#avogadro's constant
Text
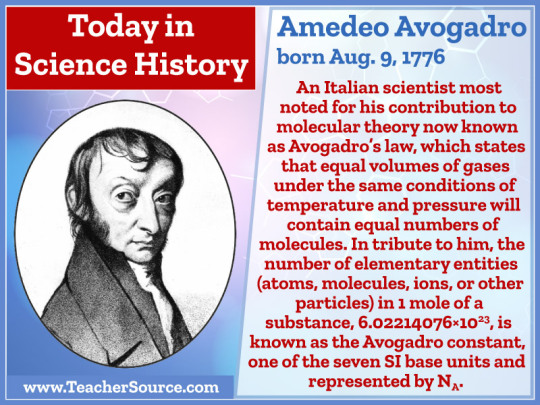
Amedeo Avogadro was born on August 9, 1776. An Italian scientist, most noted for his contribution to molecular theory now known as Avogadro's law, which states that equal volumes of gases under the same conditions of temperature and pressure will contain equal numbers of molecules. In tribute to him, the number of elementary entities (atoms, molecules, ions or other particles) in one mole of a substance , 6.02214076×10^23, is known as the Avogadro constant, one of the seven SI base units and represented by NsubA.
#avogadro#amedeo avogadro#mole#avogadro's constant#avogadro's number#molecules#science#science history#science birthdays#on this day#on this day in science history
2 notes
·
View notes
Text
Number Tournament: THE AVOGADRO CONSTANT vs EULER'S NUMBER
[link to all polls]
the Avogadro constant
seed: 27 (20 nominations)
previous opponent: fourteen
class: SI defining unit
definition: the number of moles in a mole of moles
e (Euler's number)
seed: 6 (54 nominations)
previous opponent: gross
class: irrational number
definition:

#number tournament#polls#math#mathblr#avogadro constant#e#euler's number#wow NAvse looks like it could be an actual word#navse#jan misali
1K notes
·
View notes
Text
Pssst, over here, don’t tell anyone else but I have a mole you might be interested in under the cut. Careful don’t wake him.
602,214,076,000,000,000,000,000
Ok, bye
9 notes
·
View notes
Photo



i got bored at lunch and made a few more number characters
and gave 5 a horse :)
#digital art#oc#in retrospect i do find it a bit funny that i gave avogadro's constant so many layers#like.. a toolbelt over a skirt over cargo pants... dare i say.. iconic.#also.. pi is an angel :)#mostly cause of the whole circle thing#also.. this is a horse √#but like.. in the same way that a constellation looks like an animal if that makes sense
8 notes
·
View notes
Text
Gas Constant
One of the fundamental states of matter is the gaseous state, commonly known as gas. Gases possess distinct characteristics that set them apart from other states of matter. Despite being highly compressible, gases evenly distribute pressure on all sides. They lack a definite shape and volume, instead adapting to the shape of the container they occupy. Moreover, gases readily mix due to minimal interactions between their intermolecular forces.
The behavior of gases is governed by several sets of laws derived from experimental studies conducted under various conditions, including temperature, pressure, and volume. This discussion centers around a specific constant known as the gas constant (R) and its significance.
The gas constant (R) is a physical constant expressed in units of energy per temperature increase per mole. It is also referred to as the molar gas constant or universal gas constant. The value of R is equivalent to that of the Boltzmann constant, although the latter is expressed in terms of the pressure-volume product. This constant plays a crucial role in understanding the behavior of gases and their properties under different circumstances.
Ideal Gas
An ideal gas is a theoretical concept used in physics and chemistry to simplify the behavior of gases under certain conditions. It is a hypothetical model of a gas that follows specific ideal gas laws, making its behavior easy to analyze and calculate.
The behavior of real gases deviates from the ideal gas model at high pressures and low temperatures, where intermolecular forces become significant, and the volume of gas particles becomes non-negligible. However, real gases behave similarly to ideal gases under many conditions, especially at low pressures and high temperatures.
An ideal gas can be defined as the theoretical representation of a gas comprising point particles that do not reveal any alterations during intermolecular movements. The ideal gas follows all three fundamental laws as given by Charles, Avogadro, Boyle, and Gay Lussac.
V ∝ 1/P with T and n as constant (From Boyle's law)
V ∝ T with P and n as constant (From Charles law)
Finally, V ∝ n with T and n as constant (From Avogadro's law)
Combining all three of these gives V ∝ n X T/P
Mathematically, this can be expressed as,
PV = nRT
Here,
P is the pressure
V is the volume
T is the temperature
R is the Ideal Gas Constant, and
n is the amount of substance
Gas Constant Laws
The gas constant r is a vital factor for numerous principles and laws of physics. It is used in various laws as a combination of a constant and equations as a fundamental factor.
Boyle's Law: Boyle's Law is a fundamental gas law that describes the relationship between the pressure and volume of a gas at a constant temperature. It states that when the volume of a gas decreases, its pressure increases, and vice versa, as long as the temperature remains constant. In mathematical terms, Boyle's Law can be expressed as:
P ∝ 1/V
where P is the pressure of the gas, and V is its volume. The law implies that the product of the pressure and volume of a given amount of gas is constant, provided the temperature remains unchanged.
Charles's Law: Charles's Law, also known as the law of volumes, relates the volume of a gas to its temperature at constant pressure. It states that the volume of a gas will increase or decrease in direct proportion to its absolute temperature as long as the pressure remains constant. In mathematical terms, Charles's Law can be represented as:
V ∝ T
where V is the volume of the gas, and T is its absolute temperature (measured in Kelvin). As the temperature of a gas rises, its volume will expand, and conversely, as the temperature decreases, the volume will contract.
Avogadro's Law: Avogadro's Law states that under the same temperature and pressure conditions, equal volumes of different gases contain an equal number of molecules. This law is based on the idea that the volume of a gas is directly proportional to the number of molecules it contains. In mathematical terms, Avogadro's Law can be expressed as:
V ∝ n
where V is the volume of the gas, and n is the number of gas molecules. It implies that at constant temperature and pressure, the number of molecules to the volume ratio is constant for all gases.
Gay-Lussac's Law: Gay-Lussac's Law, also known as the pressure-temperature law, describes the relationship between the pressure and temperature of a gas at constant volume. It states that the pressure of a fixed amount of gas is directly proportional to its absolute temperature, provided the volume remains constant. Mathematically, Gay-Lussac's Law can be represented as:
P ∝ T
where P is the pressure of the gas, and T is its absolute temperature. This law implies that if the temperature of a gas increases, its pressure will also increase, and if the temperature decreases, the pressure will decrease, assuming the volume remains constant.
0 notes
Text
respiR
Parler Vrai = ne Rien Taire
#constante#universelle#entropie#loi#physique#gaz#parfaits#pression#volume#avogadro#température#équation#writer#french#morale#kant#transparence#confiance#humain#mots#expression#liberté#paroles en l'air#free#art#total#public#synesthésie
1 note
·
View note
Text
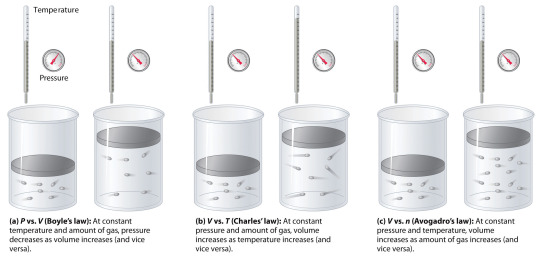
Gas Laws
A gas law is, simply put, a physical law that describes the behavior of gases. There are multiple gas laws, including the following four laws that are more well known than others:
Boyle's Law relates the pressure and volume of a gas. Published by Robert Boyle in 1662 it states that "the volume of a given mass of a gas is inversely proportional to its pressure at a constant temperature". One mathematical representation of this law is P1 x V1 = P2 x V2.
Charles' Law was published in 1787 by Jacques Charles. It relates temperature and volume and states that "volume of a given fixed mass of a dry gas is directly proportional to its absolute temperature at a constant pressure". One mathematical representation of this law is V1/T1 = V2/T2
Gay-Lussac’s Law was published by Joseph Louis Gay-Lussac in 1808 and relates pressure and temperature, stating: "The pressure exerted by a given mass and constant volume of an ideal gas on the sides of its container is directly proportional to its absolute temperature." One mathematical representation of this law is P1/T1 = P2/T2.
Finally, Avogadro's Law relates the amount and volume of a gas. It was published in 1811 by Amedeo Avogadro and states that the "volume occupied by an ideal gas at a constant temperature is directly proportional to the number of molecules of the gas present in the container." One mathematical representation of this law is V1/n1 = V2/n2.
Sources/Further reading: (Image source - LibreTexts) (NIH) (Jove) (Wikipedia)
*P - pressure; V - volume; T - temperature; n - amount of a substance
56 notes
·
View notes
Text
Random fun chemistry facts
(Some of these are "duh" for experts, but I find them really fun as a chemistry layperson anyway).
Every element after lead is radioactive.
Diamond and graphite are both forms pure carbon takes. (This is why it's so easy to create synthetic diamond, and why De Beers is shitting themselves currently. Turns out when you have the technology to create entirely new elements, even if only for seconds of observation, rearranging carbon atoms into particular configurations is trivial. But, ya know, De Beers would rather keep their slaves.)
The most radioactive naturally-occurring element is believed to be polonium, though some man-made elements are more radioactive since they have half-lives on the order of seconds. Polonium-210 is the most radioactive isotype thereof, and has the dubious honor of having been used in a political assassination.
Light behaves like both a wave and a particle at the same time, which is called wave-particle duality, and is kinda insane to think about.
"Mole day" is celebrated on October 23 every year, because written out in American conventions, it becomes 10/23, which references Avogadro's constant, 6.022*10^23. (This number equals one mole, and is used to define an amount of a substance.)
Helium is lighter than air, which is why balloons float. Also, helium can and will escape into space, making it non-recoverable, which is kind of a problem since helium is also used to cool the magnets of MRI machines.
Absolute zero (a temperature that has never actually been reached) is the point at which all molecular activity ceases/the kinetic energy disappears from the system. Even if you cooled a mass of helium to this point, it would still be a liquid instead of a solid.
Thioacetone is believed to be the worst smelling chemical in the world. It's capable of inducing nausea in people .4 km away.
Alfred Nobel, the person who the Nobel Prize is named for, invented dynamite, intended to assist in mining. There is a popular story that he was the subject of a prematurely-published obituary calling him the "merchant of death", which horrified him so much that he shifted his focus and later invented the Nobel prize to save his reputation, but this has never been verified and some think it might be apocryphal.
Some chemicals have different forms, called isomers, that still work drastically differently. The most infamous of these is thalidomide. It is a chiral (that is, like your left and right hand, it can't be superimposed over its mirror image) molecule. The "good" form, R-thalidomide, was useful in treating morning sickness, but the body would convert this to S-thalidomide since it couldn't distinguish the two. The S-thalidomide then caused an epidemic of birth defects, and thalidomide was removed from the market. It remains a popular case study for a variety of issues: isomers and interconversion, chirality, birth defects, and more.
Just some random fun facts. :)
14 notes
·
View notes
Text
༻`` 21 Jan 24 — Sunday
100 days of productivity 21/100


Studied for almost 2 hours today (chemistry and physics)! It's getting late and I've got a drivingesson tomorrow morning so I'm not pushing myself for the 2hr mark today.
I covered:
chemistry
moles, avogadro's constant & water of crystallisation
electronic configuration
bonding
physics
SI and derived units
The electromagnetic spectrum (wavelengths and order)
Not too bad of a day! Plus I made myself a snack bowl (grapes and berries, dried fruit, nuts) which I'm looking to freeze portions of and take out before studying.
#studyblr#dark academia#light academia#chaotic academia#study motivation#100dop#100 days of productivity#study inspiration#100 days of productivity challenge#chemistry#physics#studyspo#student#student life#productivity
33 notes
·
View notes
Text
Number Tournament: THE AVOGADRO CONSTANT vs FOURTEEN

[link to all polls]
the Avogadro constant
seed: 27 (20 nominations)
class: SI defining constant
definition: a constant which defines the number of particles that constitute "one mole" of a substance
14 (fourteen)
seed: 38 (14 nominations)
class: semiprime
definition: the number of days in a fortnight
488 notes
·
View notes
Text
12 notes
·
View notes
Text
Genshin Impact imagines from Tiktok : School AU
Yes, I am chronically online. And by chance it landed on all Inazuman characters to might as well make a fic out of it.
—
Person people ship you with: Gorou
“Dear [Y/N], what is Gorou to you?” The question took you aback. Of course, Yae Miko would put color into your ‘affection’ towards Gorou.
However, unlike what they all think, or at least what you think they think, Gorou is simply someone you want to take care of, like a son. You’ve ‘adopted’ some of your classmates before. You were sometime considered as the ‘mother’ of the group.
Answering her question doesn’t come easy as someone who doesn’t know you very well. You were only transferred in this class for the second time in your whole academic life so… “A friend?” An eyebrow raised followed by a pause. “I mean, he’s adorable but no, I’m not romantically attracted if that’s what you’re asking.” “Hey! Don’t talk as if I’m not here!” Gorou’s red up until his ears. You just giggled at him. He is so cute.
—
School officer that helps you cheat: Heizou Very fitting lmao.
You were going through your Chemistry test. Double checking your answers and all that when your pen decided to take a deep dive off your table. Ah shit. You were going to go pick it up and Heizou did so for you, and that’s when you noticed it. A quick glace at Heizou’s paper showed you his answer for number 35. It asked for Planck’s constant. You didn’t have an answer for it yet as both Avogadro’s number and Planck’s constant shared the number 6 as the whole number, the rest you didn’t bother to memorize but the exponents. You knew that Avogadro’s 10^23 and Planck’s is in the negatives. Heizou got it the other way around.
“Thanks.” Heizou handed you your pen before going on with his test, throwing you a suspicious look while you went back to double checking your test. Once you were done, you simply put the test on its back side and laid your head upon it to look like you’re taking a nap. You blankly stared at nothing, trying to pass time when Heizou spared you a glance just as you were about to actually nap. You handsigned, said hand hidden from the test proctor but in Heizou’s plain sight. 3. 5. C. He looked at you like he had a sudden realization and went back to the said number. Yep, he corrected it. Soon he was done with his own test. Like you, he laid his head down as if to take a nap and- he drew on his thigh. 9. 7. A. You looked at him in the eyes and he winked at you. You just smiled back. 5 minutes before the test was over you made the correction. In the end, the both of you aced the test. It isn’t like the both of you needed help but an additional point won’t hurt would it?
—
Has a crush on you: Thoma ; Your crush: Kazuha ; Your enemy: Itto
***I can’t find a simulator that does FB GCs, I’ll change this once I have access to one, sorry!
Kamisato Ayaka: Hello everyone! Please stay after class so we can discuss about the upcoming school festival!
Kujou Sara: Noted.
Kaedehara Kazuha: I’ll be there my lady.
Thoma: I’ll bring donuts for everyone!
Arataki Itto: YAY DONUTS
You: Sana ol my lady I cri Lisa babe, Good Hunter?
…
You: Oh shit 😭
Kamisato Ayato: You’re on a ‘babe’ basis with someone? Interesting…
Kamisato Ayaka: Ahehehe you won’t be a lady, you’d be a princess! 😁
Yae Miko: Oh dear, she’d be a queen instead with the way that she acts. Smart, diplomatic but domineering.
You: Nauurrr stop psychologically assessing me 😭 and yes I call my friends babe
Thoma: Will you still come to the meeting my lady? I’ll get nutty choco donuts! 😁
You: Thank you Thoma, you’re the best ❤️
You: aaaand I take it back, don’t call me my lady it’s making me cringe 😖
Arataki Itto: So… what do we call you then?
You: Stop it Itto.
—
Sana ol means hope all like, I hope [everyone] gets to be called my lady in this case.
#genshin impact#gorou x y/n#kazuha x reader#kaedehara kazuha#itto x you#thoma x reader#shikanoin heizou#heizou x reader
57 notes
·
View notes
Text
Physics "Friday" #11 [Opinion]: Okay so I have a few more problems with SI Units
Preamble: Now what Missy?
Education Level: Primary School (Y5/6)
Topic: Measuring Systems (Metrology)
Ok so this was going to be my necessary follow up to my first opinion post, Is Fahrenheit the better temperature scale?
I was meant to save it for the next time I had too much stuff to deal with on Friday and needed to write something easier. In fact, that's going to be how I do opinion posts - when I don't need to do as much a-thunkin'.
Regardless, there are of course other problems with the SI units system that many people point out. This is basically me doing it myself.
What are the SI Units?
During the french revolution, there were several attempts to metricise the existing unit system. Generating standardised units on length, volume, weight, temperature ... they even tried to decimalise time.
These units were based off physical constants:
The metre was one 10 millionth the distance from the north pole to the equator
The litre was the volume taken up by one kilogram of water
Celsius was based on the boiling/melting points of water
A kilogram was based on the weight of a specific Platinum-Iridium Alloy called the IPK (International Prototype Kilogram)
Now obviously, these weren't perfect constants. The earth's radius changed, the boiling point of water depends on the pressure of the environment, and the IPK can vary based on surrounding conditions.
This was fixed in 2019, when every SI measurement unit was defined using a universal constant rather than physical objects like a mass or the earth:
Hyperfine transition frequency of Caesium
Speed of light
Planck Constant
Charge of the Electron
Boltzmann Constant
Avogadro's Constant
Luminous Efficacy of 540 THz radiation
This gives our seven SI units:
Time (Second)
Distance (Meter)
Mass (Kilogram)
Charge (Columb)
Temperature (Kelvin)
Amount (Mole)
Luminous Intensity (Candela)
Problem #1: Unit-less units
There are several units that come from mathematics that appear to be unitless, like:
Angle (Radians)
Solid Angle (Steradians)
Amount (Number count)
We use this all the time like in lumens (candela steradians), angular velocity (radians per second), and number density (particles per cubic metre).
Wait ... amount was already listed as an SI quanity - it was a mole wasn't it?
That's exactly it. A mole is just defined as 6.022×10²³ objects of something. This is where we experience our first problem.
Why is radian and steradian not considered a unit in it's own right but a mole is?
Radians are defined as being one metre per metre. The angle required to create an arc with a length of 1 metre given a radius of one metre.

Image Credit: BYJU's
This comes from the formula S=Rθ where circular arc length (S) is equal to the radius (R) multiplied by angle in radians (θ).
The problem with this, is that the radian is still an arbitrary dimension. We could've chosen our standard angle unit to be a degree, and all we need to do is change the definition of the sine function and the above arc length definition:
S=kθR were kθ is dimensionless and k = 1 rad⁻¹
It's almost as if, while the radian is dimensionless, it still behaves and acts like a unit. Much like how we can redefine everything and make the unit for length be a foot instead, we can do the same with angle.
You may object, as S=Rθ looks a lot more fundamental than adding an extra unneeded constant. But it's like if we were to use natural units, where the speed of light is equal to 1, making E = mc² actually E = m.
This doesn't mean that m is somehow 'fundamental' when we define it in terms of energy. All we've done is redefine our unit system in a way that makes a few constants equal to one.
The same thing goes for radians.
A little side-note on Intensity
You could argue that this also applies to relative intensity units like Magnitude or Bels. However, I'd argue that they don't count as their unit systems are defined as being relative. The strength of a signal or luminosity is just choosing a unit system of power.
For example, relative magnitude is based on the luminosity of the sun. Sound/Signal amplitude is based on watts or volts.
Problem #2: Redundant Units
Many of the quantities in the SI unit system are technically redundant. Temperature can just be redefined as energy density, mass can be redefined as energy, etc.
What I'm concerned with is one unit in particular: the Candela - what exactly is it?
I often find that a lot of people attempting to explain the SI unit system often brush over this unit, even though it feels rather important given it's called 'Luminous Intensity'.
But hold on, isn't luminosity just about energy production? After all, radiation is just energy, and it's emitted as a form of energy.
A candela is equal to one lumen per steradian, where a lumen is the total luminous intensity of an object that emits light everywhere. Candelas concern luminous intensity from a specific solid angle view of the object.
Watts, the measurement of power and bolometric luminosity, measure the total (or bolometric) luminosity of an object at all wavelengths of light.
Lumens, on the other hand, represent the power produced by an object after each wavelength is passed through some filtering function that accounts for how the human eye sees light. This weighting function is determined by the ISO.
Of course, our eyes only see light within a particular range, and inside of that range, different wavelengths appear more intense to us because we pick it up more.
But this fails to recognise that this is still just a glorified unit of power. Just because it's weighted based on some function, shouldn't change what unit is necessarily uses. Hence it is technically redundant as it can be defined as a combination of other SI units - similar to radians.
Problem #3: When the unit system isn't used
I mentioned it in the last post, but there are a lot of occasions where the standard SI units aren't used. The worst culprit I feel is Astronomy, with Physics only having issues in lieu of SI vs. Natural units vs. Electron/Atomic Units. Again here's the list:
My beloved SI units
CGS Units
Whatever the fuck a Jansky is
Don't even start with natural units I can't live without big G
"Ampere in CGS units is g1/2 cm3/2 s−2"
Solar Luminosity/Mass of Sun
Angstroms (like please can we just use nanometers?)
How many Jupiters or Earths fit into this cloud of gas?
The vomit of parallax units i.e. AU, pc, Mpc, arcseconds, radians
Steradians (Solid angles can be finicky)
Logarithms, logarithms everywhere!
Hubble's constant being in km/s/Mpc but then having to turn that into Hz or per year - like can someone please acknowledged how cursed this is?
When you do Kepler's 3rd law on Mercury and realise it doesn't work (because you forgot Einstein existed) ... so no units end up working
ADUs and/or whatever you get when you deal with telescope outputs
Sidereal time, J2000, etc.
Sky Coordinates (it always depends on the telescope mount)
(the last two are new entries into the list I forgot!)
A lot of these units are very, very, annoying to handle. Often at times because they are just so unnecessary. We have scientific notation for a reason - so why are we using Jansky? Why ever use the mass of planets unless if we are talking about specifically exoplanets?
It can especially be annoying when in astronomy course subjects, unit conversions make up like 50% of the work and 50% of the error in calculations.
And the biggest problem, of course, is the CGS units system. I hate it. So much. From what I know CGS is used simply because it appears as more correctly "scaled" for a lot of astronomical processes.
However, the problem is that it just adds extra conversion factors into every equation. Now I have to remember big G in both CGS units as well as in SI or solar units etc. And it doesn't jive with a lot of other astronomical units.
J2000 is also rather annoying - why are we using the Julian calendar at all? Shouldn't we try and strive for using a more accurate year instead? Because what happens is that every four years the calendar shifts by a day.
The "Ampere in CGS units is g1/2 cm3/2 s-2" is a direct quote from one of my professors. And it also makes very little sense - because it says that the coulomb and ampere units are actually redundant.
However, CGS units aren't usually the only thing we use. Sometimes we put things in Gaussian CGS units, where we define the coulomb's constant (kₑ) as being 1. Thus, similar to natural units, we can define a statCoulomb in terms of our three base units:
1 statC = 1 g1/2⋅cm3/2⋅s−1.
Now this is where we find our ampere definition! But wait ... this is materially different to the real ampere. In fact, we can determine the difference between coulombs and statcoulombs:
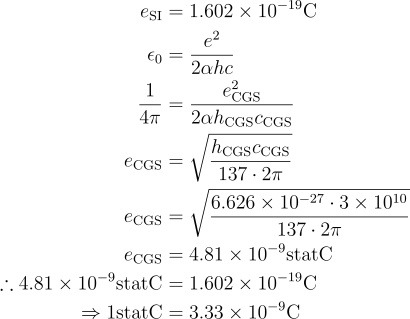
So they are different! And thus, after several paragraphs, my lecturer is wrong and they shouldn't've used the Ampere but a different stat ampere.
So what would I do?
Well, the big problem with all unit systems is that they are just equivalent to eachother dimensionally.
Because of all the seperate equations surrounding each of the SI units, we can define any SI unit in terms of any other SI unit combination just by setting certain constants to one.
So there's quite a lot of units that we could me missing.
But here's how I would do it ...
There are five unique SI units:
Distance - Metre
Time - Second
Mass - Kilogram
Charge - Coulomb
Temperature - Kelvin
There are also three dimensionless/special units:
Amount - Mole
Angle - Radian
Luminosity - Lumen
These three units are special as they can be expressed as dimensionless constants or in terms of other units, however we want them to be expressed in our own specific units.
Solid Angle is just determined from square radians where 1 sr = π/4 rad² (ratio of square and circle of same diameter).
The constants which define these unique units are:
Hyperfine transition frequency of Caesium
Speed of light
Planck Constant
Charge of the Electron
Boltzmann Constant
For the non-unique units we have:
Avogadro's Constant
Pi
Luminous Efficacy of 540 THz radiation
Conclusion
This post did end up taking longer than I expected ... much longer. Nevertheless it was fun to do!
As stated in the start, opinion posts are for those Fridays where things aren't as easy as spending 2-3 hrs writing a post. Because when you want to argue a point you have a little more passion behind it.
Anywho. Did you know that only 5% of people who read this post actually follow this channel?. SMASH THAT FUCKING LIKE AND SUBSCRIBE. Brought to you by Raid Skillspace VPN™.
Feedback's always welcome. Come and debate me you cowards. Follow if you like it ... or don't.
#physics friday#stem#academics#stemblr#science#astronomy#si units#Finding hashtags for metrology is a bit difficult
10 notes
·
View notes
Text
It's 11:24pm, I have a Chem test tomorrow, and now I'm feeling like a kiwi bird.
Half of my processor says 'The number of moles of a substance can be found by dividing the mass by the molar mass, and there are 6.022x10^23 particles in one mol, based on the Avogadro's constant' and the another half of my brains say 'mmm worm in grass,,peck peck'
send help this is not efficient.
2 notes
·
View notes
Text
I think I may be studying a bit too much 😭
I just tried triviaverse on Netflix and got to round 3, one of the questions was "which constant is smaller? A) Planck's or B) Avogadro" n I ACTUALLY SAIF OUT LOUD "Finally! An easy question!"
#I registered what I said after I'd answered like bro tf did I just say?!#like ok I'm close-ish to getting a Bachelor's in Physics but holy shit that was instinctual#Dy talks
1 note
·
View note
