#carbonyl structures
Explore tagged Tumblr posts
Photo


#organic chemistry#ochem#o-chem#o chem#ochem 1#ochem 2#organic chemistry 1#organic chemistry 2#mcat organic chemistry#orgo#reactions#orgo reactions#chemical reactions#orgo mcat#mcat orgo#ochem reactions#carbonyl group#carbonyl#carbonyls#carbonyl group structures#carbonyl structures
14 notes
·
View notes
Note
what would you considdr to be cursed science?
Fundamental (non-structure determination) NMR and EPR spectroscopy, most forms of microwave spectroscopy, anything involving quadruple+ bonds, most toxicology (naturally), deep-sea oceanography, energetics research, volcanic geology, anything involving nickel carbonyl, obviously all plasma physics, any study of lyssaviruses (including the rabies virus) or bats in general, anything involving mercury, meteorology. Statistical mechanics.
35 notes
·
View notes
Photo

Degradable polyethylene plastics from the nonalternating terpolymerization of ethylene, CO, and polar monomers
In a study published in the journal National Science Review and led by Dr. Zhongbao Jian (State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, CAS), E/CO/PM terpolymerizations were carried out with seven palladium catalysts, which were strictly non-alternating (>99%) with Pd4.
Polar monomers included acrylates, acrylic acid, vinyl ethers, vinyl acetate and acrylonitrile. High molecular weight linear polyethylene with the low content of isolated carbonyl group (selectivity > 99%) and polar functional group was developed.
The molecular structure of the resulting polymer was analyzed in detail, and the microstructure of the E/CO/PM copolymer was clearly characterized by IR, nuclear magnetic resonance spectroscopy (1H/13C/2-D NMR) and 13CO labeling technology.
Read more.
#Materials Science#Science#Polyethylene#Catalysts#Palladium#Polymers#Plastics#Materials Characterization
14 notes
·
View notes
Text
Aldehydes and ketones are very reactive due to the polarity and structure of the carbonyl group (figure 21.3).

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#aldehyde#ketone#reactive#carbonyl#polarity#electron density
3 notes
·
View notes
Text
Lasso peptides are a really cool family of peptides that have a very unique knotted structure and are chronically understudied and poorly understood. Lasso peptides get their name from the way the N-terminus wraps back around the peptide chain and forms an isopeptide bond with the carbonyl of a Glu/Asp residue, forming a literal lasso like shape:

This is a deceptively tricky reaction to study, let alone replicate. At the minute, most studies done on identifying and isolating these molecules use overexpression, chemical or even biosynthetic strategies are inaccessible, which is a real shame because the unusual topologies of these peptides gives them excellent protease resistance and much better pharmacokinetics than linear, or even standard cyclic peptides.
More detailed images of the peptides reveal some interesting quirks of how they adopt this unique shape:

This stick model on the left show the whole lasso peptide, with the section forming the ring highlighted in green. The sequence that is ‘lasso’d’ so to speak is quite interesting, and is shown on the right. The C terminal tryptophan-phenylalanine motif is common among lasso peptides and acts as a ‘stopper’ and provides a, frankly massive, steric barrier to unfolding the lasso. The encapsulated section is comprised of glycine which is pretty easy to rationalise because glycine doesn’t have a side chain, thus making it the most suitable for threading through a ring. I’ve also highlighted the proline, which is also common among lasso peptides, it is though that the proline is important to preorganising the lasso before the ring is completed, proline is a much more restricted amino acid than the others and often adopts this role as a conformational guide.
Its worth remembering just how tight this whole arrangement is, threading a peptide though a 7 residue ring is no small feat, and here’s the space filling model to highlight that fact:

There is no wiggle room at all, and that is key to understanding why these things are so hard to make, they incur a huge entropic and steric penalties for being in these tightly wound conformations and aren’t offsetting that by forming covalent bonds or massive protein scale stabilising interactions. As such I think they’re pretty remarkable and definitely worth studying more.
References:
1. Tan, S., Moore, G., and Nodwell, J. (2019) Put a bow on it: Knotted antibiotics take center stage. Antibiotics 8, 117.
2. Liu, T., Ma, X., Yu, J., Yang, W., Wang, G., Wang, Z., Ge, Y., Song, J., Han, H., Zhang, W., Yang, D., Liu, X., and Ma, M. (2021) Rational generation of lasso peptides based on biosynthetic gene mutations and site-selective chemical modifications. Chemical Science 12, 12353–12364.
3. Structure, bioactivity, and resistance mechanism of streptomonomicin, an unusual lasso peptide from an understudied halophilic actinomycete. Chemistry & Biology. Cell Press.
4 notes
·
View notes
Text
Exploring the Global Aldehydes Market: Key Players and Market Dynamics
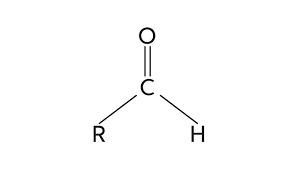
The aldehydes market is a segment of the chemical industry that deals with the production and distribution of a class of organic compounds known as aldehydes. These compounds are characterized by the presence of a carbonyl group (C=O) bonded to a hydrogen atom and a carbon atom in their chemical structure. Aldehydes find widespread applications in various industries, thanks to their unique properties and versatile reactivity.
In terms of market overview, the aldehydes market has been experiencing steady growth in recent years. This growth can be attributed to the increasing demand for aldehydes in industries such as pharmaceuticals, agriculture, food and beverages, and cosmetics. Aldehydes serve as crucial intermediates in the synthesis of various chemicals and are essential in the production of fragrances, flavor enhancers, and pharmaceuticals.
The growth in the aldehydes market industry can be primarily attributed to the expansion of these end-user industries. For instance, the pharmaceutical industry relies heavily on aldehydes for the synthesis of a wide range of drugs and active pharmaceutical ingredients (APIs). Additionally, the food and beverage industry utilizes aldehydes for flavor enhancement and preservation purposes, further driving market growth.
The aldehydes market is also influenced by evolving industry trends. One significant trend is the increasing emphasis on green chemistry and sustainable practices. Many companies in the aldehydes sector are adopting environmentally friendly production processes, such as catalytic hydrogenation, to reduce the environmental impact of their operations. This trend aligns with the growing awareness of environmental issues and the need for more eco-friendly chemical manufacturing.
Another noteworthy trend is the constant innovation and development of novel aldehyde derivatives with enhanced properties. This innovation is driven by the demand for higher-quality products in various industries. Researchers and manufacturers are continuously exploring new applications and synthesizing aldehydes tailored to meet specific industry requirements, which contributes to market expansion.
In conclusion, the aldehydes market is a dynamic segment within the chemical industry, driven by the increasing demand from various end-user industries. As industries continue to grow and evolve, the market is expected to witness further advancements, particularly in sustainable production methods and novel aldehyde derivatives, to meet the changing needs of consumers and businesses alike.
2 notes
·
View notes
Text
𝐄𝐮𝐫𝐨𝐩𝐞 𝐂𝐲𝐜𝐥𝐨𝐡𝐞𝐱𝐚𝐧𝐨𝐧𝐞 𝐌𝐚𝐫𝐤𝐞𝐭: 𝐊𝐞𝐲 𝐈𝐧𝐬𝐢𝐠𝐡𝐭𝐬 & 𝐓𝐫𝐞𝐧𝐝𝐬-IndustryARC™
The Europe Cyclohexanone Market size is forecasted to reach US$1.2 billion by 2027, after growing at a CAGR of 4.5% during the forecast period 2022-2027.
𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐑𝐞𝐩𝐨𝐫𝐭 𝐒𝐚𝐦𝐩𝐥𝐞
Cyclohexanone (C₆H₁₁O) is a key industrial solvent and chemical intermediate with a wide range of applications in various industries. It is classified as a ketone due to its molecular structure, which consists of a six-carbon chain with a carbonyl group (C=O) attached to one of the carbons. Cyclohexanone is highly valued for its ability to dissolve a variety of substances and its role in the synthesis of key chemicals.
𝐊𝐞𝐲 𝐓𝐫𝐞𝐧𝐝𝐬
𝐑𝐢𝐬𝐢𝐧𝐠 𝐃𝐞𝐦𝐚𝐧𝐝 𝐢𝐧 𝐍𝐲𝐥𝐨𝐧 𝐏𝐫𝐨𝐝𝐮𝐜𝐭𝐢𝐨𝐧:
The Europe Cyclohexanone market is experiencing sustained growth, driven primarily by the increasing demand for nylon and its derivatives. As industries like automotive and textiles expand, the need for nylon-6 and nylon-6,6 is pushing up cyclohexanone consumption, especially in countries like Germany and France.
𝐒𝐮𝐬𝐭𝐚𝐢𝐧𝐚𝐛𝐢𝐥𝐢𝐭𝐲 𝐚𝐧𝐝 𝐆𝐫𝐞𝐞𝐧 𝐂𝐡𝐞𝐦𝐢𝐬𝐭𝐫𝐲:
Environmental concerns are pushing manufacturers to adopt more sustainable practices. Bio-based cyclohexanone production is gaining traction as an eco-friendly alternative to traditional petroleum-based methods.
𝐈𝐧𝐜𝐫𝐞𝐚𝐬𝐞𝐝 𝐀𝐩𝐩𝐥𝐢𝐜𝐚𝐭𝐢𝐨𝐧 𝐢𝐧 𝐂𝐨𝐚𝐭𝐢𝐧𝐠𝐬 𝐚𝐧𝐝 𝐀𝐝𝐡𝐞𝐬𝐢𝐯𝐞𝐬:
Cyclohexanone's role as a solvent in paints, coatings, and adhesives is expanding due to its excellent ability to dissolve a variety of resins. With the growth of the construction and automotive industries, demand for high-performance coatings and adhesives continues to rise.
𝐒𝐡𝐢��𝐭 𝐓𝐨𝐰𝐚𝐫𝐝𝐬 𝐀𝐮𝐭𝐨𝐦𝐚𝐭𝐢𝐨𝐧 𝐚𝐧𝐝 𝐀𝐝𝐯𝐚𝐧𝐜𝐞𝐝 𝐌𝐚𝐧𝐮𝐟𝐚𝐜𝐭𝐮𝐫𝐢𝐧𝐠:
The Europe Cyclohexanone market is seeing a shift toward advanced manufacturing technologies and automation. This trend is improving production efficiency, reducing costs, and meeting the growing demand for high-quality cyclohexanone in various industrial sectors.
𝐄𝐱𝐩𝐚𝐧𝐝𝐢𝐧𝐠 𝐀𝐩𝐩𝐥𝐢𝐜𝐚𝐭𝐢𝐨𝐧𝐬 𝐢𝐧 𝐏𝐡𝐚𝐫𝐦𝐚𝐜𝐞𝐮𝐭𝐢𝐜𝐚𝐥𝐬 𝐚𝐧𝐝 𝐂𝐨𝐬𝐦𝐞𝐭𝐢𝐜𝐬:
Cyclohexanone's use in pharmaceuticals and cosmetics is increasing as demand for active ingredients and specialty fragrances grows. The compound is being utilized more in the formulation of certain pharmaceuticals and cosmetic products, which is opening new markets within the chemical industry.
0 notes
Text
Effective Strategies for JEE Chemistry Preparation

Chemistry is one of the most important subjects for your Joint Entrance Examination (JEE), significantly impacting your overall score. However, it is usually considered a subject where you can score high marks using the correct strategies. Contrary to Physics or Mathematics, Chemistry requires a mix of memory-based and conceptual learning, so developing a thorough plan for efficient preparation is essential.
Joining the best coaching for JEE in Bangalore can also provide structured guidance, helping you grasp both the theoretical and practical aspects of Chemistry. We'll discuss ways to assist you in mastering JEE Chemistry, focusing on solid foundations, using the correct tools, and maximizing your study time.
1. Know the JEE Chemistry Syllabus
Before you begin your preparation, familiarise yourself with the JEE Chemistry syllabus. Inorganic chemistry is broken down into three parts: Physical Chemistry, Organic Chemistry, and chemistry. Each of them needs a different approach.
Physical Chemistry: The subject involves problem-solving and numerical calculations. Concepts such as thermodynamics, chemical kinetics, and electrochemistry are the most important topics. Learning formulas and solving problems is crucial to mastering this part.
Organic Chemistry: Explores reactions and the way molecules function. The topics covered include hydrocarbons, carbonyl compounds, and alcohols, which require profound conceptual understanding. Regularly practising reactions is essential to being successful.
Chemistry inorganic chemistry is primarily a process of learning concepts like Periodic Table trends, Coordination Compounds, and bonding. Revisions that are systematic and brief notes are highly beneficial in keeping this knowledge.
If you can comprehend what you are studying, you can concentrate on more critical areas on the test.
2. Create study plans that include milestones.
A well-organized study schedule is vital to understanding the extensive JEE Chemistry syllabus. Separate your study time into monthly and weekly targets, and make sure you allocate enough time for all three areas of Chemistry. The topics you choose to focus on are based on their importance for the JEE test.
For instance, Physical Chemistry topics often require more time to practice math problems, whereas Inorganic Chemistry may involve quick review sessions focusing on learning the most important concepts. A balanced timetable between these two areas will ensure that every chemistry aspect is left unattended.
It's crucial to include frequent revisions in your schedule to keep topics you've studied previously fresh in your thoughts. Revising notes, solving problems, and revisiting complex issues will improve your knowledge and retention.
3. Focus on Conceptual Clarity
The importance of establishing solid foundations in chemistry cannot be overemphasised. Although physical chemistry relies on a solid knowledge of concepts and formulas, organic and inorganic chemistry requires detailed comprehension of the structure and reactions.
To master physical chemistry, spend ample time learning the fundamentals behind formulas. For example, knowing the fundamentals of thermodynamics instead of simply remembering equations will assist you in solving complicated numerical issues more efficiently. Finding the most complex problems feasible is crucial to sharpening your abilities.
For organic chemistry, focus on studying reaction mechanisms, not simply learning about reactions. Once you know what causes and how specific reactions happen, it becomes much easier to predict the behaviour of the compounds. Drawing the mechanisms and experimenting with responses will help in retention.
Regular memorisation using flashcards or notes may help you remember the latest trends and concepts when studying inorganic chemistry. Making a habit of reviewing the notes daily can improve memory retention over the long term.
If you're searching for professional guidance for your JEE preparation, the JEE, the most effective tutoring in Bangalore, is the best coaching JEE located in Bangalore, providing a systematic strategy to help students understand complex Chemistry concepts and simplify the learning process.
4. Use the Right Study Materials
The study material you choose can significantly impact the quality of your JEE Chemistry preparation. Inorganic NCERT textbooks form the basis for studying JEE Chemistry, mainly inorganic chemistry. Ensure you read NCERT carefully because it covers nearly all essential subjects in a simple structure.
Alongside NCERT, the reference books are beneficial for deeper understanding and practice. The recommended books that are suitable for JEE Chemistry are:
Physical Chemistry: "Physical Chemistry" by P.W. Atkins or O.P. Tandon
Organic Chemistry: "Organic Chemistry" by Morrison and Boyd or "Organic Chemistry" by O.P. Tandon
Inorganic Chemistry: "Concise Inorganic Chemistry" by J.D. Lee or O.P. Tandon
The books offer complete explanations, practice exercises and questions for advanced levels that will prepare you to take JEE Main and Advanced.
For those who don't have access to classes in offline format, Online JEE coaching in Bengaluru is a great resource. These platforms offer structured videos, live courses, and doubt-clearing sessions, allowing you to learn chemistry at a higher level from home.
5. Daily Practice and Mock Tests with Regular Practice
Tests on mocks are essential for JEE Chemistry preparation. They do not just aid in time management but will also familiarise you with the test pattern and the questions you will encounter in the exam. Try to complete the test at least once every week.
Mock tests are a great way to test your skills and pinpoint weak areas. After every test, review the answers in depth to discover and correct your mistakes. Whether it's solving mathematical questions within Physical Chemistry or revising reaction mechanisms in Organic Chemistry, constant analysis is crucial.
Many coaching institutions and online platforms offer mock tests that simulate the JEE examination conditions, allowing you to test your skills under the pressure of real-time. This helps you build confidence to take the actual test without anxiety.
6. Time Management is Key
Time management plays an essential part in JEE preparation. Because chemistry requires theoretical learning and problem-solving, regulating time during the test is vital. Try to solve chemistry problems within a specific time limit to increase ability and precision.
While you study, allocate the time to various chemistry sections depending on your strengths and weaknesses. For example, if you discover organic chemistry is more challenging, take more time rewriting reactions and tackling issues. If physical Chemistry numerical problems take too long, focus on increasing your problem-solving speed.
A well-planned time management system ensures you can cover all areas adequately and permits you to revise before the test.
7. Be Consistent With Revision
Consistent revision is essential to mastering chemistry. Make a revision plan that lets you review complex subjects regularly. In the case of inorganic chemicals, discuss flashcards or notes concisely to refresh chemical reactions and periodic trends. Reviewing reaction mechanisms periodically regarding organic chemistry ensures you don't lose these over time.
Regular revision and practice are crucial when the date for the exam is near. When you are ready, working through the previous year's question papers will help you better understand the exam's format and the type of questions you might be facing.
The best JEE coaching in Karnataka will also offer you a systematic revision plan and frequent mock tests to ensure that you remain on course and can effectively revise during the last months before the test.
Conclusion
Preparing to take JEE Chemistry requires a strategic method that balances conceptual knowledge, practice, and constant revision with these methods to maximise the chances of scoring well at JEE Chemistry. From knowing the syllabus and developing a study plan, using the appropriate tools, and managing your time efficiently, each stage plays a vital part in the preparation.
Through self-study or joining a coaching institute, remaining committed and following a disciplined strategy will help you succeed in taking the JEE Chemistry exam.
0 notes
Text
Chem Practical Viva Question
Give one example each for acidic, basic, and neutral organic compounds. - Acidic: Phenol - Basic: Aniline - Neutral: Benzaldehyde
What do you mean by 5% HCl solution? - 5ml HCl + 95ml distilled water
Which type of compounds are water soluble? - Polar compounds and organic compounds with low molecular weights.
Which type of compounds are ether soluble? - Non-polar compounds
A compound soluble in 5% HCl solution is ___ in nature. - Basic
A compound soluble in 5% NaOH and 5% NaHCO3 can be a ---. - Carboxylic acid
A compound which is soluble in 5% NaOH, but not 5% NaHCO3 can be a ___. - Phenol
A compound which does not dissolve in 5% HCl, 5% NaOH, and 5% NaHCO3 is a ___ compound. - Neutral
The compound which chars in concentrated H2SO4 is a ___. - Carbohydrate
Ether is a ___ solvent. - Non-polar.
----------------------------------------------------
A compound in which -OH group is directly attached to a benzene ring - Phenol
Phenols are weakly ___ in nature - Acidic
Phenols are soluble in 5% ___ - NaOH
The characteristic identification test for phenols is ___ - Neutral FeCL3 Test
The product formed by the reaction of phenol with phthalimide is _ - Phenolphthalein
In the preparation of azo-dye, the reaction involved during the addition of phenol to benzene diazonium chloride is ___ - Coupling
The -OH group in phenol acts as an ___ directing group - o,p-
The brominating agent used in the preparation of bromo derivative is ___ - Br2/Acetic Acid
The product of bromo derivative from phenol is ___. - 2,4,6-Tribromophenol.
Structure of β-naphthol:

---------------------------------------
Amines are ___ in nature - Basic
Amines are soluble in 5% ___ solution - HCl
Among amines, azo-dye test is given by ___ amines. - Primary aromatic
The two reactions involved in the azo-dye test are ___ and ___ - Diazotization and Coupling
The product formed when aniline undergoes acetylation is ___ - Acetanilide
The acetylating agen used in the preparation of acetyl derivative is ___. - Acetic anhydride.
-------------------------------------
What are carbonyl compounds? - Compounds which have a carbonyl group.
Carbonyl compounds are ___ in nature - Neutral
The reagent used in the identification of carbonyl compounds is - 2,4-DNP
Expand 2,4-DNP. - 2,4-Dinitrophenyl hydrazine
Chemical name of Tollen's reagent is ___ - Ammonical silver nitrate.
Fehling's A is composed of ___ - CuSO4
Fehling's B is composed of ___ - Sodium potassium tartarate
Schiff's reagent is ___ - P-Rosaniline hydrochloride
Silver mirror is formed by ___ - Aldehyde
The red color precipitate formed during Fehling's test is ___ - Cuprous oxide (Cu2O)
Give an example of aliphatic aldehyde - Acetaldehyde
Give an example of aromatic aldehyde - Benzaldehyde
Acetophenone is an ___ - Aromatic ketone
Acetone is an example of ___ - Aliphatic ketone
---------------------------------------------------
What are carbohydrates? - Polyhydroxy aldehydes and ketones.
What are reducing sugars? - Sugars which reduce Tollen's reagent and Fehling's solution.
Give an example of an aldohexose - glucose
Carbohydrates undergo ___ in the presence of sulphuric acid. - Charring
Glucose is soluble in water because ___ - of the hydrogen bonds that form between the water molecules and the OH groups.
Why are carbohydrates coluble in 5% HCl, 5% NaOH, and 5% NaHCO3 solutions? - Because these solutions contain 95% water.
Molisch's reagent is composed of ___ - 10% α-naphthol solution
Glucose does not restore the pink color of Schiff's reagent. - True
Fructose is a ketohexose, but still it can reduce Fehling's solution and Tollen's reagent. - True
The reagent used in osazone formation is ___ - Phenyl hydrozene
-------------------------------------------
What is acetylation? - The replacement of hydrogen with an acetyl group
Give two examples of acetylating agents - Acetic anhydride and acetyl chloride.
Which compounds can be acetylates? - The compounds having an active hydrogen atom such as alcohols, phenols, and amines (when a hydrogen atom is attacked to more electonegative atoms like oxygen or nitrogen.)
What is the use of acetylation in this reaction? - Acetylation is done to protect the amino groups, when aniline has to be treated with strong acids.
---------------------------------------------------------------------
Define oxidation - The addition of oxygen, the removal of hydrogen, and or the loss of electrons.
What is the starting material used in this preparation? - Benzaldehyde.
Mention two oxidizing agents? - KMnO4 and K2Cr2O7.
What is the role of a water condenser? - It condenses the vapors and prevents the wastage of starting materials.
-----------------------------------------------------------------
Define esterification - The reaction between a carboxylic acid and an alcohol in the presence of Conc. H2SO4 to give ester is called esterification.
What is the role of sulphuric acid in this reaction? - Catalyst and dehydrating agent.
How is a liquid separated from the liquid reaction mixture? - By using a separation funnel.
Why does the ester form the upper later during separation? - Due to its lower density.
0 notes
Text
Formation and Non Formation of Nitrosamine
Nitrosamine
Nitrosamine or more specifically n-nitrosamine refers to a molecule containing the nitroso functional group (R-N=O). Any compound containing the secondary amine functional group is expected to react with nitrosating agents to produce nitrosamine. These molecules are of concern because nitrosamine impurities are probable human carcinogens.
Formation of Nitrosamines
• Presence of Secondary Amines: Nitrosamines are formed when secondary amines, which contain a lone pair on the nitrogen atom, react with nitrosating agents. The lone pair on the nitrogen is crucial for the reaction, as it allows the formation of the nitroso functional group (R-N=O).
• Concentration and Reactivity: The likelihood and extent of nitrosamine formation depend on several factors, including the concentration of the secondary amine and the nitrosating agent present. Additionally, the structural characteristics of the amine can influence the reaction rate, determining how readily nitrosamines are formed.
• Environmental Conditions: Certain environmental conditions, such as pH level and temperature, can also influence the formation of nitrosamines. These conditions can affect the reactivity of the amines and the nitrosating agents, potentially facilitating or hindering the formation process.
Reaction


Why Nitrosamines Do Not Form on Amide Groups?
Electron-Withdrawing Properties: Amides contain a carbonyl group (C=O) attached to the nitrogen. This carbonyl group has electron-withdrawing properties, which reduce the reactivity of the nitrogen's lone pair. Essentially, the electron density around the nitrogen is decreased, making it less likely to engage in reactions with nitrosating agents.
Lower Reactivity: Due to the electron-withdrawing effect of the carbonyl group, amides exhibit significantly lower reactivity towards common nitrosating agents compared to secondary amines. This diminished reactivity is why nitrosamine impurities are not typically formed from amide groups.

Why nitrosamines do not form on Imidazole ?
Imidazole is a five-membered aromatic molecule containing two annular nitrogen atoms. Both nitrogen atom of imidazole are part of the aromatic ring the nitrogen NH group is secondary amine, but it is non-basic in nature as the lone pair of electrons is part of the aromatic structure.
For a non-basic nitrogen it is difficult or not possible to form nitrosamine, and if it does, it cannot have the same carcinogenic mechanism as other typical nitrosamines.
Imidazole's unique structure, especially the involvement of its nitrogen's lone pair in the aromatic system, makes it an exception in terms of basicity and reactivity compared to more conventional secondary amines. These differences significantly reduce the likelihood of nitrosamine formation and suggest a divergent potential mechanism for carcinogenicity, setting imidazole and its derivatives apart from the typical concerns associated with nitrosamines.
#N-Nitroso Aceclofenac#N-Nitroso Atenolol#N-Nitroso Atomoxetine#N-Nitroso Benazepril#N-Nitroso Betahistine#N-Nitroso Bisoprolol#N-Nitroso Brinzolamide#N-Nitroso Bupropion#N-Nitroso Ciprofloxacin#N-Nitroso Dabigatran Etexilate#N-Nitroso Desloratadine#N-Nitroso Diclofenac#N-Nitroso Elagolix#N-Nitroso Enalapril#N-Nitroso Safinamide#N-Nitroso Prilocaine#N-Nitroso Vonoprazan#N-Nitroso Silodosin#N-Nitroso Duloxetine#N-Nitroso Folic acid#N-Nitroso Propranolol#N-Nitroso Paroxetine#N-Nitroso Perindopril#N-Nitroso Vortioxetine#N-Nitroso Meglumine#N-Nitroso Nortriptyline#N-Nitroso-Rasagiline
0 notes
Text
42399-49-5
The crystal structures of racemic and (2S,3R)-(-)-3-hydroxy-2-(4-methoxyphenyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)- one (C16H15NO3S) have been determined in order to compare the interactions between molecules of the same and opposite chirality. The enantiomeric associations observed in these two crystal structures are analysed, relating the differences to those found in the equivalent diastereomers, (2R, 3R) and/or (2S, 3S). Single-crystal X-ray diffraction data were collected at low temperature with Cu Kα radiation (λ = 1.54184 A). Optically active: monoclinic, space group C2, with a = 24.726(3), b = 5.2426(5), c =12.0726(12) A, β - 112.979(9)°, V = 1440.8(5) A3, Z = 4, Dx= 1.389 g cm-3, μ = 20.35 cm-1, the refinement on 2918 observed reflections gave R=0.0271. Racemic: monoclinic, space group P21/n, with a = 13.308(3), b = 4.8474(8), c = 22.130(4) A, β = 91.782(14), V= 1426.9(5) A3, Z=4, Dx= 1.403 g cm-3, μ= 20.54 cm-1, refined to R = 0.0318 for 2753 observed reflections. An intramolecular hydrogen bond between the hydroxy and carbonyl groups appears to stabilize the benzothiazepinone ring in the (P,2S,3R) boat conformation with the hydroxy and methoxyphenyl substituents in equatorial positions. In both crystal structures two N-H...O hydrogen bonds connect the molecules into dimers. In the optically active compound the two molecules are related by a twofold axis, in the racemate by an inversion centre. The racemate contains an additional hydrogen bond which is reflected by its higher melting enthalpy compared with the optically active compound. The difference in the chiral discrimination in the solutions of the cis-and trans-diastereomers does not appear to have its origin in the strong (O-H...O, N-H...O) hydrogen bonds, but rather in the weak (C-H...O) interactions. Acta Chemica Scandinavica 1996. https://www.lookchem.com/CASDataBase_42399-49-5.htm
0 notes
Text
Carbonyl Iron Prices | Pricing | Trend | News | Database | Chart | Forecast
Carbonyl iron is a highly pure form of iron produced through the carbonyl process, which involves the decomposition of iron pentacarbonyl. This material is widely used in industries such as electronics, pharmaceuticals, and metallurgy, driving demand for it in various applications. Due to its specialized production process and high-quality standards, the price of carbonyl iron can be subject to fluctuations based on several factors, including raw material costs, production capacity, market demand, and global economic conditions.
One of the primary factors influencing the price of carbonyl iron is the cost of raw materials. Iron ore, being the main raw material for producing carbonyl iron, plays a crucial role in determining the overall cost structure. When the price of iron ore rises, the cost of producing carbonyl iron increases, subsequently leading to higher market prices. Additionally, the availability of high-quality iron ore can impact the price, as shortages or limitations in supply can result in higher production costs and reduced availability of carbonyl iron. This supply chain dependency creates volatility in carbonyl iron prices, particularly during periods of heightened geopolitical tension or trade restrictions.
Get Real Time Prices for Carbonyl Iron: https://www.chemanalyst.com/Pricing-data/carbonyl-iron-1588
Market demand is another critical driver of carbonyl iron prices. Industries such as electronics, pharmaceuticals, and powder metallurgy rely heavily on carbonyl iron due to its unique properties, including high purity and controlled particle size distribution. As these industries grow, the demand for carbonyl iron increases, often leading to upward pressure on prices. For instance, the electronics industry uses carbonyl iron in magnetic cores, inductors, and transformers, while the pharmaceutical sector employs it as a source of iron in supplements. Any surge in production within these sectors typically translates to an increase in the price of carbonyl iron due to heightened demand. Conversely, when there is a slowdown in industrial activities or reduced demand in end-use industries, prices may stabilize or decline.
Production capacity also plays a significant role in determining carbonyl iron prices. As carbonyl iron is produced through a specialized process that requires advanced technology and expertise, production facilities are often limited to specific regions or manufacturers. When production capacities are limited, and demand remains strong, prices are likely to increase. On the other hand, if manufacturers expand their production capabilities or new entrants join the market, prices could experience downward pressure due to increased supply. The balance between supply and demand is thus a key determinant of price trends in the carbonyl iron market.
Another aspect affecting carbonyl iron prices is the energy cost associated with its production. The carbonyl process is energy-intensive, requiring significant amounts of heat and energy for the decomposition of iron pentacarbonyl. As global energy prices fluctuate, so too do the costs of producing carbonyl iron. Rising energy costs, whether due to geopolitical issues, disruptions in the supply of energy sources, or shifts in environmental regulations, can contribute to higher carbonyl iron prices. Manufacturers may pass these increased costs onto consumers, leading to price hikes in the final product. Alternatively, a decline in energy costs may help to lower production expenses, potentially stabilizing or reducing carbonyl iron prices.
Global economic conditions also play a pivotal role in shaping the price trends of carbonyl iron. During periods of economic growth and expansion, industrial activities tend to increase, driving higher demand for materials such as carbonyl iron. This, in turn, can push prices upward as industries require more of the material for their processes. In contrast, during economic downturns, demand for carbonyl iron may decrease as industries scale back production or delay projects, leading to lower prices. Economic factors such as inflation, currency fluctuations, and changes in trade policies can further influence carbonyl iron prices on a global scale.
Additionally, technological advancements and innovations in manufacturing processes can impact the pricing dynamics of carbonyl iron. New production techniques that enhance efficiency or reduce waste may help lower production costs, resulting in more competitive pricing in the market. Conversely, technological challenges or limitations in the production process can contribute to higher costs, especially if manufacturers need to invest in upgrading equipment or meeting stricter quality standards.
Environmental regulations and sustainability initiatives are becoming increasingly important in industries that produce carbonyl iron. As governments and organizations push for cleaner production methods and reduced emissions, manufacturers may face additional compliance costs, which can be reflected in the final price of carbonyl iron. For example, the carbonyl process involves the use of carbon monoxide, a hazardous gas, which must be carefully managed to minimize environmental impact. Stricter environmental regulations or carbon pricing mechanisms could lead to higher production costs, thereby affecting carbonyl iron prices. On the flip side, innovations that reduce the environmental footprint of carbonyl iron production could help lower costs and improve price competitiveness.
In the global market, competition among producers can also influence carbonyl iron prices. When multiple manufacturers are vying for market share, prices may become more competitive as suppliers seek to attract buyers through lower pricing strategies. This competitive dynamic can result in price fluctuations, especially in regions where there are several producers of carbonyl iron. However, in markets with fewer suppliers or where there is a high degree of consolidation among manufacturers, prices may remain more stable or experience upward pressure due to the limited competition.
In conclusion, carbonyl iron prices are shaped by a complex interplay of factors, including raw material costs, market demand, production capacity, energy prices, global economic conditions, technological advancements, environmental regulations, and market competition. Each of these elements can exert varying degrees of influence on price trends, making the carbonyl iron market highly dynamic and sensitive to both external and internal forces. Understanding these factors is crucial for industries that rely on carbonyl iron, as they navigate the fluctuations in pricing to manage costs and maintain competitiveness in their respective markets.
Get Real Time Prices for Carbonyl Iron: https://www.chemanalyst.com/Pricing-data/carbonyl-iron-1588
Contact Us:
ChemAnalyst
GmbH - S-01, 2.floor, Subbelrather Straße,
15a Cologne, 50823, Germany
Call: +49-221-6505-8833
Email: [email protected]
Website: https://www.chemanalyst.com
#Carbonyl Iron#Carbonyl Iron Prices#Carbonyl Iron Price#Carbonyl Iron News#Carbonyl Iron Monitor#Carbonyl Iron Supply and Demand
0 notes
Text
MIT chemists explain why dinosaur collagen may have survived for millions of years
New Post has been published on https://sunalei.org/news/mit-chemists-explain-why-dinosaur-collagen-may-have-survived-for-millions-of-years/
MIT chemists explain why dinosaur collagen may have survived for millions of years

Collagen, a protein found in bones and connective tissue, has been found in dinosaur fossils as old as 195 million years. That far exceeds the normal half-life of the peptide bonds that hold proteins together, which is about 500 years.
A new study from MIT offers an explanation for how collagen can survive for so much longer than expected. The research team found that a special atomic-level interaction defends collagen from attack by water molecules. This barricade prevents water from breaking the peptide bonds through a process called hydrolysis.
“We provide evidence that that interaction prevents water from attacking the peptide bonds and cleaving them. That just flies in the face of what happens with a normal peptide bond, which has a half-life of only 500 years,” says Ron Raines, the Firmenich Professor of Chemistry at MIT.
Raines is the senior author of the new study, which appears today in ACS Central Science. MIT postdoc Jinyi Yang PhD ’24 is the lead author of the paper. MIT postdoc Volga Kojasoy and graduate student Gerard Porter are also authors of the study.
Water-resistant
Collagen is the most abundant protein in animals, and it is found in not only bones but also skin, muscles, and ligaments. It’s made from long strands of protein that intertwine to form a tough triple helix.
“Collagen is the scaffold that holds us together,” Raines says. “What makes the collagen protein so stable, and such a good choice for this scaffold, is that unlike most proteins, it’s fibrous.”
In the past decade, paleobiologists have found evidence of collagen preserved in dinosaur fossils, including an 80-million-year-old Tyrannosaurus rex fossil, and a sauropodomorph fossil that is nearly 200 million years old.
Over the past 25 years, Raines’ lab has been studying collagen and how its structure enables its function. In the new study, they revealed why the peptide bonds that hold collagen together are so resistant to being broken down by water.
Peptide bonds are formed between a carbon atom from one amino acid and a nitrogen atom of the adjacent amino acid. The carbon atom also forms a double bond with an oxygen atom, forming a molecular structure called a carbonyl group. This carbonyl oxygen has a pair of electrons that don’t form bonds with any other atoms. Those electrons, the researchers found, can be shared with the carbonyl group of a neighboring peptide bond.
Because this pair of electrons is being inserted into those peptide bonds, water molecules can’t also get into the structure to disrupt the bond.
To demonstrate this, Raines and his colleagues created two interconverting mimics of collagen — the one that usually forms a triple helix, which is known as trans, and another in which the angles of the peptide bonds are rotated into a different form, known as cis. They found that the trans form of collagen did not allow water to attack and hydrolyze the bond. In the cis form, water got in and the bonds were broken.
“A peptide bond is either cis or trans, and we can change the cis to trans ratio. By doing that, we can mimic the natural state of collagen or create an unprotected peptide bond. And we saw that when it was unprotected, it was not long for the world,” Raines says.
“This work builds on a long-term effort in the Raines Group to classify the role of a long-overlooked fundamental interaction in protein structure,” says Paramjit Arora, a professor of chemistry at New York University, who was not involved in the research. “The paper directly addresses the remarkable finding of intact collagen in the ribs of a 195-million-old dinosaur fossil, and shows that overlap of filled and empty orbitals controls the conformational and hydrolytic stability of collagen.”
“No weak link”
This sharing of electrons has also been seen in protein structures known as alpha helices, which are found in many proteins. These helices may also be protected from water, but the helices are always connected by protein sequences that are more exposed, which are still susceptible to hydrolysis.
“Collagen is all triple helices, from one end to the other,” Raines says. “There’s no weak link, and that’s why I think it has survived.”
Previously, some scientists have suggested other explanations for why collagen might be preserved for millions of years, including the possibility that the bones were so dehydrated that no water could reach the peptide bonds.
“I can’t discount the contributions from other factors, but 200 million years is a long time, and I think you need something at the molecular level, at the atomic level in order to explain it,” Raines says.
The research was funded by the National Institutes of Health and the National Science Foundation.
0 notes
Text
MIT chemists explain why dinosaur collagen may have survived for millions of years
New Post has been published on https://thedigitalinsider.com/mit-chemists-explain-why-dinosaur-collagen-may-have-survived-for-millions-of-years/
MIT chemists explain why dinosaur collagen may have survived for millions of years


Collagen, a protein found in bones and connective tissue, has been found in dinosaur fossils as old as 195 million years. That far exceeds the normal half-life of the peptide bonds that hold proteins together, which is about 500 years.
A new study from MIT offers an explanation for how collagen can survive for so much longer than expected. The research team found that a special atomic-level interaction defends collagen from attack by water molecules. This barricade prevents water from breaking the peptide bonds through a process called hydrolysis.
“We provide evidence that that interaction prevents water from attacking the peptide bonds and cleaving them. That just flies in the face of what happens with a normal peptide bond, which has a half-life of only 500 years,” says Ron Raines, the Firmenich Professor of Chemistry at MIT.
Raines is the senior author of the new study, which appears today in ACS Central Science. MIT postdoc Jinyi Yang PhD ’24 is the lead author of the paper. MIT postdoc Volga Kojasoy and graduate student Gerard Porter are also authors of the study.
Water-resistant
Collagen is the most abundant protein in animals, and it is found in not only bones but also skin, muscles, and ligaments. It’s made from long strands of protein that intertwine to form a tough triple helix.
“Collagen is the scaffold that holds us together,” Raines says. “What makes the collagen protein so stable, and such a good choice for this scaffold, is that unlike most proteins, it’s fibrous.”
In the past decade, paleobiologists have found evidence of collagen preserved in dinosaur fossils, including an 80-million-year-old Tyrannosaurus rex fossil, and a sauropodomorph fossil that is nearly 200 million years old.
Over the past 25 years, Raines’ lab has been studying collagen and how its structure enables its function. In the new study, they revealed why the peptide bonds that hold collagen together are so resistant to being broken down by water.
Peptide bonds are formed between a carbon atom from one amino acid and a nitrogen atom of the adjacent amino acid. The carbon atom also forms a double bond with an oxygen atom, forming a molecular structure called a carbonyl group. This carbonyl oxygen has a pair of electrons that don’t form bonds with any other atoms. Those electrons, the researchers found, can be shared with the carbonyl group of a neighboring peptide bond.
Because this pair of electrons is being inserted into those peptide bonds, water molecules can’t also get into the structure to disrupt the bond.
To demonstrate this, Raines and his colleagues created two interconverting mimics of collagen — the one that usually forms a triple helix, which is known as trans, and another in which the angles of the peptide bonds are rotated into a different form, known as cis. They found that the trans form of collagen did not allow water to attack and hydrolyze the bond. In the cis form, water got in and the bonds were broken.
“A peptide bond is either cis or trans, and we can change the cis to trans ratio. By doing that, we can mimic the natural state of collagen or create an unprotected peptide bond. And we saw that when it was unprotected, it was not long for the world,” Raines says.
“This work builds on a long-term effort in the Raines Group to classify the role of a long-overlooked fundamental interaction in protein structure,” says Paramjit Arora, a professor of chemistry at New York University, who was not involved in the research. “The paper directly addresses the remarkable finding of intact collagen in the ribs of a 195-million-old dinosaur fossil, and shows that overlap of filled and empty orbitals controls the conformational and hydrolytic stability of collagen.”
“No weak link”
This sharing of electrons has also been seen in protein structures known as alpha helices, which are found in many proteins. These helices may also be protected from water, but the helices are always connected by protein sequences that are more exposed, which are still susceptible to hydrolysis.
“Collagen is all triple helices, from one end to the other,” Raines says. “There’s no weak link, and that’s why I think it has survived.”
Previously, some scientists have suggested other explanations for why collagen might be preserved for millions of years, including the possibility that the bones were so dehydrated that no water could reach the peptide bonds.
“I can’t discount the contributions from other factors, but 200 million years is a long time, and I think you need something at the molecular level, at the atomic level in order to explain it,” Raines says.
The research was funded by the National Institutes of Health and the National Science Foundation.
#Animals#atom#atomic#atoms#author#bones#carbon#change#chemistry#dinosaur#double#electrons#Evolution#explanation#form#Forms#fossil#Fossils#Foundation#Fundamental#Health#how#interaction#it#life#ligaments#Link#mit#molecules#muscles
0 notes
Text
OTBCH: A Deep Dive into Its Properties and Applications
In fragrance chemistry, certain compounds are renowned for their ability to transform scents into captivating olfactory experiences. One such compound is OTBCH (4-tert-Butylcyclohexyl acetate), a versatile chemical widely used in the fragrance industry. With its unique odor profile, distinct chemical structure, and varied applications, OTBCH is a key player in perfumery and other industries. This article explores the characteristics of OTBCH, its odor profile, applications, and how Eternis, a leading supplier, plays a vital role in providing high-quality OTBCH.
Understanding the Chemical Structure of OTBCH
OTBCH, chemically known as 4-tert-Butylcyclohexyl acetate, belongs to the family of cyclohexyl esters. Its molecular structure features a cyclohexane ring (a six-carbon ring) substituted with a tert-butyl group (a branched four-carbon chain) and an acetate group (a carbonyl group bonded to an oxygen atom, followed by a methyl group). This unique structure gives OTBCH its distinct properties, including stability and solubility in various solvents.
The cyclohexane ring provides a robust backbone, ensuring structural integrity, while the tert-butyl group enhances hydrophobicity, making OTBCH compatible with a variety of organic solvents. The acetate group contributes a subtle sweetness, further enhancing its utility in fragrance compositions.
The Odor Profile of OTBCH
OTBCH is highly valued for its distinctive odor profile, characterized by a soft, woody, and slightly sweet aroma, reminiscent of sandalwood and freshly cut timber. This elegant and comforting scent blends well with other fragrance components, making it suitable for a wide range of olfactory compositions. The mild sweetness imparted by the acetate group adds complexity, making OTBCH a versatile ingredient in creating both simple and sophisticated fragrances.
In perfumery, OTBCH is typically used as a base note due to its moderate volatility and excellent tenacity. Its ability to linger on the skin or fabric makes it an effective fixative, extending the life of fragrances and ensuring the scent evolves gradually. With a low odor threshold, OTBCH imparts its aroma even at low concentrations, making it a cost-effective ingredient for perfumers.
Applications of OTBCH in Fragrance and Beyond
The versatility of OTBCH extends beyond its use in perfumes. Its woody and sweet scent makes it ideal for personal care products such as lotions, soaps, and shampoos. Its stability and compatibility with other fragrance ingredients allow it to blend seamlessly into complex formulations, providing depth and richness.
OTBCH is also used in household items like air fresheners, candles, and cleaning products, where its warm, woody aroma enhances the ambiance of living spaces. Its mildness and non-reactive nature make it suitable for products intended for sensitive skin, broadening its application in the beauty and personal care industry.
Beyond its direct use in fragrance products, OTBCH is valuable in flavor and fragrance chemistry research. Its distinct odor profile and chemical properties make it a standard reference compound for studies exploring the interactions between scent molecules and their impact on overall fragrance composition.
Eternis: Expertise in Supplying High-Quality OTBCH
Eternis, a globally recognized leader in specialty chemicals, is known for supplying high-quality OTBCH to the fragrance industry. Their commitment to excellence, innovation, and sustainability sets them apart as a trusted supplier. Eternis utilizes advanced manufacturing techniques to produce OTBCH that meets the rigorous quality standards required by perfumers and cosmetic manufacturers.
The production processes at Eternis are meticulously designed to ensure consistency and purity in every batch of OTBCH. From sourcing raw materials to the final product, each step is carefully monitored to maintain the desired olfactory characteristics and chemical stability. This quality assurance ensures that perfumers can rely on Eternis’s OTBCH to deliver the exact aroma profile needed for their creations.
Eternis is also committed to sustainable practices, making them a responsible choice for companies seeking to minimize their environmental footprint. Their eco-friendly manufacturing processes reduce waste and energy consumption, aligning with the growing demand for sustainable and ethically produced fragrance ingredients. This commitment to sustainability, coupled with their expertise in chemical production, positions Eternis as a leader in the fragrance industry.
Conclusion
OTBCH (4-tert-Butylcyclohexyl acetate) is a versatile and essential ingredient in the world of fragrance, known for its unique odor profile and wide range of applications. Its soft, woody, and sweet scent makes it valuable in perfumes, personal care products, and household items, enhancing the sensory experience. With its low odor threshold and excellent fixative properties, OTBCH remains a staple in creating long-lasting and complex fragrances.
Eternis’s expertise in producing high-quality OTBCH ensures that perfumers and manufacturers have access to a reliable and consistent supply of this important compound. Their commitment to quality, innovation, and sustainability makes them a preferred partner in the fragrance industry, providing the foundation for crafting elegant and enduring scents. As the demand for unique and captivating fragrances grows, OTBCH continues to be a key ingredient, offering warmth and sophistication to everyday life.
0 notes
Text
Must-Study Chapters for JEE: A Comprehensive Guide

Mechanics: The bedrock of physics, covering kinematics, laws of motion, work, energy, power, rotational motion, and gravitation.
Electrodynamics: A conceptually rich section encompassing electrostatics, current electricity, magnetism, and electromagnetic induction.
Modern Physics: Relatively easier with topics like atomic structure, nuclear physics, dual nature of matter, and semiconductors.
Optics: Crucial for engineering applications, covering ray and wave optics.
Thermodynamics and Kinetic Theory: Bridges physics and chemistry, covering laws of thermodynamics, Carnot engine, entropy, and heat transfer.
Chemistry
Physical Chemistry: Numerically oriented, focusing on mole concept, thermodynamics, kinetics, electrochemistry, ionic equilibrium, and solutions.
Inorganic Chemistry: Factual and memory-based, covering periodic table, chemical bonding, coordination compounds, p-block, d-block, and f-block elements.
Organic Chemistry: Concept-heavy, emphasizing general organic chemistry, hydrocarbons, alcohols, phenols, ethers, carbonyl compounds, and amines.
Mathematics
Calculus: The foundation for higher mathematics, covering limits, continuity, differentiability, applications of derivatives, definite and indefinite integrals, and differential equations.
Algebra: Core of JEE math, encompassing quadratic equations, sequences and series, matrices and determinants, complex numbers, and probability.
Coordinate Geometry: Often considered easy, covering straight lines, circles, parabolas, ellipses, and hyperbolas.
Trigonometry: Versatile and applicable across topics, covering trigonometric ratios, identities, heights and distances, and inverse functions.
Vectors and 3D Geometry: Can be challenging but highly scoring with practice, covering vector algebra and three-dimensional geometry.
Tips for Effective Preparation
Understand Concepts: Don't just memorize formulas; grasp the underlying principles.
Practice Regularly: Solve previous year papers and mock tests consistently.
Focus on Weak Areas: Identify and strengthen your weak points.
Revise Consistently: Regular revision is crucial for retention.
Stay Updated: Keep track of exam pattern changes and syllabus modifications.
0 notes