#epigenetic reversal
Explore tagged Tumblr posts
Note
I actually tend to hc that all the Links are descended from another at some point.
However, what do you think about epigenetics being a viable reason for such?
You mean epigenetics being the reason they all are similar? Or that epigenetics is the reason they're all descendants of each other? I'm not sure I understand the question.
However, considering that epigenetics regards the condensed-ness (that's not a word but oh well) of the histones and the DNA wrapped around them and that most epigenetic changes are extremely reversible due to being caused by environmental stimuli (the only epigenetic changes that are permanent being CpG methylations in gene promoters, because those control cell differentiation.), I don't think epigenetics would leave a permanent mark on all Links as a whole. If Twilight Princess was Ocarina of Time's grandson or great-grandson, then that would be the only time I could see epigenetics playing a role in more than one Link. However, I think the rest of them are too far apart in the bloodline to have any real epigenetic effect on each other. Generally, epigenetic changes have been reversed after two or three generations, unless whatever stimuli that caused the original modification was persistent. (We used diabetic rats and poor/proper dieting for this study, and research on psychological epigenetics is still very new)
Although now that you mention this, I am curious to see how epigenetics would play a role in BotW/TotK's children.
I hope that answers the question!
For me I definitely hc that Skyward, Ocarina, Twilight Princess and BotW/TotK being all relatives (BotW being descended from TP literally being a foundational piece in my lore). I just don't know enough about the rest of them to decide for the rest of them.
#sunset talks biology#legend of zelda#my stuff#thanks for the ask!#if a CpG methylation somehow got applied somewhere where acytylation would typically have occurred then maybe#I know PTSD causes an epigenetic nightmare#but it remains that epigenetic modifications are quite reversible#even with their heritability#so I'm not sure any permanent changes would occur#Good job you#I had to consult my notes for this one#hmm maybe I should consider genetic counseling as a career....
6 notes
·
View notes
Text
If we are going to reverse the mental illness epidemic in America, we need research on mild, long-term aluminum chelation protocols funding by NIH: deferoxamine (aka desferoxamine), intranasal insulin, silicic acid, cilantro, and glutathione enhancers. And we need to stop inject aluminum into ourselves. Join me in calling on Dr. Jay Bhattacharya to have his Program Officers write calls for proposals for this urgent area of clinical research to reverse iatrogenic disease caused by injected aluminum.
Aluminum (Al) is a highly abundant metal with no known physiological role in the human body. Despite its frequent use in industrial, pharmaceutical, and consumer products, aluminum is a known neurotoxin. Chronic exposure has been implicated in the pathogenesis of multiple neuropsychiatric and neurodegenerative disorders. While aluminum toxicity was once dismissed as irrelevant due to assumed poor absorption and rapid clearance, a growing body of research shows that aluminum bioaccumulates in vulnerable tissues (Tomljenovic et al., 2013) , particularly the brain, and may be a major avoidable risk factor in the development of Alzheimer’s disease (AD; Armstrong et al., 2019), autism spectrum disorder (ASD; Boretti, 2021; Roe, 2022), dialysis encephalopathy (Alfrey et al., 1978; Mach et al. 1988), and other cognitive and behavioral disorders such as acute aluminum intoxication, which requires chelation therapy.
Aluminum exerts its neurotoxicity through several well-documented mechanisms:
Oxidative Stress: Aluminum promotes the generation of reactive oxygen species (ROS), lipid peroxidation, and oxidative DNA damage, leading to mitochondrial dysfunction and neuronal death.
Mitochondrial Disruption: Aluminum impairs mitochondrial energy metabolism and promotes apoptotic pathways, contributing to neurodegeneration.
Metal Mimicry and Ion Disruption: By mimicking essential ions such as Ca²⁺ and Fe³⁺, aluminum disrupts neuronal signaling, membrane potentials, and ferritin iron storage systems.
Epigenetic Modulation: Aluminum alters DNA methylation patterns, histone acetylation, and the expression of microRNAs (e.g., miR-29a/b, miR-124; Aschne et al., 2024), contributing to dysregulated amyloid precursor protein (APP) and tau pathology in Alzheimer’s disease (Kandimalla et al., 2016;Huat et al., 2019).
Autophagy Dysfunction: Aluminum impairs neuronal autophagy, compromising the clearance of misfolded proteins such as β-amyloid and hyperphosphorylated tau (Sanajou et al., 2023; Makhdoomi et al., 2023).
8 notes
·
View notes
Text
Took advantage of a prompt to draw up a Bluff AU (Role Reversal) Hero Shigaraki

(I do mean Shigaraki and not Shimura)
I don't know what his hero name is yet, but he was the UA Principal's personal student (and apparently handpicked before that, if rumours are anything to go by). He's hailed by the higher-ups as an example of society being able to completely turn around a family's perspective - he's a hero, his father was a crabby salaryman before he was (allegedly) killed for his old family connections, and his grandmother was an anarchistic villain. To society at large, though, he's just a nice young up-and-coming saviour.
Other ramblings under the cut.
With the Bluff AU I've been trying to balance out things that are reflected and things that are altered and things that are the same, because IMO one of the main ways a lot of role reversals fall apart is that they try to one-to-one switch characters/allegiances. ANYWAY I thought it'd be funny if Bluff!Shigaraki still thought Aizawa was kind of cool, because this version of Shigaraki is honestly pretty ordinary on the outside whereas Bluff!Aizawa has rabies. Metaphorically. Probably.
More seriously, AfO is still doing his evil little grooming thing and his evil little Backup Body thing, only instead of stoking Shigaraki's hate, he's been instilling some idea of the "natural hero", which is basically a mix of actual heroics, obedience, and aggressively blotting out anything upsetting. This Shigaraki remembers that when his family was attacked by villains, he manifested his quirk out of stress and heroically did his best to save them, which sadly failed - but he still made the situation better! Exactly how much of that narrative is true is incredibly questionable, and he probably knows it deep down but that's Bad Thought Territory and he doesn't go there :)
(The hands aren't in his possession; they're in AfO's possession while he keeps them safe because Shigaraki isn't legally cleared to keep human remains at his residence. This is not sketchy at all.)
I figure his support weapon thing works in a similar way to Mirio's DNA infused suit, only it's specifically DNA with the epigenetic makeup associated with the cells on his fingers, which essentially allows it to work like one Really Long Hand. The end is kind of dotted with DNA-including and DNA-excluding zones so at any given time there's probably the requisite five points to activate, and there's a cap that can go over it so it can safely be used for, like. Hitting things you don't want turned into a dust slurry.
8 notes
·
View notes
Text
Updated: April 29, 2025
Reworked Character #13: Allen O'Neil
POTENTIAL TRIGGER WARNING: Viewer discretion is advised due to references to death, drug addiction, crime, and torture.
Real name: Alister Titus O’Neil Sr.
Esper title: Avatar of Peak Physicality, Cheating Death, and Assimilation
Aliases: Conqueror of the Staircase of Dead Bodies, Iron Cavalryman from Hell, Immortal Cacodemon, and Ghost Sergeant
Occupation: Sergeant of the Rebel Army, right-hand man of General Morden, Lance Corporal of the Marine Corps (formerly), and a high-ranking peacekeeping troop of the Regular Army (formerly)
Retirement plans: Open up an animal shelter and live the rest of his days in the countryside
Special skills: Armed and close quarters combat, crisis management, defensive tactics, and brainwashing
Esper abilities: He possesses superhuman strength, endurance, and stamina, demonstrated by his ability to effortlessly lift the main combat vehicles of the Rebel Army, withstand extreme physical stress, and carry the Jupiter King without its tracks without displaying any visible signs of exhaustion. His body is physically resilient to shotgun blasts and a single pineapple grenade or Stielhandgranate explosion. His body contains a silver-hued regenerative agent that rapidly repairs damaged cells and tissues, giving his blood a metallic grey sheen. When wrath consumes him, his skin erupts into a fiery crimson, radiating intense heat and morphing into a semi-scaly texture.
He possesses two unique, blubber-coated, kidney-shaped organs in his thighs, which are a pale pink streaked with bronze. These organs are connected to his esophagus by two trachea-like purplish-grey tubes. The kidney-shaped organs secrete a blood-streaked, sticky substance similar to intravascular and seminal fluids that gives birth to earwig-like pale brown creatures, approximately the length of a human arm. Each creature features bronze and crimson streaks, forest green antennae, and black centipede-like legs and forceps. The trachea-like tubes are lined with resilient, mucus-coated hair follicles, providing the creatures with a moderate layer of protection against blades and enhancing their ability to adhere to hosts.
These creatures serve as vectors for mental assimilation, allowing him to recruit individuals to General Morden’s cause. To achieve this, they inject neuroactive agents into a host's bloodstream by biting into a major vein and tightly latching onto their skin. Once established, the creatures transmit electrical signals to the brain, inducing brainwashing and instilling an unwavering sense of loyalty. This can only be reserved once the parasitic creature has been surgically removed, allowing the effects of these neuroactive agents to degrade slowly over a five-day period.
He's virtually immortal due to a biological self-resurrection process, which allows him to fully restore his psyche and physical body to optimal health. Upon death, his regenerative agents kick into overdrive, rapidly repairing minor and major wounds, replenishing energy in his blood, and revitalising his white blood cells to sustain their viability. His brain remains active, sending electrical impulses that stimulate cellular regeneration, tissue reorganisation, maintain blood circulation, reverse organ failure, and induce wakefulness. It can even correct genetic damage, reactivate dormant cellular functions through epigenetic reprogramming, reboot the immune system to eliminate pathogens and cancer cells, and restore cognitive function and memory. After each resurrection, his memories of his previous death are foggy, and his once-healed injuries leave lingering stiffness for a few days.
Hobbies: Whittling, extreme off-roading, frequenting pet shops, and hanging out with his family whenever he has the time to do so
Likes: Social justice, blowing stuff up, insulting his adversaries, and messing around with the Rebel Gigant
Dislikes: Being viewed as a simple-minded and ignorant assault captain, the Regular Army, insubordination, and orcas
Favourite food: Pompano en papillote and bread pudding with vanilla whiskey sauce
Favourite drink: Cognac
Sexuality: Heterosexual
Gender: Male
Age: 38 (in 2022), 44 (in 2028), 46 (in 2030), 48 (in 2032), 50 (in 2034), 57 (in 2041), 59 (in 2043), 60 (in 2044), and 63 (in 2047)
Blood type: B+
Weight: 254 lbs. (115 kg)
Design: He’s a 6’ 7” (200.66 cm) American mesomorph of Creole and Scottish descent with an inverted triangle build, rippling muscles, and sloping shoulders. He has honey-hued skin and striking asymmetrical eyes—amber on the right with a luxor gold pupil and mahogany on the left with a foggy white glaze. His face features subtle wrinkles, including forehead lines, frown lines, and nasolabial folds. Allen has slightly drooping pointed ears, skin that's somewhat taut against his spine, razor-sharp canines, a shaved head, and a full dark chocolate beard.
He has a tattoo on his left upper arm of a pygmy rattlesnake coiled around the base of a human skull with sparkling rubies embedded in the eye sockets. He has a few battle scars: one that starts from the centre of his right cheek to the side of his forehead, resembling a fiery comet flying upwards; three healed bullet wounds on his left deltoid; half of his left ring finger is missing; and a large, blotchy patch of scarred flesh on the right side of his abdomen.
His outfit consists of gunmetal grey wristbands, a burnt sienna armband displaying the Rebel Army insignia, and mud- and blood-stained laurel green army cargo pants tucked into spike-soled silver-white combat boots. He carries an old, cherished photograph of himself, his wife, their two children, and their two chestnut-fronted macaws securely tucked away in the right pocket of his army cargo pants. Allen wears a gold-buckled rifle green belt, complemented by a sheath for his Bowie knife and a gunmetal grey waist pack secured at the back, containing pipe bombs and a sprig of heather for good luck. He wears three black bandoliers, two forming an X-shape across his chest and one draped above his belt, holding ammunition for his M240 Bravo Machine Gun. He wears a burgundy cord necklace, showcasing a shark tooth as its centrepiece. The shark tooth is flanked by two canine teeth with three circular blue garnet beads positioned between each canine tooth.
During the Survival Island Occupation, he temporarily wore the same outfit that commanding officers wear in the Regular Army. He also donned a chin-length reddish-blonde wig with blunt cut bangs and soft waves.
He owns a personal Di-Cokka, twice the size of the original, its greenish-brown coat bearing battle scars—dented and scratched from a few bullet impacts. Besides transportation, he also uses it for storage, keeping portable ammo boxes and two extra weapons on hand: a M202 FLASH and a Mossberg 500 shotgun.
Super Devil form: He’s a 21’ 3” (647.7 cm) humanoid with an exaggerated musculature and smooth, rock hard rifle green skin. His head, marked by a pronounced underbite, has a canine-like appearance, with the skin tightly stretched over his skull. A wet gunmetal grey nose, reminiscent of a bear's, protrudes prominently, while his beard has grown into an unruly goatee that cascades down to the centre of his chest. He has twitching muscles, gleaming greenish-brown claws and talons, and six arms covered in decaying flesh and bulging veins, each ending in heavily scarred hands with only three fingers. He has the same eyes, ears, razor-sharp canines, and scars as the original, but his armpits and back are covered in coarse dark chocolate hair.
Two rows of stalagmite-like gilt-brass spikes run along his back, while two lengthy, glistening tentacles—each spanning the height of two adult males—protrude from the back of his deltoids. These tentacles boast a crimson hue and are adorned with mucus-coated laurel green feelers on their underside. Allen’s tail resembles that of a pygmy rattlesnake, while his waist is encircled by a bronze intestinal cord that passes through the bored temples of fifteen yellow-stained, shark-toothed human skulls. Each skull features either ruby or blue garnet eyes, arranged in an alternating pattern. His legs are feathered in a plumage that transitions from reddish-brown to burnt sienna, culminating in powerful silver-white ostrich feet.
Character summary: Allen is a proud and charismatic leader, renowned for his persistence in battle and unshakeable loyalty to those he holds dear, including his family, closest comrades, and General Morden. He passionately advocates against political corruption and systemic injustices, fearlessly standing up for what's right and recognizing it as his social responsibility to create a better world. He’s capable of compassion, but only extends his support, kindness, and sympathy to those he considers trustworthy friends and admires for their bravery and integrity. He can be somewhat forgetful, particularly when recalling complex instructions or distinguishing between appropriate and inappropriate times to use bladed weapons, such as when preparing food.
He derives great satisfaction from catching his foes off guard or disrupting their plans, gaining a strategic upper hand on the battlefield. He's ruthless and pragmatic, revelling in the suffering he inflicts on those who work for corrupt organisations and/or oppose General Morden. He relishes battling his enemies, particularly his arch-rivals, the Peregrine Falcons Squad and the S.P.A.R.R.O.W.S. His passion for frontline combat is why he chooses to remain a Sergeant, the highest rank that allows him to engage in daily battles. He displays subtle admiration for individuals who demonstrate impressive tactical expertise and skillful combat abilities.
He often rushes into battle with reckless abandon, yet occasionally takes a more measured and calculated approach. He frequently taunts his enemies, using psychological warfare to erode their resolve and sow seeds of self-doubt. He skillfully cultivates loyalty among Morden's followers by subtly undermining their critical thinking and independence, making them receptive to indoctrination. Through this manipulation, Allen implants new thoughts, reshapes attitudes, and alters values and beliefs to align with Morden's ideology. Occasionally, he spares new recruits from brainwashing if he detects genuine sympathy for Morden's cause or notices that they don't see the point in questioning the ideology and practices of the Rebel Army.
He strongly dislikes it when people underestimate his intelligence and make jokes about him having "stupid genes". When mocked for his lack of sophistication, he's quick to retaliate, scolding those who underestimate him, and will even resort to violence or intimidation if he feels provoked. Despite being a belligerent and ignorant individual, he can be surprisingly calm and composed during the heat of battle or when he isn't on the battlefield. He never fears his enemies due to a fear of being seen as a coward, but he will show prudent caution if there's a genuine reason to be concerned. He has always had a fondness for winter and working in snowy conditions, drawn to the cleanness of fresh snow and the challenge of battling through bitter cold, which he sees as a worthy obstacle to overcome during intense combat.
His phenomenal physicality and combat skills are matched only by the size of his ego. He frequently belittles those he doesn’t trust and makes crass, demoralising, and dehumanising remarks about his adversaries. While Allen’s superiority complex often gets the better of him, he’s a seasoned soldier who can endure more physical and mental punishment than his weaker comrades. However, he doesn’t view himself as superior to his family, close friends, most trusted comrades, General Morden, and the law. Besides his strength, stubbornness, and gusty courage, the only thing that keeps him alive is his devotion and unrelenting will to return home to his loving wife, Henrietta, and their children, Nancy and Allen Jr.
He deeply loves Henrietta and cherishes every moment with her, yet he can't help but find her intimidating due to her stern demeanour and piercing gaze. He loves Allen Jr. deeply, but their relationship is strained by his son's blunt criticism of his shortcomings and, more painfully, his alliance with the Rebel Army's sworn enemy. He recognizes that Allen Jr. views his actions as morally misguided, driven by an unwavering loyalty to General Morden. Additionally, he's aware that his son is troubled by his own superiority complex and impulsive decisions on the battlefield, which have repeatedly put his life at risk and led to past fatalities. Nevertheless, he does his best to support his son's military career and love life, while also respecting his capabilities as a fighter. He does notice that Allen Jr. doesn’t listen to him at times, which frustrates him. However, he chooses not to verbally address the issue, fearing it may further strain their already estranged relationship.
He shares a cordial relationship with General Morden, often bonding over drinks and swapping stories, especially after missions. Beneath Morden's tough exterior, he recognizes his struggles with alcoholism and the emotional toll of losing his family, and genuinely empathises with him. He looks up to him as a great leader due to his caring and respectful nature towards his soldiers, exceptional intelligence, and strong sense of justice. He vows to protect him at all costs, support the growth of his military strength, and ensure the success of his plans.
He harbours intense disdain for Abul Abbas, regarding him as a moronic nuisance and a poor excuse of a commanding officer. He shares the view of many Rebel soldiers and commanders that Abul is intolerable and unfit to serve their cause. Allen often mocks Abul's peculiar sense of smell, jokingly referring to it as "horny cognition" to annoy him. Despite Abul's incompetence, Allen O'Neil is genuinely surprised that he has managed to befriend General Morden, Sagan, and Logan. However, he refrains from complaining, deeming it pointless to waste his breath on someone he strongly hates.
He’s a good friend of Sagan, admiring her independence, resilience, and exceptional leadership abilities, which rival General Morden's in terms of tactical expertise and charisma. He treats her with the same respect he has for Morden, acknowledging her superior military rank and significant contributions to the Rebel Army's tactical prowess. He often goes out drinking with Sagan, assists her with her plans, helps implement her rigorous training programs for Rebel Army cadets, and playfully engages with her joking attitude.
He views Logan as somewhat infuriating and intimidating at times, yet treats him with equal respect alongside Sagan and Morden, recognizing his senior rank and crucial role in the Rebel Army's strategic planning and financial management. He admires Logan's easy-going attitude and willingness to offer him valuable advice on navigating more difficult tactical situations.
Backstory: Alister Titus O’Neil Sr. was born on November 17, 1984 in New Orleans, Louisiana, United States. He doesn’t remember his parents very well, as he didn’t spend much time with them, but he can recall his father being an Scottish-American chef and his Creole mother working as a construction worker. His parents instilled in him the importance of loyalty to loved ones and friends as well as the confidence to embrace his unique capabilities without shame. Tragically, at just 7 years old, his world was shattered when his mother was killed during a devastating robbery at a local convenience store. The loss proved too much for his father, who abandoned him, leaving him alone and frightened in a neighbourhood plagued by crime and bad influences.
Luckily for him, he was taken in by his uncle who, despite struggling with a fentanyl addiction and deep-seated feelings about systemic injustices, selflessly took him in. His uncle went above and beyond to raise him like the son he never had, teaching him essential school subjects and instilling in him a strong sense of social responsibility and courage to stand up against injustices. After his uncle's tragic death from a fentanyl overdose when Allen was just 13, he faced a harsh new reality and this pivotal event awakened his esper abilities. With no support system, he turned to a local gang for protection and began carrying blades for self-defence. As he gained more experience on the streets and honed his fighting skills, he gradually moved to firearms, progressively handling smaller weapons before moving on to larger ones. Following his revelation dream at 15, he dedicated himself to mastering his esper abilities.
At 16, Allen faced legal consequences for aggravated assault and burglary, leading to time in a juvenile detention centre. The Regular Army, recognizing his esper status, quickly intervened and offered guidance, aiming to mould him into a skilled soldier. The Regular Army offered him a stable home in a secure area of Scotland, aiming to provide a safer environment that would steer him away from a life of crime. With their therapeutic and parental support, he made a decision to turn his life around. Allen volunteered for social justice initiatives and animal welfare organisations to improve himself and give back to the community.
At 19, while working at an animal shelter, he met his future wife, Henrietta. A brief conversation about their future aspirations sparked a romance between them. As they went on multiple dates, their connection grew stronger. A year later, Allen proposed, and to his delight, she accepted. Soon after getting married, they welcomed their son, Allen Jr. Upon discovering a recruitment poster for the Regular Army, he felt a surge of purpose and a strong desire to fight for justice. This newfound passion prompted him to quit his old job and enlist in the Regular Army as a peacekeeping troop at the age of 22. After serving five years, he transitioned to the Marine Corps. Two days before his 30th birthday, he and Henrietta celebrated the birth of their daughter, Nancy. Their family grew again four years later with the adoption of two chestnut-fronted macaws, Shirley and Kingsley.
Allen's life took a dramatic turn when Donald Morden rescued him from a near-fatal confrontation with a notorious gang of terrorists and high-risk criminals, who had collaborated with pirates, in a volatile region of South Africa. Grateful for Morden's heroism, he offered his support and loyalty, forming a strong bond as a trusted friend and ally. A year later, following a successful mission against a dangerous criminal organisation bent on destabilising government authorities, Morden introduced Allen to Sagan and Logan. He formed strong bonds with Sagan and Logan, but their demanding schedules rarely allowed for downtime together.
He rose through the ranks to become a high-ranking peacekeeping troop in the Regular Army under Donald Morden's command, while also serving as a Lance Corporal in the Marine Corps. Renowned for his exceptional combat skills, toughness, and devotion to family, he was once known for his cool attitude. As Morden's most trusted soldier and the only esper under his command, he frequently received the most complex missions, which he executed with great success and enthusiasm. He always enjoyed fighting for Morden, just as he enjoyed unwinding with Henrietta, Nancy, and Allen Jr. after a tough day.
He played a crucial role in the Arms Deal Barrage, fighting as a soldier against the remnants of the Serapion Fellowship. Alongside fellow combatants, including Morden, Sagan, and Logan, he discovered the harsh reality of corruption within the Regular Army, a truth carefully concealed from the public. A small part of him yearned to defy them for their corruption, but Morden and Tequila cautioned against it, warning he'd land on their hit list. He faced off against 1st Lieutenant Wired in a fierce battle, alone and unsupported, yet he developed a hint of mutual respect for his opponent's tactical prowess and proficiency in grenades and armed combat.
After Morden defected from the Regular Army, Allen followed him out of loyalty and disappeared from public view for several years. During this period, Allen played a crucial role in building the Rebel Army's forces by brainwashing and training new recruits alongside Morden. He also contributed to the development of tactical plans with Logan and Morden and collaborated with Sagan on clandestine missions to raid weapon facilities and acquire advanced Tuatha Dé Danann technology.
When General Morden reemerged as the founding leader of the Rebel Army and launched his coup d'état, he stood by him, playing a pivotal role in the insurgency. Tasked with defending the supply warehouse at the summit of Käthehirt Valley, Allen single-handedly annihilated the troops brave enough to tackle the treacherous mountain. However, when Marco, Tarma, Tequila, Gimlet, and Red Eye ascended the mountain, they encountered Allen and were forced to engage him in battle. Gimlet and Red Eye managed to press onward, choosing to confront the Rebel Infantry instead of fighting their former comrade. After being burned alive and shot in the head for good measure, Allen was presumed dead.
Nevertheless, he would later be revived through his biological self-resurrection process and participate in the ambush on the Regular Army, Peregrine Falcons Squad, and S.P.A.R.R.O.W.S. troops. Under Morden's command, he brutally tortured and executed many of Marco’s and Tarma's comrades and friends. He played a major role in Tarma's torture, verbally exploiting his emotional sensitivity and insecurities about his academic intelligence. He also carried out General Morden's order to sever Marco's left arm.
Allen resurfaced in a secret Rebel Army base hidden in the Siberian frozen tundra, where he once again aided Morden in his plans for global conquest, this time in alliance with the Pipovulaj. When Regular Army troops infiltrated the base, Allen attempted to eliminate them but was ultimately defeated by Marco, Eri, Tarma, and Fio. In the heat of combat, Allen unleashed a ferocious strike, brutally severing Tarma's right forearm. His lifeless body plummeted down a snowy cliff and was devoured by a giant orca. However, a nearby patrol of Pipovulaj troops recovered his newly resurrected remains after Marco's team departed to confront General Morden. This traumatic incident has left him with a lingering fear and hatred towards orcas, whom he now perceives as viewing humans solely as prey. The experience has also filled him with disgust, haunted by the memory of the orca's inner anatomy.
After the Extraterrestrial Alliance Clash, Allen, Sagan, Logan, and some Rebel Infantrymen and cadets, along with a few Morden sympathisers, hatched a plan for a revenge attack on the South Pacific training island owned by the Peregrine Falcons Squad. Their aim was to install and house the earthquake-causing weapon, the Cabracan, which was jointly built by the Rebel Army, Pipovulaj, and Amadeus Syndicate, in their new base of operations. This was necessary as their previous bases had been raided.
To achieve this, Allen and Sagan disguised themselves as drill instructors and led a group of Regular Army and Peregrine Falcons Squad cadets to a remote island in the South Pacific. Unbeknownst to the cadets, Allen had secretly brainwashed them, implanting a false sense of security and focusing their minds solely on training. Their sinister intention was to use the cadets and any Intelligence Agency agents on the island as hostages and test subjects for the experimental simian and mantis transformation serums developed by Doctor Amadeus.
Once Division 6 and the uncaptured cadets infiltrated the main base, he and his platoon of land troops would engage in a fierce clash with Allen Jr., Walter, and Dilovar. He would discover that his son had joined the archenemy of the Rebel Army, a revelation that had been previously kept secret from him. He felt a deep sense of betrayal, but he knew it would be rash to turn on his son simply because of their differing allegiances. With a heavy heart, he accepted the fact that Allen Jr. had chosen to serve the Regular Army, and it seemed unlikely that he would ever switch sides. Before he could retreat with his surviving soldiers, Walter and Dilovar took his life in a furious act of retribution for their fallen comrades. Fortunately, Sagan was able to recover his body, which was undergoing a biological self-resurrection process.
#writerscorner#creative writing#writing#iron eclipse au#death tw#crime tw#torture tw#metal slug#snk#gaming community#rework#redesign#name#alias#job#skills#abilities#power#hobby#likes and dislikes#food#sexuality#gender#age#blood type#weight#personality#backstory#allen o'neil
14 notes
·
View notes
Text

🤍 NEW SUBLIMINAL: 'blessed genetics ♡ epigenetics & unstoppable DNA (w/ omni formula)'
youtube
i worked soooo hard on this one y'all… i had to read scientific research and papers on all things epigenetics and anti-aging to create this omnipotent baby. oh, did i mention that this is the first time i feature my omnipotence formula in a video? learn more about it here: https://www.hunkystephaudio.com/formulas
blessed, superior genes, heeeere we come! enjoy!!! 💗 some of the benefits are:
✨reverse aging at a cellular level ✨blessed DNA ✨epigenetic reprogramming ✨ endless cellular regeneration & sooo much more!!
🤍 my official subliminal store is packed with new subs: https://hunkystephaudio.com/shop 🤍 new year, new you! get your own personalized, high-quality custom subliminal / bundle! for info & prices: [email protected]
#law of attraction#subliminal messages#subliminals#loa#hunky steph audio#law of assumption#self concept#affirmations#subliminal#loass angel#loass states#loa tips#loassblog#loassumption#loa tumblr#loablr#loa blog#loass#law of assumption blog#law of vibration#subliminal maker#subliminal user#subconscious reprogramming#subliminal audio#subconscious#Youtube
12 notes
·
View notes
Text
Epigenetics is one of my fav fields there and it's so cool... you know why some diseases are almost impossible to treat? Well, every cell has your entire DNA in it, every cell has access to the instructions to produce every single protein your body makes.. but they don't do it, gene expression is extremely regulated
And it may be frustrating to hear that well, technically, your muscle cells can become neurons on paper, but they don't ! This is where stem cells come in, the medical holy grail... think of every cell in the body as eevelutions, they all evolved from eevee but they can't evolve back nor into other types, stem cells would be that eevee, they can turn into other types of cells, the unfortunate part is that stem cells are usually only found in embryos and in ovules making them difficult to harvest
And if you know me, and you know where I'm going with this. In 2013, while scientists where trying to discover how leprosy, an inmobile bacteria, they stumbled upon (and I quote from them) "biological alchemy" for it turns out, leprosy can reverse cells states in other words, leprosy can turn cells into stem cells, which in turn it manipulates to become muscle/bone cells, these new cells then move to their respective tissues, carrying leprosy with them
Which is like, insane? We discovered that other bacteria such as H. pylori can also do something similar, but how cool is that?? It's been 11 years though, researchers have been trying to understand how it can convert them to further be able to cure neurological diseases soon like Alzheimer and Parkinson
10 notes
·
View notes
Text


Epigenetics and politics
Our ability, as humans, to use intellect to our advantage is indisputable. However, our success is clearly not all we might hope for. In spite of this, a popular view is that all our problems can be overcome by intellect. Many people find the belief attractive because our intellect distances us from other animals and particularly those primates to which we are most closely related. Although it is overlooked that all these living species have existed far longer than we have without intellect. The demarcation has helped foster a belief in the general supremacy of intellect. The illusion of the great importance of intellect stems from our evolution. We feel, as individuals, we are numero uno. As long as collectively we were not, that worked fine. Now that we have become so, the belief is clearly to our detriment.
A misconception about intellect is that it evolved for the purpose of organising ourselves socially. There are no good reasons for thinking this and, if anything, the reverse is true. Our intellect has evolved as a biological device for working out and implementing survival strategies for our reproductive success as individuals. The logical conclusion has been to manipulate our environment on such a scale as to seriously alter it. Although our success or failure as a species can be seen to depend on the power of our intellects the view is not entirely correct. The greater part of our behaviour is not controlled by our intellects but is genetically determined in the same way as it is for other animals. Generally, people do not wish to understand the genetic control because it conflicts with their view of their intellectual supremacy.
Evidence for thinking there are unseen genes controlling our behaviour is overwhelming. The majority of the genes are not discreet units acting in a simple Mendelian fashion but are complex groups, polygenes, interacting with one another. They underpin all behaviour that is common to everyone and work within the constraints imposed by our environment. An obvious example of the effect of the environment is growth in body size which is dependent on both inheritance and diet. Intellect is genetically determined. It is purposeful and governed by self-interest. Those genes giving individuals the capacity for reason, for example, have been an evolutionary success. In conjunction with other genes they give individuals and their immediate kin who also bear them increased reproductive potential. Genes are not to help the people next door unless, of course, they are kin. A hard reality is that if these neighbours are not kin they are then normally viewed, from the point of view of genes, as something that is potentially exploitable. The interests of the genes and their bearers, our thinking bodies, cannot be separated.
Our behaviour, then, has evolved to increase our chances of survival not as a species but as individuals. Since species are composed of interbreeding individuals many of the characteristics will be common to species. Other genes to those controlling intellect and reason are important in determining general social behaviour. This behaviour was incorrectly thought by Kropotkin to have evolved for the benefit of the species. In defence of Kropotkin, our understanding of what is social is confused. Social insects such as ants and bees living in a colony are closely related to one another genetically. Again, tribes or small communities of humans consist of closely related individuals. The individuals, whether insect or human, under these conditions behave in an altruistic manner towards one another because this is in their gene's self-interest. With humans, the larger the group the less closely related individuals become. This must be true of people living in cities, for example. Social behaviour, then, evolved for the benefit of related individuals and their offspring. What is difficult to explain is why in modern societies people tend to behave as though they are genetically closely related when they are not.
Although all human behaviour is controlled by genes for the benefit of individuals, some behaviour is less gene specific than others. This indirect genetic control has been referred to by sociobiologists as epigenetic. The point at which epigenetic control merges with purely cultural behaviour, which is not genetically controlled, is fuzzy. Where this boundary lies is the nature versus nurture debate. It is a concern about the degree to which free will exists. Specifically, it is to what extent are morals, the way we think we should behave, determined by intellect. If there is a problem here it is to some extent contrived. Hume, the philosopher, pointed out that our moral sense revolves round how we use 'is' and 'ought'. Many of our cultural beliefs are based on what we think human behaviour ought to be rather than what it really is. Again, Huxley, the biologist, pointed out the paradox that cultural morality is opposed to natural morality or what is now known as epigenetic behaviour. The proponents of nurture, the ought faction, have fudged the issue for what appears to be a very good reason. They stand to gain from the confusion. Convincing people of how they ought to behave can be very self-advantageous. This cultural trick is the basis of the anarchist 'conspiracy theory' of politics.
#Africa#anthropology#England#English politics#epigenetics#field trip#genetics#Kenya#Kenyan politics#Malawi#Malawian politics#Uganda#Ugandan politics#Zimbabwe#Zimbabwean politics#music#Pëtr Kropotkin#poetry#Ruth Finnegan#The Raven#travel#africa#african politics#anarchism#anarchy#anarchist society#geopolitics#resistance#autonomy#revolution
4 notes
·
View notes
Text
Abstract
Therapeutic applications of synthetic mRNA were proposed more than 30 years ago, and are currently the basis of one of the vaccine platforms used at a massive scale as part of the public health strategy to get COVID-19 under control. To date, there are no published studies on the biodistribution, cellular uptake, endosomal escape, translation rates, functional half-life and inactivation kinetics of synthetic mRNA, rates and duration of vaccine-induced antigen expression in different cell types. Furthermore, despite the assumption that there is no possibility of genomic integration of therapeutic synthetic mRNA, only one recent study has examined interactions between vaccine mRNA and the genome of transfected cells, and reported that an endogenous retrotransposon, LINE-1 is unsilenced following mRNA entry to the cell, leading to reverse transcription of full length vaccine mRNA sequences, and nuclear entry. This finding should be a major safety concern, given the possibility of synthetic mRNA-driven epigenetic and genomic modifications arising. We propose that in susceptible individuals, cytosolic clearance of nucleotide modified synthetic (nms-mRNAs) is impeded. Sustained presence of nms-mRNA in the cytoplasm deregulates and activates endogenous transposable elements (TEs), causing some of the mRNA copies to be reverse transcribed. The cytosolic accumulation of the nms-mRNA and the reverse transcribed cDNA molecules activates RNA and DNA sensory pathways. Their concurrent activation initiates a synchronized innate response against non-self nucleic acids, prompting type-I interferon and pro-inflammatory cytokine production which, if unregulated, leads to autoinflammatory and autoimmune conditions, while activated TEs increase the risk of insertional mutagenesis of the reverse transcribed molecules, which can disrupt coding regions, enhance the risk of mutations in tumour suppressor genes, and lead to sustained DNA damage. Susceptible individuals would then expectedly have an increased risk of DNA damage, chronic autoinflammation, autoimmunity and cancer. In light of the current mass administration of nms-mRNA vaccines, it is essential and urgent to fully understand the intracellular cascades initiated by cellular uptake of synthetic mRNA and the consequences of these molecular events.
4 notes
·
View notes
Text
Introducing Creative Society: A Glimpse into Hyper Advanced Body Regeneration Biotechnology
Creative Society is a global project that aims to develop a society where the potential and creativity of every individual is paramount. The group has supporters in more than 100 countries and organizes online events to discuss the concept and model of the creative society in all spheres of human life.
Creative Society has unveiled a video that exhibits a prospective sophisticated biotechnology, which would enable individuals to customize their physical traits.
This biotechnology technology would offer users many options and autonomy over their body, such as eliminating fat, enhancing muscle mass, and optimizing bones and ligaments. The video illustrates how the technology could significantly prolong life span and preserve the user’s body in a robust and youthful state. You can view a comprehensive demonstration of this technology by following the link provided below.
youtube
Creative Society vision is to develop a holistic care system that would enable users worldwide to personalize their preferences and access them from anywhere in the world. Additionally, the technology could abolish diseases, without any detrimental impacts while also significantly extending lifespan.
One of the most promising applications of biotechnology in general is on the subject of anti-aging, which aims to stop or reverse the aging process by targeting its underlying molecular mechanisms. Aging is associated with various epigenetic changes that alter gene expression and cellular function over time. By using techniques such as partial reprogramming with Yamanaka factors, researchers hope to reset the epigenetic clock and rejuvenate cells and tissues . Other approaches include using CRISPR gene editing to correct mutations that accumulate with age, or using senolytics to eliminate senescent cells that contribute to inflammation and tissue damage. These anti-aging therapies could potentially and significantly extend human lifespan.
The following five scientific studies exemplify the feasibility and actuality of reversing human aging through various interventions.
(1) Biological age of humans reversed by years in groundbreaking study .... https://www.independent.co.uk/news/science/biological-clock-ageing-turn-back-reverse-study-new-a9094261.html. (2) The ‘Benjamin Button’ effect: Scientists can reverse aging in ... - CNN. https://www.cnn.com/2022/06/02/health/reverse-aging-life-itself-scn-wellness/index.html. (3) Scientist Discovers Aging Clock to Speed and Reverse Aging | Time. https://time.com/6246864/reverse-aging-scientists-discover-milestone/. (4) Anti-Ageing Research: Ageing in Human Cells Reversed 30 Years in New .... https://www.bloomberg.com/news/articles/2022-04-07/researchers-reverse-ageing-in-human-cells-by-30-years-study. (5) Reverse Aging: Study Finds Hyperbaric Oxygen Chamber Slows Aging. https://www.popularmechanics.com/science/health/a34730692/study-reverse-aging-in-humans/.
Furthermore there is abundant scientific evidence that validates the idea that biotechnology can facilitate fat reduction, muscle augmentation, and bone and ligament reinforcement. However, these interventions are not as efficacious or convenient as the ones Creative Society has envisioned for the future. You can access the links to some of the research papers that substantiate this idea below:
1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6279907/
2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4016236/
3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2804956/
4. https://www.mdpi.com/2304-8158/12/6/1218
If you're keen on delving deeper into Creative Society and their mission to bring about a better future, you can visit their official website at https://creativesociety.com/, where you can also participate in their online events and contribute your own ideas. By joining forces, we can use biotechnology and creativity to mold the future according to our aspirations.
I trust that you found this article insightful and gained fresh knowledge about the vast potential of biotechnology. To acquire further information about the potential of biotechnology, I recommend watching the video that presents Creative Society's vision for future biotechnology at
youtube
Article by Michael Wichkoski
#regenerationcapsule #creativesociety #biotechnology #michaelwichkoski
2 notes
·
View notes
Text
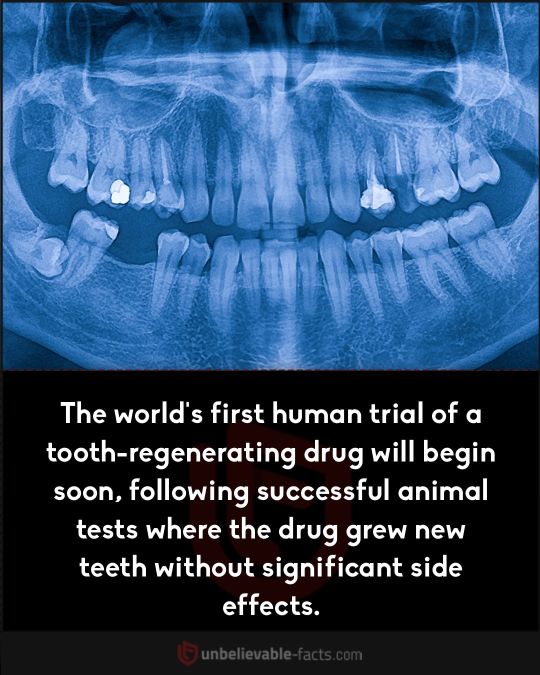
i was obsessed with the molecular bio / epigenetics approach to stopping/reversing aging for a bit and unfortunately it likely will not be a thing in any of our lifetimes due to just how much goes into it.
between telomeres and removing stress induced epigenetic stuff and a whole host of other factors, science just doesn’t move THAT quick to make reversing aging a possibility in the next 7-10 decades.
that dental regeneration stuff looks hype though i’m very excited to see that come to fruition since it’s far more feasible with current stem cell tech (assuming legal/ethical barriers don’t hold it back)
95K notes
·
View notes
Text
I’ve heard the phrase repeated so many times: “mRNA can’t change your DNA.” And yes, in a strict sense, that’s technically true. RNA doesn't just reverse-engineer itself into your genetic code like some viruses can. But if you stop there, you miss something much more subtle and potentially far more important.
Let me take you on a quick journey into the fascinating and slightly alarming world of epigenetics, where mRNA doesn’t rewrite the genetic blueprint, but it may well change which pages get read.Upgrade to paid
A new paper published on March 25th, titled “Persistent Epigenetic Memory of SARS-CoV-2 mRNA Vaccination in Monocyte-Derived Macrophages,” caught my attention. On the surface, it’s being celebrated. Researchers are saying, “Look, this is how mRNA vaccines train your immune system.” But as I read deeper, alarm bells started ringing.
They weren’t just talking about antibodies or T cells. This study revealed something else: the mRNA vaccine alters how certain immune cells—specifically macrophages—access and activate parts of the DNA. Not by changing the genetic sequence, but by unlocking sections of it and leaving them propped open for months.
4 notes
·
View notes
Text
The Ultimate Guide to Gary Brecka 10x Success

In today’s fast-paced world, the pursuit of peak performance—mentally, physically, and professionally—is more important than ever. At the forefront of this movement is Gary Brecka, a man whose name has become synonymous with personal transformation, optimized health, and biological potential. With decades of experience and a revolutionary approach grounded in genetics, Gary Brecka Biologist is not just helping people improve their lives—he’s completely redefining what’s possible.
Welcome to the ultimate guide to Gary Brecka 10x success—a journey that could help you unlock your true potential from the inside out.
Who is Gary Brecka?
Before diving into his 10x system, it’s important to understand the man behind the movement. Gary Brecka is a human biologist with over 20 years of clinical experience in analyzing blood work and DNA to forecast longevity and improve quality of life. He began his career working with life insurance companies, analyzing the data of the sick and the dying. But that work left him unfulfilled—he wasn’t saving lives, just measuring how long people had left.
Determined to change that, Gary Brecka Biologist transitioned to using his expertise to help people take control of their own health destinies. His philosophy? Your body is already telling you everything you need to know—you just need the right tools to listen.
What is Gary Brecka 10x?
At the heart of his methodology lies Gary Brecka 10x, a proprietary health optimization system rooted in functional medicine, epigenetics, and real-time biometric analysis. The “10x” concept is simple: improve every facet of your life—energy, focus, fitness, mood, longevity—tenfold by understanding and hacking your own biology.
Rather than offering generic health advice, Gary Brecka 10x is hyper-personalized. Using DNA testing, blood markers, and lifestyle audits, Brecka and his team create customized wellness protocols designed to optimize the body at the cellular level. Think of it as a blueprint for your best possible self.
The Science Behind the Method
What sets Gary Brecka Biologist apart from others in the health and wellness industry is his scientific rigor. He’s not just promoting a trendy supplement or crash diet; he’s diving deep into the genetic and biochemical makeup of each client.
For example, Gary Brecka focuses on something known as methylation—a biological process that affects everything from mood regulation to detoxification and even DNA repair. Many people have genetic mutations (such as MTHFR) that impair methylation. Without addressing this, even the best health routines can fall flat.
That’s why Gary Brecka 10x includes personalized supplementation based on genetic deficiencies, custom nutrition plans, and guided lifestyle changes. The results? Often dramatic, as detailed in numerous Gary Brecka Reviews from everyday individuals and celebrities alike.
Real Results: What Do the Gary Brecka Reviews Say?
If you're wondering whether this is all hype, the growing library of Gary Brecka Reviews says otherwise. From professional athletes to high-level executives and people simply looking to regain their energy, the testimonials are overwhelmingly positive.
One user reported shedding 30 pounds in four months and reversing chronic fatigue that plagued her for years. Another shared how a tailored protocol allowed them to ditch their antidepressants after discovering key nutrient imbalances in their DNA.
These Gary Brecka Reviews are more than anecdotal—they’re case studies in how understanding your genetic and biological fingerprint can radically change your health and life. It's this track record of tangible, reproducible success that’s turned Gary Brecka Biologist into a trusted name in personal optimization.
The Partnership with Gary Brecka Cardone Venture
The reach and impact of Gary Brecka 10x wouldn’t be what it is today without the strategic partnership with Gary Brecka Cardone Venture. When Gary teamed up with Grant Cardone—real estate mogul and 10x business guru—their combined vision of health and performance took off.
Gary Brecka Cardone Venture bridges science with entrepreneurial spirit. Through this collaboration, the 10x health methodology has been made accessible to a wider audience—from business owners seeking peak mental performance to entire corporate teams looking for a productivity edge.
This venture also paved the way for a multi-platform education system, including masterclasses, live events, and digital programs that integrate both business and biological success strategies.
The 10x Protocol: What to Expect
So what exactly happens when you sign up for Gary Brecka 10x? While the program is customized, the core process generally includes:
1. DNA and Blood Testing
Everything starts with data. Gary Brecka Biologist uses cutting-edge tests to assess your genetic makeup, vitamin levels, hormone balance, and more.
2. Detailed Analysis
Next, a team of experts interprets the results, identifying nutrient deficiencies, inflammation markers, and potential risks.
3. Personalized Protocol
You’ll receive a comprehensive wellness plan. This can include:
Customized supplements
Targeted diet strategies
Sleep and recovery optimization
Biohacking tools (like red light therapy, grounding, or breathwork)
4. Ongoing Support
Many Gary Brecka Reviews highlight the ongoing coaching as a key benefit. You’re not left alone to figure things out—you're guided every step of the way.
Why Now is the Time to 10x Your Health
More than ever, people are searching for sustainable, personalized ways to enhance their well-being. With rising chronic illness rates, skyrocketing stress, and decreasing vitality, the cookie-cutter solutions of the past simply don’t work.
That’s where Gary Brecka Biologist shines. His science-backed approach doesn’t just put a Band-Aid on your symptoms—it goes upstream to fix the root cause. Whether you’re looking to boost your energy, sharpen your focus, or simply feel like yourself again, Gary Brecka 10x offers a proven roadmap.
And thanks to the power of Gary Brecka Cardone Venture, that roadmap is now more accessible and scalable than ever before.
Is Gary Brecka 10x Right for You?
If you’ve tried every diet, supplement, or fitness trend and still feel stuck, it may be time to take a different approach. The magic of Gary Brecka lies in his ability to personalize—there are no one-size-fits-all plans here.
Still not sure? Here are some questions to ask yourself:
Do you often feel fatigued despite “doing everything right”?
Are you taking supplements but not seeing results?
Do you want to prevent disease rather than treat it?
Are you ready to invest in understanding your body?
If you answered yes to any of these, it may be time to explore what Gary Brecka Biologist can do for you.
Final Thoughts: Your Health is Your Edge
In the world of entrepreneurship, business, and even family life, your greatest asset isn’t your money, your time, or even your skills—it’s your health. When your body and mind are firing on all cylinders, everything else becomes easier.
The beauty of Gary Brecka 10x is that it doesn’t just aim to optimize your body—it aims to 10x your life. With the science of Gary Brecka Biologist, the real-life transformations in Gary Brecka Reviews, and the scalable success platform of Gary Brecka Cardone Venture, this is more than a health program. It’s a movement toward human excellence.
0 notes
Text
Longevity & Anti-Aging Breakthroughs

The Latest Research on Longevity and Anti-Aging
Longevity & Anti-Aging Breakthroughs. The pursuit of a longer, healthier life has fascinated humanity for centuries. In recent years, advances in science and medicine have brought us closer than ever to unlocking the secrets of longevity. From cellular therapies to cutting-edge genetic research, here are some of the most promising breakthroughs in the field of longevity and anti-aging. 1. Senolytics: Clearing Out Aged Cells Senolytics are drugs designed to target and eliminate senescent cells—damaged cells that no longer divide and contribute to aging and chronic inflammation. Longevity & Anti-Aging Breakthroughs. By clearing these cells, researchers have observed improved tissue function and extended lifespans in animal models. Human trials are underway, showing potential for treating age-related diseases like osteoarthritis and Alzheimer's. 2. NAD+ Restoration Nicotinamide adenine dinucleotide (NAD+) is a molecule vital for cellular energy and DNA repair, but it declines with age. Supplements such as NMN (nicotinamide mononucleotide) and NR (nicotinamide riboside) are being studied for their ability to boost NAD+ levels. Early research suggests they may improve mitochondrial function, slow aging processes, and enhance cognitive performance. 3. Epigenetic Reprogramming Epigenetic changes—how genes are expressed without altering the DNA sequence—play a crucial role in aging. Scientists are exploring partial cellular reprogramming to "reset" cells to a younger state. This approach has shown promise in animal studies, rejuvenating tissues and even reversing signs of aging in cells. 4. Caloric Restriction Mimetics Caloric restriction has long been associated with extended lifespan. Now, researchers are developing caloric restriction mimetics—compounds that mimic the benefits of fasting without requiring a reduced food intake. Drugs like rapamycin and metformin are under investigation for their longevity-enhancing effects, with some showing potential to delay age-related decline. 5. Gut Microbiome Optimization The gut microbiome has emerged as a critical factor in aging. An imbalance in gut bacteria can contribute to inflammation, metabolic disorders, and weakened immunity. New therapies aim to optimize the microbiome through probiotics, prebiotics, and even fecal microbiota transplants to promote healthy aging. 6. AI and Personalized Longevity Plans Artificial intelligence is revolutionizing how we approach aging. By analyzing genetic, biometric, and lifestyle data, AI-driven platforms can create personalized health and longevity plans. These tools help predict disease risks, recommend interventions, and monitor progress in real time, offering a tailored approach to anti-aging. 7. Gene Editing and CRISPR CRISPR and other gene-editing tools are being used to target genetic factors involved in aging and age-related diseases. Although still in early stages, gene therapy holds promise for correcting mutations that cause premature aging syndromes and improving longevity by repairing damaged DNA.
The Road Ahead
Longevity & Anti-Aging Breakthroughs. While many of these breakthroughs are still in experimental stages, the future of longevity science is auspicious. As research continues to accelerate, the goal of living longer and living better, free from age-related diseases, may become a reality for future generations. In short, Aging is no longer seen as inevitable, but as a process that can be studied, slowed, and potentially reversed. Read the full article
#AgingResearch#AIinHealthcare#Anti-Aging#Artificialintelligence#Biotechnology#FutureofMedicine#GeneTherapy#GutHealth#Health#Health&Wellness#LifeExtension#Longevity#MedicalInnovation#NAD+Boosters#PersonalizedMedicine#Science#Senolytics
0 notes
Text
The Future of Anti-Ageing and Longevity: Breakthroughs in Healthspan Extension
The anti-ageing and longevity industry is evolving rapidly, with groundbreaking research pointing to ways we can not only live longer but better. New advances in gene therapy, stem cell treatments, and epigenetic reprogramming are paving the way for revolutionary interventions that could one day halt or even reverse aspects of ageing.
Among the most promising discoveries are senolytics—compounds that selectively eliminate old, dysfunctional cells—and personalized medicine based on DNA analysis. These innovations aim to reduce age-related diseases, improve cellular function, and boost immune resilience. While still in development, they represent the cutting edge of longevity science.
Lifestyle still plays a major role. Integrating biohacking tools like red light therapy, cold plunges, and wearable tech helps monitor and optimize ageing markers in real time. The future of anti-ageing and longevity lies in combining these technologies with traditional wellness practices to create a smarter, healthier, and longer life.
0 notes
Text
How does epigenetics influence gene expression?
How Does Epigenetics Influence Gene Expression?
Since you’re here, we’re gonna skip the textbook language and get straight into the good stuff — the kind that makes sense, sticks in your brain, and maybe even makes you say, “Wait… that’s kinda cool.” — First: What Is Epigenetics? Okay, so you know you’ve got genes, right? They’re like tiny instruction manuals made of DNA that tell your body how to build everything — eye color, how tall you are, whether your hair gets curly in humidity — all that. But here’s the wild twist: just having a gene doesn’t mean it’s “turned on” or active. Some genes stay silent, like they’re muted. Others are fully blasting, telling your body to grow, react, or even heal. Now here’s where epigenetics jumps in. 🧬 Epigenetics is like the system that decides which genes get turned on or off — without changing your actual DNA. So your DNA is the playlist. Epigenetics is the DJ deciding which songs to play and which ones to skip. — So… How Does It Do That? Great question. Here’s the lowdown: Epigenetics controls gene expression using little chemical “tags” or switches. These are like post-it notes your body sticks on your DNA to say: - “Hey, use this gene!” - “Nah, ignore this one.” - “Only use this if things get stressful.” Two of the most common ways this happens: - DNA Methylation: This is when a tiny molecule (called a methyl group) attaches to DNA and blocks a gene from being used. Think of it like a piece of tape over the play button. - Histone Modification: DNA is wrapped around proteins called histones. If these histones are relaxed, the gene is open and ready to be read. If they’re squeezed tight, the gene is hidden and can’t be used. These changes don’t mess with the gene itself — they just change how easy it is for your body to read and use that gene. —


What Triggers These Changes? This is the part that’ll blow your mind: Things like your environment, diet, stress levels, sleep, exercise, exposure to chemicals — even your childhood experiences — can all affect your epigenetics. Yep. What you do in life can literally tell your genes how to behave. 😲 Example: - If you’re constantly stressed, your body might switch on genes related to inflammation or anxiety. - If you eat well, your body might switch on genes that help with cell repair and energy use. And it’s not just about you. Some epigenetic changes can be passed down to your kids — meaning your lifestyle today might affect your future children. (No pressure!) — Real-Life Stuff That Makes It Easier to Understand 🍼 Identical twins: They have the exact same DNA, but over time they can look different or have different health issues. Why? Because their epigenetics change based on their life choices and environments. 🧪 Cancer research: Some cancers are caused by genes being wrongly switched on or off. Scientists are studying epigenetics to reverse that — like turning a “bad” gene off. 💪 Muscle memory: Epigenetics might help explain why your body “remembers” workouts and gets stronger faster the next time around. — Why It Matters in 2025 In today’s world, we’re not just treating diseases — we’re learning how to prevent them by understanding what triggers them at the gene level. Epigenetics is helping scientists personalize medicine, design better diets, and even figure out how trauma affects generations. It’s like science finally admitted: genes aren’t your destiny — they’re just the script. You, your life, your choices? You’re the editor. — 💡 TL;DR — In the Simplest Words: - Your genes are blueprints. - Epigenetics decides which blueprints to use. - It turns genes on/off using chemical tags. - Environment, lifestyle, and even emotions can influence those tags. - It affects how your body reacts, grows, heals — and even what you pass on to your kids. — 📌 Disclaimer: This easy version is meant to help you understand the concept better. If your exam or teacher expects a textbook explanation and you write this one instead, we’re not responsible if it affects your marks. Use this for understanding, not copy-pasting. — 🔗 Related Articles from EdgyThoughts.com: What If Your Memories Could Change Your DNA 2025 https://edgythoughts.com/what-if-your-memories-could-change-your-dna-2025 What If Emotions Leave Physical Marks on Your Genes 2025 https://edgythoughts.com/what-if-emotions-leave-physical-marks-on-your-genes-2025 🌐 External Resource: Want the full breakdown with scientific terms? Check out the Wikipedia page: https://en.wikipedia.org/wiki/Epigenetics — Read the full article
#effectsofepigeneticsonfuturegenerations#environmentandgeneexpression#epigeneticchangesduetostress#epigeneticseasyguide#epigeneticsexplainedsimply2025#epigeneticsforhighschoolstudents#epigeneticsvsgenetics2025#geneexpressionandlifestyle#geneswitchesexplainedsimply#genesturnedonandoff#histonemodificationbasics#howepigeneticsaffectshealth#howepigeneticsworksforstudents#howlifestyleinfluencesdna#howyourhabitsaffectyourgenes#isgeneexpressionreversible#personalizedmedicineandepigenetics#reallifeexamplesofepigenetics#whatisdnamethylation2025
0 notes
Text
Global Epigenetics Market to Grow at a CAGR of 16.1% Driven by Advances in Personalized Medicine and Rising Disease Prevalence
Market Overview
The global epigenetics market is projected to grow at a compound annual growth rate (CAGR) of 16.1% during the forecast period. The global epigenetics market is expanding at a remarkable rate. Advancements in research, coupled with the increasing prevalence of diseases like cancer, neurological disorders, and cardiovascular conditions, have heightened interest in epigenetic therapies and diagnostics.
Key Growth Drivers
Rising Prevalence of Chronic Diseases: Conditions such as cancer, diabetes, and cardiovascular diseases are closely linked to epigenetic alterations. The need for effective treatments based on epigenetic mechanisms is increasing as these diseases continue to rise.
Advances in Epigenetic Research: Breakthroughs in gene editing tools, such as CRISPR, and the growing understanding of DNA methylation, histone modification, and non-coding RNA have unlocked new possibilities in epigenetic-based therapies.
Personalized Medicine: Epigenetics plays a critical role in personalized medicine, allowing for more targeted treatments based on a person’s genetic makeup and the expression of their genes. This trend is pushing the demand for epigenetic products and services.
Government Funding and Research Initiatives: The growing allocation of government funds toward epigenetic research and public health initiatives has significantly accelerated innovation and the commercialization of epigenetic therapies.
Growing Awareness of Preventive Healthcare: Epigenetic testing for early detection of predispositions to certain diseases is becoming more mainstream. This trend is contributing to the demand for epigenetic diagnostics in both clinical and research settings.
Major Applications of Epigenetics
Epigenetics is poised to impact several industries, particularly healthcare and agriculture. Here’s a closer look at some key areas:
Oncology: Epigenetics is crucial for understanding how cancer develops and progresses. Epigenetic therapies, including DNA methylation and histone modification inhibitors, are emerging as powerful treatments for various cancers.
Neurological Disorders: Epigenetic changes are increasingly being linked to conditions like Alzheimer’s, Parkinson’s, and Huntington’s disease. Researchers are exploring how epigenetic drugs could reverse or slow down the progression of these diseases.
Cardiovascular Diseases: Epigenetics is helping researchers understand how lifestyle factors and environmental influences contribute to heart disease, paving the way for preventive treatments.
Agriculture and Animal Breeding: In agriculture, epigenetic modifications are being explored to enhance crop yield, pest resistance, and disease tolerance. Similarly, epigenetics is used in animal breeding to improve traits such as growth rate, fertility, and disease resistance.
Segment Insights
Epigenetic Drugs and Therapies: This segment includes the development of drugs targeting epigenetic changes, such as inhibitors of DNA methyltransferase and histone deacetylase inhibitors, which are used in the treatment of cancers and other conditions.
Epigenetic Diagnostics: Epigenetic testing technologies are used to detect biomarkers for disease risk, cancer diagnostics, and determining patient responses to treatments. Companies are developing assays that can identify specific epigenetic changes tied to disease progression.
Research and Services: This includes contract research organizations (CROs) and laboratories offering services in epigenetic research, including genome-wide studies, biomarker discovery, and clinical trials.
Competitive Landscape
The epigenetics market is competitive, with a mix of established pharmaceutical companies, biotechnology firms, and research institutions. Key players in the market include Illumina, Thermo Fisher Scientific, Agilent Technologies, EpiCypher, and Zymo Research Corporation. These companies are leading the way with innovative epigenetic products, diagnostic tools, and research services, while also forming strategic partnerships to drive growth in the emerging epigenetic therapeutics space.
Regional Insights
North America is the largest market for epigenetics, driven by robust healthcare infrastructure, extensive research funding, and the presence of leading biotech companies. The United States, in particular, has been a hub for epigenetic research and clinical applications.
Europe follows closely, with countries like Germany and the UK investing heavily in genetic research and epigenetic therapies.
Asia-Pacific is expected to grow rapidly, particularly in China and India, due to the increasing adoption of advanced technologies, expanding healthcare infrastructure, and rising interest in genetic research and precision medicine.
Challenges Facing the Epigenetics Market
Despite its promising growth, the epigenetics industry faces several challenges:
High Costs: The cost of developing epigenetic-based therapies and diagnostics can be prohibitively expensive, limiting their accessibility, especially in emerging markets.
Regulatory Hurdles: Epigenetic therapies are relatively new, and navigating the regulatory landscape for approval can be complex and time-consuming.
Ethical Considerations: The potential for epigenetic modifications to alter human DNA raises ethical concerns, particularly regarding germline editing and its long-term effects.
Future Outlook
The epigenetics market is poised for significant expansion, driven by breakthroughs in genomics, rising disease prevalence, and the growing demand for personalized medicine. The increasing focus on the role of the environment and lifestyle in gene expression is expected to fuel demand for epigenetic therapies, diagnostics, and research services in the coming years.
Conclusion
The epigenetics industry's future looks promising, with innovations and trends likely to shape its landscape significantly. Companies that adapt to these changes and leverage new technologies will be best positioned to capitalize on the expanding market. For a more in-depth analysis and future predictions, consider exploring the full market report by Mordor Intelligence.
For a detailed overview and more insights, you can refer to the full market research report by Mordor Intelligence
0 notes