#first plant genome to be sequenced
Explore tagged Tumblr posts
Text
Seeing UV colors is common for butterflies, but in some species, it is a female-only power

So, you may or may not know that many butterflies can actually see in UV light. It is very cool and I'm definitely not jealous that they get extra colors. It's helpful to them because many flowers have UV patterns on them (invisible to us) that let the butterflies know that they're a good source of food. The plants get pollinated and the butterflies get to eat. Everybody wins. This is a simulated version of what butterflies might see when they look at a flower.

Some butterflies, such as the zebra longwing pictured above, only display this trait in females. Because of this, male and female butterflies will tend to visit different types of flowers. But scientists have just recently figured out how this difference came to be, evolutionarily speaking.
Obviously many species have sexually dimorphic traits, some more prominently than others. There are also cases in which one sex develops a trait that is just... less useful than the other, like this case with the UV vision. Almost all butterflies can see in the UV spectrum, so it follows that at some point in the evolutionary line the male zebra longwing butterflies lost that particular ability. There are multiple ways that this sort of thing can happen, and the article covers them briefly, but after sequencing the genome for these butterflies they found that none of those previously seen explanations were the case.
Basically, we already know the gene that causes UV vision in butterflies. It is called the opsin gene. In zebra longtail butterflies, this gene occurs on the chromosome W, which is the female sex chromosome. That means that sometime in history, this gene just jumped from a normal chromosome onto the female-only chromosome, and has locked the male butterflies out of this ability ever since. This is the first time we have seen a gene do a jump like that, and it is pretty cool.
But anyway, appreciate the girlpower of zebra longwing butterflies getting all the UV vision, and take a look at the study! It's free to read, which is really nice to see.
#science#stem#science side of tumblr#stemblr#biology#biochemistry#butterfly#butterflies#insects#bugs#entomology#animals#zoology
528 notes
·
View notes
Text



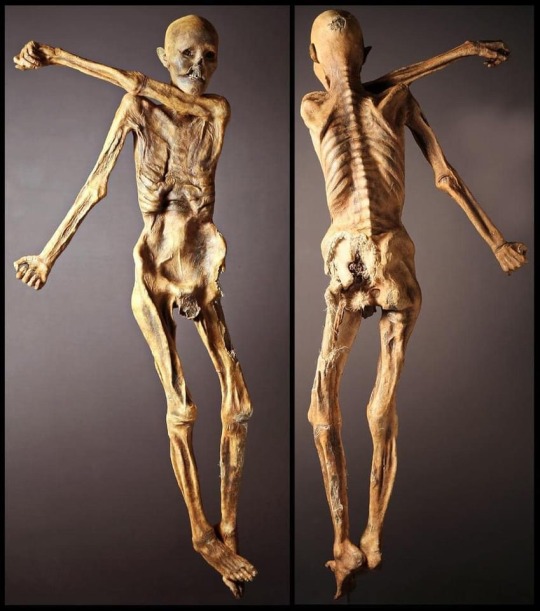

ID: a Facebook post by Em Jay:
“Do any of you remember when I was posting about the recent scientific revelation that Cheddar Man was actually very dark-skinned and how pale skin is soooo much of a newer phenomenon (according to studies, pale skin began appearing in the human genome roughly 4,000 years ago as opposed to the previous assumption of 40,000 years ago) than originally surmised? A new genome sequencing study adds the famous 'Otzi the Iceman' to the list of incorrectly reconstructed (referring to the long-haired, pale-skinned rendering of him found in the Italian museum next to his real remains) ancient humans, as it has been revealed he was dark skinned and balding! The initial discovery of Otzi the Iceman in 1991 (on the Italian side of the Italian/Austrian border) was of enormous import for the scientific community for several reasons; Otzi is the oldest 'wet mummy' yet found and the clothes and equipment he was unearthed with are incomprehensibly unique as no other organic material from the Copper Age has survived. He also became popular for his 61 tattoos, which are the oldest preserved tattoos known to date. I absolutely love studies/revelations like this because (borrowing a lovely sentiment from co-author of the study Johannes Krause) they truly reflect our own biases in assuming what a person from that time looked like, and to use my own words, challenges many of us to re- examine the appearance of our ancient human ancestors in general. "The Iceman's new genome also reveals he had male-pattern baldness and much darker skin than artistic representations suggest. Genes conferring light skin tones didn't become prevalent until 4,000 to 3,000 years ago when early farmers started eating plant-based diets and didn't get as much vitamin D from fish and meat as hunter-gathers did, Krause says.
“As Ötzi and other ancient people's DNA illustrate, the skin color genetic changes took thousands of years to become commonplace in Europe. 'People that lived in Europe between 40,000 years ago and 8,000 years ago were as dark as people in Africa, which makes a lot of sense because [Africa is] where humans came from," he says. "We have always imagined that [Europeans] became light-skinned much faster. But now it seems that this happened actually quite late in human history!" (excerpt in quotations from Science News article by Tina Hesman Seay) Below are photos of Otzi, the first taken in 1991 shortly after he was discovered by 2 hikers, his naturally mummified body after he was carefully unearthed from the ice and his incorrect/false rendering with pale skin of 2011, and I hope to return to add a correct/more accurate rendering of him if/when a new one is made!”
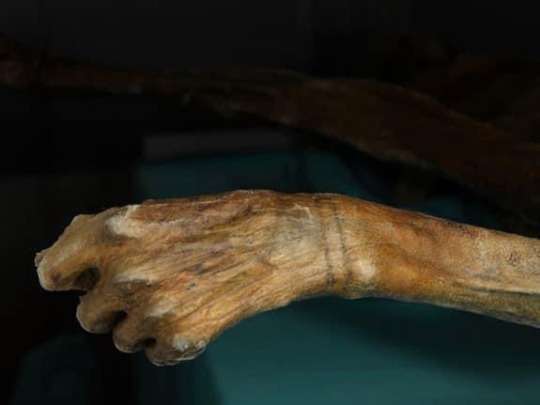
Photos show 1) a pair of light-skinned, brown-haired hikers with brown beards, dressed in very 1980s clothing, with the exposed body of Otzi in situ in the ice where they found his body; 2) two photographs of Otzi’s preserved body from the top and back, 3) a close-up photo of Otzi’s preserved hand 4) an inaccurate reconstruction of Otzi in life, showing him as a light-skinned white man.
#otzi#otzi the iceman#historical racism#scientific racism#prehistoric humans#human physiology#whiteness is a construct and a really shitty one we should do everything possible to denaturalise and deconstruct#whiteness is a construct#male pattern baldness
44 notes
·
View notes
Text

A snip, a splice : Power of rDNA Technology
Deoxyribonucleic acid (DNA), the blueprint of life, holds the secrets to the intricate workings of every living organism. But what if we could manipulate this blueprint, adding, removing, or tweaking its code? This revolutionary concept forms the core of recombinant DNA (rDNA) technology, a powerful tool that has transformed biology and medicine.
The story starts in the early 1970s with two brilliant scientists; Stanley Cohen at Stanford University and Herbert Boyer at the University of California, San Francisco. Cohen, a microbiologist, had been studying plasmids – small circular DNA molecules found in bacteria. Boyer, a biochemist, was an expert on restriction enzymes – molecular scissors that could cut DNA at specific sequences. Their collaboration proved groundbreaking. They envisioned combining these tools to create the first ever recombinant DNA molecule. Cohen provided the plasmids, which would act as vectors to carry foreign DNA into host cells. Boyer, on the other hand, used restriction enzymes to cut both the plasmid and the desired foreign DNA, allowing them to be pieced together. Through meticulous experimentation, they successfully created the first recombinant DNA molecule, forever altering the course of biology.
Cohen and Boyer's work wouldn't have been possible without the earlier discoveries of restriction enzymes. These "molecular scissors" were independently identified by three separate research groups in the 1960s. Werner Arber in Switzerland, along with Hamilton Smith and Daniel Nathans in the US, unraveled the role of restriction enzymes in bacterial defense mechanisms. These enzymes helped bacteria defend against invading viruses by cutting up their foreign DNA. Recognizing the potential of these "genetic scalpels," the groundwork was laid for their application in rDNA technology.
Here's a simplified breakdown of the rDNA process:
Isolation of DNA: The journey starts with isolating DNA from a donor organism.
Cleavage with Restriction Enzymes: Specific enzymes cut the DNA at defined sequences.
Selection of Vector: A carrier molecule (often a plasmid) is chosen to transport the recombinant DNA.
Ligation: The DNA fragments and vector are stitched together using DNA ligase, an enzyme.
Transformation: The recombinant DNA enters a host cell (usually bacteria or yeast).
Selection and Expression: The transformed cells are selected, and the gene of interest is expressed, leading to the desired protein production.
Since its inception, rDNA technology has played a pivotal role in several groundbreaking advancements. Let's take a whirlwind tour through some of the most significant moments in R-DNA history:
1978: Birth of Insulin on the Factory Floor: Scientists achieved a feat of genetic engineering by using R-DNA to produce human insulin in bacteria. This marked a turning point for diabetics, offering a readily available and more consistent source of this life-saving hormone.
1980s: Gene Wars and the Rise of GMOs: The 1980s saw the development of genetically modified organisms (GMOs). Plants were engineered with genes for insect resistance or herbicide tolerance, sparking debates about the safety and ethics of this technology. R-DNA research continues to be at the forefront of discussions regarding genetically modified foods.
1990s: The Human Genome Project Sets Sail: This ambitious international project aimed to sequence the entire human genome. R-DNA techniques played a crucial role in deciphering the 3 billion letters of our genetic code, opening doors for personalized medicine and a deeper understanding of human health and disease.
2000s: Gene Therapy Takes Center Stage: The first successful gene therapy trials for inherited diseases like severe combined immunodeficiency (SCID) took place. R-DNA technology offered a glimmer of hope for treating genetic disorders by introducing healthy genes to replace defective ones.
2010s and Beyond: CRISPR Takes Over: The emergence of CRISPR-Cas9, a revolutionary gene editing tool based on R-DNA principles, has ushered in a new era of genetic manipulation. With unprecedented precision, scientists can now edit genes in various organisms, holding immense potential for gene therapy, crop improvement, and even the eradication of diseases.
But with great power comes great responsibility, and R-DNA raises a host of ethical concerns.Tinkering with the building blocks of life carries the risk of unintended consequences. Engineered genes could escape and disrupt ecosystems, or modified organisms could have unforeseen health effects. The ability to edit human genes opens the door to designer babies, raising questions about social equity and the potential misuse of the technology for eugenics.
Who Controls the Tools? Access to R-DNA technology could be restricted to wealthy nations or corporations, exacerbating existing inequalities. Biosecurity is also a concern, as the technology could be misused for bioterrorism. Creating entirely new organisms forces us to confront what it means to be "natural." Should we modify plants and animals for human benefit, or preserve their original forms? R-DNA technology is a powerful tool, and we must have open discussions about its ethical implications. Scientists, policymakers, and the public all need to be involved in shaping the future of this technology. As we move forward, open dialogue and collaboration between scientists, policymakers, and the public are crucial to ensure the safe and ethical application of this powerful technology.
The journey of rDNA technology is a testament to human ingenuity and its potential to reshape our world. From decoding the secrets of life to creating solutions for healthcare, agriculture, and beyond, rDNA technology continues to evolve, promising a future filled with exciting possibilities.
#science sculpt#life science#science#molecular biology#biology#biotechnology#artists on tumblr#dna#double helix#genetics#recombinant#genetic engineering#insulin#research#education#learning#academics#scientific research#scientific illustration#medical science#scifi#daily dose of science#scientific advancements#scientific tools#medical school
16 notes
·
View notes
Text
Excerpt from this story from The Revelator:
As the environment shifts — due to climate change, habitat destruction, or other threats — we can often observe some of the ways that wildlife responds. Populations may decline. Individual animals may move. Some species may alter their behavior.
But at the same time, scientists warn, wild plants or animals may experience harder-to-detect changes — for example, alterations to their genomes, the very DNA that defines them.
It requires a sophisticated genetics laboratory to see these otherwise invisible changes at first, but they may have important implications for populations’ futures.
How exactly can threats such as climate change and habitat loss have hidden effects on a species’ genetic code? Two studies on California birds, both published in the past year, illustrate the potential — both beneficial and problematic.
The endangered southwestern willow flycatcher (Empidonax traillii extimus), ranging from California east to New Mexico and Colorado, depends on rapidly disappearing riparian habitats. As those riverbanks dry up, scientists began to wonder how the birds have adapted. They found the answers by looking to the past.
As the environment shifts — due to climate change, habitat destruction, or other threats — we can often observe some of the ways that wildlife responds. Populations may decline. Individual animals may move. Some species may alter their behavior.
But at the same time, scientists warn, wild plants or animals may experience harder-to-detect changes — for example, alterations to their genomes, the very DNA that defines them.
It requires a sophisticated genetics laboratory to see these otherwise invisible changes at first, but they may have important implications for populations’ futures.
How exactly can threats such as climate change and habitat loss have hidden effects on a species’ genetic code? Two studies on California birds, both published in the past year, illustrate the potential — both beneficial and problematic.

In summer 2023 a group of scientists published a study comparing the genomes of flycatcher specimens collected in the San Diego around the turn of the 20th century — taxidermied birds preserved in museums — with those of contemporary birds, using blood samples collected from individuals captured across willow flycatchers’ breeding range today.
The study was only possible due to rapid advances in technology.
“Until recently, it was very difficult to sequence historical specimens across their entire genome,” says Sheela Turbek, a postdoctoral fellow at Colorado State University who led the project. “DNA tends to degrade over time, and older specimens can have really low DNA concentrations.”
The results surprised Turbek and her colleagues: San Diego flycatchers’ genetic diversity has increased over time.
Most notably, this increased diversity included areas of the genome linked with climate adaptation.
According to the study, it appears the San Diego birds have bred with flycatchers originally from populations in other areas of the West, which may have moved in response to local habitat losses. And as natural selection has acted on this increased diversity, the San Diego birds’ genomes have shifted away from those of neighboring populations, potentially making the local birds better suited for life in a wetter, more humid environment being shaped by climate change.
6 notes
·
View notes
Note
Hiya!
📝
For any character you want, please and thank you.
Here are some of my headcanons for Mark “Ik” Ikagami (Real Genius)!
From the Seattle area, probably a Virgo Five other Marks in the dorm his first year, so went by Ikagami which was eventually shortened to Ik by Chris Big fan of motorcycles, used to ride mopeds before upgrading Has to buy a size up for his Harley Davidson shirts bc Chris keeps stealing them Decent pingpong player(based on his line in the script “I'm depressed. Why did I listen to my parents? I should have become a ping-pong pro.”) Major/Master is in Bio Chemistry(from the script "MARK IKAGAMI, better know as ICK. Ick is a bio-chemist who spends a great deal of time in his lab experimenting with various fun compounds that do things ranging from making artificial ice to increasing memory.") Has a strong interest in agricultural genetics, especially in radiations effects on plant DNA He'd be delighted to learn about the new research into the mathematical link to genetic mutations. Also by whole genome sequencing and genetic algebra. Stims by drawing fractals and the Fibonacci spiral.(I'm sorry did ANYONE think that these folk were neurotypical?) Like looking for math on nature Incredibly sarcastic but in such an utterly deadpan way that no one is entirely sure if it was sarcasm. Hell of a poker face. Can only be beaten by Mitch counting cards Y'all know that tweet about getting away with stealing chairs by saying they were for a math meet next door? Yeah, that's him No one expects him to be just as much of a being of chaos as Chris, but he is~

I just think he's neat!
#real genius#Mark Ikagami#thanks for the ask!#sorry my RG brain rot took over~#I really need a tag for stuff I write#I have more but I need to organize my nonsense#I'm such a nerd I cite sources for my headcanons
3 notes
·
View notes
Text
"Tree of Life" connects every living organism on Earth - CBS News
""Tree of Life" connects every living organism on Earth - CBS News" https://www.cbsnews.com/amp/news/scientists-unveil-first-complete-tree-of-life/
The Black History Channel and The Black on Black Love Movement explains the entire world history through science. The Human Genome project concluded it's evidence of sequencing the entire human genome and all life forms on earth and this scientific experiment was completed in 2003.
#black love#black positivity#black africans#black history#science#evolution#science side of tumblr#atheism#african atheism#black women
12 notes
·
View notes
Text
Magiautotrophs
When Olua is deep into star-feeding, magic floods the atmosphere, and the air fills with critters who feed on it. It's so thick with life, certain macrofauna swim the sky throughout summernoon. Many microorganisms have developed ways essentially eat magic. These magisynthetic processes are fundamentally different from photo- or chemosynthetic ones, leading to a new set of trophic categories for Oluan microbes: Magiotrophs. An organism may get their energy, their electrons, or both from magic. An organism who does both and obtains carbon via CO2 fixation would be fully termed a magiomagiautotroph -- magiotroph for short. Though the convention is to shorten a -troph to its first (and sometimes third) prefix, a non-magisynthetic organism who nonetheless oxidizes magic for electrons will often be called a magitroph anyway, due to the novelty and contextual significance of the concept.
Side note
Though plenty of species use magic in myriad ways, including various energy-boosting strategies, no known eukaryote has figured out how to use magic directly as food, the way plants use sunlight. Magic, by definition, is poorly understood, so it is yet unknown why this is a power exclusive to the micros.
One popular hypothesis posits the ability originated in domain Alcana and spread via horizontal gene transfer to some other species; among alcans with fully sequenced genomes, over 90% have genes that code for magisynthetic biomachinery. Same and similar genes have been found in several bacteria and archaea (some of which are relative newcomers to Olua). Trace amounts of these genes have even been found in lichens. Though the magisynthetic section of archaea and bacteria is much smaller, that they possess these sequences at all implies remarkable things for interdominal HGT, and the role magic may play in the process.
Second side note
There is emerging evidence certain alcan lineages have evolved ways to avoid cleaving oxygen during carbon fixation, likely due to the sheer abundance of atmospheric oxygen and magiparticles' resemblance to it. Big if true.
(Is it possible some of these microbes have complexes unrelated to Rubisco at all?)
#worldbuilding#olua#biology#microorganisms#magic#unreality#that's right boiz olua's got midichlorians >:333#Proud of this one.
3 notes
·
View notes
Text
Bananas Are At Risk of Extinction, But Scientists Have a Plan
A Fungus That Can Infect Over 100 Different Plants is Devastating the Popular Fruit.
— By Laura Baisas | August 16, 2024

Fighting a fungal pathogen on the molecular level is key to the banana’s survival. Deposit Photos
The bright bananas dotting your fruit bowl are in some serious trouble. A popular type of banana is facing extinction from a fungal pathogen. The disease Fusarium wilt of banana (FWB) blocks the flow of nutrients to the fruit and makes it wilt. During the 1950s, the pathogen wiped out commercial banana crops and made one species–Gros Michel bananas–functionally extinct.
But not all is lost for this colorful fruit. New research from an international team of scientists has pinpointed the molecular mechanisms behind the microbe that destroys bananas and it opens the door to new treatments and strategies against the pathogen. The findings are detailed in a study published August 16 in the journal Nature Microbiology.
What Is Hurting Bananas?
The crop failures are due to a fungal pathogen with a very long name–Fusarium oxysporum f.sp. Cubense (Foc) tropical race 4 (TR4). Abbreviated as Foc TR4, this fungus decimated several banana crops in the 1950s and wiped out one entire species, but bananas aren’t the only plants at risk.
“As a species complex, Fusarium oxysporum can infect over 100 different plant hosts,” study co-author and University of Massachusetts Amherst molecular biologist Li-Jun Ma tells Popular Science.”

Fusarium wilt of banana is currently decimating the Cavendish banana—the world’s most popular commercially available banana. Once present in a banana field, the fungus cannot be eradicated, making future production of Cavendish bananas almost impossible. Credit: A. Viljoen
Part of this virulence comes down to its genome and the ways it can change. According to Ma, each Fusarium oxysporum genome can be divided into two parts–a core genome and an accessory genome. The core genome does all of the main housekeeping functions of keeping the genome going. An accessory genome is then free to vary from strain to strain and can handle specialized functions–including the ability to infect a specific plant.
Understanding how the pathogen and its genome work on a molecular level is key for developing ways to combat it and prevent more banana species from going extinct.
Not Your Grandparents’ Bananas–Or Fungus
Over 50 years ago, the first victims of this fungal war were the Gros Michel bananas. Largely in response to banana wilt, the Cavendish variety was bred to be a disease-resistant replacement and is the most popular type of commercially available banana today. This worked for a while, but by the 1990s, there was another outbreak of banana wilt that spread from Southeast Asia to Central America.
Ma and her team have spent the last decade studying how TR4’s genome works to combat the new outbreak of banana wilt in the Cavendish banana. Surprisingly, they found that it is actually not derived from the same pathogen that wiped out crops in the ‘50s.
“We now know that the Cavendish banana-destroying pathogen TR4 did not evolve from the race that decimated the Gros Michel bananas,” Ma said in a press release accompanying the study. “TR4’s genome contains some accessory genes that are linked to the production of nitric oxide, which seems to be the key factor in TR4’s virulence.”
Harmful Gasses
In this new study, Ma and co-authors from institutions in the United States, China, and South Africa sequenced and compared 36 different Foc strains from all over the world. These strains include the ones that attack Gros Michel bananas. The sequences revealed that the Foc TR4 that is responsible for the current outbreak of banana wilt. It also uses some accessory genes for two purposes when invading a host. These genes both produce and detoxify fungal nitric oxide.
“As expected, we found accessory sequences in the TR4 genome that contribute to its virulence, including the production of the harmful gas, nitric oxide, that facilitates the host invasion,” says Ma.
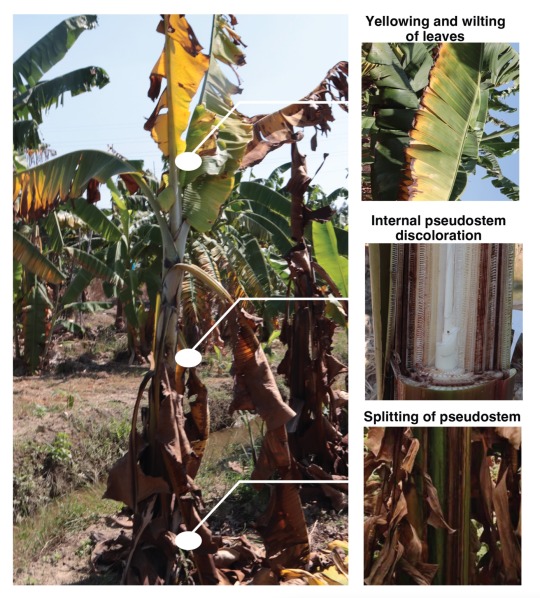
External Symptoms of FWB in Cavendish bananas. Credit: Zhang et al.
The team still doesn’t know how this gas specifically contributes to disease infestation in Cavendish banana. However, they were able to determine that the virulence of Foc TR4 was greatly reduced if the two genes that control nitric oxide production were eliminated.
“Identifying these accessory genetic sequences opens up many strategic avenues to mitigate, or even control, the spread of Foc TR4,” Yong Zhang, study co-author and a postdoctoral researcher at UMASS Amherst, said in a statement.
‘Always Remember To Say Thank You To a Farmer’
In future research, the team would like to better understand how the fungus can produce such a harmful gas without hurting itself. They would like to test various ways to interrupt the production of nitric oxide and explore genes that can take the gas away before it damages plant cells.
Importantly, work like this raises awareness about the dangers of monocropping in agriculture and relying on a single species.
“Growing a single cultivar of any crop, also called monoculture agricultural production, provides breeding ground for the development of pathogens,” says Ma. “To help increase the demand for diverse bananas in the market, we can intentionally pick up different varieties of bananas from the shelf. We can support local producers by shopping locally.”
It is also a lesson in the importance of valuing the time and effort of those who produce all of the food we put on our tables.
“We, consumers, should appreciate that bananas or other fruits/vegetables do not grow from grocery stores,” says Ma. “There are tremendous efforts to bring food to our tables and feed our bodies. Always remember to say thank you to a farmer when you see one with dirt and soil all over his/her body.”
2 notes
·
View notes
Note
hey so i saw you reblog that tiktok about STIs and you said you were a microbiologist, and now i'm super curious about how tracking down pathogens from thousands of years ago works! can you shed any light on that?
Hi! Thanks for the ask!
My knowledge is super rusty, it's what I studied at university but truth be told I'm not a professional by any means lol. I just still love pathogens and evolution. Hopefully I can give you enough info to do further reading if you want, just be aware what I say may have errors or be outdated:
Some viruses can integrate themselves into the host's genome. As a species we have thousands of viral genes that have become embedded in our DNA. It's not in the bits that program for our cells, but the "intergenic" DNA - the DNA between functional genes. (Only about 10% of our genes code for proteins, the rest is often described as "junk" but there are still possible uses. Research is ongoing.)
We can also date genes based on the rate of natural mutations being pretty steady over many generations. This is particularly true in intergenic DNA since it doesn't undergo pressure from natural selection - it's like carbon dating but for DNA. So that gives them an estimation of how old an integrated viral genome is based on how mutated it is. (I think.)
They've also probably found evidence of certain pathogens in ancient human remains, such as fossils or frozen remains from the most recent ice age. I'm pretty sure syphilis can damage bones, especially it's congenital ie: you're born with it/contract it at birth. (Googling syphilis can turn up some pretty grim images, so be warned.)
You can also trace when two organisms split into separate species using a molecular clock. It works best in closer-related species because the differences are more apparent, and I think it's more common in research on eukaryotes (animals, plants, fungi) than bacteria/viruses, but I'm not sure. Basically, as mutations naturally occur over a species' history, the proteins making up various cells also change as a result. These can be very minor changes but it can be compared on the level of both amino acid chains and the sequence of DNA that acts as the blueprint for it. Like the first example, the steady rate of mutation means you can calculate how far back they diverged. The only problem with using this for pathogens is that since they have a very short generation time, new species can evolve at a much faster rate. It takes humans about 15 years to sexually mature - some bacteria can replicate in 20 minutes! So I'm not sure if this is used for researching diseases of the distant past, but maybe. Essentially you take a strain of a pathogen from humans and another strain of the same pathogen that is found in animals, and compare their proteins/DNA. If they are very similar they diverged recently; if they are very different, it was a long time ago.
I hope that makes sense and serves as a jumping off point. I miss studying this stuff but life has taken me on a different path. There's all sorts of ingenious methods and technologies researchers are using! They've probably got even more since I graduated 6 years ago.
2 notes
·
View notes
Photo
An image of an Arabidopsis thaliana leaf, which is used as a model organism in plant biology research — and was the first plant to have its entire genome sequenced.
Wikimedia Commons
#wikimedia commons#photographer#arabidopsis thaliana#autumn leaf#micro photography#electron microscope#nature
5 notes
·
View notes
Text
So last week shared a reflection on a science paper with you guys and I thought it went pretty well. So tonight, let’s talk photosynthesis.
I’m mostly going to be discussing this 2018 paper, “Dating phototrophic microbial lineages with reticulate gene histories.” However, since I’ve also taken classes that discussed this issue at length, I’ll be pulling in some outside knowledge too.
Most people that took high school bio probably remember that chloroplasts, the organelles responsible for photosynthesis in plant cells, have two photosystems: photosystem II (discovered second, but first in sequence) and photosystem I. What you may or may not know is that these photosystems have a really convoluted evolutionary history.
“But wait!” you (hypothetically) say. “These two photosystems are so similar! Isn’t gene duplication the simplest, most parsimonious explanation?” Nope. Probably not
Gene duplication seems like it would be the obvious candidate for How Chloroplasts Ended Up With Two Photosystems, but alas, there’s a pretty big mountain of phylogenetic evidence that this is not the case. There are at least five groups of photosynthetic bacteria that have a homolog for photosystem II OR photosystem I, but not both. None of these are oxygen producing. In other words, both photosystems are distinctive and neither seems to have emerged based on the other. This throws a wrench in things.
Stem-lineage cyanobacteria are considered to be the MRCA (most recent common ancestor) of chloroplasts: all plants and photosynthetic microbes are descended from them as endosymbionts. Only cyanobacteria and the subsequent lineages (with both photosystems) use oxygen as the terminal electron acceptor in photosynthesis, meaning that they can produce oxygen. Cyanobacteria were responsible for the Great Oxidation Event.
So okay, it stands to reason that cyanobacteria acquired the photosystems from Somewhere else. The questions are: 1) When 2) From what organisms 3) Vertical or horizontal acquisition?
The most commonly accepted model right now is the “Fusion model,” in which cyanobacteria acquired photosystems II and I from different, relatively distant photosynthetic organisms.
The article I linked above takes a molecular clock approach to these questions. For those unfamiliar: the molecular clock is a useful metric because of neutral evolution, the process by which synonymous mutations (mutations that don’t affect the protein sequence due to the degeneracy of the aa code) occur in genomes at a predictable rate. Molecular clocks are “calibrated” using fossils whose ages are known. We can use the molecular clock to help date evolutionary events and build phylogenies.
The authors of this paper used a maximum likelihood statistical approach to construct a phylogeny for the major groups of early photosynthetic bacteria. The sequence data they used came from ribosomal proteins. The end result looks like this:
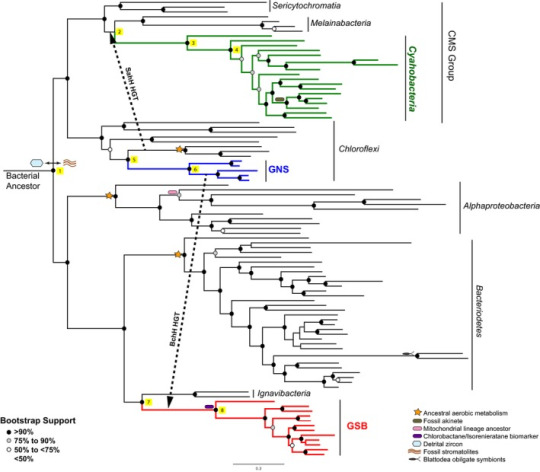
(Note: “Bootstrap support” is basically the percent chance that you’ve arrived at a particular branch/bifurcation nonrandomly. There's more to it than that, but I'm not gonna go into it here. That's the important bit.)
These authors assert that stem cyanobacteria likely received photosystem II genes via HGT from green non-sulphur bacteria (as seen above) prior to the Great Oxidation Event. They don’t think that something similar happened with photosystem I genes, however. Maybe those arose from within cyanobacteria themselves? Or a now extinct microbial line? Something else? It’s an open question.
It’s also worth pointing out that eukaryotic plant cells arose as a result of endosymbiosis with cyanobacteria. HOWEVER, this was also likely really convoluted. I’m not gonna go into this part in great detail, but there were at least five (but probably more like a dozen) separate endosymbiotic uptakes, leading to primary plastids. Additional uptakes of red and green algae led to secondary plastids. It’s awesome.
Okay! Whew. It’s been a little while since I took this class, so I hope all of that is right. Do check out the paper I linked, if you want, particularly if you’re a stats person.
And like. This is so cool you guys.
“God of the Gaps” is a logical fallacy in which people use things that science can’t yet explain as proof of God’s existence. I’ve heard people use God this way to try to explain these areas in evolutionary history where open questions still exist. How did abiogenesis, life from non-living matter, occur? We’ve got a lot of compelling biochemistry, but since there isn’t one simple answer, I’ve seen people handwave it away and just say God. More relevant here, how did oxidative photosynthesis evolve? There are still a lot of questions, and I do believe that they are questions that we can eventually, reasonably hope to answer.
What I really love is to see God IN the gaps. I love these sort of transitional, liminal phases in life’s history where we can see modern forms beginning to take shape. “Endless forms most beautiful,” Darwin wrote, just as God said “It is good;” but what about when “it” wasn’t exactly assembled yet? What about the time before the Great Oxidation Event when the stem cyanobacteria had just one photosystem?
That, I think, is where I see God hovering over the face of the deep. In deep time, I see him present with creation, the only one there to observe as genes that would oxidize our planet were transferred from one microbe to another, somewhere there in the deep.
#i need a tag for weekly science rambles#how about#endless forms most beautiful#i'll go back and tag the other one in a bit#block it if you're not interested#all truth is God's truth#pontifications and creations
4 notes
·
View notes
Text
Oh hey, this is my field!
I'm gonna go on a long rant about all the minutia of studying mycorrhizal fungi, feel free to ignore
This definitely covers a ton of the problems with current mycorrhizal research, but it also gets even weirder when you consider that in addition to mycorrhizal fungi, there are also billions of rhizobacteria that ALSO span the gamut from mutualist to parasite AND interact with the mycorrhizal fungi in specific and unpredictable ways.
So there's broadly two approaches to studying these relationships- experimental and observational. Nothing new for ecology, but how do you observe what's going on in the soil? Currently the only methods for identifying mycorrhizal fungi are through root clearing and staining (which can only get you information on colonization rates and proportions of the big categories of root endophytes - arbuscular mycorrhizal fungi (AMFs), dark septate endophytes (DSEs), and fine root endophytes (FREs)) and through genome sequencing (which can identify down to species, but there's a LOT of stuff living in the soil, and the noise is incredibly high. Also, just because it shows up in your genome assay doesn't mean it's also actually interacting with the plant at all- it might just live there), and both of these methods require destructive sampling of the plant's roots. So how do you test how the relationship between the plant-fungal partners changes over the life of a plant?
For observational studies it's extremely hard to isolate the effects of root symbionts from the cacophony of other growth impacts- sunlight, water, air quality, competition, herbivores, nutrient differences (that can vary widely within a single meter!), so observational field studies essentially can only look at large trends and test weak effect sizes.
So how do we test this experimentally instead?
First, experiments require some degree of substrate sterilization because there's an absolutely incredible amount of bacteria and fungi in the dirt, water, air, our hands, etc. So not only do we need to sterilize soil, but ideally we also need to keep these plants sterile (except the inoculum) for the entire test period- anywhere from a couple weeks to a couple months (full life experiments would be ideal, but who's paying for that greenhouse space for years-decades?). Soil sterilization itself is a poorly supported method for a number of reasons.
1. What soil do you use? Wild collected soil has a higher bioload and is impossible to fully sterilize for your control, potting mix or custom sand mixtures have an extremely different nutrient/compositional profile to wild soils
2. How do you sterilize soil without changing the nature of the soil? Current methods involve autoclave sterilization, which involves moderately high temperature (~130°C) and ~2atm pressure. This is enough to kill exposed living cells, but not well protected bacteria, or any organism that's capable of going into a dormant spore state. Heating the soil hot enough to kill these changes the chemistry of the soil itself. Don't even consider chemical or radioactive sterilization, because those will irreversibly alter the soil in even worse ways.
3. How do you keep soil sterile from contamination in the long term? Like I already mentioned, water, air, and every surface are covered in contaminants that can only really be prevented in the top level sterile protocol labs. That's expensive as hell, and the time scale has to be reduced even more to minimize risk of contamination
So, essentially one cannot both sterilize the soil AND maintain it's chemical and physical characteristics. So we've gotta deal with extra noise there too. We've got to accept that the soil will never be sterile, so we just have to make sure that our inoculant has the strongest effect, so that we can detect it with our math later!
So uh. How.
Several studies on mycorrhizal inoculation methods suggest that colonization rates in experimental studies are different than in the wild, and that colonization rates differ somewhat proportionally to the amount of inoculum used, so we need to add enough inoculum that there's colonization occurring, but also each replicate needs to have the same amount of inoculum- and the control needs the same inoculum, but sterile (otherwise you're adding tons of free nutrients that are going to ruin any chance of driving microbial effects).
So what even is our inoculum? There are generally two approaches: single strain inoculation and full soil inoculation. Both have significant strengths and extreme weaknesses.
Single strain inoculation will give you the least noise - outside of the inevitable noise from contamination, you're only measuring the effects of the one species of microbe. But that means you have to isolate the microbe you want to study. There's a couple methods for this, but generally you grow plants with known inoculants for a couple generations, inoculating the next generation with the roots of its parents, and then once a viable candidate has been produced, you can do fun agar plate streaking to get it down to a handful (almost impossible to just one) of strains. Great. Now you just make a nutrient broth and pour it into each replicate's pot- several times to improve chances of inoculation.
Great! Let's say we do all this and run the experiment successfully and get our results, maybe there's a growth response, maybe not. What do we do with this now? If there's a positive growth impact do we tell farmers to flush their fields with inoculants? Do we encourage national parks to seminate the ground with fungi cultures? The applications of this is a tiny tiny piece of a much bigger puzzle- it's incredibly good information to have, but on its own it's virtually useless.
So what if we try to more actually represent what we see in nature? Let's just take some soil from the wild and put it on our sterilized test pots and see what growth impacts result!
Great, except like we know, a teaspoon of soil has billions of microbes in it, and not just bacteria and fungi- algae that can compete with the plants and change the nutrients in the soil, animals and protists that may eat the fungi or roots, seeds of other plants, viruses and other pathogens, and chemical contaminants from your source soil. Ok, that sounds rough but that was the point right? These are all things that wild plants have to deal with anyway, this is a better representation of nature right? Well each of the organisms in the soil also has its own panel of limiting factors that might keep it in check in the wild but not the lab- UV, competitors, macroherbivores, etc.
By the time we've accounted for all of the differences from the field, we're back in the damn field- the only place where the pressures of nature are met 1:1.
So often full soil inoculations end up introducing pathogens, producing negative growth responses, and it's pretty much impossible to say what growth response was caused by nutrients in the soil vs the microbes you're interested in vs the pathogens and opportunists in the soil.
Frick.
So what does this mean for the research on mycorrhizal fungi and soil biota in general?
Essentially we have a jigsaw puzzle with 1,000,000 pieces and we can only see like 5 of them, and the picture on the box changes at an unknown rate. This is why so many soil microbe researchers are pissed at science journalists that have overblown and misinterpreted the results of studies. There are far too many variables and far too many unknowns to be able to draw sweeping conclusions about the kumbaya nature of the woods, and we don't even know what's going on. Even the studies that found transfer of marked carbon molecules didn't take into account fucking transpiration and root exudates as a potential mechanism of transfer.
Anyway. Yeah.
We need more research, we need funding, and we need to be very careful about how we apply interpretations of the results of research.
An attempt at summarizing the controversies that embroil mycorrhizal network research:
a bunch of scientists are miffed at how the media has taken "plants communicate and distribute nutrients through the mycorrhizal network" and run with it, finding the "mother tree" thing too anthropomorphizing and too presumptive about something very poorly understood
unfortunately all of the major models for understanding the mycorrhizal network are anthropomorphizing, even the more competition-centered ones...to the point that papers discuss whether the network is a "capitalist" or a "socialist" system
other researchers, screaming STOP USING LOADED TERMS THAT PROMOTE AN ANTHROPOCENTRIC INTERPRETATION
But, setting aside the question of whether trees can "intentionally" do something or be altruistic...how do we know the plant is the one in control? Are the trees "sending" nutrients or is the fungus taking the nutrients and sending them to other trees? Wait, how do we assign agency in a system like this at all? Isn't it unscientific to assume that any part of the system, fungus or plant, is consciously acting? Wait...are they actually separate organisms with their own interests, or is it more accurate to view all the members of a mycorrhizal network as one big super-organism? (Wait, is it anthropomorphizing to consider organisms as having interests? If yes, how do we describe what's happening using language?)
Basically, yes we have demonstrated and established that nutrients move from one plant to another plant in the mycorrhizal network, including from fully grown trees to saplings, plants in sunlight to shaded plants, and other things that are definitely fun to interpret as one plant "helping" the weaker plant. However, we don't actually know the intentions of plants, so for all we know, the fungus could be doing everything. Or it could be completely stupid to describe any of it as "one individual organism in the network Intentionally Does A Thing."
Big Problem: Although a shit ton of research is being done, most research in the mycorrhizal network is done on very simple networks of 1 or 2 plant species with a handful of selected fungal inoculants in otherwise sterile laboratory settings. These conditions do not reflect the natural world at all.
in fact, experimental conditions used to study mycorrhizal networks are mostly completely unlike anything that would ever exist...you know, Outside,
most of the research pertains to agriculture and there are many demonstrated benefits, and many farmers are ALREADY using methods to promote mycorrhizal networks, but my guess is that it's not as simple as matching crops up to fungal inoculants that help them for instant 20% yield increase, at least in Real Outdoor Soil with an existing microbiome and seed bank.
Roughly speaking, 50% of mycorrhizal associations benefit seedling establishment, and the remaining 50% are themselves split halfway between "no effect" and "negative effect." Doesn't this mean that the mycorrhizal network is not always chill and altruistic?
Well, those findings might mean absolutely nothing either way, since in a field-setting plant community, there are dozens if not hundreds of fungi species (the diversity and number of specialists increases in later-successional communities) that are part of the mycorrhizal network, and through them any given seedling might be linked to a thousand different plants.
Some researchers find it puzzling how so many mycorrhizal partnerships seem to have no effect. Maybe the effect only comes online in certain conditions?
Parasitism, mutualism and commensalism aren't fixed types of relationship, and two partners in the mycorrhizal network can and do switch between the three constantly. This is another problem: the experiments don't usually follow both partners in a plant-fungal pairing to the end of their natural lives, and it's been shown that a fungus can be mutualistic early in a plant's life and later on become more parasitic (for example). Or that a fungus can be beneficial in poor soil conditions and become parasitic in rich soil conditions.
But...is this really best understood as a situational switch between types of symbiosis, or can we judge it by the net effect on both partners throughout their life spans, or...my brain is breaking
Like, a fungus that mostly decreases the fitness of the host plant, BUT becomes very helpful in the presence of extreme drought...is it a parasite or mutualistic partner?
Some researchers lean toward a source-sink model where nutrients tend to flow toward plants that are most lacking and away from plants with most abundance. This is a rough approximation of something ridiculously complicated
Plants can and do select fungal partners to pair with and reject fungi that contribute fewer benefits.
Fungi also appear capable of selectively distributing resources based on the fitness of the host, or at least they did this one experiment where the fungus was connected to two different trees and researchers ripped all the leaves off one of the trees. This caused the fungus to divert its nutrient flow to the undamaged tree (throwing in its lot with the tree most likely to survive). However, we're not sure if this would happen in a forest or other natural plant community, since in the lab, the fungus was totally dependent on the two trees for survival and there were no other participants in the network. So basically, it's kinda like those behavior studies on captive wolves?
4K notes
·
View notes
Text
Bird flu and more
A few days late (I've been busy, working on 4 books at once) but for the first time US dairy cattle are confirmed infected by the more dangerous-to-people HPAI H5N1 (bird flu) genotype D1.1. And starlings in Nevada are being culled.
This is a bit late: I’ve been working on four books at once (final proofs on three, pre-submission editing on a new one1) which—you’ll be shocked, shocked to hear!—eats into a person’s time… Spillovers and starlings A few days ago the USDA Animal and Plant Health Inspection Service (APHIS) National Veterinary Services Laboratories (NVSL) confirmed by whole genome sequence the detection of…
#avian-influenza#bird flu#d1.1#dairy cattle#H5N1#health#HPAI#influenza#nevada#news#poultry#seasonal flu#starlings
1 note
·
View note
Text
DNA Sequencing: Unlocking the Secrets of Life
In the age of modern science, one of the most fascinating advancements is our ability to read the very blueprint of life—our DNA. DNA sequencing, the process of determining the precise order of nucleotides within a DNA molecule, has transformed biological research, medical diagnostics, and even the criminal justice system. It allows scientists to understand genetic makeup at a deeper level, and has a wide range of applications, from personalized medicine to ancestry tracing.
What is DNA Sequencing?
At its core, DNA sequencing is the process by which we determine the exact order of the four nucleotides (adenine, thymine, cytosine, and guanine) in a DNA strand. This sequence encodes genetic information, providing instructions for building and maintaining an organism. By reading and understanding these instructions, we gain insights into everything from inherited traits to potential health risks.
DNA sequencing can be thought of as reading a very complex instruction manual. Each sequence is unique to the individual organism, and even small changes in the sequence can have significant effects. For instance, a single alteration in a DNA sequence could lead to a genetic disorder, while other variations might make someone more resilient to certain diseases.
Types of DNA Sequencing Technologies
Sanger Sequencing: This is one of the earliest and most accurate methods for DNA sequencing, developed by Frederick Sanger in the 1970s. Often referred to as "first-generation sequencing," it’s commonly used for smaller projects, such as sequencing single genes or small DNA fragments.
Next-Generation Sequencing (NGS): This technology revolutionized DNA sequencing by enabling high-throughput, massively parallel sequencing. NGS platforms can sequence millions of DNA fragments simultaneously, making them ideal for larger projects, like sequencing entire genomes or analyzing complex populations of microorganisms.
Third-Generation Sequencing: This includes newer methods like nanopore sequencing and single-molecule real-time sequencing, which allow scientists to sequence longer stretches of DNA in real time. This can be particularly useful for sequencing highly repetitive or complex regions of the genome.
Why is DNA Sequencing Important?
DNA sequencing has a huge range of applications, which include:
Medical Diagnostics: DNA sequencing allows doctors to pinpoint genetic mutations that may cause diseases. In cases of rare genetic disorders, sequencing can help diagnose the condition, enabling targeted treatments. For instance, the BRCA1 and BRCA2 genes can be sequenced to assess an individual's risk of developing breast or ovarian cancer.
Personalized Medicine: One of the most promising applications of DNA sequencing is personalized or precision medicine. By understanding a patient's unique genetic makeup, doctors can prescribe treatments tailored specifically for them. This can improve the efficacy of treatments, reduce side effects, and help manage chronic conditions more effectively.
Forensic Science: DNA sequencing has become a critical tool in forensic science. From solving criminal cases to identifying disaster victims, sequencing can provide accurate identification based on DNA left at a scene or recovered from remains.
Agriculture and Food Security: DNA sequencing is widely used in agriculture to develop better crop strains, improve livestock health, and even track food sources. By understanding the genetic traits of plants and animals, scientists can create more resilient crops, increase yields, and breed livestock with desired characteristics.
Ancestry and Genealogy: Ancestry services, powered by DNA sequencing, allow individuals to explore their genetic heritage. By analyzing the DNA and comparing it to known population databases, these services can provide insights into an individual's ethnic background and ancestral migration patterns.
The Process of DNA Sequencing
The process of DNA sequencing typically involves several steps:
DNA Extraction: First, DNA is extracted from cells, often through a simple swab or blood sample.
Library Preparation: The DNA is fragmented into smaller pieces, which are then prepared for sequencing. This preparation varies depending on the sequencing technology being used.
Sequencing: The DNA fragments are sequenced to determine the order of nucleotides. Depending on the technology, this can involve methods like fluorescence (Sanger sequencing) or electrical signals (nanopore sequencing).
Data Analysis: After sequencing, bioinformatics tools are used to analyze the data. The raw sequence is assembled, aligned, and compared to reference sequences to identify any genetic variations.
Interpretation: In a clinical or research setting, scientists and doctors interpret the sequence data to make conclusions about gene function, disease risk, or ancestry.
Challenges and Ethical Considerations
While DNA sequencing offers incredible potential, it also raises ethical and practical challenges:
Privacy Concerns: The accessibility of genetic information raises concerns about privacy. Who owns your genetic data, and who has the right to access it? These are critical questions as more people undergo sequencing for health and ancestry purposes.
Data Interpretation: Just because we can sequence DNA doesn’t mean we fully understand it. Interpreting the vast amount of data generated by sequencing requires sophisticated computational tools and a deep understanding of genetics.
Genetic Discrimination: There’s a risk of genetic discrimination if employers, insurers, or other parties misuse genetic information. This has led to regulations like the Genetic Information Nondiscrimination Act (GINA) in the United States, which aims to protect individuals from discrimination based on their DNA.
Equity in Access: The cost of sequencing has dropped dramatically, but access is still limited in many parts of the world. Ensuring equitable access to these technologies is essential to prevent widening disparities in health and research.
The Future of DNA Sequencing
As technology continues to improve, DNA sequencing is becoming faster, more affordable, and more accessible. In the future, it's likely that sequencing will become a routine part of medical checkups, allowing for more preventative and personalized healthcare. Imagine a world where everyone has a digital version of their genome, providing insights into their health, ancestry, and even traits like athletic ability or dietary needs.
Furthermore, as we decode more genomes from various species, we may uncover new insights into evolution, biodiversity, and the origins of life itself. Scientists are also exploring "gene editing" techniques like CRISPR, which, when combined with DNA sequencing, could potentially allow us to correct genetic mutations and cure inherited diseases.
Conclusion
DNA sequencing is one of the most transformative technologies of our time. By understanding our genetic code, we unlock the secrets of biology and open up a world of possibilities in medicine, agriculture, forensics, and beyond. As we move forward, it’s crucial to navigate the ethical and social implications of this technology carefully, ensuring that it benefits society as a whole.
0 notes
Text
Sugarcane Genome Fully Mapped for the First Time

Contemporary hybrid sugarcane, the most harvested crop worldwide in terms of tonnage, fulfills 80 percent of the world's sugar production needs. This raw sugar is then converted into additional commercially valuable products including molasses, bio-based materials, and bioethanol. Until recently, sugarcane was the last major crop remaining without a reference-quality genome. Having a highly accurate, complete genome allows for whole-genome sequencing and the production of superior bioengineered variants.
Sequencing the sugarcane genome, presents some unique challenges, beginning with the ploidy of its genome. Most modern cultivars are derived from a cross between a high sugar content, octoploid S. officinarum and the ‘wild’, more disease resistant, polyploid Saccharum spontaneum. While traditional sugarcane breeding practices created a range of cultivars that flourish in diverse environments and are pathogen-resistant, sugar yield improvements have leveled off in recent years. Limiting factors included lengthy breeding cycles, lack of genetic diversity in breeding populations, and the sheer complexity of the sugarcane genome (approximately 114 chromosomes).
In March 2024, an international group of scientists announced a breakthrough by combining various techniques in mapping sugarcane's genetic code. Published in Nature, the research was undertaken under the United States Department of Energy Joint Genome Institute (JGI). It included critical work performed at the Lawrence Berkeley National Laboratory in California and the HudsonAlpha Institute for Biotechnology in Alabama. International partners included several Australian, French, and Czech agencies and academic institutions, including the University of Queensland's ARC Centre of Excellence for Plant Success in Nature and Agriculture. Together, they focused on mapping R570, a sugarcane hybrid cultivar that scientists have employed for many years in researching sugarcane genetics.
Sugarcane's genome is complex due to its large size and a feature known as polyploidy, or the presence of many copies of chromosomes. The human genome has around three billion base pairs of DNA. By contrast, sugarcane contains 10 billion base pairs. Compounding the issue, many sections of the sugarcane DNA are identical, both internally and across various chromosomes. This creates obstacles when seeking to reassemble tiny DNA segments and reconstruct the genetic blueprint. Among the next-generation genetic sequencing techniques employed in piecing together this complex genetic code was PacBio HiFi, a sequencing approach that enables longer DNA section sequences to be accurately mapped.
With a complete reference genome, scientists can compare sugarcane's genes and transcription pathways with other extensively studied crops, from the biofuel feedstocks miscanthus and switchgrass to sorghum. Understanding sugarcane alongside other crops provides valuable insight into how each unique gene impacts traits of interest. A particular focus is understanding which genes are highly expressed during sugar production. Another emphasis is on identifying those genes that boost disease resistance. An example from a recent study involved singling out a location in the genome that contains all the genes that infer resistance to the commercially destructive fungal pathogen brown rust.
Having a complete genetic picture of R570 will make it much easier for researchers to identify those genes that control various traits and, in the process, improve yields. Not only will this increase the amount of sugar harvested from a limited land area, but it will also maximize other uses, such as employing bagasse, or residues remaining after sugarcane pressing, as a feedstock for bioproducts and biofuels. The genome has been made available to the public via Phytozome, the plant portal of the federally funded Joint Genome Institute.
0 notes
Text
Effective traps in bladderworts may come at the cost of genome size
Effective traps in bladderworts may come at the cost of genome size https://ift.tt/tSwL0M3 Species of carnivorous bladderwort, butterwort and sundew plants harbor some of the smallest genomes found in flowering plants. Researchers have long wondered what genetic or environmental factors contribute to these miniature plant genomes and now, a team of scientists discovered that a single mutation correlates with and drives the downsizing of genomes across some carnivorous species within the Lentibulariaceae family. The results were recently published in Annals of Botany. Mitochondria are the energy-producing organelles within cells. The researchers focused on a mutation in a gene called cytochrome c oxidase (COX), which codes for an enzyme critical for mitochondria to generate energy through cellular respiration. They hypothesized that the COX mutation boosts mitochondrial efficiency, providing an advantage for carnivorous plants that rely on suction traps to catch prey. However, the mutation may come at a cost by increasing production of damaging molecules called reactive oxygen species (ROS) as a byproduct of respiration. To test this idea, the team based in Masaryk University compiled genome size data and chromosome measurements for over 100 species across the three genera that make up the carnivorous Lentibulariaceae family: Genlisea, Pinguicula and Utricularia. They also isolated and analyzed the sequence of the COX gene from each species to identify whether they carried the ancestral or mutated version. Using statistical analyses, the researchers assessed if patterns in the data supported the COX mutation driving smaller plant genomes over evolutionary time. Their findings provide compelling evidence that the COX mutation contributes to genome downsizing in these carnivorous plants. Species with the mutated COX gene consistently had smaller genomes and chromosomes compared to those retaining the ancestral sequence. Phylogenetic modeling also revealed the genomes of COX mutation carriers trended towards becoming progressively smaller as an evolutionary response. The researchers believe increased ROS levels resulting from the COX mutation overwhelm plants’ DNA repair abilities, leading to higher rates of genomic deletions over generations. As nonessential regions gradually removed, plant genomes shrink in size. While enhancing mitochondrial function benefits carnivorous traps, this genetic tweak comes at the cost of destabilizing the genome. Our findings indicate that the whole genus Utricularia and sections Recurvatae and Genlisea from the genus Genlisea harbour CC or CS mutations in the COX gene… The observation that these Lentibulariaceae lineages tend to evolve smaller genome sizes compared to those with the ancestral LS state (genus Pinguicula, Genlisea section Tayloria) aligns with the hypothesis that changes in the COX sequence elevate ROS production, increasing DNA damage and fostering deletion-biased DNA repair, culminating in genome contraction. By uncovering how a single mitochondrial mutation impacts genome evolution, this study sheds light on the complex genetic and environmental trade-offs shaping species. It illustrates how even subtle changes to cellular processes can cascade into altering an organism’s entire genome blueprint over evolutionary timescales. The work also highlights carnivorous plants as intriguing genetic models and underscores mitochondrial mutations as an underappreciated driver of genome change. Continued exploration of this unique system may reveal further unexpected findings. READ THE ARTICLE Zedek F., Šmerda J., Halasová A., Adamec L., Veleba A., Plačková K. and Bureš P. (2024) “The smallest angiosperm genomes may be the price for effective traps of bladderworts” Annals of Botany. Available at: https://doi.org/10.1093/aob/mcae107 The post Effective traps in bladderworts may come at the cost of genome size appeared first on Botany One. via Botany One https://botany.one/ October 09, 2024 at 03:30PM
1 note
·
View note