#guangxin
Explore tagged Tumblr posts
Text



Wangxian Township, Guangxin District, Shangrao City, Jiangxi Province, China
@ Hwang199 Photography
#art#design#stairwell#stairway#architecture#staircase#stairs#staircases#china#wangxian#guangxin#shangrao city#jiangxi#scenic#landscape#hwang199#fairy#cliff#mountains
1K notes
·
View notes
Text











joy of life
I'll swap out all the people working at Guangxin Palace and report this to Father.
#joy of life#joy of life 2#庆余年#庆余年2#cdrama#perioddramaedit#cdramanet#cdramaedit#gifshistorical#chineseartistsinc#cdramagifs#cdramasource#asiandramanet#perioddramasource#onlyperioddramas#gzh
83 notes
·
View notes
Text

Researchers identify unique phenomenon in Kagome metal
In traditional Japanese basket-weaving, the ancient "Kagome" design seen in many handcrafted creations is characterized by a symmetrical pattern of interlaced triangles with shared corners. In quantum physics, the Kagome name has been borrowed by scientists to describe a class of materials with an atomic structure closely resembling this distinctive lattice pattern. Since the latest family of Kagome metals was discovered in 2019, physicists have been working to better understand their properties and potential applications. A new study led by Florida State University Assistant Professor of Physics Guangxin Ni focuses on how a particular Kagome metal interacts with light to generate what are known as plasmon polaritons—nanoscale-level linked waves of electrons and electromagnetic fields in a material, typically caused by light or other electromagnetic waves. The work was published in Nature Communications.
Read more.
11 notes
·
View notes
Text
It’s the most fundamental of processes — the evaporation of water from the surfaces of oceans and lakes, the burning off of fog in the morning sun, and the drying of briny ponds that leaves solid salt behind. Evaporation is all around us, and humans have been observing it and making use of it for as long as we have existed.
And yet, it turns out, we’ve been missing a major part of the picture all along.
In a series of painstakingly precise experiments, a team of researchers at MIT has demonstrated that heat isn’t alone in causing water to evaporate. Light, striking the water’s surface where air and water meet, can break water molecules away and float them into the air, causing evaporation in the absence of any source of heat.
The astonishing new discovery could have a wide range of significant implications. It could help explain mysterious measurements over the years of how sunlight affects clouds, and therefore affect calculations of the effects of climate change on cloud cover and precipitation. It could also lead to new ways of designing industrial processes such as solar-powered desalination or drying of materials.
The findings, and the many different lines of evidence that demonstrate the reality of the phenomenon and the details of how it works, are described today in the journal PNAS, in a paper by Carl Richard Soderberg Professor of Power Engineering Gang Chen, postdocs Guangxin Lv and Yaodong Tu, and graduate student James Zhang.
The authors say their study suggests that the effect should happen widely in nature— everywhere from clouds to fogs to the surfaces of oceans, soils, and plants — and that it could also lead to new practical applications, including in energy and clean water production. “I think this has a lot of applications,” Chen says. “We’re exploring all these different directions. And of course, it also affects the basic science, like the effects of clouds on climate, because clouds are the most uncertain aspect of climate models.”
A newfound phenomenon
The new work builds on research reported last year, which described this new “photomolecular effect” but only under very specialized conditions: on the surface of specially prepared hydrogels soaked with water. In the new study, the researchers demonstrate that the hydrogel is not necessary for the process; it occurs at any water surface exposed to light, whether it’s a flat surface like a body of water or a curved surface like a droplet of cloud vapor.
Because the effect was so unexpected, the team worked to prove its existence with as many different lines of evidence as possible. In this study, they report 14 different kinds of tests and measurements they carried out to establish that water was indeed evaporating — that is, molecules of water were being knocked loose from the water’s surface and wafted into the air — due to the light alone, not by heat, which was long assumed to be the only mechanism involved.
One key indicator, which showed up consistently in four different kinds of experiments under different conditions, was that as the water began to evaporate from a test container under visible light, the air temperature measured above the water’s surface cooled down and then leveled off, showing that thermal energy was not the driving force behind the effect.
Other key indicators that showed up included the way the evaporation effect varied depending on the angle of the light, the exact color of the light, and its polarization. None of these varying characteristics should happen because at these wavelengths, water hardly absorbs light at all — and yet the researchers observed them.
The effect is strongest when light hits the water surface at an angle of 45 degrees. It is also strongest with a certain type of polarization, called transverse magnetic polarization. And it peaks in green light — which, oddly, is the color for which water is most transparent and thus interacts the least.
Chen and his co-researchers have proposed a physical mechanism that can explain the angle and polarization dependence of the effect, showing that the photons of light can impart a net force on water molecules at the water surface that is sufficient to knock them loose from the body of water. But they cannot yet account for the color dependence, which they say will require further study.
They have named this the photomolecular effect, by analogy with the photoelectric effect that was discovered by Heinrich Hertz in 1887 and finally explained by Albert Einstein in 1905. That effect was one of the first demonstrations that light also has particle characteristics, which had major implications in physics and led to a wide variety of applications, including LEDs. Just as the photoelectric effect liberates electrons from atoms in a material in response to being hit by a photon of light, the photomolecular effect shows that photons can liberate entire molecules from a liquid surface, the researchers say.
“The finding of evaporation caused by light instead of heat provides new disruptive knowledge of light-water interaction,” says Xiulin Ruan, professor of mechanical engineering at Purdue University, who was not involved in the study. “It could help us gain new understanding of how sunlight interacts with cloud, fog, oceans, and other natural water bodies to affect weather and climate. It has significant potential practical applications such as high-performance water desalination driven by solar energy. This research is among the rare group of truly revolutionary discoveries which are not widely accepted by the community right away but take time, sometimes a long time, to be confirmed.”
Solving a cloud conundrum
The finding may solve an 80-year-old mystery in climate science. Measurements of how clouds absorb sunlight have often shown that they are absorbing more sunlight than conventional physics dictates possible. The additional evaporation caused by this effect could account for the longstanding discrepancy, which has been a subject of dispute since such measurements are difficult to make.
“Those experiments are based on satellite data and flight data,“ Chen explains. “They fly an airplane on top of and below the clouds, and there are also data based on the ocean temperature and radiation balance. And they all conclude that there is more absorption by clouds than theory could calculate. However, due to the complexity of clouds and the difficulties of making such measurements, researchers have been debating whether such discrepancies are real or not. And what we discovered suggests that hey, there’s another mechanism for cloud absorption, which was not accounted for, and this mechanism might explain the discrepancies.”
Chen says he recently spoke about the phenomenon at an American Physical Society conference, and one physicist there who studies clouds and climate said they had never thought about this possibility, which could affect calculations of the complex effects of clouds on climate. The team conducted experiments using LEDs shining on an artificial cloud chamber, and they observed heating of the fog, which was not supposed to happen since water does not absorb in the visible spectrum. “Such heating can be explained based on the photomolecular effect more easily,” he says.
Lv says that of the many lines of evidence, “the flat region in the air-side temperature distribution above hot water will be the easiest for people to reproduce.” That temperature profile “is a signature” that demonstrates the effect clearly, he says.
Zhang adds: “It is quite hard to explain how this kind of flat temperature profile comes about without invoking some other mechanism” beyond the accepted theories of thermal evaporation. “It ties together what a whole lot of people are reporting in their solar desalination devices,” which again show evaporation rates that cannot be explained by the thermal input.
The effect can be substantial. Under the optimum conditions of color, angle, and polarization, Lv says, “the evaporation rate is four times the thermal limit.”
Already, since publication of the first paper, the team has been approached by companies that hope to harness the effect, Chen says, including for evaporating syrup and drying paper in a paper mill. The likeliest first applications will come in the areas of solar desalinization systems or other industrial drying processes, he says. “Drying consumes 20 percent of all industrial energy usage,” he points out.
Because the effect is so new and unexpected, Chen says, “This phenomenon should be very general, and our experiment is really just the beginning.” The experiments needed to demonstrate and quantify the effect are very time-consuming. “There are many variables, from understanding water itself, to extending to other materials, other liquids and even solids,” he says.
“The observations in the manuscript points to a new physical mechanism that foundationally alters our thinking on the kinetics of evaporation,” says Shannon Yee, an associate professor of mechanical engineering at Georgia Tech, who was not associated with this work. He adds, “Who would have thought that we are still learning about something as quotidian as water evaporating?”
“I think this work is very significant scientifically because it presents a new mechanism,” says University of Alberta Distinguished Professor Janet A.W. Elliott, who also was not associated with this work. “It may also turn out to be practically important for technology and our understanding of nature, because evaporation of water is ubiquitous and the effect appears to deliver significantly higher evaporation rates than the known thermal mechanism. … My overall impression is this work is outstanding. It appears to be carefully done with many precise experiments lending support for one another.”
The work was partly supported by an MIT Bose Award. The authors are currently working on ways to make use of this effect for water desalination, in a project funded by the Abdul Latif Jameel Water and Food Systems Lab and the MIT-UMRP program.
0 notes
Text
How light can vaporize water without the need for heat
New Post has been published on https://sunalei.org/news/how-light-can-vaporize-water-without-the-need-for-heat/
How light can vaporize water without the need for heat

It’s the most fundamental of processes — the evaporation of water from the surfaces of oceans and lakes, the burning off of fog in the morning sun, and the drying of briny ponds that leaves solid salt behind. Evaporation is all around us, and humans have been observing it and making use of it for as long as we have existed.
And yet, it turns out, we’ve been missing a major part of the picture all along.
In a series of painstakingly precise experiments, a team of researchers at MIT has demonstrated that heat isn’t alone in causing water to evaporate. Light, striking the water’s surface where air and water meet, can break water molecules away and float them into the air, causing evaporation in the absence of any source of heat.
The astonishing new discovery could have a wide range of significant implications. It could help explain mysterious measurements over the years of how sunlight affects clouds, and therefore affect calculations of the effects of climate change on cloud cover and precipitation. It could also lead to new ways of designing industrial processes such as solar-powered desalination or drying of materials.
The findings, and the many different lines of evidence that demonstrate the reality of the phenomenon and the details of how it works, are described today in the journal PNAS, in a paper by Carl Richard Soderberg Professor of Power Engineering Gang Chen, postdocs Guangxin Lv and Yaodong Tu, and graduate student James Zhang.
The authors say their study suggests that the effect should happen widely in nature— everywhere from clouds to fogs to the surfaces of oceans, soils, and plants — and that it could also lead to new practical applications, including in energy and clean water production. “I think this has a lot of applications,” Chen says. “We’re exploring all these different directions. And of course, it also affects the basic science, like the effects of clouds on climate, because clouds are the most uncertain aspect of climate models.”
A newfound phenomenon
The new work builds on research reported last year, which described this new “photomolecular effect” but only under very specialized conditions: on the surface of specially prepared hydrogels soaked with water. In the new study, the researchers demonstrate that the hydrogel is not necessary for the process; it occurs at any water surface exposed to light, whether it’s a flat surface like a body of water or a curved surface like a droplet of cloud vapor.
Because the effect was so unexpected, the team worked to prove its existence with as many different lines of evidence as possible. In this study, they report 14 different kinds of tests and measurements they carried out to establish that water was indeed evaporating — that is, molecules of water were being knocked loose from the water’s surface and wafted into the air — due to the light alone, not by heat, which was long assumed to be the only mechanism involved.
One key indicator, which showed up consistently in four different kinds of experiments under different conditions, was that as the water began to evaporate from a test container under visible light, the air temperature measured above the water’s surface cooled down and then leveled off, showing that thermal energy was not the driving force behind the effect.
Other key indicators that showed up included the way the evaporation effect varied depending on the angle of the light, the exact color of the light, and its polarization. None of these varying characteristics should happen because at these wavelengths, water hardly absorbs light at all — and yet the researchers observed them.
The effect is strongest when light hits the water surface at an angle of 45 degrees. It is also strongest with a certain type of polarization, called transverse magnetic polarization. And it peaks in green light — which, oddly, is the color for which water is most transparent and thus interacts the least.
Chen and his co-researchers have proposed a physical mechanism that can explain the angle and polarization dependence of the effect, showing that the photons of light can impart a net force on water molecules at the water surface that is sufficient to knock them loose from the body of water. But they cannot yet account for the color dependence, which they say will require further study.
They have named this the photomolecular effect, by analogy with the photoelectric effect that was discovered by Heinrich Hertz in 1887 and finally explained by Albert Einstein in 1905. That effect was one of the first demonstrations that light also has particle characteristics, which had major implications in physics and led to a wide variety of applications, including LEDs. Just as the photoelectric effect liberates electrons from atoms in a material in response to being hit by a photon of light, the photomolecular effect shows that photons can liberate entire molecules from a liquid surface, the researchers say.
“The finding of evaporation caused by light instead of heat provides new disruptive knowledge of light-water interaction,” says Xiulin Ruan, professor of mechanical engineering at Purdue University, who was not involved in the study. “It could help us gain new understanding of how sunlight interacts with cloud, fog, oceans, and other natural water bodies to affect weather and climate. It has significant potential practical applications such as high-performance water desalination driven by solar energy. This research is among the rare group of truly revolutionary discoveries which are not widely accepted by the community right away but take time, sometimes a long time, to be confirmed.”
Solving a cloud conundrum
The finding may solve an 80-year-old mystery in climate science. Measurements of how clouds absorb sunlight have often shown that they are absorbing more sunlight than conventional physics dictates possible. The additional evaporation caused by this effect could account for the longstanding discrepancy, which has been a subject of dispute since such measurements are difficult to make.
“Those experiments are based on satellite data and flight data,“ Chen explains. “They fly an airplane on top of and below the clouds, and there are also data based on the ocean temperature and radiation balance. And they all conclude that there is more absorption by clouds than theory could calculate. However, due to the complexity of clouds and the difficulties of making such measurements, researchers have been debating whether such discrepancies are real or not. And what we discovered suggests that hey, there’s another mechanism for cloud absorption, which was not accounted for, and this mechanism might explain the discrepancies.”
Chen says he recently spoke about the phenomenon at an American Physical Society conference, and one physicist there who studies clouds and climate said they had never thought about this possibility, which could affect calculations of the complex effects of clouds on climate. The team conducted experiments using LEDs shining on an artificial cloud chamber, and they observed heating of the fog, which was not supposed to happen since water does not absorb in the visible spectrum. “Such heating can be explained based on the photomolecular effect more easily,” he says.
Lv says that of the many lines of evidence, “the flat region in the air-side temperature distribution above hot water will be the easiest for people to reproduce.” That temperature profile “is a signature” that demonstrates the effect clearly, he says.
Zhang adds: “It is quite hard to explain how this kind of flat temperature profile comes about without invoking some other mechanism” beyond the accepted theories of thermal evaporation. “It ties together what a whole lot of people are reporting in their solar desalination devices,” which again show evaporation rates that cannot be explained by the thermal input.
The effect can be substantial. Under the optimum conditions of color, angle, and polarization, Lv says, “the evaporation rate is four times the thermal limit.”
Already, since publication of the first paper, the team has been approached by companies that hope to harness the effect, Chen says, including for evaporating syrup and drying paper in a paper mill. The likeliest first applications will come in the areas of solar desalinization systems or other industrial drying processes, he says. “Drying consumes 20 percent of all industrial energy usage,” he points out.
Because the effect is so new and unexpected, Chen says, “This phenomenon should be very general, and our experiment is really just the beginning.” The experiments needed to demonstrate and quantify the effect are very time-consuming. “There are many variables, from understanding water itself, to extending to other materials, other liquids and even solids,” he says.
“The observations in the manuscript points to a new physical mechanism that foundationally alters our thinking on the kinetics of evaporation,” says Shannon Yee, an associate professor of mechanical engineering at Georgia Tech, who was not associated with this work. He adds, “Who would have thought that we are still learning about something as quotidian as water evaporating?”
“I think this work is very significant scientifically because it presents a new mechanism,” says University of Alberta Distinguished Professor Janet A.W. Elliott, who also was not associated with this work. “It may also turn out to be practically important for technology and our understanding of nature, because evaporation of water is ubiquitous and the effect appears to deliver significantly higher evaporation rates than the known thermal mechanism. … My overall impression is this work is outstanding. It appears to be carefully done with many precise experiments lending support for one another.”
The work was partly supported by an MIT Bose Award.
0 notes
Text
How light can vaporize water without the need for heat
New Post has been published on https://thedigitalinsider.com/how-light-can-vaporize-water-without-the-need-for-heat/
How light can vaporize water without the need for heat


It’s the most fundamental of processes — the evaporation of water from the surfaces of oceans and lakes, the burning off of fog in the morning sun, and the drying of briny ponds that leaves solid salt behind. Evaporation is all around us, and humans have been observing it and making use of it for as long as we have existed.
And yet, it turns out, we’ve been missing a major part of the picture all along.
In a series of painstakingly precise experiments, a team of researchers at MIT has demonstrated that heat isn’t alone in causing water to evaporate. Light, striking the water’s surface where air and water meet, can break water molecules away and float them into the air, causing evaporation in the absence of any source of heat.
The astonishing new discovery could have a wide range of significant implications. It could help explain mysterious measurements over the years of how sunlight affects clouds, and therefore affect calculations of the effects of climate change on cloud cover and precipitation. It could also lead to new ways of designing industrial processes such as solar-powered desalination or drying of materials.
The findings, and the many different lines of evidence that demonstrate the reality of the phenomenon and the details of how it works, are described today in the journal PNAS, in a paper by Carl Richard Soderberg Professor of Power Engineering Gang Chen, postdocs Guangxin Lv and Yaodong Tu, and graduate student James Zhang.
The authors say their study suggests that the effect should happen widely in nature— everywhere from clouds to fogs to the surfaces of oceans, soils, and plants — and that it could also lead to new practical applications, including in energy and clean water production. “I think this has a lot of applications,” Chen says. “We’re exploring all these different directions. And of course, it also affects the basic science, like the effects of clouds on climate, because clouds are the most uncertain aspect of climate models.”
A newfound phenomenon
The new work builds on research reported last year, which described this new “photomolecular effect” but only under very specialized conditions: on the surface of specially prepared hydrogels soaked with water. In the new study, the researchers demonstrate that the hydrogel is not necessary for the process; it occurs at any water surface exposed to light, whether it’s a flat surface like a body of water or a curved surface like a droplet of cloud vapor.
Because the effect was so unexpected, the team worked to prove its existence with as many different lines of evidence as possible. In this study, they report 14 different kinds of tests and measurements they carried out to establish that water was indeed evaporating — that is, molecules of water were being knocked loose from the water’s surface and wafted into the air — due to the light alone, not by heat, which was long assumed to be the only mechanism involved.
One key indicator, which showed up consistently in four different kinds of experiments under different conditions, was that as the water began to evaporate from a test container under visible light, the air temperature measured above the water’s surface cooled down and then leveled off, showing that thermal energy was not the driving force behind the effect.
Other key indicators that showed up included the way the evaporation effect varied depending on the angle of the light, the exact color of the light, and its polarization. None of these varying characteristics should happen because at these wavelengths, water hardly absorbs light at all — and yet the researchers observed them.
The effect is strongest when light hits the water surface at an angle of 45 degrees. It is also strongest with a certain type of polarization, called transverse magnetic polarization. And it peaks in green light — which, oddly, is the color for which water is most transparent and thus interacts the least.
Chen and his co-researchers have proposed a physical mechanism that can explain the angle and polarization dependence of the effect, showing that the photons of light can impart a net force on water molecules at the water surface that is sufficient to knock them loose from the body of water. But they cannot yet account for the color dependence, which they say will require further study.
They have named this the photomolecular effect, by analogy with the photoelectric effect that was discovered by Heinrich Hertz in 1887 and finally explained by Albert Einstein in 1905. That effect was one of the first demonstrations that light also has particle characteristics, which had major implications in physics and led to a wide variety of applications, including LEDs. Just as the photoelectric effect liberates electrons from atoms in a material in response to being hit by a photon of light, the photomolecular effect shows that photons can liberate entire molecules from a liquid surface, the researchers say.
“The finding of evaporation caused by light instead of heat provides new disruptive knowledge of light-water interaction,” says Xiulin Ruan, professor of mechanical engineering at Purdue University, who was not involved in the study. “It could help us gain new understanding of how sunlight interacts with cloud, fog, oceans, and other natural water bodies to affect weather and climate. It has significant potential practical applications such as high-performance water desalination driven by solar energy. This research is among the rare group of truly revolutionary discoveries which are not widely accepted by the community right away but take time, sometimes a long time, to be confirmed.”
Solving a cloud conundrum
The finding may solve an 80-year-old mystery in climate science. Measurements of how clouds absorb sunlight have often shown that they are absorbing more sunlight than conventional physics dictates possible. The additional evaporation caused by this effect could account for the longstanding discrepancy, which has been a subject of dispute since such measurements are difficult to make.
“Those experiments are based on satellite data and flight data,“ Chen explains. “They fly an airplane on top of and below the clouds, and there are also data based on the ocean temperature and radiation balance. And they all conclude that there is more absorption by clouds than theory could calculate. However, due to the complexity of clouds and the difficulties of making such measurements, researchers have been debating whether such discrepancies are real or not. And what we discovered suggests that hey, there’s another mechanism for cloud absorption, which was not accounted for, and this mechanism might explain the discrepancies.”
Chen says he recently spoke about the phenomenon at an American Physical Society conference, and one physicist there who studies clouds and climate said they had never thought about this possibility, which could affect calculations of the complex effects of clouds on climate. The team conducted experiments using LEDs shining on an artificial cloud chamber, and they observed heating of the fog, which was not supposed to happen since water does not absorb in the visible spectrum. “Such heating can be explained based on the photomolecular effect more easily,” he says.
Lv says that of the many lines of evidence, “the flat region in the air-side temperature distribution above hot water will be the easiest for people to reproduce.” That temperature profile “is a signature” that demonstrates the effect clearly, he says.
Zhang adds: “It is quite hard to explain how this kind of flat temperature profile comes about without invoking some other mechanism” beyond the accepted theories of thermal evaporation. “It ties together what a whole lot of people are reporting in their solar desalination devices,” which again show evaporation rates that cannot be explained by the thermal input.
The effect can be substantial. Under the optimum conditions of color, angle, and polarization, Lv says, “the evaporation rate is four times the thermal limit.”
Already, since publication of the first paper, the team has been approached by companies that hope to harness the effect, Chen says, including for evaporating syrup and drying paper in a paper mill. The likeliest first applications will come in the areas of solar desalinization systems or other industrial drying processes, he says. “Drying consumes 20 percent of all industrial energy usage,” he points out.
Because the effect is so new and unexpected, Chen says, “This phenomenon should be very general, and our experiment is really just the beginning.” The experiments needed to demonstrate and quantify the effect are very time-consuming. “There are many variables, from understanding water itself, to extending to other materials, other liquids and even solids,” he says.
“The observations in the manuscript points to a new physical mechanism that foundationally alters our thinking on the kinetics of evaporation,” says Shannon Yee, an associate professor of mechanical engineering at Georgia Tech, who was not associated with this work. He adds, “Who would have thought that we are still learning about something as quotidian as water evaporating?”
“I think this work is very significant scientifically because it presents a new mechanism,” says University of Alberta Distinguished Professor Janet A.W. Elliott, who also was not associated with this work. “It may also turn out to be practically important for technology and our understanding of nature, because evaporation of water is ubiquitous and the effect appears to deliver significantly higher evaporation rates than the known thermal mechanism. … My overall impression is this work is outstanding. It appears to be carefully done with many precise experiments lending support for one another.”
The work was partly supported by an MIT Bose Award.
#air#albert einstein#applications#artificial#atoms#change#clean water#climate#climate change#climate models#climate science#Cloud#clouds#Color#Community#Companies#complexity#conference#container#course#data#Desalination#details#devices#Discoveries#effects#electrons#energy#engineering#Explained
0 notes
Text
Chemical Evolution: Exploring the Dynamics of Thiochemicals Market By 2023 to 2030

The global thiochemicals market is expected to reach US$ 2.72 billion by 2030, growing at a CAGR of 2.5% from 2023 to 2030. This growth is driven by increasing demand for thiochemicals in various applications, including oil and gas, animal nutrition, polymers and chemicals, and other industries.
Dimethyl sulfoxide (DMSO) is the most widely used thiochemical, accounting for over 50% of the market share. DMSO is used in a variety of applications, including pharmaceuticals, personal care products, and industrial solvents. Other important thiochemicals include thioglycolic acid and esters, which are used in the production of plastics, rubber, and other materials.
Discover Our Expert Analysis with Our Sample Report! https://absolutemarketresearch.com/Global-Thiochemicals-Market/1818/request-sample
The Thiochemicals market is witnessing significant growth, driven by increased demand across diverse industries such as petrochemicals, pharmaceuticals, and agriculture. Thiochemicals play a crucial role in various applications, including catalysts, mining, oil and gas, and water treatment. As industries continue to embrace sustainable practices, the demand for eco-friendly thiochemicals is also on the rise.
Absolute Market Research has emerged as a frontrunner in the Thiochemicals market, leveraging its expertise in research and development, state-of-the-art manufacturing facilities, and a commitment to sustainability. Our innovative thiochemical solutions are designed to enhance efficiency, reduce environmental impact, and meet the stringent quality standards demanded by our customers.
Thiochemicals are a broad class of chemical compounds that contain sulfur. They are used in a wide variety of applications, including:
Food and agriculture: Thiochemicals are used in the production of fertilizers, pesticides, and herbicides. They are also used to make food additives, such as preservatives and flavorings.
Pharmaceuticals: Thiochemicals are used to make a variety of drugs, including antibiotics, antifungal drugs, and anti-inflammatory drugs.
Personal care products: Thiochemicals are used in the production of soaps, shampoos, and cosmetics.
Industrial products: Thiochemicals are used in the production of rubber, plastics, and textiles. They are also used in the production of lubricants, coolants, and adhesives.
Key Takeaways:
The global thiochemicals market is projected to reach US$ 2.72 billion by 2030, growing at a CAGR of 2.5% from 2023 to 2030.
The growth of the market is driven by increasing demand from various applications such as oil and gas, animal nutrition, polymers and chemicals, and others.
Dimethyl sulfoxide (DMSO) is the largest type of thiochemicals, accounting for over 40% of the market share.
Asia Pacific is the largest market for thiochemicals, followed by North America and Europe.
Regional Outlook:
Asia Pacific is expected to remain the largest market for thiochemicals throughout the forecast period, driven by strong demand from China, India, and Japan.
North America is expected to be the second-largest market, driven by the growing demand from the oil and gas industry.
Europe is expected to be the third-largest market, driven by the demand for thiochemicals in various industrial applications.
Key Players:
Arkema Group (France)
Bruno Bock Chemische Fabrik GmbH & Co. KG (Germany)
Chevron Phillips Chemical Company (USA)
Daicel Corporation (Japan)
Dr. Spiess Chemische Fabrik GmbH (Germany)
Hebei Yanuo Bioscience Co. Ltd. (China)
Hohhot Guangxin Chemical Trade Co. Ltd. (China)
Merck KGaA (Germany)
Taizhou Sunny Chemical Co. Ltd. (China)
TCI Chemicals (Japan)
Toray Fine Chemicals Co. Ltd. (Japan)
Zhongke Fine Chemical Co. Ltd. (China)
Segmentation:
The global thiochemicals market is segmented by type, application, and region.
By Type:
Dimethyl sulfoxide (DMSO)
Thioglycolic acid and ester
Others
By Application:
Oil and gas
Animal nutrition
Polymers and chemicals
Others
By Region:
Asia Pacific
North America
Europe
South America
Middle East and Africa
0 notes
Text

Wangxian Township, Guangxin District, Shangrao City, Jiangxi Province, China @ Hwang199 Photography via: tumblr.com/upstairsdownstairsandinbetween
1 note
·
View note
Text
Hydroxy Benzo Nitrile (HBN) Market Segmentation, Research Methodology And Revenue Growth Forecast Till 2030
Hydroxy Benzo Nitrile (HBN) Marketresearch report looks at the main drivers impacting global growth as well as the opportunities, problems, and threats that the market's major competitors are now dealing with. A vendor's business overview, total sales (financial), market opportunities, global presence, realized sales and realized revenue, market share, pricing, facilities and industry capabilities, SWOT analysis, and product launches are just a few examples of the data that makes up the competitive landscape for the keywords market. The study is accompanied by an Excel datasheet suite that contains quantitative information from each of the report's stated numerical forecasts.
The global Hydroxy Benzo Nitrile (HBN) market share is growing as a result of numerous causes. These elements, according to the most recent MRFR research, include an increase in smartphone ownership for video conferencing, mobile gaming, and video streaming, the creation of online applications for entertainment, media, online food delivery, and navigation, and the creation of creative solutions.
Browse More Details On This Report at - https://www.businessresearchinsights.com/market-reports/hydroxy-benzo-nitrile-hbn-market-106163
The Major Key Players Listed in Heart Rate Monitoring Devices Market Report are:
Hangzhou Xinyuanzhong Chemical Co., Ltd.
Yongtong Co., Ltd.
Changzhou Huihe Chemical
Anhui Guangxin Agrochemical Co., Ltd
Alzchem
Xiangyang Yujue Chemical
Pingyuan Xinda Chemical
Tianchen Chemical
Hubei Yuanhuan Industrial Investment
Jiangxi Yangpu Biotechnology Co., Ltd.
Some of the key questions answered in this report:
What is global? Sales Value, Production Value, Consumption Value, Import and Export of the Hydroxy Benzo Nitrile (HBN) Market?
What application/end user or product type can look for incremental growth prospects?
What are the different sales, Marketing and sales channels in the global industry?
What are the key market trends influencing the growth of the Hydroxy Benzo Nitrile (HBN) Market?
What are the market opportunities, market risk and market overview of the Hydroxy Benzo Nitrile (HBN) Market?
What are the key drivers, restraints, opportunities and challenges of the Hydroxy Benzo Nitrile (HBN) Market and how are they expected to be affect the market?
How big is the market for Hydroxy Benzo Nitrile (HBN) at regional and country level?
Contact Us:
Business Research Insights
Phone:
US: (+1) 424 253 0807
UK: (+44) 203 239 818
Our Other Reports: -
Magnetic Treadmills Market Analysis Report
ONH Analyzer Market Industry Revenue
MS Polymer Hybrid Adhesives and Sealants Market Share
Exposed Fastener Panel Systems Market Share
PC and PMMA Composite Sheet Market Share
Streetwear Market Share
Bar Loaders Market Share
Order Picker Machines Market Share
Multiple Launch Rocket Systems (MLRS) Market Share
High Purity Solvent Market Share
0 notes
Text
Texas mogul is branded 'treacherous' over 'deal of the century' which saw him sell 130,000 acres of farmland to Chinese billionaire
David Frankens, of Lufkin, East Texas, has sparked fury among local ranchers after he sold swathes of land to Sun Guangxin, a former Chinese military captain
Local realtors claim Frankens made 'millions of dollars' in profit from the trades, in which he would buy the land before selling it on to Sun within the same day
The Texan businessman has since been accused by one of his former ranch managers of cornering him in his office and punching him in the head
A Texan real estate mogul allegedly made 'millions of dollars' by selling vast swathes of local farmland to a Chinese billionaire with close ties to Beijing.
David Frankens, from Lufkin, East Texas, scored the 'deals of the century' when flipping the land at around twice its market value to Sun Guangxin, a former captain in the Chinese military, local realtors told DailyMail.com.
The trades have sparked fury among ranchers in Val Verde County, where Sun bought more than 130,000 acres of farmland for an estimated $110million between 2016 and 2018.
A report written by former CIA officials, seen by DailyMail.com, suggested the Chinese billionaire could be considered a national security risk by US authorities due to his extensive links to the Chinese Communist Party (CCP).
One local landowner described the Frankens' actions as 'treacherous'.

1 note
·
View note
Text
‘Treacherous’: Texas Real Estate Mogul Sells 130K Acres to Chinese Billionaire
Breitbart By Amy Furr September 3, 2023 A Texas real estate mogul from Lufkin reportedly sold a large amount of local farmland to a Chinese billionaire identified as Sun Guangxin, who has links to the Chinese Communist Party (CCP). Realtors in the area told the Daily Mail that David Frankens made millions of dollars via the deal with Sun, who was previously a captain in the Chinese military, the…

View On WordPress
0 notes
Text
Why So Many States Want to Ban China From Owning Farmland#States #Ban #China #Owning #Farmland
Fourteen states prohibit or restrict foreign ownership of private agricultural land, but that number may soon grow. ROBYN BECK / AFP via Getty Images The spy balloon spotted over Montana wasn’t the first recent incident to spark fears about national security and espionage in the U.S. Only a few years ago, a Chinese billionaire named Sun Guangxin planned to build a wind farm on part of 140,000…

View On WordPress
0 notes
Text
0 notes
Text
Administrative geography of Western Han (22,109) etc

Yang揚州
Yuzhang豫章郡
Gan贛
Nanye南野
Jing 荊州
Guiyang桂陽郡
Chen郴
Linwu臨武
Nanping南平
Guiyang桂陽
Yangshan陽山
Qujiang曲江
Hankuang含洭
Chengyang湞陽
Lingling零陵郡
Lingling零陵
Yingdao營道
Shi'an始安
Yingpu營浦
Lingdao泠道
Jiao交州
Nanhai南海郡
Panyu番禺
Boluo博羅
Zhongsu中宿
Longchuan龍川
Sihui四會
Yulin鬱林郡
Bushan布山
Anguang安廣
Alin阿林
Zhongliu中留
Guilin桂林
Tanzhong潭中
Cangwu蒼梧郡
Guangxin廣信
Xiemu謝沐
Gaoyao高要
Fengyang封陽
Linhe臨賀
Duanxi端溪
Fengcheng馮乘
Fuchuan富川
Lipu荔浦
Mengling猛陵

Jiao交州
Nanhai南海郡
Jieyang揭陽
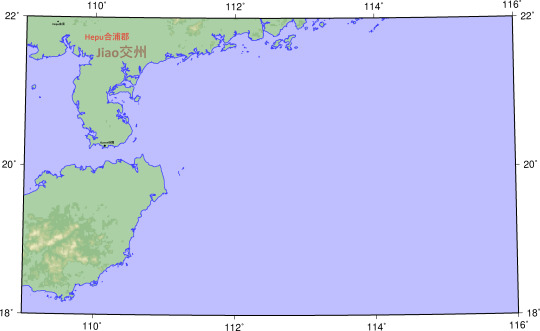
Jiao交州
Hepu合浦郡
Xuwen徐聞
Hepu合浦
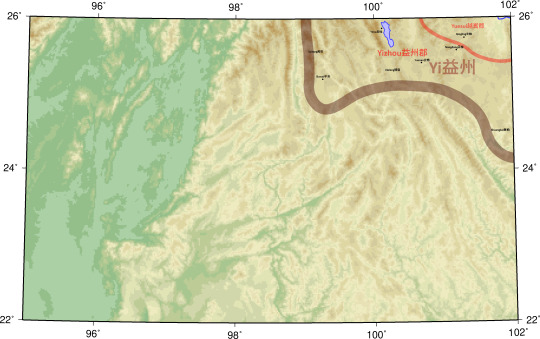
Yi益州
Yuesui越嶲郡
Qingling青蛉
Yizhou益州郡
Shuangbai雙柏
Xielong邪龍
Yeyu葉榆
Buwei不韋
Yunnan雲南
Suitang嶲唐
Nongdong弄棟
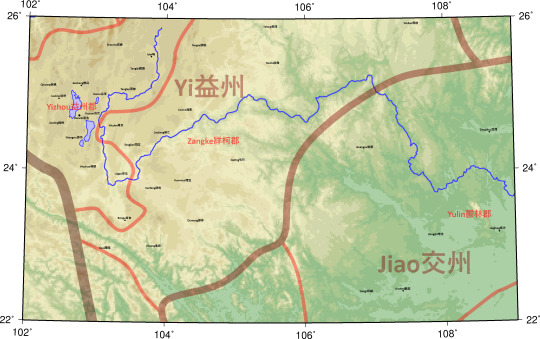
Yi益州
Yizhou益州郡
Dianchi滇池
Tonglao同勞
Tonglai銅瀨
Lianran連然
Yuyuan俞元
Shouma收靡
Guchang穀昌
Qinzang秦臧
Mei味
Kunze昆澤
Lügao律高
Bengu賁古
Wuzhuo毋棳
Shengxiu勝休
Jianling健伶
Zangke牂柯郡
Xunfeng鐔封
Louwo漏臥
Tongban同��
Tanzhi談指
Yuanwen宛溫
Wulian毋斂
Yelang夜郎
Wudan毋單
Loujiang漏江
Xisui西隨
Dumeng都夢
Tangao談稿
Jinsang進桑
Quting句町
Jiao交州
Yulin鬱林郡
Guangyu廣郁
Linchen臨塵
Dingzhou定周
Zengshi增食
Lingfang領方
Yongji雍雞
County locations and ancient rivers, lakes, and shorelines from The Historical Atlas of China.
I have intentionally stuck to the Hanshu where it differs from the Historical Atlas.
6 notes
·
View notes