#azide chemistry
Explore tagged Tumblr posts
Note
HI FELLOW CHEMISTRY NERD HERE PLEASE RAMBLE ABOUT AZIDES I LOVE AZIDES
Ok! So I haven’t actually done much with asides since organic chemistry two years ago, BUT while I was refreshing my memory on their properties I found the loveliest of papers…
and I think that sums up azide chemistry very nicely.
Fav paragraph has to go to:
In the first case, the combination of sodium azide and acid affords hydrazoic acid. Hydrazoic acid is both acutely toxic (mouse LD50 = 22 mg/kg) (3) and a powerful explosive; in its neat form, hydrazoic acid is more explosive than TNT and orders of magnitude less stable. (4) The first scientists to isolate hydrazoic acid (Curtius and Radenhausen, in 1891) (5) found that “the blast of 50 mg was sufficient to disintegrate the apparatus to dust” and when a subsequent 700 mg batch “exploded spontaneously”, it seriously injured the coauthor (Radenhausen) and the shock wave from the explosion shattered every glass vessel nearby. There is no safe quantity when dealing with neat hydrazoic acid.
Even if this is diluted to hell, evaporation of hydrazoic acid followed by condensation will make it explosive all over. It does not require oxygen or a spark to explode.
So anyway, azides are fun :)
1 note
·
View note
Text
Oxidation numbers calculated by the rules on p. 491 can have fractional values (for example, the nitrogen atoms in the ionic compound sodium azide, NaN3 (see figure 12.2), have an oxidation number of -⅓).

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#oxidation#calculation#fraction#nitrogen#sodium#sodium azide#air bag
0 notes
Text
The MoFe protein can then reduce the l numerous substrates (Table 13.4), although under natural conditions it reacts only with N2 and H+.

"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#nitrogenase#redox#oxidation#reduction#inorganic chemistry#nitrogen fixation#nitrous oxide#azide#acetylene#hydrogen#atp#molybdenum#iron
0 notes
Text
PJO Meta (1 of ??)
DISCLAIMER: I don't mean to offend anyone. I am a ship and let ship person. Please note that criticism =/= hate. Lastly, if you're a solangelo shipper who doesn't wish to hear any criticism regarding their ship, please scroll away.
Ever since it's first appearance, solangelo has been a massive fan favourite. I, however, don't have a favourable opinion of the ship. Here is a brief explanation of my reasons for the same. (I apologise for my inarticulation)
Characterization:-
Nico-
Honestly Nico's writing in the trials of Apollo was terrible even by the usual character assassination standards- in the hidden oracle, he legitimately read as a gag charater who served no purpose apart from being "Will Solace's Goth/Emo Boyfriend". Things were hardly better in Tower of Nero, where he was basically just "Will Solace's traumatized Goth/Emo Boyfriend" who happens to be lazy PTSD rep.
Will Solace-
Can't say much. He was never a character of his own, and probably never will be. His substance as a character is comparable to the quantitative mass of an electron in Kilograms (9.11x 1/10³¹) i.e, negligible.
Nico's Healing Arc (or lack of the same )-
After what was essentially two series worth of fanfic-esque trauma bombing, Nico should have received a proper healing arc. Unfortunately, there was no such arc for him (courtesy of solangelo), because Rick has this earth-shattering, inspired-by-Disney idea that relationships can fix everything. So guess what? Nico's years' worth of emotional baggage, pain and trauma simply volatalize into oblivion .
Some shippers may argue "Oh but he got better off page"-but that it is bad writing per se; Nico's struggles, grief, fears, unhealthy coping mechanisms etc. are more pivotal and relevant to his character than a nascent, one dimensional relationship with an insignificant side character. He is much more than just his sexuality, after all.
Cliché and one dimensional:-
Solangelo is firmly rooted in the "opposites attract" trope, which is simply unrealistic in nature.
Also, newsflash: "Opposites attract" is a law specific to physics and chemistry; It's all fun and games till people who are poles apart sit down to take serious decisions, or share opinions. Friction between them can be rather intense.
(Oh! wait... I think Nico was described to be a Nitride anion {or was he Azide? No, I think he was Nitride} and Will was a Tungsten cation. So, they are basically Tungsten Nitride... Boy! They have so much chemistry between them/s)
Anyways, I totally understand why people prefer jasico, valdangelo, percico, nicobaster etc...
#nico di angelo#pjo fandom#anti solangelo#anti will solace#percy jackson#jason grace#jasico#percico#pernico#leo valdez#valdangelo#pjo hoo toa#percy jackon and the olympians#the lost hero#heroes of olympus#blood of olympus#the hidden oracle#tower of nero#nicobaster#alabaster torrington#Tagging these ships as their fans are probably the only people who'll understand#the trials of apollo#rr crit#pjo meta
112 notes
·
View notes
Text
I am putting my whole brain through chemistry hell with what little I remember from high school chemistry to understand how nitroglycerin works (thanks to one whole thread that's just meant to be silly and goofy, and also ignoring that the substance he makes is described as "nitroglycerin-like"). And man, when I tell you... Katsuki simply has to be built different to deal with his Quirk.
For one, it's a good thing that the nitroglycerin only produces from his hands. For two, remember that nitroglycerin is only slightly soluble with water - which also checks out with how Horikoshi draws the sweat beads sometimes, because they're not exactly combining with the nitroglycerin he makes.
But that likely means the nitroglycerin Katsuki makes is... Quite literally pure.
(This got long, so the rest is under the read more!)
Basic fundamentals to understand about nitroglycerin: it is both flammable and combustible. Wikipedia (bc this shit is the only thing making it make sense) says that "flammable applies to combustible materials that ignite easily and thus are more dangerous and more highly regulated". This is because of nitroglycerin's extremely reactive nature, prone to explosion via shock, friction, heat, and flame.
It is a class A explosive under OSHA, which is defined as: "possessing, detonating, or otherwise maximum hazard; such as dynamite, nitroglycerin, picric acid, lead azide, fulminate of mercury, black powder, blasting caps, and detonating primers".
Its HMIS diamond scaffold is as follows (based on this):
Health: 2 (can cause temporary incapacitation or residual injury)
Flammability: 3 (can be ignited under almost all ambient temperature conditions)
Instability: 4 (readily capable of detonation or explosive decomposition or explosive reaction at normal temperatures and pressures)
Special: N/A
Solid nitroglycerin (at 13°C or lower) will destabilise and explode if melted too quickly. A bottle of pure liquid nitroglycerin (14°C-50°C) will explode if it's dropped on the ground. It will begin to decompose at 50-60°C and explode at a temperature of 218°C. Its decomposition has an exothermic reaction, thus it can literally ignite itself. Nitroglycerin does not need oxygen to explode, because it has enough oxygen molecules in its chemical formula.
Its chemical formula in question is C3H5N3O9. The products after exploding are 3 CO2 + 2.5 H2O + 1.5 N2 + 0.5O...
A clean equation looks something like:
4 C3H5N3O9 -> 12 CO2 + 10 H20 + 6 N2 + O2.
Heat liberated from a nitroglycerin explosion exceeds temperatures of 5,000°C. the detonation wave from this reaction reaches a velocity of 7,280-7,700 metres per second, and creates the development of 20,000 atmospheres of pressure. Its explosive energy density sits at about 6.23 kJ/g, which is one of the higher power outputs in terms of explosive molecules.
So what does this mean for Bakugou Katsuki?
Well, simply put, he does not need a substantial amount of nitroglycerin on his palms to create detonations. The tiny sparks he creates are arguably miniscule doses of nitroglycerin that he's spontaneously igniting.
The method of ignition is thankfully obvious: he can heat his palms. He can shock the nitroglycerin on his palms with this rapid heat, thus spurring the molecules into the desired reaction.
It also explains how Katsuki needs to store sweat so he may create larger explosions later on - it's likely that he doesn't typically produce enough nitroglycerin from his palms to immediately justify any large-scale detonations that he desires. So, to achieve this, he has to work harder and sweat more to garner enough of the substance for the result he's after.
In the realm of BNHA, it's pretty clear that Katsuki's explosions don't exhibit the exothermal properties of nitroglycerin, or we would be seeing some very devastating burns left behind. While I think that he is ultimately capable of heat-related damage, his explosions overall are more force-based. I think his ultimate moves (Howitzer in particular) utilise the full force of nitroglycerin's reaction... Maybe, sometimes, reduced to varying degrees depending on the person he's fighting (plus the use of support items, skill, etc.).
For instance, compare his standard Howitzer Impact used against Todoroki in the sports festival to the Cluster-boosted Howitzer against Shigaraki in the final war:
While it was an ultimate move used on Todoroki, he was less experienced at the time, likely hindering its effectiveness. (I want to say he dialled it back so it wouldn't, you know, cause irreparable damage... But it's possible that inexperience trumped its overall power, because he wanted to win with everything he had.)
Then, against Shigaraki, it was so much force that it quite literally moved floating U.A. across the sky. He combined his support gear, his experience, and the amount of nitroglycerin stored to achieve this level of force.
As a further note, because of nitroglycerin's extreme reactivity and explosive properties, it cannot really produce a flame of its own. Because Katsuki's explosions seem to be more force-based on top of that, he is not necessarily capable of setting things alight... (Seen in the training camp arc, where he tried and failed to start a fire, instead just exploding the wood.)
As for Katsuki's natural resistances to his own Quirk (of which I have described before, but I want to reiterate):
Larger explosion sound levels can be anywhere between 120 decibels and 210 decibels... And a human's "safe hearing" level is about 85 decibels. In my headcanon, Katsuki has a natural adaptation which means he can withstand higher decibels than others, but that does not make him immune - so while he can tolerate large-scale explosion sounds in an open area, especially with him at the centre point, he would suffer if it were the same explosion in an enclosed area.
The aforementioned heating on his palms are only a part of Katsuki's adaptation to his Quirk; the skin is also significantly thicker on his palms and fingers, making his tolerance for touching hot things at a higher level. Like... Much, much higher. It accommodates for when nitroglycerin requires 218°C to explode in a controlled environment. It's why he can handle hot things out of the oven, and why he doesn't know what burns on his hands feel like.
The musculature on his arms and shoulders also have a natural level of strength to withstand the force of his quirk, but this isn't impervious either. He's had to train hard and build on that natural strength to best withstand the higher outputs of force.
Exposure to pure nitroglycerin for any individual is ill-advised, but with manga logic and creative liberties, I don't think anyone who wants to hold Katsuki's hand should have to worry. (I'm 99% sure that he washes his hands thoroughly after working out, anyway.) As for Katsuki himself, I think he does exhibit some symptoms of a nitroglycerin overdose when he's pushed himself way past his limit... But these episodes are incredibly rare, and often very short-lived.
The final notes:
Constant detonation of nitroglycerin means Katsuki has a bit of a smoky smell. Past that, there is also a faint caramel smell.
Also do not taste the nitroglycerin (for whatever reason there is to lick his hand or something? Which Katsuki will literally kill you for doing that, because what the fuck). It does not taste as nice as it smells.
#💥 ⸍ ii. headcanon.#long post /#/ man that's a lot#/ my hands are starting to get cold af but this covers most of it?#/ i think. main thing is that his explosions are more force > heat
10 notes
·
View notes
Text
i've just leared that my chemistry ia may be fatal or cause permament damage to my genetic material... what a wonderful day to be alive!
tbh i am not going anywhere near sodium azide, 7 in chemistry isn't worth it
#ib#ib diploma#ib diploma programme#ibdp#ibdp student#international baccalaureate#international baccalaureate diploma programme#homework#grades#chemistry#chemistry hl#chemistry ib
2 notes
·
View notes
Text
Back at it again with another funky way to make funky peptides, gotta admit I have a type. The authors didn't provide a scheme but I whipped one up anyways :)
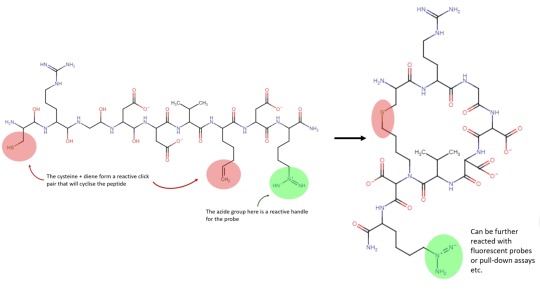
This time the approach is to forgo the traditional cyclisation methods (chemical ligation or disulphide bridging) and instead cyclise the peptide via a click reaction. So, what is a click reaction and why bother? Well click chemistry is the nickname given to a series of biorthogonal (meaning it doesn’t interfere with biological systems) azide-alkyne coupling reactions developed by Bertozzi, Meldal and Sharpless, its kinda a big deal and those folks won the 2022 Nobel prize for their work. There are a few reasons it would be useful to cyclise a peptide with click chemistry, a) its an orthogonal reaction which means that previously inaccessible functional groups could be included in cyclic peptides, b) a lot of ink has been spilled optimising, developing and expanding click chemistry so there’s a massive array of options and flexibility for future development, c) the click reaction produces a far more stable product that disulphide bridging and d) its not confined to the N/C terminus like chemical ligation usually is.
So with the background out of the way, what did these folks actually do? Well the paper is describes a protocol for creating cyclic peptide probes; i.e. a cyclic peptide that will bind to a target and is functionalised with a reactive handle so it can be visualised via a fluorophore. The paper is detailing a quite specific protocol, and what I think likely happened is that these folks were trying to create probes for their other research, but they realised the method they came up with was so effective that they should really publish it as a protocol (this would not be unusual).
But anyways, onto the chemistry. To create cyclic probes the researchers incorporated 2 orthogonal click reactivities into the linear sequence, a thiol-ene reaction to generate the cycle and a classical azide-alkyne for the probe functionality. The scheme does all the talking really (and was a bitch to draw) but nonetheless I think that it’s a neat demonstration of the expanding tools available for synthesising tricky peptides and versatility of click chemistry.
References:
LeValley PJ , Ovadia EM , Bresette CA , Sawicki LA , Maverakis E , Bai S , Kloxin AM . Design of functionalized cyclic peptides through orthogonal click reactions for cell culture and targeting applications. Chem Commun (Camb). 2018 Jun 19;54(50):6923-6926. doi: 10.1039/c8cc03218a. PMID: 29863200; PMCID: PMC7433322.
10 notes
·
View notes
Text

Alkyne PEG | Glycomindsynth Glycomindsynth is high purity Alkyne PEG with multifunctional groups for your research. Alkyne PEG (Polyethylene Glycol) linkers are specialized compounds used in chemical and biological research. Here's a breakdown of their key aspects and uses: Structure and Chemistry: PEG (Polyethylene Glycol): A hydrophilic polymer that is often used to increase solubility, stability, and reduce immunogenicity of compounds. Alkyne Group: A functional group featuring a carbon-carbon triple bond (–C≡C–). This group is commonly used in click chemistry reactions due to its ability to react with azides in a highly selective and efficient manner.To know more, please visit our website: https://glycomindsynth.com/sub_cat_list/Alkyne%20PEG/PEG%20Linkers Call on : 9766253311 Address - Glyco Mindsynth Pvt Ltd 3rd floor, Plot No 144A, Sector 7, PCNTDA MIDC, Bhosari, Pune-411026 (Maharashtra) INDIA
0 notes
Text
Growing Antibody-Drug Conjugates Market Owing to Rising Demand for Targeted Cancer Therapy.

Antibody-drug conjugates (ADCs) are a type of bioconjugate consisting of monoclonal antibodies that are attached by chemical linkers to highly potent anti-cancer payloads. ADCs selectively target antigens that are highly expressed on tumor cells while sparing normal tissues through the use of antibodies. Linkers attached between the antibody and cytotoxic drug allow for the drug to be delivered unchanged until it reaches the intended tumor site, minimizing harm to healthy cells. ADCs have demonstrated clinical efficacy in treating various cancers including lymphoid malignancies, breast cancer, and solid tumors.
The Global Antibody-Drug Conjugates Market is estimated to be valued at US$ 5.38 Bn in 2024 and is expected to exhibit a CAGR of 14% over the forecast period 2023 to 2030. Key Takeaways: Key players operating in the Antibody-Drug Conjugates are AstraZeneca PLC, Daiichi Sankyo Company, Limited, Novasep, ADC Therapeutics SA, Alentis Therapeutics AG, F. Hoffmann-La Roche, Gilead Sciences, Inc., AbbVie Inc., Biosion USA, Inc., Astellas Pharma Inc., Duality Biologics (Suzhou) Co. Ltd., BioNTech SE, LaNova Medicines Ltd., Bliss Biopharmaceutical, Eisai Co., Ltd., ProfoundBio, Pfizer, Inc., ImmunoGen Inc., Mersana Therapeutics Inc., Sorrento Therapeutics Inc., Oxford BioTherapeutics Ltd, and Takeda Pharmaceutical Company Ltd. Growing demand for targeted cancer therapy with minimal side effects is expected to drive significant growth of the ADC market over the forecast period. Additionally, ongoing technological advancements in linker chemistry, increasing pipeline products and approvals are further fueling the market growth. Market Trends: The ADC market is witnessing increasing adoption of cleavable linkers that are stable in circulation but rapidly release the drug payload intracellularly upon internalization into target tumor cells. Additionally, the development of novel conjugation technologies such as DBCO-azide click chemistry is allowing for site-specific conjugation without effect on bioactivity and efficacy of ADCs. Market Opportunities: The significant opportunities in the ADC market are in developing ADCs for liquid and solid tumor indications with unmet medical needs. Additionally, optimization of physiochemical properties of molecules to improve pharmacokinetics is another key area that ADC developers are increasingly focusing on to enhance therapeutic index and efficacy of ADCs.
#Antibody Drug Conjugates Market Share#Antibody Drug Conjugates Market Growth#Antibody Drug Conjugates Market Analysis
0 notes
Text
Alfa Chemistry Newly Announces Offering of Click Chemistry Reagents: Azides, Terminal Alkynes, and Copper-Chelating Ligands
http://dlvr.it/T1Gg6N
0 notes
Text
Alfa Chemistry Newly Announces Offering of Click Chemistry Reagents: Azides, Terminal Alkynes, and Copper-Chelating Ligands
http://dlvr.it/T1GbvF
0 notes
Link
Organic Chemistry: 100 Must-know Mechanisms by Roman Valiulin, ISBN-13: 978-3110608304 [PDF eBook eTextbook] Publisher: De Gruyter (April 20, 2020) Language: English 230 pages ISBN-10: 3110608308 ISBN-13: 978-3110608304 Organic chemistry at a glance: straightforward and easy-to-grasp depiction of 100 crucial mechanisms in organic chemistry. This book summarizes 100 essential mechanisms in organic chemistry ranging from classical such as the Reformatsky Reaction from 1887 to recently elucidated mechanism such as the copper(I)-catalyzed alkyne-azide cycloaddition. The reactions are easy to grasp, well-illustrated and underpinned with explanations and additional information. Audience: Students, Organic Chemists. Author information: Roman Valiulin, Cambridge, USA. What makes us different? • Instant Download • Always Competitive Pricing • 100% Privacy • FREE Sample Available • 24-7 LIVE Customer Support
0 notes
Text
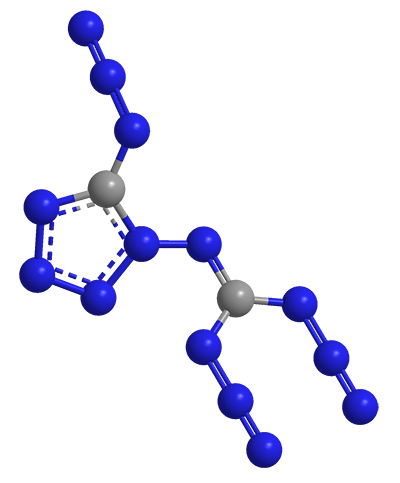
A beautiful molecule with a beautiful name: Azidoazide Azide
Molecular formula: C2N14
(Blue - Nitrogen, Gray - Carbon)
It explodes if:
Touched
Moved
Dispersed in solution
Exposed to bright light
Or even if
Left undisturbed on a glass plate
Source:
#chemistry#cool molecules#honestly why do i relate to this molecule#this is my *spirit molecule* now#cool science#science
15 notes
·
View notes
Text
Enzyme-Degradable Peptides in Click Chemistry Drug Delivery
https://www.lifetein.com/blog/enzyme-degradable-peptides-in-click-chemistry-drug-delivery/ As methods of medicine advance, targeted drug delivery becomes a more appealing and achievable option over its non-selective counterpart. It can focus solely on increasing therapeutic concentration in the target area while greatly eliminating any exposure to healthy tissue, and thus drastically lowering side effects as well. The effective and simple mechanisms of click chemistry are a great way to design payloads for these targeted drug delivery methods. With the use of enzyme-degradable peptides in click chemistry drug delivery, lasting therapeutics can remain in the system for local sustained release over time as well. Enzyme-degradable peptides for sustained drug delivery The team at Rutgers focused on a two-phase method to set up the targeted drug delivery. First, ROS-sensitive PEGDA and acrylate-PEG-azide are aimed at the target area, driven by elevated free radical levels. Once the pretargeting is complete, a payload tethered to DBCO is delivered and captured via azide-DBCO reactions. Enzyme-degradable peptides were provided by LifeTein and incorporated into both steps for ongoing release of the captured payloads. The results showed success in the models tested, with the initial dosage still effective in capturing the payload several days later. This system demonstrated the versatility of a two-phase method, where long term effects are even avoided further by the incorporation of enzyme degradable peptides. The proof of concept displayed here has great promise for the future of drug delivery, and just goes to show how applicable click chemistry is to even more fields. Emily T. DiMartini, Kelly Kyker-Snowman & David I. Shreiber (2023) A click chemistry-based, free radical-initiated delivery system for the capture and release of payloads, Drug Delivery, 30:1, DOI: 10.1080/10717544.2023.2232952
0 notes
Text
Fluorescent Labeling of #RNA and DNA on the Hoogsteen Edge using Sulfinate Chemistry [Method]
We have devised a single pot, low-cost method to add azide groups to unmodified nucleic acids without the need for enzymes or chemically modified NTPs. This involves reacting an azide-containing sulfinate salt with the nucleic acid, leading to replacement of C-H bonds on the nucleobase aromatic rings with C-R, where R is the azide-containing linker derived from the original sulfinate salt. With the addition of azide functional groups, the modified nucleic acid can easily be reacted with any alkyne-labeled compound of interest, including fluorescent dyes as shown in this work. This methodology enables the fluorescent labeling of a wide variety of nucleic acids, including natively folded RNAs, under mild conditions with minimal effects upon biochemical function and #ribozyme catalysis. To demonstrate this, we show that a pair of labeled complementary ssDNA oligonucleotides (oligos) can hybridize to form dsDNA, even when labeled with multiple fluorophores per oligo. In addition, we also demonstrate that two different group II introns can splice when pre-labeled internally with fluorophores using our method. Broadly, this demonstrates that sulfinate modification of RNA is compatible with #ribozyme function and Watson-Crick pairing, while preserving the labile backbone. http://rnajournal.cshlp.org/cgi/content/short/rna.079679.123v1?rss=1&utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
Had a dream that naruto decided to do this new jutsu where he turned into sodium azide but then it started raining and he rapidly was phase changing into a gas and diffusing and sasuke had to perform a jutsu to sequester naruto and prevent him from diffusing away
#i haven’t taken chemistry in years I don’t even remember what an azide is why did I dream this ??#cade.txt
64 notes
·
View notes