#ethanoic acid
Explore tagged Tumblr posts
Text
Table 23.1 lists several of the unbranched aliphatic carboxylic acids found in the biological world (e.g. figure 23.1), along with the common names of each.


"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#aliphatic#carboxylic acid#70s#1670s#17th century#formica#ants#venom#formic acid#acetic acid#propionic acid#butyric acid#valeric acid#caproic acid#caprylic acid#capric acid#lauric acid#myristic acid#palmitic acid#stearic acid#arachidic acid#methanoic acid#ethanoic acid#propanoic acid#butanoic acid#pentanoic acid
4 notes
·
View notes
Text
An example of a Fischer esterification, treating acetic acid with ethanol in the presence of concentrated sulfuric acid gives ethyl acetate, a common solvent (figure 23.4), and water:
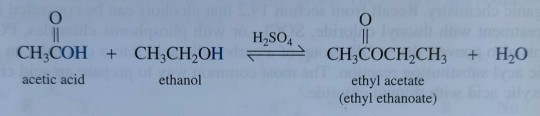

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#fischer esterification#acetic acid#ethanol#sulfuric acid#ethyl acetate#ethyl ethanoate#water#chemical reactions#glue#nail polish remover
0 notes
Text
where did the two extra carbons come from
0 notes
Text
Answer : Click on the above link...
0 notes
Text
Question:
How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Answer:
Difference in physical properties ...
0 notes
Text
The Global Ethanoic Acid market is anticipated to rise at a considerable rate during the forecast period, between 2023 To 2030. In 2022, the market is growing at a steady rate and with the rising adoption of strategies by key players, the market is expected to rise over the projected horizon.
0 notes
Text
Rules: Without naming them, post a gif of ten of your favorite films, then tag 10 people to do the same!
I was tagged by @belovedgoofball










This was oddly harder than it could've been, in part due to some movies not having gifs, and me lacking the skill to make them lol. Anyways, i tag @ultraskull1000 @lotsagold @cowboy-in-training @beacon-of-chaos @pokidragon @ethanoic-acids @sassmasterqueen @vaspider @i-need-a-better-url @athenasdragon
#royal announcement#tag game#i want to do these more bc they are harmless and kinda fun when you embrace them
4 notes
·
View notes
Text
Acetic Acid Market - Forecast(2024 - 2030)
Acetic Acid Market Overview
Acetic Acid Market Size is forecast to reach $14978.6 Million by 2030, at a CAGR of 6.50% during forecast period 2024-2030. Acetic acid, also known as ethanoic acid, is a colorless organic liquid with a pungent odor. The functional group of acetic acid is methyl and it is the second simplest carboxylic acid. It is utilized as a chemical reagent in the production of many chemical compounds. The major use of acetic acid is in the manufacturing of vinyl acetate monomer, acetic anhydride, easter and vinegar. It is a significant industrial chemical and chemical reagent used in the production of photographic film, fabrics and synthetic fibers. According to the Ministry of Industry and Information Technology, from January to September 2021, the combined operating revenue of 12,557 major Chinese garment companies was US$163.9 billion, showing a 9% increase. Thus, the growth of the textile industry is propelling the market growth for Acetic Acid.
Report Coverage
The “Acetic Acid Market Report – Forecast (2024-2030)” by IndustryARC, covers an in-depth analysis of the following segments in the Acetic Acid industry.
By Form: Liquid and Solid.
By Grade: Food grade, Industrial grade, pharmaceutical grade and Others.
By Application: Vinyl Acetate Monomer, Purified Terephthalic Acid, Ethyl Acetate, Acetic Anhydride, Cellulose Acetate, Acetic Esters, Dyes, Vinegar, Photochemical and Others
By End-use Industry: Textile, Medical and Pharmaceutical, Oil and Gas, Food and Beverages, Agriculture, Household Cleaning Products, Plastics, Paints & Coating and Others.
By Geography: North America (the USA, Canada and Mexico), Europe (the UK, Germany, France, Italy, Netherlands, Spain, Russia, Belgium and the Rest of Europe), Asia-Pacific (China, Japan, India, South Korea, Australia and New Zealand, Indonesia, Taiwan, Malaysia and the Rest of APAC), South America (Brazil, Argentina, Colombia, Chile and the Rest of South America) and the Rest of the World (the Middle East and Africa).
Request Sample
Key Takeaways
The notable use of Acetic Acid in the food and beverages segment is expected to provide a significant growth opportunity to increase the Acetic Acid Market size in the coming years. As per the US Food and Agriculture Organization, world meat production reached 337 million tonnes in 2019, up by 44% from 2000.
The notable demand for vinyl acetate monomer in a range of industries such as textile finishes, plastics, paints and adhesives is driving the growth of the Acetic Acid Market.
Increase in demand for vinegar in the food industry is expected to provide substantial growth opportunities for the industry players in the near future in the Acetic Acid industry.
Acetic Acid Market Segment Analysis – by Application
The vinyl acetate monomer segment held a massive 44% share of the Acetic Acid Market share in 2021. Acetic acid is an important carboxylic acid and is utilized in the preparation of metal acetates and printing processes, industrially. For industrial purposes, acetic acid is manufactured by air oxidation of acetaldehyde with the oxidation of ethanol, butane and butene. Acetic acid is extensively used to produce vinyl acetate which is further used in formulating polyvinyl acetate. Polyvinyl acetate is employed in the manufacturing of plastics, paints, textile finishes and adhesives. Thus, several benefits associated with the use of vinyl acetate monomer is boosting the growth and is expected to account for a significant share of the Acetic Acid Market.
Inquiry Before Buying
Acetic Acid Market Segment Analysis – by End-use Industry
The food and beverages segment is expected to grow at the fastest CAGR of 7.5% during the forecast period in the Acetic Acid Market. Acetic Acid is also known as ethanoic acid and is most extensively used in the production of vinyl acetate monomer. Vinyl acetate is largely used in the production of cellulose acetate which is further used in several industrial usage such as textiles, photographic films, solvents for resins, paints and organic esters. PET bottles are manufactured using acetic acid and are further utilized as food containers and beverage bottles. In food processing plants, acetic acid is largely used as cleaning and disinfecting products. Acetic acid is extensively used in producing vinegar which is widely used as a food additive in condiments and the pickling of vegetables. According to National Restaurant Association, the foodservice industry is forecasted to reach US$898 billion by 2022. Thus, the advances in the food and beverages industry are boosting the growth of the Acetic Acid Market.
Acetic Acid Market Segment Analysis – by Geography
Asia-Pacific held a massive 41% share of the Acetic Acid Market in 2021. This growth is mainly attributed to the presence of numerous end-use industries such as textile, food and beverages, agriculture, household cleaning products, plastics and paints & coatings. Growth in urbanization and an increase in disposable income in this region have further boosted the industrial growth in this region. Acetic acid is extensively used in the production of metal acetates, vinyl acetate and vinegar which are further utilized in several end-use industries. Also, Asia-Pacific is one of the major regions in the domain of plastic production which provides substantial growth opportunities for the companies in the region. According to Plastic Europe, China accounted for 32% of the world's plastic production. Thus, the significant growth in several end-use industries in this region is also boosting the growth of the Acetic Acid Market.
Acetic Acid Market Drivers
Growth in the textile industry:
Acetic Acid, also known as ethanoic acid, is widely used in the production of metal acetate and vinyl acetate which are further used in the production of chemical reagents in textiles, photographic films, paints and volatile organic esters. In the textile industry, acetic acid is widely used in textile printing and dyes. According to China’s Ministry of Industry and Information Technology, in 2020, textile and garment exports from China increased by 9.6% to US$291.22 billion. Also, according to the U.S. Department of Commerce, from January to September 2021, apparel exports increased by 28.94% to US$4.385 billion, while textile mill products rose by 17.31% to US$12.365 billion. Vinyl acetate monomer is utilized in the textile industry to produce synthetic fibers. Thus, the global growth in demand for textiles is propelling the growth and is expected to account for a significant share of the Acetic Acid Market size.
Schedule a call
Surge in use of vinegar in the food industry:
The rapid surge in population along with the adoption of a healthy and sustainable diet has resulted in an increase in demand for food items, thereby increasing the global production level of food items. As per US Food and Agriculture Organization, in 2019, global fruit production went up to 883 million tonnes, showing an increase of 54% from 2000, while global vegetable production was 1128 million tonnes, showing an increase of 65%. Furthermore, world meat production reached 337 million tonnes in 2019, showing an increase of 44% from 2000. Acetic acid is majorly used in the preparation of vinegar which is further widely utilized as a food ingredient and in personal care products. Vinegar is used in pickling liquids, marinades and salad dressings. It also helps to reduce salmonella contamination in meat and poultry products. Furthermore, acetic acid and its sodium salts are used as a food preservative. Thus, the surge in the use of vinegar in the food industry is boosting the growth of the Acetic Acid Market.
Acetic Acid Market Challenge
Adverse impact of acetic acid on human health:
Acetic Acid is considered a strong irritant to the eye, skin and mucous membrane. Prolong exposure to and inhalation of acetic acid may cause irritation to the nose, eyes and throat and can also damage the lungs. The workers who are exposed to acetic acid for more than two or three years have witnessed upper respiratory tract irritation, conjunctival irritation and hyperkeratotic dermatitis. The Occupational Safety and Health Administration (OSHA) reveals that the standard exposure to airborne acetic acid is eight hours. Furthermore, a common product of acetic acid i.e., vinegar can cause gastrointestinal tract inflammatory conditions such as indigestion on excess consumption. Thus, the adverse impact of Acetic Acid may hamper the market growth.
Buy Now
Acetic Acid Industry Outlook
The top 10 companies in the Acetic Acid Market are:
Celanese Corporation
Eastman Chemical Company
LyondellBasell
British Petroleum
Helm AG
Pentoky Organy
Dow Chemicals
Indian Oil Corporation
Daicel Corporation
Jiangsu Sopo (Group) Co. Ltd.
Recent Developments
In March 2021, Celanese Corporation announced the investment to expand the production facility of vinyl portfolio for the company’s acetyl chain and derivatives in Europe and Asia.
In April 2020, Celanese Corporation delayed the construction of its new acetic acid plant and expansion of its methanol production by 18 months at the Clear Lake site in Texas.
In October 2019, BP and Chian’s Zhejiang Petroleum and Chemical Corporation signed MOU in order to create a joint venture to build a 1 million tonne per annum Acetic Acid plant in eastern China.
Key Market Players:
The Top 5 companies in the Acetic Acid Market are:
Celanese Corporation
Ineos Group Limited
Eastman Chemical Company
LyondellBasell Industries N.V.
Helm AG
For more Chemicals and Materials Market reports, please click here
#Acetic Acid Market#Acetic Acid Market Share#Acetic Acid Market Size#Acetic Acid Market Forecast#Acetic Acid Market Report#Acetic Acid Market Growth
2 notes
·
View notes
Text
mixed copper oxide and ethanoic acid and thought of my oc when it turned into an ectoplasm-looking substance. i need to be put down
2 notes
·
View notes
Text
i shouldnt be allowed in chem labs i think .guess who inhaled a bunch of fumes because we were making esters and i thought that would be a good way to tell if id poured any out with the other solvents by accident (the other solvents were concentrated sulphuric acid and ethanoic acid)(Smelt Bad)(Head Went Weird)
3 notes
·
View notes
Note
this was a while ago but i remembered this morning and wondered whether you might like to see the time we made ethanoic acid in class!!


it smelt very strongly of vinegar (shocking, i know)
the blue stuff in the pear-bottomed flask is carcinogenic :)
:00 chemicals!!! (That cause cancer but are Such A Shade Of Blue!!! :00)
#sorry this has sat in my inbox for forever lol#ask#aro-mantia-muscaria#chemicals!!!#that smell like vinegar!!#:00
3 notes
·
View notes
Text
As an example of a Fischer esterification, treating acetic acid with ethanol in the presence of concentrated sulfuric acid gives ethyl acetate, a common solvent (figure 23.4), and water:


"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#fischer esterification#esterification#acetic acid#ethanol#sulfuric acid#ethyl acetate#solvent#water#ethyl ethanoate#chemical reactions
2 notes
·
View notes
Link
2 notes
·
View notes
Text
Answer:
(i) Ethanoic acid (CH3COOH)
0 notes
Text
Most Important Topics in Science Class 10

Most Important Topics in science class 10
Science in Class 10 serves as the foundation for advanced studies and helps students develop a scientific temperament. With a vast syllabus, identifying and focusing on the most important topics is essential for effective preparation. Here’s an in-depth look at the key topics from each branch of science—Physics, Chemistry, and Biology—along with tips for mastering them.
Physics
1. Light: Reflection and Refraction
Reflection: Laws of reflection, image formation by plane and spherical mirrors.
Refraction: Laws of refraction, refractive index, image formation by lenses.
Key Diagrams: Ray diagrams for mirrors and lenses.
Numerical Problems: Mirror formula, lens formula, magnification.
Why It’s Important: This topic lays the groundwork for optics in higher studies and is highly scoring due to numerical and diagram-based questions.
2. The Human Eye and the Colourful World
Structure and functioning of the human eye.
Defects of vision and their corrections: Myopia, hypermetropia, presbyopia.
Atmospheric refraction: Twinkling of stars, advanced sunrise and delayed sunset.
Dispersion of light: Formation of a rainbow.
Why It’s Important: Questions often focus on applications and real-life phenomena, making it a favorite among examiners.
3. Electricity
Electric current, potential difference, Ohm’s law.
Resistance: Factors affecting resistance, resistivity.
Series and parallel combinations of resistors.
Heating effect of electric current, power, and energy.
Why It’s Important: This topic combines theory with problem-solving, offering students an opportunity to score through well-practiced numericals.
4. Magnetic Effects of Electric Current
Magnetic field and its representation.
Magnetic field due to a current-carrying conductor, solenoid, and circular loop.
Electromagnetic induction: Fleming’s left-hand and right-hand rules.
Why It’s Important: Understanding electromagnetism is crucial for grasping advanced topics in physics and electrical engineering.
Chemistry
1. Chemical Reactions and Equations
Types of chemical reactions: Combination, decomposition, displacement, double displacement, oxidation, and reduction.
Balancing chemical equations.
Why It’s Important: It’s the fundamental topic in chemistry, often linked to other chapters.
2. Acids, Bases, and Salts
Properties of acids and bases.
Reactions of acids and bases with metals, non-metals, and each other.
pH scale and its importance.
Common salts: Preparation, properties, and uses.
Why It’s Important: This chapter’s practical applications make it a favorite for experiments and real-life application-based questions.
3. Metals and Non-Metals
Physical and chemical properties of metals and non-metals.
Reactivity series and displacement reactions.
Corrosion and its prevention.
Why It’s Important: This chapter forms the basis for metallurgy and is important for application-based questions.
4. Carbon and its Compounds
Covalent bonding, hydrocarbons, functional groups.
Homologous series.
Properties and uses of ethanol and ethanoic acid.
Soaps and detergents.
Why It’s Important: Organic chemistry begins here, and questions often revolve around reasoning and chemical equations.
5. Periodic Classification of Elements
Modern periodic table: Position of elements, trends in properties (atomic size, ionization energy, etc.).
Why It’s Important: It simplifies the study of elements and their properties, a stepping stone for advanced chemistry.
Biology
1. Life Processes
Nutrition: Autotrophic and heterotrophic nutrition.
Respiration: Aerobic and anaerobic.
Transportation in humans and plants.
Excretion in humans and plants.
Why It’s Important: This is a high-weightage chapter that covers fundamental biological functions.
2. Control and Coordination
Nervous system: Structure and function in humans.
Hormones in animals and plants.
Tropic movements in plants.
Why It’s Important: Questions often test conceptual understanding of body mechanisms.
3. How do Organisms Reproduce?
Modes of reproduction: Asexual (binary fission, budding) and sexual.
Human reproductive system.
Reproductive health.
Why It’s Important: This chapter is frequently tested in exams due to its relevance to health and population studies.
4. Heredity and Evolution
Mendel’s experiments and laws of inheritance.
Evolution: Speciation, natural selection, and fossil records.
Why It’s Important: Concepts of heredity and evolution are not only important for exams but also fascinating to learn.
5. Our Environment
Ecosystem: Components and functions.
Food chains and food webs.
Waste management: Biodegradable and non-biodegradable.
Why It’s Important: This chapter connects theoretical biology to environmental concerns.
6. Management of Natural Resources
Conservation of forests, wildlife, and water resources.
Sustainable practices.
Why It’s Important: Questions from this chapter often test awareness of sustainability and conservation.
Tips for Effective Preparation
Understand Concepts: Focus on understanding rather than rote memorization. Science relies heavily on concepts.
Practice Diagrams: Practice and label diagrams accurately. They fetch direct marks in exams.
Solve Numericals: Especially in physics and chemistry, numericals are crucial. Regular practice ensures speed and accuracy.
Revise Regularly: Keep revising important formulas, chemical equations, and biological processes.
Take Mock Tests: Solve sample papers and previous years’ question papers to understand the exam pattern and time management.
Use Mnemonics and Tricks: For memorizing the periodic table, reactivity series, and biological processes, create mnemonics or memory aids.
Focus on NCERT: NCERT textbooks are the most important resource. Read them thoroughly and solve all in-text and exercise questions.
Use Reference Books Judiciously: After completing NCERT, refer to additional books like Lakhmir Singh & Manjit Kaur for better clarity.
Conclusion
Class 10 Science is a mix of theoretical understanding and practical application. By focusing on the topics mentioned above, practicing regularly, and staying consistent, you can score well in the exam while building a strong foundation for future studies. Remember, science is not just about passing exams but also about exploring the wonders of the natural world. Happy learning!
0 notes
Text
What are the Chemical Properties of Carbon Compounds?
Carbon is an extraordinary element, forming the foundation of life and a vast array of compounds essential to modern living. In this blog, we explore carbon and its compounds, focusing on the Class 10 Science syllabus, to help students understand its significance and properties.
The Unique Nature of Carbon
1. Elemental Properties
Carbon, represented by the symbol C, is a non-metal with an atomic number of 6. It belongs to Group 14 of the periodic table and has a versatile nature due to its small size and ability to form covalent bonds.
2. Catenation
One of carbon’s most remarkable properties is catenation—the ability to form long chains, branched structures, or rings by bonding with itself. This property is the basis of the diversity of organic compounds.
3. Tetravalency
Carbon has four valence electrons, forming four covalent bonds with other atoms. This property binds carbon with elements such as hydrogen, oxygen, nitrogen, and even metals, forming countless compounds.
Types of Carbon Compounds
Carbon compounds are broadly classified into two categories:
1. Organic Compounds
Organic compounds are those containing carbon bonded with hydrogen and often other elements. Examples include methane (CH4), ethanol (C2H5OH), and glucose (C6H12O6).
Functional Groups in Organic Compounds
Functional groups define the chemical properties of organic compounds. Examples include:
Hydroxyl group (-OH) in alcohols.
Carboxyl group (-COOH) in acids.
Aldehyde group (-CHO) and Ketone group (>C=O).
2. Inorganic Compounds
Inorganic compounds of carbon include carbonates, bicarbonates, and oxides of carbon such as CO (carbon monoxide) and CO2 (carbon dioxide).
Allotropes of Carbon
Carbon exists in different structural forms, known as allotropes. The major allotropes include:
Diamond – A hard, transparent crystal where carbon atoms are bonded in a tetrahedral structure.
Graphite – A soft, black material with layers of hexagonally arranged carbon atoms.
Fullerenes – Molecules of carbon arranged in hollow spheres, such as C60 (Buckminsterfullerene).
Amorphous Carbon – Includes coal, charcoal, and lampblack.
Chemical Properties of Carbon Compounds
1. Combustion
Carbon and its compounds, like hydrocarbons, burn in oxygen to produce carbon dioxide, water, and energy. This property makes them valuable as fuels.
2. Oxidation
Carbon compounds can undergo oxidation to form other compounds. For example, ethanol can be oxidized to ethanoic acid.
3. Addition Reactions
Unsaturated hydrocarbons (alkenes and alkynes) undergo additional reactions in the presence of catalysts.
4. Substitution Reactions
In saturated hydrocarbons (alkanes), a hydrogen atom can be replaced by other atoms, such as halogens.
Versatile Nature of Carbon Compounds
Carbon compounds play a crucial role in various industries and daily life. Here are some key examples:
Fuels – Petrol, diesel, natural gas, and coal.
Polymers – Plastics like polyethylene and PVC.
Medicines – Many drugs contain organic carbon compounds.
Food – Carbohydrates, proteins, and fats are all carbon-based.
Applications in Daily Life
Carbon compounds are essential to:
Clothing – Synthetic fibers like polyester.
Construction – Graphite in electrodes.
Environment – Understanding greenhouse gases like CO2.
The study of carbon and its compounds offers a fascinating insight into the building blocks of life and technology. By understanding the properties, reactions, and uses of carbon compounds, Class 10 students can appreciate their importance in science and daily life. With a solid grasp of these concepts, students can tackle both academic challenges and real-world applications.
For students seeking additional guidance in mastering these topics, Tutoroot Online Tuition Classes provide personalised learning experiences, expert educators, and comprehensive resources to help you excel in Class 10 Science. Join us today to enhance your learning journey!
0 notes