#ffdca
Explore tagged Tumblr posts
Text
OK, I keep seeing people get this wrong, and it really irks me, so I'm making this post:
Kinder Surprise Eggs are banned in the United States. A lot of you probably knew this already, and this fact is indeed true. What is not true is the assumption I see almost everyone then take: that we did it intentionally, i.e. we banned Kinder Surprise after they were invented due to safety concerns. This is then (anecdotally, I admit) usually followed by some claim that either American children are too stupid to keep themselves from choking on the toy inside or that adults are simply too worried about children potentially choking and decided to then ban them. (Bonus points if the person also mentions our lack of gun control or school shootings in the same breath!)
Except, the second part of that claim, that we intentionally banned Kinder Surprise after they came out, is simply false.
The Federal Food, Drug, and Cosmetic Act (FFDCA) of 1938 bans confectionery that "has partially or completely imbedded therein any nonnutritive object" (21 U.S.C. § 342(d)(1)). You will note, as stated above, that the FFDCA was passed in 1938. Kinder Surprise was released by Ferrero in 1974. Kinder Surprise came 36 years after the FFDCA. I don't think it's much of a stretch to say that the small toy inside Kinder Surprise Eggs isn't nutritive, and therefore when they were launched, they were already by default banned by the FFDCA.
So no, we don't have some sort of special vendetta out for Kinder Surprise Eggs, they were simply already illegal in the US from the start. There is, however, a country which has specifically targeted Kinder Surprise Eggs since they've released:
Chile, in 2016, banned Kinder Surprise, along with a multitude of other sugary foods in an effort to curb Chile's rapidly growing childhood obesity rate. Their specific issue with Kinder Surprise was that the toy is, as they called it, a "promotional gadget" (source). According to Ley 20606, "La venta de alimentos especialmente destinados a menores no podrá efectuarse mediante ganchos comerciales... como regalos, concursos, juegos u otro elemento de atracción infantil" (Ley 20606, Artículo 6, Párrafo 3, source in Spanish). Or, as a rough translation, "The sale of food specifically intended for children may not be carried out through the means of commercial hooks... such as gifts, contests, games, or other elements intended to attract children."
I'm not really sure how I want to end this post. I guess the main thing to take away is whenever you come across any claims similar to the one I addressed in this post, maybe do some research into why what they're making fun of is the case. (Another example I could talk about is how lots of people just assume someone randomly decided there should be 5,280 feet in a mile through the means of, in the words of one notable Tumblr post, "a drunk mathematician rolling dice." The short answer to this is there's 5,280 feet in a mile for the same reason there's exactly 2.54 centimeters in an inch.)
I think that's about it. End post.
#sorry this post is kinda all over the place it kinda started off as a rant#i was pretty pissed at the start of writing this post but i calmed down over the 45 minutes it took me to research and write it lol#also i did not edit this and barely even proofread it at all so i hope it makes sense#even though this is partially a rant its still ok to reblog#actually i encourage reblogging this if you want because even if at least one person learns something new from this post#i shall consider it a success#text#not aro
12 notes
·
View notes
Text
Tragic Lessons: The Elixir Sulfanilamide Incident and the Birth of Drug Safety Regulation

In 1937, the US-based Massengil pharmaceutical company's chemists developed a new sulfonamide oral preparation named "Elixir Sulfanilamide." At that time, there was no requirement for safety review before launching new drugs. Consequently, this untested drug went on sale in September of that year. In October, the American Medical Association received reports of fatalities caused by the medication.

In November, the US Food and Drug Administration (FDA) intervened, recalling the drug. The investigation found that "ethylene glycol," used as a solvent in the drug, was the main culprit of poisoning. Ethylene glycol is highly toxic to mammals, with a minimum lethal dose (LD₅₀) of 786 mg/kg in humans. This issue, easily detectable through a simple animal experiment, resulted in over 100 deaths that fall, with 30% being children.
The following year, under immense public pressure, the US Congress passed the landmark "Federal Food, Drug, and Cosmetic Act" (FFDCA), mandating safety reviews for all new drugs before market approval and granting the FDA regulatory oversight authority.
#fda#us fda#drug safety#drug#ethylene glycol#chemblr#stemblr#science#molecule#chemistry#kingdraw#incidence#toxicity
5 notes
·
View notes
Text
Senator Cindy Hyde-Smith (R-Miss.) and U.S. Representative August Pfluger (R-Texas-11) today led 13 Senators and 54 Representatives in signing an amicus brief supporting a lawsuit challenging the Food and Drug Administration’s approval and deregulation of chemical abortion drugs.
The brief, authored by Americans United for Life, has been filed in the case of Alliance for Hippocratic Medicine v. FDA, which is pending before the Northern District of Texas. The brief spotlights how the FDA’s actions have disregarded the law and put the lives and health of women and girls in danger. It follows a Jan. 26 letter to the FDA led by Hyde-Smith that was signed by 77 Senators and Members of Congress.
“The FDA’s reckless decisions to approve and deregulate chemical abortion drugs put the profits and political agenda of the abortion industry over the law and abundant evidence that abortion drugs present harm to women, girls, and their unborn babies,” Senator Hyde-Smith said.
The amicus brief makes three primary arguments that demonstrate how the FDA exceeded its authority by subverting the law and public policy with its approval and deregulation of harmful chemical abortion drugs. The brief contends the FDA endangered the health of women and girls when it:
Failed to adhere to the drug approval process in the Federal Food, Drug, and Cosmetic Act (FFDCA)
Unlawfully waived the pediatric study requirement under the Pediatric Research Equity Act
Violated federal law by permitting mail-order chemical abortion pills
24 notes
·
View notes
Text
Biorational Pesticides Market Overview, Growth Factors, Opportunities, and Top Companies
The biorational pesticides market size is expected to expand from USD 7.5 billion in 2023 to USD 15.1 billion by 2028, with a compound annual growth rate (CAGR) of 15.2% during this period. Growing awareness of the harmful impacts of synthetic chemical pesticides on ecosystems, biodiversity, and human health has driven a shift toward more environmentally friendly alternatives. Regulatory authorities are increasingly supporting biorational pesticides due to their lower toxicity and reduced environmental persistence compared to synthetic chemicals, which is fueling market growth.

Biorational Pesticides Market Drivers: Government Initiatives on Chemical Pesticide Bans and Public Awareness Programs
The detrimental impact of chemical pesticides on soil, the environment, and water bodies globally has driven a growing focus on promoting biopesticides in agriculture. This push includes awareness campaigns and supportive policies that encourage private sector involvement. Regions like South America, Asia Pacific, and Europe are witnessing rapid market growth, thanks to initiatives that promote the use and production of biorational pesticides among farmers and producers.
In North America, the Environmental Protection Agency (EPA) regulates bioinsecticides, biofungicides, and bionematicides—key categories of biopesticides—overseeing their registration and monitoring their effects on human health and the environment. The sale and distribution of biopesticides are governed by the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), which ensures food and feed remain residue-free under the Federal Food, Drug, and Cosmetic Act (FFDCA).
In India, the government has integrated bioinsecticides into its Integrated Pest Management (IPM) approach, which combines cultural, mechanical, and biological pest control techniques. Central Integrated Pest Management Centers (CIPMCs) promote this strategy, along with the judicious use of chemical pesticides when necessary, through programs like Farmers Field Schools and training initiatives. These government efforts play a pivotal role in advancing the biorational pesticides market, especially bioinsecticides, by underscoring the importance of sustainable pest management practices.
Biorational Pesticides Market Opportunities: Advancements in microbial research undertaken by key players across regions
Companies like Bayer AG (Germany) have made significant advancements in microbial and RNA interference (RNAi) technology, creating promising opportunities in the biorational pesticides market. Major industry players in crop protection have invested heavily in research, leading to the use of biological signals to trigger RNAi-specific genes. This innovation has the potential to enhance disease and pest resistance while improving crop yield and quality. A key development is the focus on creating sprayable RNAi products for biological crop protection. For example, Monsanto Company (before its acquisition by Bayer) received EPA approval in 2017 for a genetic engineering technology that uses RNA interference to combat insect pests. Additionally, Corteva Agriscience (US) secured licenses for two insect traits from Monsanto, including an RNAi rootworm trait. The growing adoption of this technology in the industry offers a novel solution, especially for managing rootworm traits.
What are the key benefits of using microbials in biorational pesticide production?
The growth of the biorational pesticides market is driven by the use of microbials such as bacteria, fungi, viruses, and protozoa to create environmentally sustainable pest control solutions. These microorganisms work through specific modes of action, effectively targeting pests while reducing the impact on the environment and non-target species. They are formulated into various products, including microbial-based insecticides, fungicides, and nematicides.
For example, Bacillus thuringiensis (Bt), a well-known microbial insecticide, highlights the market's commitment to eco-friendly solutions. Bt produces insect-toxic proteins that specifically target certain pests without posing risks to humans, animals, or beneficial insects. Bt-based products, widely used in agriculture and mosquito control programs around the world, illustrate the market's shift toward sustainable pest management practices.
Asia Pacific's Biorational Pesticides Market Expected to Experience the Highest CAGR During the Forecast Period.
The Asia Pacific region is expected to experience the highest Compound Annual Growth Rate (CAGR) in the market from 2023 to 2028, driven by the increasing adoption of eco-friendly pest control solutions. Japan's "Organic Village" initiative, spearheaded by the Ministry of Agriculture, Forestry and Fisheries (MAFF), aims to boost the organic farming share to 25 percent by 2050. This initiative reflects a strategic push toward organic practices, likely fueling the demand for biorational pesticides in the region.
Similarly, China's “14th Five-Year Plan for National Economic and Social Development” underscores a growing focus on organic and sustainable agriculture. The plan highlights efforts to enhance green agricultural standards and strengthen certification for green food, organic products, and geographical indications. This commitment to eco-friendly farming is expected to increase demand for biorational pesticides in China, positively impacting the growth trajectory of the Asia Pacific market.
Top Biorational Pesticides Companies:
The key players in this include BASF SE (Germany), Bayer AG (Germany), UPL (India), FMC Corporation (US), Syngenta AG (Switzerland), Novozymes A/S (Denmark), Sumitomo Chemical Co., Ltd (Japan), Pro Farm Group Inc (US), Koppert (Netherlands), Valent BioSciences LLC (US), Gowan Company (US), Certis Biologicals (US), Biobest Group (Belgium), BIONEMA (UK), and Vestaron Corporation (US).
Key Questions Addressed by the Biorational Pesticides Market Report:
Which are the major companies in the biorational pesticides market? What are their major strategies to strengthen their market presence?
What are the drivers and opportunities for the biorational pesticides market?
Which region is expected to hold the highest market share?
Which are the key technology trends prevailing in the biorational pesticides market?
What is the total CAGR expected to be recorded for the biorational pesticides market during 2023-2028?
Schedule a call with our Analysts to discuss your business needs: https://www.marketsandmarkets.com/speaktoanalystNew.asp?id=57324225
#Biorational Pesticides Market#Biorational Pesticides#Biorational Pesticides Market Size#Biorational Pesticides Market Share#Biorational Pesticides Market Growth#Biorational Pesticides Market Trends#Biorational Pesticides Market Forecast#Biorational Pesticides Market Analysis#Biorational Pesticides Market Report#Biorational Pesticides Market Scope#Biorational Pesticides Market Overview#Biorational Pesticides Market Outlook#Biorational Pesticides Market Drivers#Biorational Pesticides Industry#Biorational Pesticides Market Companies
0 notes
Text
Employees and managers can learn about food safety and hygiene standards through professional training programs, such as food safety training. Regular training sessions are required for businesses in the food service sector. Whether they work in food processing, packing, storing, or growing, this is unaffected.
Participants in this program learn about laws, rules, and guidelines about food safety, including:
FDA Hazard Analysis Critical Control Point (HACCP)
Federal Food, Drug, and Cosmetic Act (FFDCA)
Food Safety Modernization Act (FSMA)
#online food safety awareness training#food safety awareness training#food safety awareness training online#online food safety training#food safety training course
0 notes
Text
youtube
What are Regulatory Compliances for Healthcare Products?
Description:
As in every industry, the healthcare industry is also ruled by certain regulations that determine what is legal and illegal. Every country has certain regulations the healthcare industry needs to follow while implementing healthcare software solutions.
Here are 6 major healthcare regulations:
1. Health Insurance Portability and Accountability Act HIPAA - USA
2. The Federal Food, Medicine, and Cosmetic Act- FFDCA - USA
3. Food and Medicine Administration FDA - USA
4. Health Information Technology for Economic and Clinical Health Act HITECH - USA
5. Personal Information Protection and Electronic Documents Act PIPEDA -Canada
6. General Data Protection Regulation GDPR — The European Union -European Union
Before you decide to hire healthcare app developers, you must check whether they have a history of non-compliance or legal action being taken against them.
EMed HealthTech is your best bet if you want to create a system that adheres to all healthcare regulatory compliance standards. Contact Us to hire healthcare software developers at emedhealthtech.com
View Source:
1 note
·
View note
Photo

CUSTOM COSMETICS THAT FIT PRODUCT REGULATIONS Creating a new cosmetic product can be daunting when it comes to finding all applicable regulations, handling the required paperwork, and implementing the necessary quality tests. Sourcing-Lab helps you find your way through cosmetic rules and quality assurance requirements. We ensure your beauty product is safe for use and that regulatory paperwork and quality tests are taken care of. Get to know more and get started with your cosmetic regulation: http://www.sourcing-lab.com #seenthemost #depechemode #cosmeticregulation #FFDCA #fooddrugandcosmeticact #CPNP ##cleanbeauty #parabens #cosmeticsafety #cleanmakeup #safecosmetics #safemakeup #cleanmakeup #veganmakeup #cosmeticingredientreview #cosmeticresearch #cosmeticchemistry #IFRA #cosmetictoxicology https://www.instagram.com/p/CgX4y4xLOnp/?igshid=NGJjMDIxMWI=
#seenthemost#depechemode#cosmeticregulation#ffdca#fooddrugandcosmeticact#cpnp#cleanbeauty#parabens#cosmeticsafety#cleanmakeup#safecosmetics#safemakeup#veganmakeup#cosmeticingredientreview#cosmeticresearch#cosmeticchemistry#ifra#cosmetictoxicology
0 notes
Text
Nonrth America Scar Treatment Market - Size, Share, Outlook, and Opportunity Analysis, 2019 - 2027
Healing of damaged tissues or wounds may create marks on the skin. These marks are referred as scars. Burn injury or other trauma condition, including surgery can also lead to a scar. Scars may be raised or recessed, different in color or texture from the surrounding healthy tissue, or particularly noticeable due to their size, shape, or location. Increasing incidents of burns is expected to boost growth of North America scar treatment market over the forecast period. For instance, according to the Centers for Disease Control and Prevention (CDC), 1.1 million burn injuries require medical attention in the U.S. annually and around 10,000 people in the U.S. die annually of burn-related infections. Increasing number of accidents and surgeries is also expected to boost growth of the market. For instance, according to 2018 Fatal Motor Vehicle Crashes: Overview by National Highway Traffic Safety Administration, a U.S. federal agency, 10,511 deaths were registered in the U.S. in 2018. Request a Sample Copy @ https://www.coherentmarketinsights.com/insight/request-sample/3305 U.S. held dominant position in North America scar treatment market in 2018, accounting for 87.9% share in terms of value, followed by Canada. High cost of laser treatment is expected to hamper growth of North America scar treatment market over the forecast period. For instance, according to the American Society of Plastic Surgeons, the average cost for laser skin resurfacing, which includes scar treatment, is US$ 2,071 for an ablative laser, whereas the average price for non-ablative laser treatment is US$ 1,144. Moreover, scar treatment can lead to several side effects, which are also expected hinder growth of the market. Side effects of ablative laser resurfacing include temporary burning discomfort, oozing, crusting, ulceration, erythema, edema, acneiform eruptions, eczematous dermatitis, and infections. Side effects of non-ablative laser include transient erythema, blistering, crusting, scarring, post-inflammatory hyperpigmentation, and post-treatment purpura lasting for up to one week. Get Free PDF Brochure of Research Report: https://www.coherentmarketinsights.com/insight/request-pdfhttps:// Increasing demand for prescription products for scar treatment is expected to offer lucrative growth opportunities for players in North America scar treatment market. Majority of the populace in North America prefer to consult qualified dermatologists and opt for prescribed products rather than using over-the-counter cosmetic products for the treatment of scars. Moreover, technological advancements in the treatment of organ scarring is also expected to boost growth of the market. For instance, in December 2019, in the journal Cell Reports, researchers from the University of California Los Angeles reported development of accurate model of organ scarring using stem cells in a lab. Topical sub segment in product type segment in North America scar treatment market was valued at US$ 2,222.8 Mn in 2018 and is expected to reach US$ 3,061.4 Mn by 2027 at a CAGR of 3.6% during the forecast period. The demand for silicon-based products is increasing in the market for scar treatment. This is owing to the ability of these products to reduce the size and darkness of scars and protect the wound from moisture and further infection. Moreover, silicone gel sheets are biocompatible (with extended wear time), repositionable, resistant to microbial growth and hydrophobicity, and are effective in treatment of burn scars, post-surgical scars, and wound scars. The market is also witnessing increasing adoption of ablative laser resurfacing (ALR) and non-ablative fractional lasers (NAFL) as the approach can offer improvement in the pigmentation and thickness of surgical scars, atrophic scars, hypertrophic scars, and hypopigmented scars. Moreover, use of ALR is effective for the treatment of traumatic and surgical scars. : U.S. • As stated by FDA, silicon sheeting for scar management is classified as class 1 device, which is considered the safest classification and does not require prescription and certification outside the U.S. • The device is exempt from the premarket notification procedures • Scar sheets are subject to general controls such as good manufacturing techniques, manufacturer’s registration with the FDA, proper branding and labelling, and general reporting procedures • According to FDA, in case of lasers, the manufacturers of electronic radiation emitting products that are sold in the U.S. are expected to comply to the Federal Food, Drug and Cosmetic Act (FFDCA), Chapter V, Subchapter C - Electronic Product Radiation Control guidelines
Major players operating in North America scar treatment market include, Smith & Nephew Inc., Merz Inc., Enaltus LLC, Oculus Innovative Sciences, Inc., CCA Industries, Cynosure, Inc., Avita Medical, Lutronics, Janssen Biotech, Inc., Luminus Inc., Syneron Medical Ltd., and NutraMarks, Inc. Key players in the market are focused on product launch and assessment to expand their product portfolio. For instance, in June 2019, AVITA Medical, a regenerative medicine company, announced new preliminary RECELL Autologous Cell Harvesting Device (RECELL System) data at the 24th annual World Congress of Dermatology Meeting. The RECELL system is used in the treatment of vitiligo and facial acne scars. Key players in the market are focused on adopting inorganic growth strategies to expand their product portfolio. For instance, in December 2019, Janssen Biotech, Inc. entered a definitive agreement to acquire all rights to the investigational compound bermekimab – an anti-IL-1alpha monoclonal antibody for the treatment of atopic dermatitis and hidradenitis suppurativa— from XBiotech Inc. Browse Full Report: www.coherentmarketinsights.com/market-insight/north-america-scar-treatment-market-3305
About us: Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity. Contact Us: Name: Mr. Shah Phone: US +12067016702 / UK +4402081334027 Email: [email protected] Visit our Blog: https://hospitalhealthcareblog.wordpress.com/
1 note
·
View note
Text
Best Baby Formula Of 2022 – Forbes Health
Best Baby Formula Of 2022 – Forbes Health
The Federal Food, Drug, and Cosmetic Act (FFDCA) defines infant formula as: “A food which purports to be or is represented for special dietary use solely as a food for infants by reason of its simulation of human milk or its suitability as a complete or partial substitute for human milk.” Baby formula is the primary food option for infants in the first 12 months of life who aren’t nursing or…

View On WordPress
0 notes
Text
What is Veterinary Medicines?
Veterinary to help increase animal capacity and growth as well
Veterinary drugs were not talked about in this setting before the mid 1990s. It was proposed in the European Union (EU) in the start of the 1990s to recognize drugs with very low focuses in the climate and which consequently would be no danger to the climate on the one side and medications that could happen in adequately high fixations to cause weakening of the climate. The last option gathering of medications ought to be inspected further by setting up stepwise an ecological gamble appraisal (ERA). This has been reached and the EU has embraced to require ERA for all new veterinary medications from 1 January 2004.
Presentation
CVM doesn't assess grumblings for pesticides and creature antibodies. These protests ought to be shipped off the Environmental Protection Agency (EPA) or the USDA. The CVM will acknowledge wellbeing data for creature takes care of, creature gadgets, human medications utilized in creatures, and supported creature drugs. Obligatory detailing of wellbeing grievances by the organization are just expected for endorsed creature medications to incorporate cured takes care of. Creature sedated feeds might be blended inaccurately or given to some unacceptable species, for example, monensin planned for cows is taken care of to ponies. Then again, creatures feeds might be defiled with normal or man-made poisons, like aflatoxin and polychlorinated biphenyls (PCBs), with the potential for genuine outcomes in people and the objective species.
Despite the fact that, there are no administrative prerequisites for the firm to report post-endorsement security data for creature gadgets, CVM will make an administrative move when essential. Pre-market endorsement isn't needed: the FDA doesn't need formal pre-market endorsement for gadgets utilized in veterinary medication (510(k) of the FFDCA). Firms that make radiation discharging gadgets need to enlist their items under the radiological wellbeing guidelines, regulated by the Center for Devices Radiological Health. FDA has administrative oversight over veterinary gadgets and can make a proper administrative move assuming that a veterinary gadget is misbranded, mislabeled, or contaminated. It is the obligation of the maker or potentially wholesaler of these articles to guarantee that these creature gadgets are protected, compelling, and appropriately named.
cow medicine
cow heat medicine
cow milk increase medicine
cow fever medicine
buffalo medicine
horse medicine
0 notes
Text
Black Nitrile Gloves: Versatility At Its Best
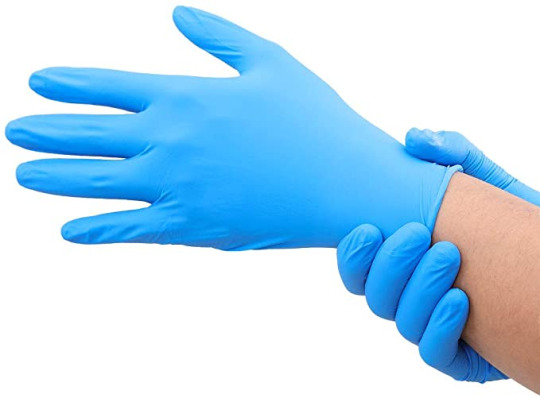
We are fully aware that there are different types of gloves available in the market and each variety has its own relevance and functions to serve. The black nitrile gloves too are considered to be an epitome of versatility as they offer outstanding resistance when they are used against a myriad range of chemicals as well as animal fats. The black nitrile gloves are also found to be compliant with the standards put forth by FFDCA and hence can be used in food service too. These gloves are made out of 100% synthetic polymer so that there are no concerns that people face like when they use the latex gloves.
Whenever gloves are worn, they have to fit in very snugly with the way our hand is. Otherwise our entire concentration is shifted towards this ill fitting gloves and work takes a beating. Another major advantage with black nitrile gloves is that they fit in very snugly and give an incomparable comfortable fit to the wearer. And when people get involved in heavy duty work there is every chance that the gloves might get punctured. But, the nitrile gloves are found to be resistant to this kind of puncturing when compared to their vinyl or latex counterparts. They exhibit three times better resistance for puncturing than the vinyl or latex gloves.
The nitrile disposable gloves offer high performance under any kind of work conditions and come in with industrial grade. They are dyed in black as technicians and mechanics prefer this color so that the dirt is hid well and cuts across a cleaner picture when they have to meet people. The color can very easily camouflage oil, grease, toners and different kinds of materials they work with which leave residues on the gloves and dark stains on the gloves. Offering superior comfort is of course the main function of these gloves. In addition to comfort, they also offer great strength that is coupled with tactile sensitivity. The gloves are textured so as to give enhanced dexterity to the wearer.
There are different sizes that are available in the nitrile disposable gloves segment and one can find medium, large, XL and 2XL sizes. You may be wondering from where you can purchase these gloves. Any shop that houses safety equipment and safety supplies will be having these different types of gloves too for you to choose from. More here guanti in nitrile
And, many thanks to the advancement of internet and technology, we now do not have to get out of our homes in order to shop. When we shop at online stores and add items to our cart and make the payment through reliable payment gateways, you can get the items shipped within a few business days.
1 note
·
View note
Text
Infant Formula Milk Powder Market Size, Share With Top Companies, Region Forecast 2021-2027
The Infant Formula Milk Powder Market report is latest report published by Fusion Market Research which provides comprehensive information, overview of the demands and describe Impact of Covid-19 on the market during the forecast period 2021–2027.
Get Free Sample Report @ https://www.fusionmarketresearch.com/sample_request/2015-2025-Global-Infant-Formula-Milk-Powder-Market/15020
Infant formula milk powder is a manufactured food designed and marketed for feeding to babies and infants, usually prepared for bottle-feeding or cup-feeding from milk powder (mixed with water). The U.S. Federal Food, Drug, and Cosmetic Act (FFDCA) defines infant formula as “a food which purports to be or is represented for special dietary use solely as a food for infants by reason of its simulation of human milk or its suitability as a complete or partial substitute for human milk”. Market Segment as follows: Key Companies IBM (USA) Unisys (USA) Fujitsu (JP) Key Types z Systems ClearPath Dorado Systems GS21 Series Key End-Use Financial Services Public Affairs Commercial Operation Others
Request For Discount @ https://www.fusionmarketresearch.com/request_discount/2015-2025-Global-Infant-Formula-Milk-Powder-Market/15020
Table Of Content
1 Industrial Chain Overview CHAPTER 1 MARKET OVERVIEW 1.1 Market Definition and Segment 1.1.1 Product Definition 1.1.2 Product Type 1.1.3 End-Use 1.1.4 Marketing Channel 1.2 Major Regions 1.2.1 Europe Market Size and Growth Figure Europe Infant Formula Milk Powder Market Size and Growth Rate, 2015E-2020F (Million USD) Figure Europe Infant Formula Milk Powder Market Forecast and Growth Rate, 2020E-2025F (Million USD) 1.2.2 America Market Size and Growth Figure America Infant Formula Milk Powder Market Size and Growth Rate, 2015E-2020F (Million USD) Figure America Infant Formula Milk Powder Market Forecast and Growth Rate, 2020E-2025F (Million USD) 1.2.3 Asia Market Size and Growth Figure Asia Infant Formula Milk Powder Market Size and Growth Rate, 2015E-2020F (Million USD) Figure AsiaInfant Formula Milk Powder Market Forecast and Growth Rate, 2020E-2025F (Million USD) 1.2.4 Oceania Market Size and Growth Figure Oceania Infant Formula Milk Powder Market Size and Growth Rate, 2015E-2020F (Million USD) Figure Oceania Infant Formula Milk Powder Market Forecast and Growth Rate, 2020E-2025F (Million USD) 1.2.5 Africa Market Size and Growth
CHAPTER 9 GLOBAL MAJOR COMPANIES LIST 9.1 IBM (USA) 9.1.1 IBM (USA) Profile Table IBM (USA) Overview List 9.1.2 IBM (USA) Products & Services 9.1.3 IBM (USA) Company Dynamics & News 9.1.4 IBM (USA) Business Operation Conditions Table Business Operation of IBM (USA) (Sales Revenue, Sales Volume, Price, Cost, Gross Margin) 9.2 Unisys (USA) 9.2.1 Unisys (USA) Profile Table Unisys (USA) Overview List 9.2.2 Unisys (USA) Products & Services 9.2.3 Unisys (USA) Company Dynamics & News 9.2.4 Unisys (USA) Business Operation Conditions Table Business Operation of Unisys (USA) (Sales Revenue, Sales Volume, Price, Cost, Gross Margin) 9.3 Fujitsu (JP) 9.3.1 Fujitsu (JP) Profile Table Fujitsu (JP) Overview List 9.3.2 Fujitsu (JP) Products & Services 9.3.3 Fujitsu (JP) Company Dynamics & News 9.3.4 Fujitsu (JP) Business Operation Conditions
…
Continue…
ABOUT US :
Fusion Market Research is one of the largest collections of market research reports from numerous publishers. We have a team of industry specialists providing unbiased insights on reports to best meet the requirements of our clients. We offer a comprehensive collection of competitive market research reports from a number of global leaders across industry segments.
CONTACT US
Email:
Phone:
+(210) 775-2636 (USA)
+(91) 853 060 7487 (APAC)
https://ipsnews.net/business/2021/06/14/cold-chain-solutions-market-2021-industry-analysis-size-share-growth-trends-and-forecast-to-2027/
https://ipsnews.net/business/2021/11/23/blockchain-identity-software-market-2021-industry-analysis-size-share-growth-trends-and-forecast-to-2027/
https://ipsnews.net/business/2021/06/15/headless-compression-screws-market-2021-applications-and-swot-analysis-to-2027/
0 notes
Text
Cosmetic Laser Market Size will Grow at a Robust Pace through 2026
Transparency Market Research (TMR) has published a new report titled “Cosmetic Lasers Market - Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2018–2026.” According to the report, the global cosmetic laser market was valued at US$ 1,455.1 Mn in 2017. It is anticipated to expand at a CAGR of 13.7% from 2018 to 2026.
Expansion of the cosmetic laser market can be attributed to a rise in medical tourism among people to avail high-quality treatment at affordable rates in emerging economies of Asia Pacific and Latin America. Further, continual growth of the cosmetic industry is projected to augment the global cosmetic laser market between 2018 and 2026.
Request a PDF Sample - https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=53928
Rise in Medical Tourism
Significant expansion of the medical tourism industry is expected to present a bright prospect for the growth for the cosmetic laser market. Countries such as India, Thailand, Brazil, Mexico, and Turkey have emerged as essential hubs for medical tourism in recent times. Advanced technology, better quality of care, faster access to several procedures, and lower rates of procedures are a few of the drivers of medical travel. It was estimated that in 2017, more than 1.4 million U.S. citizens travelled to other countries to seek health care services. The medical tourism industry has been growing at a rate of 15% to 25%. It is estimated that in 2017, 14-16 million medical tourists traveled abroad to seek medical attention. Affordable and attractive rates of laser treatment in such medical tourism friendly countries has been a major driver for the cosmetic laser market. The cosmetic laser market is further anticipated to expand at a robust rate during the forecast period, owing to a rise in the number of medical tourists across the globe.
Enquiry before Buying Cosmetic Laser Market Report - https://www.transparencymarketresearch.com/sample/sample.php?flag=EB&rep_id=53928
Growth of Cosmetic Industry
The cosmetic industry has expanded at a high growth rate over several years. Rise in awareness and increase in disposable incomes are a few factors propelling the industry. According to the American Society of Plastic Surgeons (ASPS), the number of cosmetic procedures rose by 115% since 2000, with more than 15.9 million cosmetic procedures being performed in the U.S. in 2015. The number of non-surgical procedures performed in 2017 exhibited a hike of 4.2% since the past year. In the U.S., the number of procedures conducted for laser skin resurfacing was a staggering 31,892 in 2017. Rise in preference for minimally invasive cosmetic procedures and cosmetic procedures in general is expected to present lucrative expansion opportunities for the cosmetic laser market in the coming years.
Request For Discount - https://www.transparencymarketresearch.com/sample/sample.php?flag=D&rep_id=53928
Stringent Safety Regulations for Cosmetic Lasers to Restrain Market
The cosmetic laser segment is governed by numerous rules and regulations to properly establish a control system and thereby ensure safe, hazard-free treatment option for patients. In the U.S., manufacturers of electronic devices emitting radiation are obligated to comply with certain regulations such as the Federal Food, Drug and Cosmetic Act (FFDCA), Cosmetic Act (FD&C Act), and Title 21- Code of Federal Regulations (21 CFR) among others. The existence of regulations further restricts the number of individuals that own and use such products in the U.S.. Moreover, as cosmetic lasers are a type of medical device, compliance to medical device regulations makes the protocols for aesthetic lasers extremely strenuous.
Asia Pacific Market to Expand at a Rapid Pace
In terms of revenue, the cosmetic laser market in Asia Pacific is projected to expand at a CAGR of 14.1% during the forecast period. Increase in awareness regarding the advantages of minimally invasive cosmetic procedures is expected to boost the cosmetic lasers market. Furthermore, favorable government laws supporting medical tourism, attractive procedure rates, and rise in population in this region is expected to present attractive opportunities for the cosmetic laser market in the coming years.
Buy now Cosmetic Laser Market Report - https://www.transparencymarketresearch.com/checkout.php?rep_id=53928<ype=S
Key Players in the Cosmetic Lasers Market Launch New Product to Boost Growth
The report also provides profiles of leading players operating in the global cosmetic laser market. These include Aerolase Corp., Shanghai Fosun Pharmaceutical?Group?Co., Ltd., Solta, Medical, Cutera, Hologic Inc., Lumenis, Sciton, Inc., SharpLight Technologies Inc, Syneron Medical Ltd., and El.En. S.p.A.. The cosmetic laser market has witnessed several acquisitions and mergers over the past several years. In July 2017, Apax Partners, a private equity advisory firm acquired Syneron Candela for US$ 400 Mn. The deal led to the establishment of Syneron Candela as a privately held company. This deal was expected to positively drive Syneron Candela’s growth at a global level. Similarly, in March 2017, Hologic, Inc. incorporated the services of Cynosure, Inc. for an agreement worth US$ 1.65 Bn. The deal led to the entry of Hologic, Inc. into the medical aesthetics industry and is expected to further boost the growth of the company in the coming years. In May 2013, Fosun Pharmaceutical Co., Ltd.’s subsidiary Sisram Medical acquired Alma Laser for US$ 240 Mn. Such mergers and acquisitions in the cosmetic laser industry have led to substantial growth in the industry over the past years. The trend is expected to continue during the forecast period.
More Trending Reports by Transparency Market Research:
https://www.prnewswire.com/news-releases/rise-in-the-demand-for-personalized-medicines-to-propel-the-drug-discovery-outsourcing-market-says-tmr-301326948.html
https://www.prnewswire.com/news-releases/rising-demand-for-needle-free-vaccines-to-drive-innovation-in-the-respiratory-virus-vaccines-market-tmr-301332746.html
https://www.prnewswire.com/news-releases/surgical-microscopes-market-revenue-to-reach-us-1-3-billion-by-2027--manufacturers-focus-on-performance-and-portability-of-products-for-innovation-transparency-market-research-301336425.html
About Us
Transparency Market Research is a global market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants, use proprietary data sources and various tools and techniques to gather, and analyze information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact
Transparency Market Research, 90 Sate Street, Suite 700, Albany, NY 12207 Tel: +1-518-618-1030 USA - Canada Toll Free: 866-552-3453 Website: https://www.transparencymarketresearch.com/
0 notes
Text
Goat Milk Infant Formula Market by Forecast From 2021 to 2027 With Covid-19 Impact Analysis and Future Business Opportunities
Goat Milk Infant Formula Market 2021-2027
A New Market Study, Titled “Goat Milk Infant Formula Market Upcoming Trends, Growth Drivers and Challenges” has been featured on fusionmarketresearch.
Description
This global study of the Goat Milk Infant Formula market offers an overview of the existing market trends, drivers, restrictions, and metrics and also offers a viewpoint for important segments. The report also tracks product and services demand growth forecasts for the market. There is also to the study approach a detailed segmental review. A regional study of the global Goat Milk Infant Formula industry is also carried out in North America, Latin America, Asia-Pacific, Europe, and the Near East & Africa. The report mentions growth parameters in the regional markets along with major players dominating the regional growth.
Goat Milk Infant Formula, also known as breast milk of goat milk formula. It is a manufactured food designed and marketed with fresh goat’s milk as the main raw material for feeding to babies and infants, usually prepared for bottle-feeding or cup-feeding from goat milk powder (mixed with water) or liquid (with or without additional water). The U.S. Federal Food, Drug, and Cosmetic Act (FFDCA) defines infant formula as “a food which purports to be or is represented for special dietary use solely as a food for infants by reason of its simulation of human milk or its suitability as a complete or partial substitute for human milk”.
Request a Sample Report @ https://www.fusionmarketresearch.com/sample_request/(COVID-19-Version)-Global-Goat-Milk-Infant-Formula-Market/12939
The report offers detailed coverage of Goat Milk Infant Formula industry and main market trends with impact of coronavirus. The market research includes historical and forecast market data, demand, application details, price trends, and company shares of the leading Goat Milk Infant Formula by geography. The report splits the market size, by volume and value, on the basis of application type and geography.
First, this report covers the present status and the future prospects of the global Goat Milk Infant Formula market for 2015-2024. And in this report, we analyze global market from 5 geographies: Asia-Pacific[China, Southeast Asia, India, Japan, Korea, Western Asia], Europe[Germany, UK, France, Italy, Russia, Spain, Netherlands, Turkey, Switzerland], North America[United States, Canada, Mexico], Middle East & Africa[GCC, North Africa, South Africa], South America[Brazil, Argentina, Columbia, Chile, Peru].
At the same time, we classify Goat Milk Infant Formula according to the type, application by geography. More importantly, the report includes major countries market based on the type and application. Finally, the report provides detailed profile and data information analysis of leading Goat Milk Infant Formula company.
Key Companies DGC Danone Ausnutria Dairy Baiyue youlishi YaTai-Precious Red Star Guanshan MilkGoat Herds Fineboon Jinniu Shengfei ShengTang Holle FIT Vitagermine
Market Segment as follows: By Region Asia-Pacific[China, Southeast Asia, India, Japan, Korea, Western Asia] Europe[Germany, UK, France, Italy, Russia, Spain, Netherlands, Turkey, Switzerland] North America[United States, Canada, Mexico] Middle East & Africa[GCC, North Africa, South Africa] South America[Brazil, Argentina, Columbia, Chile, Peru]
Market by Type First class Second class Third class
Market by Application 0~6 months baby 6~12 months baby 1~3 years baby
Ask Queries @ https://www.fusionmarketresearch.com/enquiry.php/(COVID-19-Version)-Global-Goat-Milk-Infant-Formula-Market/12939
Table of Contents
Part 1 Market Overview 1.1 Market Definition 1.2 Market Development 1.2.1 Current Situation 1.2.2 Aspects of COVID-19 Impact 1.3 By Type Table Type of Goat Milk Infant Formula Figure Global Goat Milk Infant Formula Market Share by Type in 2019 1.4 By Application Table Application of Goat Milk Infant Formula Figure Global Goat Milk Infant Formula Market Share by Application in 2019 1.5 By Region Figure Global Goat Milk Infant Formula Market Share by Region in 2019 Figure Asia Goat Milk Infant Formula Market Share by Region in 2019
Part 3 Global Market Status and Future Forecast 3.1 Global Market by Region Table Global Goat Milk Infant Formula Market by Region, 2015-2019 (Million USD) Figure Global Goat Milk Infant Formula Market Share by Region in 2019 (Million USD) Table Global Goat Milk Infant Formula Market by Region, 2015-2019 (Volume) Figure Global Goat Milk Infant Formula Market Share by Region in 2019 (Volume) Table Price List by Region, 2015-2019 3.2 Global Market by Company Table Global Goat Milk Infant Formula Market by Company, 2015-2019 (Million USD) Figure Global Goat Milk Infant Formula Market Share by Company in 2019 (Million USD) Table Global Goat Milk Infant Formula Market by Company, 2015-2019 (Volume) Figure Global Goat Milk Infant Formula Market Share by Company in 2019 (Volume) Table Price List by Company, 2015-2019 3.3 Global Market by Type Table Global Goat Milk Infant Formula Market by Type, 2015-2019 (Million USD) Figure Global Goat Milk Infant Formula Market Share by Type in 2019 (Million USD) Table Global Goat Milk Infant Formula Market by Type, 2015-2019 (Volume) Figure Global Goat Milk Infant Formula Market Share by Type in 2019 (Volume) Table Price List by Type, 2015-2019 3.4 Global Market by Application Table Global Goat Milk Infant Formula Market by Application, 2015-2019 (Million USD) Figure Global Goat Milk Infant Formula Market Share by Application in 2019 (Million USD) Table Global Goat Milk Infant Formula Market by Application, 2015-2019 (Volume) Figure Global Goat Milk Infant Formula Market Share by Application in 2019 (Volume) Table Price List by Application, 2015-2019 3.5 Global Market by Forecast Figure Global Goat Milk Infant Formula Market Forecast, 2020-2025 (Million USD) Figure Global Goat Milk Infant Formula Market Forecast, 2020-2025 (Volume)
…
Part 9 Market Features 9.1 Product Features 9.2 Price Features 9.3 Channel Features 9.4 Purchasing Features
Part 10 Investment Opportunity 10.1 Regional Investment Opportunity 10.2 Industry Investment Opportunity PART 11 Coronavirus Impact 11.1 Impact on Industry Upstream 11.2 Impact on Industry Downstream 11.3 Impact on Industry Channels 11.4 Impact on Industry Competition 11.5 Impact on Industry Obtain Employment Part 12 Conclusion
Continue…
ABOUT US :
Fusion Market Research is one of the largest collections of market research reports from numerous publishers. We have a team of industry specialists providing unbiased insights on reports to best meet the requirements of our clients. We offer a comprehensive collection of competitive market research reports from a number of global leaders across industry segments.
CONTACT US
PH : +(210) 775-2636
0 notes
Text
Edible Insects Market Competitive Landscape, Segmentation | Regional Analysis by 2026
Global Market Insights Inc. has announced the launch of “Edible Insects Market Report” to its collection of market research reports. This comprehensive study enumerating the latest price trends and pivotal drivers rendering a positive impact on the industry landscape. The Edible Insects market study outlines the analysis of all the inclusive segments, along with the market size, Y-O-Y growth analysis & structure of the overall industry. The Edible Insects Market is expected to rise with an impressive CAGR and generate the highest revenue by 2026.
According to Global Market Insights Inc. Edible Insects market size was accounted for USD 112 Million in 2019 and is projected to grow at a CAGR of 47% with the global market size of USD 1.5 Billion in 2026.
Request for Sample Copy at: https://www.gminsights.com/request-sample/detail/501
Further, the Edible Insects report is inclusive of the competitive analysis of this vertical in addition to the market share analysis and the contribution of the prominent players toward the overall industry. The study includes the analysis of prominent players and also covers the new entrants, including all the information suitable for the clients to make strategic business decisions in the industry.
Competitive landscape:
The Edible Insects report highlights the numerous strategic initiatives, such as new business deals and collaborations, mergers & acquisitions, product launches, joint ventures, and technological developments implemented by the leading players to set a firm foot in the market. Hence, this section is inclusive of the company profiles of the key players, total revenue, product sales, product pricing, profit margins, sales & distribution channels.
Key Players List: HaoCheng Mealworms, Agriprotein Technologies, Kreca, EnviroFlight, Chapul, Exo Protein, Thailand Unique, Six Foods, Gathr Foods, Crowbar Protein, Crik Nutrition, Nutribug, Crickers, and Bugsolutely.
Geographical Analysis:
The Edible Insects business intelligence report analyzes the market in terms of market reach and consumer bases in the market’s key geographical regions. The Edible Insects market can be categorized into North America, Asia Pacific, Europe, Latin America, and the Middle East & Africa based on geography. All of the regions are examined based on price, gross margin, revenue, growth rate, production, import/export, and sales. Here, the size and CAGR of the regional markets are also provided. This section of the report precisely evaluates the presence of the Edible Insects market in the major regions. It determines the market share, market size, revenue contribution, sales network of each regional segment.
Table Of Content
Chapter 1 Methodology & Scope
1.1 Methodology
1.2 Market definitions
1.3 Market estimation & forecast parameters
1.4 Data Sources
1.4.1 Primary
1.4.2 Secondary
1.4.2.1 Paid Sources
1.4.2.2 Public Sources
Chapter 2 Executive Summary
2.1 Edible insects industry 3600 synopsis, 2015 - 2026
2.1.1 Business trends
2.1.2 Regional trends
2.1.3 Product trends
2.1.4 Application trends
Chapter 3 Edible Insects Industry Insights
3.1 Industry segmentation
3.2 Industry landscape, 2015 - 2026
3.3 Industry ecosystem analysis
3.3.1 Distribution channel analysis
3.3.1.1.1 Collaboration/Partnership
3.3.1.1.2 E-commerce (Online)
3.3.1.1.3 Retailers
3.3.1.1.4 Direct
3.3.1.1.5 Street vendor and local shops
3.3.2 Vendor matrix
3.4 Innovation & sustainability
3.5 Regulatory landscape
3.5.1 European Food Safety Agency (EFSA)
3.5.2 EU
3.5.3 Codex Alimentarius
3.5.4 Federal Food, Drug, and Cosmetic Act (FFDCA)
3.5.5 Health Canada
3.5.6 French Federation of Producers, Importers and Distributors of Insects (FFPIDI)
3.6 Industry impact forces
3.6.1 Growth Drivers
3.6.1.1 Asia Pacific: Growing demand owing to high nutritional value and application in functional foods
3.6.1.2 Europe: Rising adoption of edible insects in food application as protein supplement
3.6.1.3 Low environmental impact
3.6.1.4 Low cost of raw materials and transportation
3.6.2 Industry pitfalls & challenges
3.6.2.1 Limited technology and lack of knowledge
3.6.2.2 Lack of regulatory clarity
3.7 Growth potential analysis, 2019
3.7.1 Addressable market potential
3.7.2 Aquaculture industry
3.7.3 Poultry industry
3.8 Porter’s analysis
3.9 Competitive landscape, 2019
3.9.1 Company market share, 2019
3.9.2 Top players overview
3.9.3 Strategy dashabord
3.10 PESTEL analysis
Chapter 4 Edible Insects Market, By Product
4.1 Key product trends
4.2 Beetles
4.2.1 Global edible insects market from beetles, 2015 - 2026, (USD Million)
4.2.2 Global edible insects market from beetles, by region, 2015 - 2026, (USD Million)
4.3 Caterpillars
4.3.1 Global edible insects market from Caterpillars, 2015 - 2026, (USD Million)
4.3.2 Global edible insects market from Caterpillars, by region, 2015 - 2026, (USD Million)
4.4 Grasshoppers, locusts & crickets
4.4.1 Global edible insects market from Grasshoppers, locusts & crickets, 2015 - 2026, (USD Million)
4.4.2 Global edible insects market from Grasshoppers, locusts & crickets, by region, 2015 - 2026, (USD Million)
4.5 Bees, wasps & ants
4.5.1 Global edible insects market from Bees, wasps & ants, 2015 - 2026, (USD Million)
4.5.2 Global edible insects market from Bees, wasps & ants, by region, 2015 - 2026, (USD Million)
4.6 Scale insects & tree bugs
4.6.1 Global edible insects market from Scale insects & tree bugs, 2015 - 2026, (USD Million)
4.6.2 Global edible insects market from Scale insects & tree bugs, by region, 2015 - 2026, (USD Million)
4.7 Others
4.7.1 Global edible insects market from Others, 2015 - 2026, (USD Million)
4.7.2 Global edible insects market from Others, by region, 2015 - 2026, (USD Million)
Chapter 5 Edible Insects Market, By Application
5.1 Key application trends
5.2 Flour
5.2.1 Global edible insects market from flour, 2015 - 2026, (USD Million)
5.2.2 Global edible insects market from flour, by region, 2015 - 2026, (USD Million)
5.3 Bar
5.3.1 Global edible insects market from bar, 2015 - 2026, (USD Million)
5.3.2 Global edible insects market from bar, by region, 2015 - 2026, (USD Million)
5.4 Snacks
5.4.1 Global edible insects market from snacks, 2015 - 2026, (USD Million)
5.4.2 Global edible insects market from snacks, by region, 2015 - 2026, (USD Million)
5.5 Others
5.5.1 Global edible insects market from others, 2015 - 2026, (USD Million)
5.5.2 Global edible insects market from others, by region, 2015 - 2026, (USD Million)
View Full Report Details at: https://www.gminsights.com/industry-analysis/edible-insects-market
About Us:
Global Market Insights Inc. is a global market research and management consulting company catering to leading corporations, non-profit organizations, universities and government institutions. Our main goal is to assist and partner organizations to make lasting strategic improvements and realize growth targets. Our industry research reports are designed to provide granular quantitative information, combined with key industry insights, aimed at assisting sustainable organizational development.
We serve clients on every aspect of strategy, including product development, application modeling, exploring new markets and tapping into niche growth opportunities.
Contact Us:
Global Market Insights Inc.
Phone: 1-302-846-7766
Toll-Free: 1-888-689-0688
Email Address: mailto:[email protected]
Read Our More Reports From Other Reputed Sources:
1. Protein Ingredients Market Value to Hit $62 Billion by 2026
2. Aquafeed Market to reach over $109 billion by 2026
3. Probiotics Market size to exceed $4.15bn by 2027
0 notes
Text
Baby Food and Formula Market Poised to Expand at a Robust Pace Over 2025
Baby food and formula market consists of soft, easy consumed food with the infant formula for the replacement of natural breast milk. Specifically, the products under baby food are majorly categorized for four to six months, whereas the infant formula is majorly for the babies under the age of twelve months and usually prepared for cup feeding or bottle feeding from powder or liquid. Specifically, The U.S. Federal Food, Drug, and Cosmetic Act (FFDCA) has defined the infant formula as a food specially represented for special dietary use for infants because of its complete or partial replacement of human milk. On the other hand, baby food comes in different varieties and tastes. It can be ready made food from the market or the homemade mashed up food.
The market of baby food and formula is a huge market and its major growth has been seen in past 5 years. It is expected to witness major growth in the next seven years owing to the increase in the growth of working women and increase in the people awareness about the product’s convenience, quality, comfort and benefits associated with it. Nowadays, parents with time have become very precise in giving proper nutrition to their child through any mode whether it’s a mashed-up food or an infant formula.
Avail Sample Report @ https://www.millioninsights.com/industry-reports/baby-food-and-formula-market/request-sample
Due to this fact parents are ready to spend any amount, but due to the increase in competition in the baby care market where numerous branded products at different price points come in parent’s attention, market is going to be stiff in future. Hence, this becomes the major restrain for new players to enter in the market. In this market key drivers are growing awareness of parent’s to partially or completely substitute the daily natural food products, increase in the number of working women population, consciousness of parents to provide nutritious rich food, and attention of parents to the growing diseases owing to the increase in pollution to food products.
According to the World Bank, between 1960 and 2013 the birth rates around the world have declined to more than 45% on an average. Majorly, due to that parents have become more critical in choosing the right baby’s food product. Companies find themselves in difficult stage where they must provide the utopian products in a stiff competition that too at a lucrative price points so that parents mind and heart can be won.
Baby infant formula is generally segmented into the three types; first being the Powder type which is prepared by mixing the scoops of powder with sterilized water and can be stored for less than 30 days in a cool place, second being the Concentrated liquid type which is majorly prepared with sterilized water in equal proportion and can be stored for less than 24 hours in a refrigerators, lastly ready to use type which do not need any water mix and can be stored for less than 24 hours in a refrigerators.
The Food and Drug Administration (FDA) has benchmarked the quality standards on the basis of nutrition level to ensure that the products are safe and supports healthy growth in infants. Due to this reason, producers of the same have set the nutritional level above the FDA set points which are different for different segments. Other than infant formula which is for babes less than 12 months of age, there are baby foods which are segmented according to the food type such as Cereals, Fruits, and Vegetables, Meats, and Sweet & Salty foods. Baby foods are produced majorly for babies four to five years old.
Baby Food and Formula sales share is highest in developed region whereas growing fastest in developing regions. Globally European region constitutes to be more than 50% sales values for baby food and more than 25% sales values for infant formula. From other developed regions North America constitutes more than 30% in the baby food and more than 40% in the infant formula. These regions is expected to constitute to account more than 30-40% each in baby food & in infant formula as they are heavily investing in producing the best quality products with utmost care to the nutritious level.
Read Complete Report With TOC @ https://www.millioninsights.com/industry-reports/baby-food-and-formula-market
The other main reason for them to remain main regions for these products is due to the drastic increase in the awareness and dependency. Other very important region is Asia Pacific which is currently accounts for more than 10% sales value in infant formula and more than 5% in the baby food. Asia Pacific region is the fastest growing region as it includes countries such as India, China, Japan and Singapore which are the fastest developing nations economically.
There is a huge transition in the working population of women. Due to this here use of baby food products and infant formula have substantially increased. Latin America has also shown the highest market growth in sales value of more than 40% in 2014 and is expected to foster same growth rate in forecasted period. Major vendors in this market are Abbott Laboratories, Danone SA, Mead Johnson & Co. LLC, Nestle SA. Other prominent vendors are Ballamy Organics, Meiji, Nurture Inc, Parent’s Choice etc.
Get in touch
At Million Insights, we work with the aim to reach the highest levels of customer satisfaction. Our representatives strive to understand diverse client requirements and cater to the same with the most innovative and functional solutions.
Contact Person:
Ryan Manuel
Research Support Specialist, USA
Email:[email protected]
0 notes