N₄sheets were found to emit fluorescence at the wavelength of 635 nm when exposed to red light in a liquid state. At this time, the released electrons induced calcium to accumulate in the cytoplasm.
Professor Suh conducted a biomedical application of this material. First, stem cells and cancer cells were cultured in a medium containing 200 μg/ml of C₃N₄sheets. After two days of testing, the material showed no cytotoxicity, making it useful as biomaterials.It was also confirmed that C₃N₄sheets act on stem cells to differentiate into osteoblasts to promote mineral formation. In this process, the osteogenic differentiation marker genes (ALP, BSP, and OCN) proliferated. Moreover, the Rux2 (Runt-related transcription factor 2), a key factor in osteoblast differentiation was also activated. This resulted in the increased osteoblast differentiation and accelerated bone formation.
#engineering#nanotechnology#technology#biology#medicine#stem cell#bone repair#human bone marrow#carbon material#photocatalytic properties#skeletal system#fractures#periodontal disease#osteogenic differentiation#stem cells#osteoblasts#mineral formation
0 notes
Text
Attracting stem cells and facilitating bone regeneration by adhesive protein
Three research teams led by Professor Hyung Joon Cha of the Chemical Engineering Department at Pohang University of Science and Technology (POSTECH), Professor Yun Kee Jo of the School of Convergence at Kyungpook National University (KNU), and Professor Sang Ho Jun of the Department of Oral and Maxillofacial Surgery at Korea University Anam Hospital together developed an osteogenic barrier coating material for dental implants that prevents the invasion of soft tissue cells, attracts osteo-progenitor cells including bone stem cells, and sustainably releases the loaded bone morphogenetic protein-2 (BMP-2), significantly facilitating bone regeneration. GBR is widely used in dental implant placement. It maintains the space for bones to grow and prevents cells other than osteogenic cells, such as fibroblasts, from populating the bone defect sites, allowing the bone to grow without interference by non-osteogenic cells. However, the GBR approach is still less likely to be successful and requires longer treatment time for those patients with insufficient bone quantity and quality. Depending on the configurations of defect sites, preventing the invasion of the non-osteogenic cells by using barrier membranes alone is not enough to significantly facilitate the bone regeneration. The joint research team first loaded BMP-2 on top of the bioengineered material where RGD peptide, cell recognitive motif that is capable of attracting cells, is fused with mussel adhesive protein (MAP) that maintains strong adhesiveness in a wet environment. The team then coated the titanium mesh (Ti-mesh) membrane with it. According to the research findings, the coated barrier membrane exhibited cell occlusivity where fibroblasts could not permeate the membrane. The team also found that it induced a high level of bone differentiation in a short period of time inside the membrane by means of high growth of mesenchymal stem cells and release of BMP-2. Application of the developed MAP-based barrier coating for guided bone regeneration to a titanium membrane in a rat calvarial defect model showed that the coating roughly doubled the speed of bone tissue regeneration. Professor Hyung Joon Cha who led the research said, “This research was conducted based on long-term research cooperation of the joint research team in the area of bone regeneration for implant placement. Its findings revealed the possibility of improving the success rate of implant treatment regardless of the bone condition.” He added that the research findings could also be applied to regenerate a variety of hard tissues. The research findings were published in the online edition of the Bioengineering & Translational Medicine, a distinguished journal in the field of bioengineering and regenerative medicine. The study was conducted as a part of the Dentistry Technology R&D Project under the Korea Health Technology R&D Project funded by the Ministry of Health & Welfare, the High Value-added Food Technology Development Program funded by the Ministry of Agriculture, Food & Rural Affairs, and the BK21 Four Program by the National Research Foundation of Korea.
0 notes
Text
Human Adipose tissue -Derived stem cells
Human Adipose tissue-derived stem cells (ADSCs), a subgroup of mesenchymal stem cells (MSCs), can be easily isolated from adipose tissues and share many of the same regenerative characteristics as other MSCs. Multipotent adipose tissue-derived stem cells have the potential to be used in regenerative medicine. These cells can be easily isolated from adipose tissue, are able to grow in vitro, and can differentiate into a variety of cell lineages. The primary application of ADSCs in regenerative medicine was mesodermal regeneration, with an emphasis on their potential for osteogenic, adipogenic, chondrogenic, and eventually cardiovascular applications.

0 notes
Text
Genes, Vol. 15, Pages 376: Expression, Polymorphism, and Potential Functional Sites of the BMPR1A Gene in the Sheep Horn
Sheep horns are composed of bone and sheaths, and the BMPR1A gene is required for cartilage and osteogenic differentiation. Therefore, the BMPR1A gene may have a function related to the sheep horn, but its relationship with the sheep horn remains unclear. In this study, we first utilized #RNA sequencing (#RNA-seq) data to investigate the expression of the BMPR1A gene in different tissues and breeds of sheep. Second, whole-genome sequencing (WGS) data were used to explore the functional sites of the BMPR1A gene. Lastly, the allele-specific expression of the BMPR1A gene was explored. Our results indicate that BMPR1A gene expression is significantly higher in the normal horn groups than in the scurred groups. Importantly, this trend is consistent across several sheep breeds. Therefore, this finding suggests that the BMPR1A gene may be related to horn type. A total of 43 Single-Nucleotide Polymorphisms (SNPs) (F-statistics > 0.15) and 10 allele-specific expressions (ASEs) exhibited difference between the large and small horn populations. It is probable that these sites significantly impact the size of sheep horns. Compared to other polled species, we discovered ten amino acid sites that could influence horn presence. By combining #RNA-seq and WGS functional loci results, we identified a functional site at position 40574836 on chromosome 25 that is both an SNP and exhibits allele-specific expression. In conclusion, we demonstrated that the BMPR1A gene is associated with horn type and identified some important functional sites which can be used as molecular markers in the breeding of sheep horns. https://www.mdpi.com/2073-4425/15/3/376?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
Electrospinning series you want to know - L-polylactic acid (PLLA)
Catalog:
1. Properties of PLLA materials
2. Application of PLLA nanofibers in tissue engineering
3. Experimental parameters of electrospun PLLA
4. Common problems and solutions
1. Properties of PLLA materials
Since there is an asymmetric carbon atom in the lactic acid molecule, which has optical activity, polylactic acid can also be divided into dextral polylactic acid (PDLA), left-handed polylactic acid (PLLA), racemic polylactic acid (PDLLA), and non optical polylactic acid (Meso PLA). PLA is an important biodegradable polymer material, characterized by non-toxic, non irritating, biodegradable absorption, high strength, good plasticity, and easy processing and molding. The degradation cycle is 2~12 months.
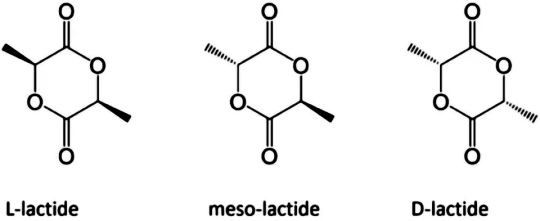
Fig. 1 Enantiomeric form of lactic acid
2. Application of PLLA nanofibers in tissue engineering
PLLA is one of the first synthetic polymer materials recognized as suitable for tissue engineering due to its non-toxic, non irritating, biodegradable absorption and high strength. The nanofiber scaffold produced by the invention can be used for specific tissue engineering applications, including bone, cartilage, blood vessel and skin tissue regeneration.

Fig. 2 Application of PLLA stent in tissue engineering
(1) Bone tissue
In bone tissue engineering, electrospinning has been widely used to prepare nanofiber scaffolds, whose structure is close to the nano collagen fibers of bone. In actual preparation, electrospun PLLA scaffold will be prepared in combination with surface modification to guide cells to differentiate into bone lineage and achieve the best bone regeneration performance.
A research team successfully modified the surface of PLLA nanofibers prepared by electrospinning through the surface deposition of osteogenic ECM. These scaffolds were then used to detect the response of mouse bone marrow stromal cells after implantation of the scaffold. The results showed that compared with pure PLLA nanofibers, the mineral growth, ALP activity and cell morphology of the modified structure were in the best state.
(2) Cartilage tissue
Although PLLA nanofiber scaffolds have shown significant effects on cartilage repair, their fine fibers lead to limited bearing capacity.
PLLA nanofiber scaffold made by phase separation and pore forming agent leaching has been proved to be the optimal scheme for cartilage repair in vivo and in vitro. This is due to the high porosity and interoperability of these scaffolds, as well as their good degradability. In addition, the appropriate size of the scaffold hole also improves the functional characteristics of cartilage, thus providing some different solutions for the regeneration and repair of cartilage tissue.
(3) Vascular tissue
Recently, a research team has made multi-layer scaffolds for vascular tissue engineering: PCL, collagen and PLLA nanofibers are used to simulate the inner membrane, middle membrane and outer membrane respectively. These nanofibers were made into three-layer tubular scaffolds by continuous electrospinning, and cultured endothelial cells and smooth muscle cells for biological activity evaluation. The final evaluation experiment results showed that the collagen in the middle layer significantly improved the adhesion and proliferation of SMC, and the proliferation of endothelial cells increased significantly with culture, indicating the non cytotoxicity of the construct.
(4) Skin tissue
In dermal fibroblasts culture in vitro, the mixed scaffold induced higher cell seeding efficiency and improved fibroblast adhesion and proliferation compared with collagen sponge. On the other hand, in vivo wound healing evaluation showed that the composite scaffold healed faster and more effectively than the collagen scaffold. In most cases, PLLA, collagen and gelatin are often combined to produce nanofiber scaffolds, which show physical and biological characteristics matching with skin substitutes.
PLLA is also used to produce multilayer scaffolds that are very similar to natural skin structures. A research team has developed a new type of double-layer scaffold, which is composed of chitosan/PCL nanofiber pad on the surface and PLLA microporous disk on the bottom. In this work, keratinocytes and fibroblasts were cultured as epidermal equivalent and dermal equivalent respectively. The final results showed that the cell proliferation in the double-layer scaffold was higher than that in the single-layer CP nano pad and PLLA microdisk. In addition, the evaluation of genes and proteins showed that the wound healing was active, which again confirmed that the double-layer scaffold could provide a suitable microenvironment for stimulating skin regeneration.
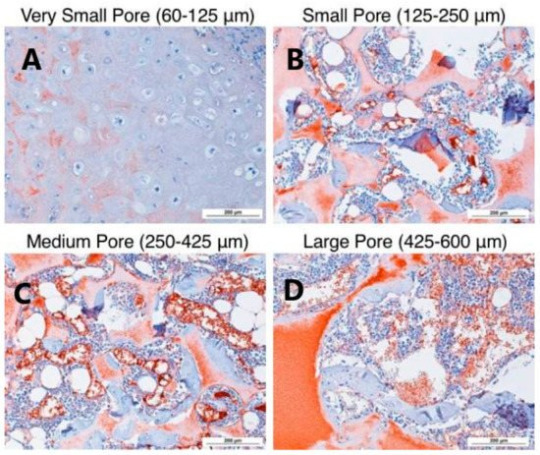
Fig. 3 Small Aperture (125-250 μ m) In vitro cartilage differentiation of human bone marrow mesenchymal stem cells induced by β - lactamase scaffold Large specific aperture (425-600 μ m) The stents in our body better support cartilage formation.
Cited by: Capuana, E; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153. https://doi.org/10.3390/polym14061153
3. Experimental parameters of electrospun PLLA
3.1 Experimental parameters for preparation of poly (L-lactide)/hydroxyapatite (PLLA/HA) nanocomposite scaffold
Material configuration: Dissolve 1g of PLLA (intrinsic viscosity 0.90-1.20dL/g) in 5mL of dichloromethane, and disperse 0.1g of nano HA powder (particle size<200nm) in the polymer solution under stirring, and transfer the obtained suspension to the medical syringe.
Electrospinning experiment parameters: positive voltage 15kV, liquid supply speed 1.5mL/h, needle 23G, spinning distance 15cm.
Reference: Rainer A, Spadaccio C, Sedati P, et al. Electrospun Hydroxapatite Functionalized PLLA Scaffold: Potential Applications in Sternal Bone Healing [J] Annals of Biomedical Engineering, 2011, 39(7):1882-1890.
3.2 Experimental parameters for preparation of PLLA/PCL oriented fiber scaffold
Material configuration: PLLA is dissolved in chloroform first, then in dimethylformamide (DMF) (4.25:0.75), while PCL is dissolved in chloroform/DMF (8:2). They were stirred for 3 hours, and then the polymer solution was loaded into the syringe by applying a positive voltage between the needle and the collector. The solution droplets leave the needle to form nanofibers and deposit at the same time. The percentage of PLLA/PCL is 47/53 wt%. The needle tip is placed 15 cm from the collector for PLLA and 20 cm for PCL, while rotating the disk at a linear rate set at 2800 rpm to collect parallel nanofibers.
Electrospinning test parameters: 5ml plastic syringe, 21 # needle, positive voltage (18 kV for PLLA, 24 kV for PCL)
Reference: Mashhadikhan M, Soleimani M, Parivar K, Yaghmaei P. ADSCs on PLLA/PCL Hybrid Nanoscaffold and Gelatin Modification: Cytocompatibility and Mechanical Properties Avicenna J Med Biotechnol. 2015 Jan-Mar; 7(1):32-8. PMID: 25926950; PMCID: PMC4388888.
3.3 Experimental parameters for preparation of pure PLLA nanofibers
Material configuration: dissolve PLLA in hexafluoroisopropanol, with the mass fraction range of 8%~10%, 20G (1/2 inch, flat mouth) stainless steel needle, the needle is connected to the positive pole of high-voltage power supply, and the receiving roller is connected to the negative pole of high-voltage power supply
Electrospinning experiment parameters: the liquid supply speed is 0.5~3mL/h (depending on the electrospinning process), the roller speed is 300 rpm, the slide travel is 20cm, and the spinning voltage is positive 15kV and negative 5kV
4. Common problems and solutions
(1) The boiling point of the solvent is low, so it cannot be spun at high temperature to avoid the blockage of the needle tip due to the rapid evaporation of the solvent.
(2) The solution shall be prepared by gradually adding particles or powders to prevent the dissolution rate from decreasing due to particle agglomeration and suspension.
0 notes
Text
Hypoxia dissociates HDAC6/FOXO1 complex and aggregates them into nucleus to regulate autophagy and osteogenic differentiation
Pubmed: http://dlvr.it/Swkjbf
0 notes
Text
Bone Morphogenetic Proteins Market Size, Current Insights and Development Trends
Rigorous research and developments on other BMP applications have become critical differentiating strategy. However, Rising Traumatic Injury Caseloads Remain Pivotal to Growth.
In its latest market analysis, ResearchMoz has revealed that the global market revenue of bone morphogenetic proteins will clock nearly US$ 704 Mn by 2027. ResearchMoz uncovers the global bone morphogenetic proteins market in detail in its report that includes the drivers, restraints, opportunities, threats and competitive topography during the forecast period.
Players Uncovering Potential Avenues in Dentistry
The utilization of bone morphogenetic proteins is not only concentrated on osteogenic regeneration. Instead, BMP producers, along with medical researchers, are conducting extensive studies in other areas, including periodontal regeneration and tooth conservation procedures. In dentistry, BMP-2 is seeing huge demand uptick in several medical procedures, including sinus lift augmentation, alveolar bone regeneration, and dental implants. Such discoveries are influencing market players to devise innovative biomaterials for BMP delivery in these potential avenues.
BMPs to Act as Possible Bridge between Bone Metabolism with Obesity
Riding the wave of meticulous Research and Development, players in bone morphogenetic proteins market are striving to determine relationship between bone metabolism and obesity as well as glucose metabolism. Studies have highlighted impact of bone morphogenetic proteins – BMP9, BMP7, BMP6, BMP4, and BMP2 – on pathophysiological process of glucose metabolism and obesity beyond bone metabolism. Furthermore, holistic understanding of impacts of bone morphogenetic proteins could help market players better understand bone metabolism in obesity and type-2 diabetes.
APAC BMP Market to Grow at Breakneck Speed
The report underscores fast-paced growth of bone morphogenetic proteins market in Asia Pacific (APAC). Increasing healthcare spending, especially in
China, India, and Singapore, and rising prominence of recombinant human bone morphogenetic proteins (rhBMP) are critical growth engines.
Besides, cost-effective healthcare services, superior hospitality, and leading-edge medical technologies (MedTech) have bolstered medical tourism in APAC. Thailand, Malaysia, and India are leading the pack in this theme.
Competitive Landscape
ResearchMOZ underscores the competitive gradient of the bone morphogenetic proteins market which would enable the new entrants to extract factual information regarding the strategies adopting by the leading players in order to dive deeper into the uncharted regions and grab their positions.
The leading players of the bone morphogenetic proteins market are
ProSpec
DePuy Synthes
Ember Therapeutics Inc
Sigma-Aldrich Corporation
R&D Systems
Cellumed Co. Ltd
Read More: https://www.beetribune.com/bone-morphogenetic-proteins-market-overview/
Key Takeaways
ResearchMoz establishes a research-based projections and information that unveils the larger picture of the global bone morphogenetic proteins market to help stakeholders to take informed decisions in order to succeed in this arena. Some of the key takeaways from the reports include-
The bone morphogenetic proteins to grow at a 2.9% CAGR between 2021 to 2027, exhibiting estimated value of nearly USD 704 million.
The sports related injuries to remain the dominant factor driving the market because of growing adoption of minimally invasive surgeries.
Osteoarthritis in geriatric population to spur the demand for bone morphogenetic proteins market.
Spinal fusion to remain the target application.
North America will maintain its supremacy over the market owing to advanced healthcare system and increasing geriatric population with degenerative bone diseases.
Table Of Content
1. Executive Summary 1.1. Global Market Outlook 1.2. Demand-side Trends 1.3. Supply-side Trends 1.4. Technology Roadmap Analysis 1.5. Analysis and Recommendations
2. Market Overview 2.1. Market Coverage / Taxonomy 2.2. Market Definition / Scope / Limitations
3. Market Background 3.1. Market Dynamics 3.1.1. Drivers 3.1.2. Restraints 3.1.3. Opportunity 3.1.4. Trends 3.2. Scenario Forecast 3.2.1. Demand in Optimistic Scenario 3.2.2. Demand in Likely Scenario 3.2.3. Demand in Conservative Scenario 3.3. Opportunity Map Analysis 3.4. Product Life Cycle Analysis 3.5. Supply Chain Analysis 3.6. Investment Feasibility Matrix 3.7. Value Chain Analysis 3.8. PESTLE and Porter’s Analysis 3.9. Regulatory Landscape 3.9.1. By Key Regions 3.9.2. By Key Countries 3.10. Regional Parent Market Outlook 3.11. Production and Consumption Statistics 3.12. Import and Export Statistics
Continue…
Download Report PDF: Bone Morphogenetic Proteins Market
0 notes
Text
Long noncoding #RNA KCNMA1-AS1 promotes osteogenic differentiation of human bone marrow mesenchymal stem cells by activating the SMAD9 signaling pathway
The human bone marrow mesenchymal stem cells (hBMSCs) undergo intense osteogenic differentiation, a crucial bone formation mechanism. Evidence from prior studies suggested an association between long noncoding #RNAs (l#ncRNAs) and the osteogenic differentiation of hBMSCs. However, precise roles and molecular mechanisms are still largely unknown. In this work, we report for the first time that l#ncRNA KCNMA1 antisense #RNA 1 (KCNMA1-AS1) plays a vital role in regulating hBMSCs' osteogenic... https://pubmed.ncbi.nlm.nih.gov/38017487/?utm_source=dlvr.it&utm_medium=tumblr&utm_campaign=None&utm_content=1RYYbE7j9SUSBe_aHniaI_J1MQIFIBbfLuFxoWdLNMNDzVVIWF&fc=None&ff=20231218003321&v=2.18.0
0 notes