#oncogenesi
Explore tagged Tumblr posts
Text
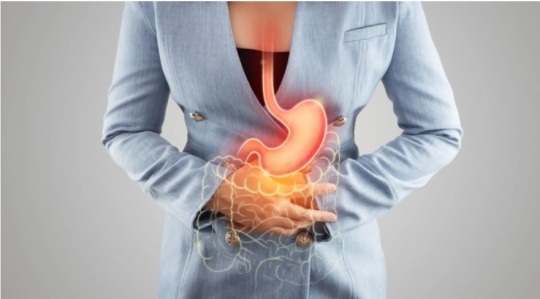
Il DNA extracromosomiale: "il nemico in casa" che può essere sfruttato per trattare i tumori più aggressivi
DNA normale e speciale: focus sul DNA extracromosomiale Il nostro DNA è solitamente immagazzinato in strutture chiamate cromosomi che si trovano in quasi tutte le cellule del corpo. Garantiscono che quando le cellule si dividono, il loro DNA venga copiato accuratamente in nuove cellule. Tuttavia, il DNA extracromosomico (ecDNA) esiste al di fuori dei cromosomi in piccoli cerchi di materiale…
#BBI-2779#carcinoma mammario#carcinoma polmonare#chemioresistenza#DNA extracromosomiale#glioblastoma#oncogenesi#proteina chinasi Chk1#sistema immunitario
0 notes
Text

Mistigram: this texty screen, a touch of Bosch at the beach, is the handiwork of @adelfaure , designed as album art for #EarthlyDelight by #Oncogenesis. This piece was included in the new music-themed MIST0224 artpack collection.
5 notes
·
View notes
Photo

Unexpected Indication
Like a burly bouncer with a hidden talent for ballet, there’s more to telomeres than meets the eye. They are repeating sections of DNA that sit at the end of strands, protecting the inner regions by degrading a little each time a cell replicates. They eventually diminish sufficiently to cause cell damage as we age. It was recently discovered that, counter to previous beliefs, these lengths of DNA can code for two small proteins, VR and GL. Researchers synthesised these to study their impacts, and then found them more frequently in cancer cells (pictured, VR in green, human cancer cells in red) and in people with telomere-related diseases. A blood test for these proteins might be a route to early cancer diagnosis, and as the levels rise over time, the potent proteins might be an indicator of a person’s biological age (a more significant indicator of health than chronological age).
Written by Anthony Lewis
Image from work by Taghreed M. Al-Turki and Jack D. Griffith
Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Image copyright held by the original authors
Research published in Proceedings of the National Academy of Science (PNAS), September 2023
You can also follow BPoD on Instagram, Twitter and Facebook
9 notes
·
View notes
Text
youtube
#SRSF12 deficiency#tumor innervation#pancreatic tumorigenesis#neural infiltration#cancer progression#metastasis#tumor microenvironment#alternative splicing#RNA regulation#oncogenesis#cancer cell proliferation#therapeutic targets#aggressive tumor phenotype#pancreatic cancer#gene expression#molecular oncology#cancer biology#neural signaling#tumor growth#cancer therapy.#Youtube
0 notes
Text
Our Research Targets as Many Traits of Cancer as Possible Through Pseudogenes
Our Research Targets as Many Traits of Cancer as Possible Through Pseudogenes @neosciencehub #neosciencehub #science #research #pseudogenes #cancer #NSH #EMBL #NCI #NIH #biology #Genomics #healthcare #DNA #oesophagel #IISER #AI #NCI #
Meet GovadaPravallika, a promising mind who has pursued her Masters in Science from the esteemed Indian Institute of Science Education and Research (IISER), Pune, one of only six such institutions in India. Currently, she is delving deeper into the realm of Cancer Biology and Genomics for her PhD. She took a leap in her world of genetics and genomics and published a radical research- “Stage II…

View On WordPress
#Biomedical innovation#Cancer research#Cellular reprogrammin#Esophageal cancer#Esophageal carcinoma#featured#Gene regulatory networks#Genomic medicine#Molecular biology#Oncogenesis#Pseudogenes#sciencenews
0 notes
Text


September 16th | Signal transduction | 26 days.
Dear Lenin,
I’ve finally gathered the courage to share these subtle updates with you. Truth be told, I was hesitant to document even a fragment of this still-undefined/ undifferentiated journey! — like a trembling bird in my palms, waiting to take flight.
Yesterday, I sat at my desk, not feeling grumpy for once, and immersed myself in clinical oncology. I pinned a few notes to the wall—I had never done that before intentionally you believe that? then I worked through a quiet decent number of questions, and they felt manageable. I tried to keep the momentum going, by diving into blood diseases but found myself wandering through the winding paths of pathology and oncogenesis. I didn’t quite finish, but today, if Allah wills, I will.
Pray for me, will you! I’ll tell you about my last week soon enough, but for now, did you know already that tissue is, indeed, the issue?
Kindley yours.
19 notes
·
View notes
Text
i managed to get about 60%-70% prepared for this exam that i have in two hours. the content of it is really cool, oncogenesis basically, a synthesis of all the molecular knowledge that i have gained so far + some more details. so! i'm excited to see how much i know/can guess, there is 0 worry in me and i wish i had had this calm for the past 5 years lol
9 notes
·
View notes
Text
The naked mole rat has been endowed by natural selection with an exceptionally long life span among rodents (over thirty years) and has a very low cancer incidence (Tian et al. 2013). Given the relative lack of predators and other external hazards in the rats’ sealed underground burrows, natural selection favored prolonged investment in tissue maintenance because it paid off in terms of reproductive success through these long lives. In terms of cancer prevention, naked mole rat fibroblasts secrete a special form of the molecule hyaluronan, a major component of the extracellular matrix in many tissues that controls cell proliferation and migration. The hyaluronan produced by naked mole rat cells is of larger molecular weight than that in humans and mice, and it accumulates at high levels throughout the rat’s tissues. Cell culture experiments showed that manipulating hyaluronan could dictate transformability: removing this high molecular weight hyaluronan made the usually cancer refractory naked mole rat cells susceptible to transformation to a more cancer-like phenotype (Tian et al. 2013). The type of hyaluronan determined how cells responded to their neighbors, with the larger hyaluronan form in naked mole rats leading to cell division arrest when cells contact other cells. If these mechanisms function similarly in tissues during oncogenesis, species-specific differences in hyaluronan could contribute to differential cancer susceptibility. Notably, this mechanism so far appears unique to naked mole rats.
5 notes
·
View notes
Text
Multiple doctors and scientists, including Dr. David Rasnick, Dr. Ryan Cole, Dr. Roger Hodkinson, and Scientist Kevin McKernan, say that covid injections cause “turbo cancers” due to immune system suppression.
The injections contain DNA plasmids with the SV40 promoter sequence, which has been associated with oncogenesis and can bind with P53, “the guardian of the genome.”
Doctors and experts report a significant increase in aggressive cancers, often in younger people, with rapid growth to Stage 3 or Stage 4, and link this phenomenon to the covid injections’ degradation of the immune system.
Multiple case reports and studies suggest a potential link between covid injections and an increased risk of cancer, including aggressive and metastatic types. Specific cases reported include colon cancer, breast cancer, skin cancer, gastric cancer, basaloid carcinoma, melanoma, adenoid cystic carcinoma and acute lymphoblastic leukaemia/lymphoblastic lymphoma, among others. Researchers propose that the vaccines may cause immune suppression, leading to accelerated cancer progression and that certain modifications in mRNA vaccines (e.g., 100% N1-methyl-pseudouridine) enhance tumour growth.
Additionally, numerous personal accounts on social media report loved ones and friends developing aggressive cancers, referred to as “turbo cancers,” shortly after receiving covid “vaccines.”
All of the above was described in a “mega thread” posted on Twitter on 30 December by Sense Receptor. The following is a copy of the thread as posted by NZDSOS.
0 notes
Text
A Comparative Study on the Biological and Magical Differences Between Homo Sapiens and Homo Sylvanis
Tiera Novrus
Department of Biology, Blackstaff Academy
Blackstaff Tower, Waterdeep, Sword Coast North
Abstract
This study explores the biological and magical mechanisms underlying the stark differences in aging between Humans (Homo sapiens) and Elves (Homo sylvanis). While Humans experience replicative senescence due to telomere shortening and oxidative stress, Elves exhibit negligible senescence, maintaining cellular functionality across centuries. This longevity is attributed to two unique factors: an advanced telomerase regulatory system and the phenomenon of cellular repurposing. Elven telomerase extends telomeres without inducing oncogenesis, a feat achieved through a mana-mediated oncostatic factor that prevents mutagenic proliferation. Additionally, Elven cells, upon reaching replicative limits, undergo functional transformation instead of entering senescence, contributing to enhanced tissue integrity and immune responses.
Elven mitochondria further distinguish themselves through their symbiotic relationship with ambient magic, which enhances mitochondrial stability and energy production while reducing oxidative damage. This study proposes that ambient mana acts as both a regulator of biological processes and a supplementary energy source, enabling Elves to sustain prolonged vitality and resilience. In contrast, Humans lack such magical adaptations, resulting in accelerated aging, chronic inflammation, and age-related tissue degeneration. These findings highlight the profound interplay between magic and biology in Elves and offer potential avenues for adapting these mechanisms to extend Human healthspan and mitigate age-related decline.
Keywords: aging, cancer, mana, epigenetic, senescence, mutagenic proliferation, mitochondria, telomeres, oncogenesis.
Introduction
The study of aging has long been a subject of fascination and inquiry, especially when comparing the lifespans and biological processes of Humans (Homo sapiens) and Elves (Homo sylvanis). While Humans experience the inevitable decline associated with aging, including cellular senescence, tissue degradation, and increased susceptibility to disease, Elves are renowned for their extraordinary longevity, often living for centuries with minimal signs of physical or cognitive decline. This divergence raises fundamental questions about the interplay between biology and magic in shaping life processes.
In Humans, aging is driven by well-documented hallmarks, such as genomic instability (genetic damage throughout life), telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. These processes collectively result in cellular damage, tissue dysfunction, and a decline in physiological resilience, ultimately contributing to morbidity and mortality. By contrast, Elves exhibit a near-complete absence of these age-related phenomena. Their cellular systems appear to maintain vitality indefinitely, raising the possibility of unique physiological adaptations
We have attempted to identify and categorize these cellular, magical and molecular differences. Key among these differences include an advanced telomerase regulatory system that prevents telomere shortening without inducing oncogenesis, and a phenomenon known as cellular repurposing, wherein senescent cells adopt new functional roles within the body.
Beyond these cellular processes, Elves’ relationship with magic introduces a compelling dimension to their biology that we, as Humans, dearly lack. Their mitochondria have specialized adaptations that allow them to absorb ambient magic, then proceed to convert mana into energy in addition to the conventional biochemical processes. This magical augmentation reduces oxidative stress and ensures the stability of mitochondrial function, a stark contrasts to the progressive deterioration observed in Human mitochondria. Furthermore, the mana-rich environments in which Elves thrive appear to serve as external supports, reinforcing their biological systems and delaying the onset of aging.
We have attempted to identify and categorize these cellular, magical, and molecular differences between Humans and Elves, focusing on both the biological underpinnings and the role of magic in their physiology. For the sake of clarity, this study will not delve into the evolutionary differences among the various subspecies of Elves. Instead, it will focus on the common biological and magical traits shared by Elves as a whole, comparing them to Humans to better understand the mechanisms underlying their longevity. By analyzing cellular processes, mitochondrial function, and the role of magic in Elven biology, this research seeks to shed light on the fundamental differences between these two species. The findings not only enhance our understanding of Elves and Humans but also open avenues for potential applications, such as mitigating age-related decline in Humans through the adaptation of Elven biological mechanisms.
Telomerase Regulation in Humans and Elves
Telomerase, an enzyme responsible for maintaining telomeres—the protective caps at the ends of chromosomes—plays a critical role in cellular replication and longevity. In Humans (Homo sapiens), telomerase activity is tightly restricted, being primarily active in germ cells, embryonic stem cells, and certain adult stem cells. This limitation minimizes the risk of oncogenesis but results in progressive telomere shortening with each cell division, a key driver of replicative senescence and tissue aging.
In Elves (Homo sylvanis), the regulation of telomerase is fundamentally different and involves the presence of a unique physical structure within their cells: the Telomeric Regulatory Complex (TRC). This organelle-like structure is a highly specialized addition to Elven cells, functioning as a central hub for telomere maintenance and repair. The TRC enables Elves to achieve remarkable longevity, but its eventual degradation and dysfunction are the primary reasons for Elven mortality due to aging.
The Telomeric Regulatory Complex (TRC)
The TRC is a nano-scale organelle embedded within the nucleus of every Elven cell. It serves as both a telomerase activator and a precision regulator, ensuring that telomerase activity is tightly controlled. The TRC's functions are enhanced by its integration with mana-responsive elements, which allow it to draw on ambient magical energy to fuel telomerase activity. Unlike in Humans, where telomerase is only sporadically active, the TRC ensures sustained telomere maintenance across all somatic cells.
However, the TRC is not immune to degradation. Over centuries, exposure to environmental stressors, oxidative damage, and mana fluctuations gradually erodes the TRC's efficiency. When the TRC can no longer sustain telomerase activity or maintain its structure, Elven cells begin to experience replicative senescence, ultimately leading to aging and death.
Oncostatic Factors Embedded in the TRC
The TRC is equipped with advanced oncostatic factors, a suite of proteins and regulatory molecules that actively suppress mutations, repair DNA damage, and eliminate pre-cancerous cells. These factors are embedded within the TRC itself, allowing Elves to maintain continuous telomerase activity without the heightened risk of oncogenesis that such activity would pose in Humans. The TRC's degradation in later life reduces the effectiveness of these oncostatic factors, contributing to a slight increase in cellular instability as Elves approach the end of their lifespans.
The Human-Elven Contrast
In Humans, the absence of a TRC leaves telomerase activity unregulated and confined to specific cell types, such as germ cells and some stem cells. This evolutionary tradeoff limits cancer risk but accelerates aging due to telomere attrition. By contrast, Elves benefit from the TRC's regulated telomerase activity and embedded oncostatic factors, allowing them to delay senescence for centuries. The eventual degradation of the TRC, however, demonstrates that even the most advanced biological systems have finite lifespans.
Implications for Human Longevity
The Elven TRC provides a fascinating blueprint for extending Human lifespans. If the TRC's structure and function could be replicated or adapted for Humans, it may be possible to extend telomere maintenance and mitigate aging-related decline. However, the challenge lies in replicating the mana-integrated processes of the TRC and preventing its degradation over time. Future studies might explore hybrid biological-magical constructs as a potential avenue for addressing these challenges.
Cellular Repurposing in Elves
One of the most remarkable features of Elven biology is their ability to repurpose senescent cells, a process that dramatically reduces the accumulation of non-functional cells in their tissues. In Humans (Homo sapiens), when cells reach replicative senescence—either due to telomere attrition or damage—they typically enter a state of permanent growth arrest. These senescent cells may persist in tissues, secreting inflammatory molecules and contributing to age-related degeneration. In contrast, Elves (Homo sylvanis) exhibit a unique biological mechanism known as cellular repurposing, in which senescent cells are systematically broken down and transformed into cells with alternative, simplified functions.
The Cellular Breakdown and Repurposing Process
When an Elven cell reaches the threshold of senescence, it undergoes a highly regulated breakdown sequence mediated by specialized organelles called repurposing vesicles. These vesicles are activated by signals from the Telomeric Regulatory Complex (TRC) and initiate a process that disassembles the senescent cell into its constituent components, such as proteins, organelles, and cytoplasmic materials.
Rather than being discarded, these components are reassembled to create new cells with reduced functional complexity. For instance:
Immune Cells: A white blood cell (e.g., a lymphocyte) that has reached senescence may be repurposed into a red blood cell. The resulting red blood cell is simpler in function, requiring less energy and replication while still contributing to tissue homeostasis.
Neurons and Glial Cells: In the nervous system, a senescent neuron may be broken down and reassembled into glial cells, which provide structural support and maintain the magical conductivity required for Elven neurological systems.
Epithelial Cells: Senescent epithelial cells from tissues like the skin may be converted into keratinocytes, which are more durable and energy-efficient, contributing to the maintenance of youthful skin.
This process not only prevents the accumulation of harmful senescent cells but also sustains tissue functionality and structural integrity for centuries.
Magical Integration in Cellular Repurposing
Elven cellular repurposing is closely linked to their unique mana-based biology. The repurposing vesicles rely on ambient magical energy to fuel the breakdown and reconstruction processes. Mana is used to stabilize cellular components during repurposing, preventing waste accumulation and oxidative damage. This ensures that the repurposed cells are as efficient and functional as their predecessors, albeit with fewer demands on cellular replication.
Comparative Perspective: Humans vs. Elves
In Humans, the absence of a repurposing mechanism means that senescent cells persist in tissues, often secreting pro-inflammatory cytokines and contributing to the chronic inflammation observed with aging. These so-called senescence-associated secretory phenotypes (SASP) exacerbate tissue degeneration and age-related diseases. Additionally, the lack of efficient recycling pathways results in greater metabolic waste and cellular inefficiencies over time.
By contrast, Elves’ ability to recycle cellular materials allows them to maintain a low metabolic burden and reduces the inflammatory response associated with aging. The continual repurposing of cells also ensures a high level of tissue homeostasis, with older tissues functioning almost as efficiently as younger ones.
The Cost of Cellular Repurposing
While cellular repurposing is a highly efficient mechanism, it is not without limits. Over the course of centuries, the repurposing vesicles themselves begin to degrade, similar to the Telomeric Regulatory Complex. This degradation leads to a gradual reduction in the effectiveness of the repurposing process. As a result, Elven tissues eventually begin to accumulate non-functional or poorly repurposed cells, which contributes to the onset of age-related decline in their final centuries of life.
Elven Mitochondria: Stability Through Magic
Mitochondria, often referred to as the powerhouses of the cell, are essential for energy production in both Humans (Homo sapiens) and Elves (Homo sylvanis). These organelles generate adenosine triphosphate (ATP) through oxidative phosphorylation, powering cellular processes and maintaining homeostasis. While Human mitochondria are highly efficient, they are also vulnerable to damage over time, with mitochondrial dysfunction being a hallmark of aging. In contrast, Elves possess mitochondria that are uniquely attuned to magic, enabling remarkable stability and functionality throughout their extended lifespans
Magical Integration in Elven Mitochondria
Elven mitochondria are infused with mana-conductive elements, specialized structures that allow them to interact with ambient magical energy. These elements serve two critical purposes:
Stabilizing Energy Production: Mana supplements the electron transport chain, reducing the reliance on oxidative processes and minimizing the production of reactive oxygen species (ROS). This significantly lowers oxidative damage, a key contributor to mitochondrial aging in Humans.
Repair and Maintenance: Mana-conductive elements enable mitochondria to self-repair by facilitating the re-synthesis of damaged proteins and lipids. This continuous maintenance prevents the accumulation of mutations in mitochondrial DNA (mtDNA) and preserves mitochondrial function over centuries.
Mana as an Alternative Energy Source
In addition to producing ATP through traditional oxidative phosphorylation, Elven mitochondria can directly convert mana into biochemical energy. This process, known as arcane phosphorylation, bypasses the need for oxygen and glucose, allowing Elves to maintain energy production even under extreme conditions, such as starvation or hypoxia. This adaptation not only enhances their survival capabilities but also reduces the metabolic burden on their bodies.
Mitochondrial-Nuclear Communication
Elven cells exhibit enhanced communication between mitochondria and the nucleus, mediated by mana-regulated signaling pathways. This ensures that the nucleus responds to mitochondrial stress signals with greater efficiency, activating repair pathways and adjusting metabolic processes in real time. By contrast, mitochondrial-nuclear communication in Humans becomes less effective with age, contributing to cellular dysfunction and systemic aging.
Human vs. Elven Mitochondria: A Comparative View
Oxidative Stress: Human mitochondria are prone to oxidative stress due to the generation of ROS during energy production. Over time, this leads to mtDNA mutations, compromised energy output, and cellular senescence. Elven mitochondria, bolstered by mana, produce significantly less ROS, maintaining stability and reducing the risk of oxidative damage.
Aging: Mitochondrial dysfunction is a key driver of aging in Humans, contributing to fatigue, reduced cellular repair, and age-related diseases. Elves, by leveraging magic to sustain mitochondrial health, delay these effects for centuries.
Adaptability: While Human mitochondria rely solely on oxygen and glucose for energy production, Elven mitochondria can seamlessly transition between traditional metabolic pathways and mana-based energy systems, providing unparalleled adaptability.
Limits of Elven Mitochondrial Stability
Despite their resilience, Elven mitochondria are not immune to aging. Over centuries, the mana-conductive elements within the mitochondria degrade, reducing their ability to utilize magic for repair and energy production. This gradual decline mirrors the degradation of the Telomeric Regulatory Complex and the repurposing vesicles, marking the beginning of cellular dysfunction and the eventual onset of aging in Elves.
Conclusions and Perspectives
The biological and magical mechanisms underpinning Elven longevity provide a fascinating contrast to Human aging, highlighting the interplay between cellular regulation, magic, and evolutionary adaptation. By examining telomerase regulation, cellular repurposing, and mitochondrial stability, we have gained insight into the intricate processes that enable Elves (Homo sylvanis) to extend their lifespans far beyond those of Humans (Homo sapiens).
Elves demonstrate exceptional biological efficiency:
The Telomeric Regulatory Complex (TRC) maintains telomere length and suppresses oncogenesis, ensuring sustained cellular function for centuries. Its eventual degradation underscores the finite nature of even advanced biological systems.
Cellular repurposing eliminates senescent cells by converting them into simpler, functional counterparts, preventing the buildup of metabolic and inflammatory waste.
Mana-integrated mitochondria stabilize energy production and minimize oxidative damage, mitigating one of the central drivers of aging in Humans.
These systems collectively illustrate how magic and biology coexist in Elves, resulting in a life cycle characterized by prolonged vitality and delayed aging. Yet, even in Elves, aging is inevitable as the very structures responsible for their longevity degrade over time.
Implications for Comparative Biology and Medicine
The study of Elven biology offers intriguing possibilities for advancing Human science and medicine:
Longevity Research: Understanding the TRC and its regulation of telomerase activity may provide strategies to extend Human lifespans or combat age-related diseases.
Anti-Cancer Therapies: The oncostatic factors within the TRC could inspire new approaches to preventing or treating cancer while minimizing cellular senescence.
Mitochondrial Stability: Insights into mana-fueled mitochondrial repair and oxidative stress reduction could inform therapies for mitochondrial diseases and metabolic disorders.
Challenges and Perspectives
While the integration of magic into Elven biology provides profound advantages, replicating such systems in Humans poses significant challenges. The dependence on mana, an energy source unique to magical organisms, makes direct application to Humans difficult without a comparable energy system. Future research might focus on developing hybrid biological-magical constructs or discovering synthetic alternatives to mana.
Elves also represent a striking example of evolutionary tradeoffs. Their reliance on magical mechanisms, while advantageous for longevity, creates vulnerabilities to mana depletion and environmental fluctuations. As such, their biology serves as a reminder that no system is without limits.
Final Thoughts
The study of Elves expands our understanding of life and aging, challenging us to rethink the boundaries of biology as we know it. By examining the interplay between magic and cellular processes, we not only deepen our knowledge of fantastical organisms but also open doors to new possibilities in medicine and biotechnological innovation. The Elven ability to harmonize the mystical and the molecular presents a tantalizing vision of what might one day be achievable in Human science and beyond.
Citations and References
Tolkien, J. R. R. (1977). The Silmarillion. George Allen & Unwin.
Tolkien, J. R. R. (1954). The Lord of the Rings. Houghton Mifflin.
Wizards of the Coast. (2014). Player's Handbook (5th ed.). Wizards of the Coast.
Wizards of the Coast. (2014). Dungeon Master's Guide (5th ed.). Wizards of the Coast.
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Kirkwood, T. B. L., & Austad, S. N. (2000). Why do we age? Nature, 408(6809), 233–238. https://doi.org/10.1038/35041682
Gaiman, N. (2017). Norse mythology. W.W. Norton & Company.
Hamilton, E. (1942). Mythology: Timeless tales of gods and heroes. Little, Brown and Company.
Clute, J., & Grant, J. (2017). The encyclopedia of fantasy. St. Martin's Press.
Csicsery-Ronay, I. (2008). The aging hero: Science fiction and the representation of time. Science Fiction Studies, 35(2), 141–157.
Ainsworth, M. D. S., & Bowlby, J. (1991). An ethological approach to personality development. American Psychologist, 46(4), 331–341.
Hayflick, L., & Moorhead, P. S. (1961). The serial cultivation of human diploid cell strains. Experimental Cell Research, 25, 585–621. https://doi.org/10.1016/0014-4827(61)90192-6
Campisi, J. (2005). Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell, 120(4), 513–522. https://doi.org/10.1016/j.cell.2005.02.003
1 note
·
View note
Text
Fattori di trascrizione: i "direttori d'orchestra" cellulari della salute umana
Fattori di trascrizione e salute umana I fattori di trascrizione sono proteine essenziali che regolano l’espressione genica, influenzando la trascrizione del DNA in RNA messaggero (mRNA), che è il primo passo della produzione di proteine. Essi si legano a sequenze specifiche di DNA, chiamate promotori o enhancer, per attivare o reprimere la trascrizione dei geni. Sono cruciali per il controllo di…
#antitumorale#apoptosi#autoimmunità#c-Myc#cellule tumorali#citochine#fattore di trascrizione AP-1#flogosi cronica#FOXO3#istone deacetilasi#metilazione#napabucasin#oncogenesi#p53#sistema immunitario#STAT-3
0 notes
Text
Resihance

Resihance
Regorafenib is a multi-kinase inhibitor used primarily for the treatment of cancer. It is particularly effective in targeting angiogenesis (the formation of new blood vessels), which plays a crucial role in cancer growth and metastasis. Below is a detailed overview of Regorafenib:
Mechanism of Action:
Regorafenib inhibits multiple protein kinases involved in tumor angiogenesis, oncogenesis, and the tumor microenvironment. Specifically, it targets:
VEGFR (Vascular Endothelial Growth Factor Receptor) – involved in blood vessel formation.
PDGFR (Platelet-Derived Growth Factor Receptor) – involved in the growth and survival of cells.
RAF kinases (including BRAF) – involved in cell proliferation and survival.
By blocking these pathways, Regorafenib reduces tumor growth and the spread of cancer.
Indications:
Regorafenib is used in the treatment of several cancers, including:
Colorectal Cancer: It is used in metastatic colorectal cancer (mCRC) that has progressed after standard therapy.
Gastrointestinal Stromal Tumors (GIST): It is prescribed for GIST after imatinib and sunitinib treatment have failed.
Hepatocellular Carcinoma (HCC): For patients with advanced liver cancer who have been previously treated with sorafenib.
Common Side Effects:
Fatigue
Hand-foot skin reaction (redness, swelling, pain in palms and soles)
Diarrhea
Hypertension (high blood pressure)
Nausea and vomiting
Abdominal pain
Decreased appetite
Weight loss
Serious Side Effects:
Liver toxicity: Regorafenib can lead to severe liver damage, including elevated liver enzymes, jaundice, and, in rare cases, liver failure.
Bleeding: Regorafenib can increase the risk of severe bleeding, especially in patients with cancer that has spread to the liver.
Cardiovascular complications: It can lead to high blood pressure and may increase the risk of heart attack or stroke.
Gastrointestinal perforation: A rare but potentially life-threatening complication.
Monitoring and Precautions:
Liver function should be monitored regularly because of the risk of liver toxicity.
Blood pressure should be checked frequently to detect any early signs of hypertension.
Skin reactions should be monitored closely, as they can affect the patient's quality of life.
Kidney function should also be assessed periodically, especially in patients at risk of kidney damage.
Pharmacokinetics:
Absorption: Regorafenib is well absorbed after oral administration but should be taken with a low-fat meal to ensure proper absorption.
Metabolism: The drug is metabolized in the liver primarily through CYP3A4, and its active metabolites also play a role in its efficacy.
Excretion: Regorafenib and its metabolites are excreted primarily through feces, with a small portion eliminated through urine.
0 notes
Text
Deciphering the Ras/MAPK Signaling Pathway in the Progression and Treatment of Hepatocellular Carcinoma_Crimson Publishers

Abstract
Hepatocellular Carcinoma (HCC) is a serious health issue and its frequency is rapidly escalating throughout the world therefore researchers have focused more attention to the Ras/MAPK signaling pathway. The signaling pathways are linked to develop tumors and the Ras/MAPK pathway is one of these pathways, activated in 60% of HCCs with poor prognosis. A number of different proteins causes the abnormal regulation of the MAPK pathway in HCC. Ras, a small GTPase and Raf are the most commonly mutated oncogene supports the critical function of this pathway in oncogenesis. The genetic mutations leading to effector molecule to permanently activated in the Ras/MAPK signaling cascades. The inappropriate activation of this pathway is primarily due to the downregulation of various Ras/MAPK pathway inhibitors including RASSF proteins, GAPs, DUSP1, Spred and Sprouty proteins. The post-transcriptional or epigenetic processes downregulate these cancer suppressor genes. The aim of current study on the primary mutations resulting in aberrant activation of Ras/MAPK pathway and their role on the initiation and progression of HCC. It also offers an update on the various inhibitors to target this central signaling pathway including various Ras, Raf, MEK inhibitors in the context of HCC. Finally, we evaluate the available options for treatment in this context.
Read more about this article:
For more articles in Novel Approaches in Cancer Study
#cancer#breast cancer#crimson cancer#open access journal#cancer open access journal#crimsonpublishers#novel approaches in cancer study
0 notes
Text
youtube
#Cancer metabolism#glycosylation#stemness#tumor microenvironment#cancer stem cells#cell signaling#metabolic reprogramming#therapeutic targets#glycan structures#immune modulation#tumor growth#cell differentiation#cancer adaptation#oncogenesis#sugar code#precision oncology#biomarker discovery#protein glycosylation#cancer resistance#oncology research.#Youtube
0 notes
Text
Pseudogenes in Esophageal Carcinoma
Unveiling the Shadows: New Frontier in Cancer Biology Cancer research is continually evolving, branching into areas previously unexplored or deemed less significant. A prime example of this evolution is the study of pseudogenes and their impact on cancer, specifically esophageal carcinoma. How do the Pseudogenes Drive Cancer Progression in the Oesophagus? Pseudogenes are DNA sequences that…
View On WordPress
#Biomedical innovation#Cancer research#Cellular reprogrammin#Esophageal cancer#Esophageal carcinoma#featured#Gene regulatory networks#Genomic medicine#Molecular biology#Oncogenesis#Pseudogenes#sciencenews
0 notes