#adenovirus vector vaccines
Explore tagged Tumblr posts
Text
How Philanthropists hijacked COVID-19
Since early 2020, some observant people have speculated about where all the money committed by the Gates Foundation in January 2020 was going. The Bill and Melinda Gates website proudly announce their work to fight covid-19 from January 2020. How did they know how Coronavirus Disease 2019 would cause a global pandemic? Defensive institutes such as the main subject of this blog, patronisingly…

View On WordPress
#adenovirus vector vaccines#Censorship#chimpanzee adenovirus#Corporate smear#COVID-19#defensive#funding media and advocacy groups#infectious bronchitis virus#Ivermectin#Merck patent#patents#Philanthropists#pigs and chickens#Pirbright Institute#propaganda#protease inhibitor#vaccines
0 notes
Text
Adenovirus Vector Vaccine Market Projected to Show Strong Growth

The Latest research coverage on Adenovirus Vector Vaccine Market provides a detailed overview and accurate market size. The study is designed considering current and historical trends, market development and business strategies taken up by leaders and new industry players entering the market. Furthermore, study includes an in-depth analysis of global and regional markets along with country level market size breakdown to identify potential gaps and opportunities to better investigate market status, development activity, value and growth patterns. Access Sample Report + All Related Graphs & Charts @: https://www.advancemarketanalytics.com/sample-report/165891-global-adenovirus-vector-vaccine-market
Major & Emerging Players in Adenovirus Vector Vaccine Market:- Creative Biolabs (United States), Sartorius AG (Germany), Lonza (Switzerland), Merck KGaA (Germany), Cobra Biologics (United States), Thermo Fisher Scientific (United States), Boehringer Ingelheim (Germany), Oxford Biomedica (United Kingdom), Advanced Bioscience Laboratories (United States). The Adenovirus Vector Vaccine Market Study by AMA Research gives an essential tool and source to Industry stakeholders to figure out the market and other fundamental technicalities, covering growth, opportunities, competitive scenarios, and key trends in the Adenovirus Vector Vaccine market. Adenovirus represents the class of the genetically diverse DNA viruses that can cause non-life-threatening infections related to eyes, respiratory system, gastrointestinal lining and other parts. These viruses represent promising results as a vector for delivering target antigens to various hosts due to excellent ability induce immune response. Due to these property, the new studies concluded positive results for use of adenovirus for both gene therapy and vaccine production. Adenovirus-based vectors shows various benefits when compared to other viral vectors such as wide range of tissue tropism, ease of genetic manipulation especially for large transgene DNA insertions, superior ability to induce robust transgene-specific T cell and antibody responses, easy production of the adenovirus based vaccines at large scale. Due to this, it has emerged as a preferred choice for delivering vaccine for both humans as well as animals.
In February 2021, the Janssen Biotech, a part of Johnson and Johnson submitted Emergency Use Authorization (EUA) to the US Food and Drug Administration (FDA), for its investigational single-dose coronavirus 2019 (COVID-19) vaccine candidate. The vaccine Ad26.COV2.S, is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilized SARS-CoV-2 spike (S) protein
In January 2021, Thermo Fisher Scientific, a company offering range of instrumentation, reagents and consumables, and software and services acquired the viral-vector manufacturing business of the Novasep, a pharmaceutical company for approximately USD 875 million in cash. This acquisition is a step towards expanding Thermo Fisher capabilities in the cell and gene vaccines and therapies worldwide. The titled segments and sub-section of the market are illuminated below: by Type (Adenovirus-based tuberculosis vaccine, Adenovirus-based HIV vaccine, Adenovirus-based influenza vaccine, Others), Application (Human, Animals), End-user (Hospitals, Ambulatory Surgical Center, Research Institutes, Others) Market Trends: Increasing investment in vaccine and drug development
Rising collaboration between the pharmaceutical companies and CROs for development of adenovirus vector vaccine
Opportunities: Emergence of adenovirus vectored vaccines for COVID-19
Production of low cost adenovirus vaccines
Market Drivers: Growing prevalence of chronic medical conditions
Rise in demand for efficient vaccines to treat infectious diseases Enquire for customization in Report @: https://www.advancemarketanalytics.com/enquiry-before-buy/165891-global-adenovirus-vector-vaccine-market Some Point of Table of Content: Chapter One: Report Overview Chapter Two: Global Market Growth Trends Chapter Three: Value Chain of Adenovirus Vector Vaccine Market Chapter Four: Players Profiles Chapter Five: Global Adenovirus Vector Vaccine Market Analysis by Regions Chapter Six: North America Adenovirus Vector Vaccine Market Analysis by Countries Chapter Seven: Europe Adenovirus Vector Vaccine Market Analysis by Countries Chapter Eight: Asia-Pacific Adenovirus Vector Vaccine Market Analysis by Countries Chapter Nine: Middle East and Africa Adenovirus Vector Vaccine Market Analysis by Countries Chapter Ten: South America Adenovirus Vector Vaccine Market Analysis by Countries Chapter Eleven: Global Adenovirus Vector Vaccine Market Segment by Types Chapter Twelve: Global Adenovirus Vector Vaccine Market Segment by Applications What are the market factors that are explained in the Adenovirus Vector Vaccine Market report?
– Key Strategic Developments: Strategic developments of the market, comprising R&D, new product launch, M&A, agreements, collaborations, partnerships, joint ventures, and regional growth of the leading competitors.
– Key Market Features: Including revenue, price, capacity, capacity utilization rate, gross, production, production rate, consumption, import/export, supply/demand, cost, market share, CAGR, and gross margin.– Analytical Tools: The analytical tools such as Porter’s five forces analysis, SWOT analysis, feasibility study, and investment return analysis have been used to analyze the growth of the key players operating in the market. Buy This Exclusive Research Here: https://www.advancemarketanalytics.com/buy-now?format=1&report=165891 Definitively, this report will give you an unmistakable perspective on every single reality of the market without a need to allude to some other research report or an information source. Our report will give all of you the realities about the past, present, and eventual fate of the concerned Market. Thanks for reading this article; you can also get individual chapter wise section or region wise report version like North America, Europe or Asia. Contact US : Craig Francis (PR & Marketing Manager) AMA Research & Media LLP Unit No. 429, Parsonage Road Edison, NJ New Jersey USA – 08837 Phone: +1 201 565 3262, +44 161 818 8166 [email protected]
#Global Adenovirus Vector Vaccine Market#Adenovirus Vector Vaccine Market Demand#Adenovirus Vector Vaccine Market Trends#Adenovirus Vector Vaccine Market Analysis#Adenovirus Vector Vaccine Market Growth#Adenovirus Vector Vaccine Market Share#Adenovirus Vector Vaccine Market Forecast#Adenovirus Vector Vaccine Market Challenges
0 notes
Text
The new nasal vaccine seems to be awesome.
@EricTopol
Two doses of a Covid nasal vaccine spray led to >50-fold increase in spike-specific secretory IgA antibodies against 10 strains of #SARSCoV2, indicative of potent mucosal immunity https://insight.jci.org/articles/view/180784
@JCI_insight
with evidence of blocking infections among health care workers who were assessed after exposure.
"At least 86.2% participants who completed 2 [nasal vaccine] doses maintained uninfected status, likely without even asymptomatic infection, for at least 3 months."
28 notes
·
View notes
Text
Reference saved in our archive
Sharing this study because 1. it's late-breaking 2. to highlight what numbers scientists mean when they say things like "highly effecatious" and "acceptable protection."
In an exploratory analysis relative vaccine efficacy of ARCT-154 vs. ChAdOx1-S against any COVID-19 from Day 36 to Day 394 was 19.8% (95% CI: 4.0–33.0).
I'm not saying this to fearmonger, I'm just saying this is why we need to keep masking, ventilating, and distancing whenever possible. At best, your vaccination has a ~1/3 chance of keeping you from contracting a *strongly symptomatic* case of covid. It also has a decent chance of keeping you from needing to visit the hospital for covid. It's a life vest, not a primary mode of protection. (It's also why we need more than 1 shot a year like the CDC tries to tell us, but that's a different rant)
Abstract This phase 3 trial compared safety, tolerability, immunogenicity and efficacy of the self-amplifying mRNA COVID-19 vaccine, ARCT-154, with ChAdOx1-S adenovirus-vector vaccine. In four centers in Vietnam adult participants aged 18‒85 years were randomly assigned to receive two doses, 28 days apart, of either ARCT-154 (n = 1186) or ChAdOx1-S (n = 1180). Both vaccines were well tolerated with similar safety and reactogenicity profiles consisting of mainly mild-to-moderate solicited adverse events and few related serious adverse events. Higher neutralizing antibody responses persisting to one-year post-vaccination after ARCT-154 compared with ChAdOx1-S were associated with a generally higher efficacy against COVID-19. In an exploratory analysis relative vaccine efficacy of ARCT-154 vs. ChAdOx1-S against any COVID-19 from Day 36 to Day 394 was 19.8% (95% CI: 4.0–33.0). Self-amplifying mRNA vaccine offers potential immunological advantages in terms of immunogenicity and efficacy over adenovirus-vector vaccine without compromising safety.
#mask up#public health#wear a mask#pandemic#wear a respirator#covid#covid 19#still coviding#coronavirus#sars cov 2
19 notes
·
View notes
Text
Mucosal adenovirus vaccine boosting elicits IgA and durably prevents XBB.1.16 infection in nonhuman primates | Nature Immunology - September 3, 2024
A mucosal route of vaccination could prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication at the site of infection and limit transmission.
We compared protection against heterologous XBB.1.16 challenge in nonhuman primates (NHPs) ~5 months following intramuscular boosting with bivalent mRNA encoding WA1 and BA.5 spike proteins or mucosal boosting with a WA1–BA.5 bivalent chimpanzee adenoviral-vectored vaccine delivered by intranasal or aerosol device. NHPs boosted by either mucosal route had minimal virus replication in the nose and lungs, respectively. By contrast, protection by intramuscular mRNA was limited to the lower airways. The mucosally delivered vaccine elicited durable airway IgG and IgA responses and, unlike the intramuscular mRNA vaccine, induced spike-specific B cells in the lungs. IgG, IgA and T cell responses correlated with protection in the lungs, whereas mucosal IgA alone correlated with upper airway protection. This study highlights differential mucosal and serum correlates of protection and how mucosal vaccines can durably prevent infection against SARS-CoV-2.
#covid#mucosal immunity#nasal vaccines#research#study#data#nasal vaccine#vaccines#good news#immunology#nature journal
5 notes
·
View notes
Text
I'm really fascinated by that new nasal SARS-CoV-2 vaccine but they still haven't explained how they'll deal with the shortened duration of protection offered by adenovirus vectors or the fact that they stop working with repeated administration - hopefully they'll look for a different delivery platform as the nasal administration is a really good idea
2 notes
·
View notes
Note
I was very optimistic about mRNA vaccines since they seem like a very safe and effective alternative to already fairly safe and mostly effective adenovirus vector vaccination, but the latest research leans towards conclusion that the most harmful part of Covid is the long-term effects of spike protein exposure, the same protein mRNA vacc. is specifically trying to produce in large amounts and then, apparently, just keeps producing even months later. That is some 4D chess bullshit, I gotta say.
Asks Received In September 2021
5 notes
·
View notes
Text
Elevate Your Biopharma Pipeline with Reliable Plasmid DNA Manufacturing
The global plasmid DNA manufacturing market size is expected to reach USD 6.70 billion by 2030, expanding at a CAGR of 21.44% from 2025 to 2030, according to a new report by Grand View Research, Inc. The rising prevalence of chronic diseases, combined with the wide application scope of plasmid DNA in the healthcare industry is expected to drive the industry growth. Plasmid DNA can be used directly for therapeutic applications, such as the production of vaccine antigens or gene therapy. Moreover, it can be utilized for various research purposes, such as gene cloning, gene mapping, etc. The rising number of patients choosing gene therapy is expected to drive industry growth in the coming years.
According to clinicaltrials.gov, the number of cell & gene therapies across global pipeline programs (Phase I to Phase III trials) increased from 289 in 2018 to 327 in 2022. Furthermore, the U.S. FDA provides constant support for innovations in the advanced therapy arena via several policies concerning product manufacturing. With increased cell and gene therapy products, the industry will see an increased need for manufacturing plasmid DNA on a larger scale. The COVID-19 pandemic is anticipated to positively impact industry growth. For instance, in September 2021, India approved the first COVID-19 DNA vaccine. It is the world’s first COVID-19 DNA vaccine manufactured in partnership with DBT-BIRAC as part of Mission COVID Suraksha.
Circular DNA is used in India’s ZyCoV-D vaccination to protect against COVID-19 infection. Furthermore, key entities in the global industry are undertaking various strategic initiatives to strengthen their market presence, which is also expected to propel growth. For instance, in June 2022, BioCina announced the expansion of the production of plasmid DNA in a new dedicated GMP suite to its offering of CDMO services. The suite has a single-use fermentation capacity of up to 300 L and appropriately scaled downstream processing machinery.
Plasmid DNA Manufacturing Market Report Highlights
MP-grade plasmid DNA manufacturing dominated the market in 2024 with a share of 86.29% and is expected to grow at the fastest CAGR over the forecast period.
The clinical therapeutics segment held the largest market share of 54.6% in 2024. Plasmid DNA is currently increasing in importance for clinical research applications in genetic vaccination and gene therapy.
The cell & gene therapy segment held the largest market share of 54.4% in 2024. This high share can be attributed to the fact that gene therapy is broadly applied in the treatment of several inherited and genetic diseases.
The cancer segment held the largest market share of 40.0% in 2024 and is expected to witness the fastest CAGR from 2025 to 2030.
The North America Plasmid DNA manufacturing market held the largest revenue share of 43.43% of the global market in 2024.
Plasmid DNA Manufacturing Market Segmentation
Grand View Research has segmented the global plasmid DNA manufacturing market on the basis of service, application, end use, and country:
Plasmid DNA Manufacturing Grade Outlook (Revenue, USD Million, 2018 - 2030)
R&D Grade
Viral Vector Development
AAV
Lentivirus
Adenovirus
Retrovirus
Others
mRNA Development
Antibody Development
DNA Vaccine Development
Others
GMP Grade
Plasmid DNA Manufacturing Development Phase Outlook (Revenue, USD Million, 2018 - 2030)
Pre-Clinical Therapeutics
Clinical Therapeutics
Marketed Therapeutics
Plasmid DNA Manufacturing Application Outlook (Revenue, USD Million, 2018 - 2030)
DNA Vaccines
Cell & Gene Therapy
Immunotherapy
Others
Plasmid DNA Manufacturing Disease Outlook (Revenue, USD Million, 2018 - 2030)
Infectious Disease
Cancer
Genetic Disorder
Others
Plasmid DNA Manufacturing Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Mexico
Europe
Germany
UK
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
China
Japan
India
South Korea
Australia
Thailand
Latin America
Brazil
Argentina
Middle East & Africa
South Africa
Saudi Arabia
UAE
Kuwait
List of Key Players
Charles River Laboratories
VGXI, Inc.
Danaher (Aldevron)
Kaneka Corp.
Nature Technology
Cell and Gene Therapy Catapult
Eurofins Genomics
Lonza
Luminous BioSciences, LLC
Akron Biotech
Order a free sample PDF of the Plasmid DNA Manufacturing Market Intelligence Study, published by Grand View Research.
0 notes
Text
Viral Vector Production Market: Supporting Gene Therapy and Vaccine Development up to 2033
Market Definition The Viral Vector Production market includes the development and manufacturing of viral vectors used as delivery mechanisms for gene therapy, vaccine production, and other advanced therapeutics. Viral vectors, such as adenovirus, lentivirus, and adeno-associated virus (AAV), are engineered to carry genetic material into cells, facilitating the treatment of genetic disorders, cancers, and infectious diseases. This market serves biotechnology, pharmaceutical, and research institutions that require viral vectors for clinical trials, therapeutic applications, and vaccine development.
To Know More @ https://www.globalinsightservices.com/reports/Viral-Vector-Production-Market
The viral vector production market is anticipated to expand from $3.8 billion in 2023 to $14.2 billion by 2033, with a CAGR of 13.8% reflecting robust growth.
Market Outlook The Viral Vector Production market is poised for substantial growth, driven by increasing demand for gene therapies, rising incidence of genetic disorders, and the ongoing development of viral-vector-based vaccines. Gene therapy has emerged as a promising approach for treating diseases with limited conventional treatment options, making viral vectors essential for delivering therapeutic genes. Additionally, advancements in cell and gene therapy research have increased the need for scalable and efficient viral vector production processes, fueling growth in the sector.
Key drivers include the expanding application of viral vectors across therapeutic areas and technological advancements in vector engineering, which enhance production efficiency and safety. Demand for viral vector manufacturing services has also surged as pharmaceutical companies prioritize partnerships and outsourcing to accelerate the development of gene-based therapies. Innovations in bioreactor technology, vector design, and purification methods are improving production capabilities, making high-quality viral vectors more accessible to a wider range of clinical and commercial applications.
Despite its potential, the market faces challenges related to complex manufacturing processes, high production costs, and stringent regulatory standards. The complexity of viral vector production requires specialized infrastructure, skilled expertise, and adherence to rigorous quality controls, which can be barriers to scalability. Addressing these challenges through process optimization, automation, and standardization will be crucial to meet rising demand effectively. As the market continues to expand, investment in infrastructure, partnerships, and advancements in production technology are expected to drive sustainable growth in the Viral Vector Production market.
Request the sample copy of report @ https://www.globalinsightservices.com/request-sample/GIS31585
0 notes
Text
The Vaccine Technologies Market is projected to grow from USD 45,405 million in 2024 to USD 104,636.38 million by 2032, at a compound annual growth rate (CAGR) of 11%.The global vaccine technologies market has undergone significant transformations over the past decade, driven by technological advancements, increased investment in research and development (R&D), and the heightened awareness of infectious diseases. Vaccines have become a cornerstone in public health, playing a pivotal role in reducing the spread of infectious diseases, improving health outcomes, and saving lives. The COVID-19 pandemic has only accelerated the growth of the vaccine technologies market, spurring further innovations and expanding its scope. This article explores the current state of the vaccine technologies market, the emerging trends, key innovations, and future prospects.
Browse the full report at https://www.credenceresearch.com/report/vaccine-technologies-market
Market Overview
The vaccine technologies market refers to the collective field of innovations, platforms, and tools used in the development, production, and distribution of vaccines. In recent years, the market has expanded significantly due to the growing prevalence of infectious diseases, the emergence of new pathogens, and the increasing global population.
The market encompasses a wide range of technologies, including traditional inactivated and live-attenuated vaccines, protein subunit vaccines, viral vector vaccines, and the more recent mRNA-based vaccines. Each of these platforms offers unique benefits, with mRNA vaccines making headlines during the COVID-19 pandemic for their rapid development, high efficacy, and ability to address new strains quickly.
According to market research, the global vaccine technologies market was valued at approximately $40 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 9% from 2021 to 2028. This growth is attributed to increased government funding for vaccine development, rising demand for immunization programs, and growing partnerships between pharmaceutical companies and academic institutions.
Key Vaccine Technologies
1. Inactivated and Live-Attenuated Vaccines Inactivated vaccines use pathogens that have been killed or inactivated, rendering them unable to cause disease, while live-attenuated vaccines use a weakened form of the pathogen. Both types have been widely used for decades and remain a foundational technology in vaccine development. Examples include vaccines for diseases like polio (inactivated) and measles, mumps, and rubella (live-attenuated).
2. Protein Subunit Vaccines Protein subunit vaccines use specific fragments of a pathogen (such as a protein) to stimulate the immune response without introducing the entire pathogen. These vaccines are considered safer for immunocompromised individuals. They have been used for diseases like hepatitis B and HPV and are considered an important technology for future developments.
3. Viral Vector Vaccines Viral vector vaccines use a harmless virus (such as an adenovirus) to deliver genetic material from the target pathogen into human cells, prompting an immune response. This technology has been employed in vaccines like the AstraZeneca and Johnson & Johnson COVID-19 vaccines, offering robust protection against infectious diseases with a relatively quick development timeline.
4. mRNA Vaccines The success of mRNA-based vaccines during the COVID-19 pandemic has brought this technology into the spotlight. mRNA vaccines work by introducing a small piece of genetic material (mRNA) that instructs cells to produce a protein from the target pathogen, thereby triggering an immune response. Companies like Pfizer-BioNTech and Moderna have pioneered the use of mRNA in vaccines, which holds potential for rapid adaptation to new strains of viruses and other diseases like cancer and HIV.
Trends Driving the Vaccine Technologies Market
1. Personalized Vaccines Personalized vaccines, tailored to an individual’s genetic profile or specific disease characteristics, represent a growing area of interest. These vaccines hold the potential for improved efficacy, particularly for diseases like cancer, where each patient’s immune response may vary.
2. Advances in Adjuvant Technologies Adjuvants are substances added to vaccines to enhance the body’s immune response. Modern adjuvant technologies are being developed to improve vaccine potency, reduce the number of doses required, and target specific populations, such as the elderly or immunocompromised.
3. Nanotechnology in Vaccine Delivery Nanoparticle-based delivery systems are being explored for their ability to enhance the stability, efficacy, and targeted delivery of vaccines. Nanotechnology can help deliver antigens more efficiently to specific cells or tissues, potentially reducing side effects and improving the immune response.
4. Global Vaccine Manufacturing Capacity The COVID-19 pandemic exposed the vulnerabilities in global vaccine supply chains. This has led to increased investment in expanding global vaccine manufacturing capacity, particularly in low- and middle-income countries, to ensure equitable access to life-saving vaccines.
Challenges and Future Prospects
While the vaccine technologies market is growing rapidly, several challenges remain. Regulatory hurdles, high development costs, and logistical issues in distribution, particularly in low-resource settings, continue to pose barriers. Additionally, vaccine hesitancy remains a global issue, with misinformation and distrust in vaccines impeding public health efforts.
Looking ahead, the vaccine technologies market is poised for continued innovation. The development of vaccines for emerging infectious diseases, advancements in next-generation vaccine platforms (such as DNA vaccines), and the exploration of therapeutic vaccines for non-infectious diseases like cancer are key areas of focus. Furthermore, increased global collaboration and investment in vaccine research will be critical in preparing for future pandemics and addressing unmet medical needs.
Segments:
Based on Technology
Conjugate Vaccines
Recombinant Vaccines
Inactivated and Subunit Vaccines
Live Attenuated Vaccines
Toxoid Vaccines
Other Vaccine Technologies
Based on Type
Monovalent Vaccines
Mulitvalent Vaccines
Based on Disease Indication
Pneumococcal Disease
Influenza
Combination Vaccines
HPV
Meningococcal Disease
Herpes Zoster
Rotavirus
MMR
Varicella
Hepatitis
DTP
Polio
RSV
Other Disease Indications
Based on Route of Administration
Intramuscular & Subcutaneous
Oral
Other Route of Administration
Based on End User
Pediatric Vaccine
Adult Vaccine
Based on the Geography:
North America
U.S.
Canada
Mexico
Europe
Germany
France
U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of the Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/vaccine-technologies-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
The Marburg Virus: A Rising Threat and Current Developments in 2024

The Marburg virus, like its close cousin Ebola virus, is in the family Filoviridae but is much more virulent. Like Ebola, it causes severe hemorrhagic fever with a devastating fatal case rate of up to 88%.
The virus was isolated following an outbreak that first occurred in the town of Marburg, Germany, among laboratory workers handling infected monkeys in 1967. On the African continent, not least in 2024, have come recent occurrences of outbreaks that proved deadly and raised the profile of the virus throughout the globe.
Understanding the Marburg Virus
MVD is contracted primarily through contact with fruit bats. This is the natural host. Human-to-human transmission occurs directly or through contact with an infected person’s bodily fluids or contaminated surfaces. Symptoms occur acutely and may include high fever, severe headache, muscular pain, vomiting, both internal and external bleeding, and other symptoms. Generally, in most affected patients, the disease runs its course rapidly with multi-organ dysfunction and death. Its incubation period is within the range of 2 to 21 days. This limits quick detection and control and hence allows the pathogen to spread quickly, especially in under-resourced healthcare setups. It is not currently available with a vaccine, but experimental treatments and supportive care can salvage a few patients.
The latest Outbreak of the Marburg Virus in 2024
In September 2024, Rwanda confirmed its first outbreak of Marburg virus disease and confirmed a case of 26 with six deaths. The specific concern of this outbreak is the high cases found among healthcare workers, which indicates the existence of great challenges to the infection control protocols.

The country’s current outbreak came after recent occurrences were experienced in neighboring states. Recently, Tanzania faced a Marburg outbreak in 2023 which led the continent to assess the spread of the virus to other parts of East Africa. Equatorial Guinea and Uganda have also faced such Marburg outbreaks in the recent past, emphasizing the need for enhanced surveillance and cooperation across borders.
Global Response and Vaccine Development
The Marburg virus is recognized globally to be a direct threat, and an important step has been taken in the development of vaccines. Breakthrough came in 2023 with a vaccine that was undergoing evaluation in human subjects; at this time, a vaccine that was considered quite promising. The experimental cAd3-Marburg vaccine developed by the NIH elicited a good response in its first human trial-that is, a one-time dose vaccine with its basis of the chimpanzee adenovirus vector tested on 40 healthy volunteers. It provoked an immune response without adverse reactions; it is a potential candidate for emergency use in future outbreaks.
It is noteworthy that the vaccine is still in the very nascent stages of development; therefore, widespread clinical trials are required before the vaccine will be ready for mass deployment. Meanwhile, the health authorities are contained with traditional methods of outbreak control, such as isolation and contact tracing, and public education about health.
What Has Been Learnt from the Ebola Outbreak?
Several aspects made the Marburg virus similar to Ebola, thus permitting health authorities to reap lessons learned from previous Ebola outbreaks to better grapple with the current situation. Among them are the creation of rapid response teams, the use of PPE among healthcare workers, and community engagement aimed at reducing the transmission. Another imperative aspect of cross-border collaboration exists because viruses such as Marburg do not respect political boundaries. In Rwanda, neighboring countries have been alerted and preparedness has been enhanced for the prevention of the virus.
Moving Forward: Obstacles and Hopes
Although the situation is grim at this moment, there’s hope. On top of accelerated vaccines and treatment, international cooperation gives a glimmer of hope in controlling future outbreaks. WHO’s involvement in Rwanda’s outbreak response further proved its commitment to reining in the impact of the virus. However, there is also a challenge. The vaccines and antiviral treatments have yet to be approved, so the containment is solely dependent on early detection, isolation, and supportive treatment, which gives resource-constrained countries a special vulnerability because they generally lack infrastructure for handling such outbreaks. Stigma in affected communities usually acts as a barrier to public health efforts since the people involved may make later diagnoses and hence are likely to allow the disease to spread by delaying medical review.
Conclusion
In summary, the Marburg virus is a highly lethal pathogen that induces severe hemorrhagic fever, which symptomatically tends to appear rapidly. Marburg virus symptoms normally begin with sudden onset fever, chills, severe headache, and muscle pain. More advanced forms of the disease are accompanied by gastrointestinal symptoms such as nausea and vomiting and diarrhea, while the worst type of the disease involves jaundice, abdominal pain, and bleeding. These symptoms often present within 2 to 21 days from exposure; therefore, early recognition is important for effective management.

Currently, there is no proven treatment for the Marburg virus; however, support care has greatly improved survival. This includes hydration with oral or intravenous fluids to correct severe fluid loss as well as treating symptoms as they arise. Studies are underway on promising therapies, such as immune treatments and drug therapies, that might one day provide some hope for interventions.
The Marburg virus, as of now, is less well known than its cousin Ebola, but it’s dangerous and could trigger enormous damage. The recent outbreak in Rwanda could stir up in everybody the memory of the dangers the virus posed and continue necessary efforts with vigilance, more research, and preparedness.
Even when there’s still promising development in the vaccine sphere, the journey to eradicate the virus remains long.”. In the meantime, the present times will demand global health systems to be ready and flexible in case there is another outbreak of this killing disease. International co-operation together with the progress of medical research will see to it that the world wards off the Marburg virus and other emerging infectious diseases. That is, if the whole world had had the right tools and strategies, then one would have been able to stop the unbridled spread of disease in its track.
Disclaimer
In this article, information related to a particular topic has been collected from various sources, the purpose of which, is only to increase the knowledge of the readers and it does not confirm the existence of any disease, particular statement, explanation, appropriateness, congruity, and information or any kind of treatment. Health Alpha does not take any responsibility for any such information.
0 notes
Text
How the Gates Foundation Hijacked COVID-19 for Profit
After much thought and a continuing stream of new evidence coming to light, I am going to do this. This is a timeline of events leading up to and surrounding the coronavirus outbreak in late 2019. I still cannot prove that the long-lasting, breath-shortening cold many people caught in December 2019 was in anyway related to COVID-19. I also cannot find information – most likely because in 2013,…
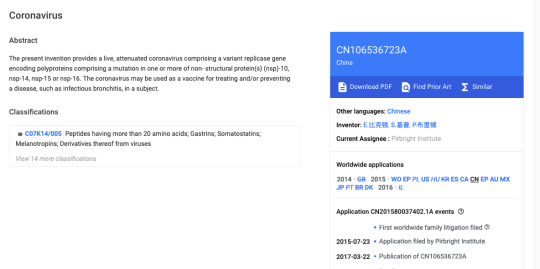
View On WordPress
#attenuated#attenuated live infections bronchitis virus#ChAdOx1#Chimp Adenovirus vector#Competition#coronavirus#Covid-19 vaccine#Dr Tess Lawrie#Erica Bickerton#Gates Foundation#human adenovirus#intellectual property#Inventor#Ivermectin#licensing#Livestock Antibody Hub#mutant spike protein#patents#pirbright institute#propaganda and censorship#Protease inhibitor#Responding to public#shutting down public speculation#viruses affecting farm animals and human health#Written Evidence to parliament
0 notes
Text
Maxanim Enhancing Laboratory Solutions for Research and Diagnosis
In a groundbreaking move within the fields of laboratory supply and biotech research, Maxanim proudly announces its integration into the esteemed Gentaur Group. This partnership marks a significant milestone in the pursuit of providing cutting-edge testing tools, reagents, and specialized solutions for laboratories across the USA and Europe. With a focus on delivering high-quality products tailored for research and diagnostic purposes, Maxanim’s inclusion within the Gentaur Group fortifies its commitment to excellence and innovation in the field.
Maxanim’s expertise lies in the provision of laboratory reagents and tools essential for a wide array of scientific endeavors, ranging from fundamental research to advanced diagnostic applications. As part of the Gentaur Group, Maxanim extends its reach and capabilities, ensuring a broader access to its comprehensive portfolio of products and services.
One of the flagship offerings by Maxanim is its extensive range of ELISA kits meticulously designed for research purposes. These kits, renowned for their reliability and accuracy, empower researchers with the tools necessary to explore various biological pathways, identify biomarkers, and unravel the mysteries of diseases. Whether unraveling the complexities of cancer biology or deciphering the mechanisms of infectious diseases, Maxanim’s ELISA kits, Panbio serve as invaluable assets in the scientific community’s quest for knowledge and breakthroughs.
The expanded portfolio of Maxanim now includes a comprehensive range of products such as Abbott, ABM Labs’ innovative tools for gene expression studies, Adeno and AAV vectors for gene therapy applications, iPSC reagents for stem cell research, Lentivectors and Retroviral vector for gene delivery systems, as well as Adenovirus vectors for vaccine development and gene transfer experiments. Additionally, Maxanim offers products from renowned suppliers such as Cusabio, Nova Lifetech plasmids, Gentarget, SBI, ABMGood, and Genprice, ensuring access to a diverse array of high-quality reagents and tools.
Furthermore, Maxanim takes pride in its prowess in manufacturing custom recombinant proteins and plasmids, catering to the specific needs and requirements of researchers and laboratories. With a keen emphasis on quality assurance and precision, Maxanim ensures that each custom product meets the highest standards of excellence, empowering scientists with the flexibility to embark on ambitious projects and push the boundaries of scientific discovery.
In addition to recombinant proteins and plasmids, Maxanim specializes in the design and production of primers, rabbit plyclonal antibodies, and mouse monoclonal antibodies. These essential tools play a pivotal role in various experimental techniques, including PCR, Western blotting, and immunohistochemistry, facilitating the detection and analysis of specific molecules with unparalleled specificity and sensitivity.
The integration of Maxanim into the Gentaur Group not only amplifies its product offerings but also reinforces its commitment to customer satisfaction and service excellence. With a dedicated team of experts and scientists, Maxanim remains steadfast in its mission to empower researchers and laboratories with the tools and resources necessary to drive groundbreaking discoveries and advancements in the realms of biotechnology and medical research.
Moreover, Maxanim’s collaboration with Gentaur Group enhances its distribution network, ensuring prompt and efficient delivery of products to laboratories across the USA and Europe. Through strategic partnerships and alliances, Maxanim endeavors to streamline the procurement process for researchers, enabling them to focus their efforts and resources on their scientific pursuits.
With Maxanim’s integration into the Gentaur Group, researchers can now benefit from a seamless procurement experience, accessing a wide range of products including ELISA kits, PCR reagents, Antybody, and quality controls like NatTtrol. Whether it’s basic research, drug discovery, or clinical diagnostics, Maxanim remains committed to providing the necessary tools and support to accelerate scientific progress and improve human health worldwide.
[Related site1] [Related site2]
0 notes
Text
Detailed Report on Upstream Bioprocessing Market | BIS Research
Upstream Bioprocessing refers to the initial phase of Biopharmaceutical production that involves the preparation and growth of cells or microorganisms to produce the desired biological product.
The Global Upstream Bioprocessing Market was valued at $250.1 million in 2023 and is expected to reach $1,639.1 million by 2033, growing at a CAGR of 20.68% between 2023 and 2033.
Upstream Bioprocessing Overview
Upstream Bioprocessing is an advanced manufacturing approach used in the production of biological products, including pharmaceuticals, biopharmaceuticals, and industrial enzymes. This method is characterized by the uninterrupted flow of materials through the production process, from the initial cultivation of cells or microorganisms to the final product purification.
Grab the free sample page click here
Key Stages involved in Upstream Bioprocessing
Cell Line Development
Media Preparation
Bioreactor Cultivator
Monitoring and Control
Key Applications for Upstream Bioprocessing
Biopharmaceutical Production
Monoclonal Antibodies
Recombinant Proteins
Vaccines
Gene Therapy- Production of viral vectors (such as lentivirus and adenovirus) for delivering genetic material in gene therapy applications.
Cell Therapy - Cultivation of stem cells and other cell types used in regenerative medicine to repair or replace damaged tissues and organs.
Agricultural Biotechnology - Development of genetically modified organisms (GMOs) and biofertilizers to enhance crop yield, pest resistance, and nutrient utilization.
Market Drivers for Upstream Bioprocessing Market
Growing demand for Biopharmaceuticals
Advancements in Biotechnology
Expansion of Personalized Medicines
Technological Innovations
Environmental Sustainability
These market drivers collectively contribute to the growing adoption and expansion of the Upstream Bioprocessing Market
Recent Developments in the Upstream Bioprocessing Market
• Waters and Sartorius expanded their partnership to develop integrated analytical tools for downstream biomanufacturing following their successful collaboration in upstream processes. • Sartorius and Repligen Corporation launched an integrated system with Biostat STR and XCell ATF for upstream process intensification.
Visit our Life Sciences and Biopharma Vertical page for better understanding
Key Players in the market
• 3M • Bio-Rad Laboratories, Inc. • Thermo Fisher Scientific, Inc. • Merck KGaA • Sartorius AG • Danaher Corporation
Key Questions Answered
Q What is the estimated global market size for the Upstream Bioprocessing Market ?
Q What future trends are expected in the Upstream Bioprocessing Market ?
Q What does the supply chain of the Upstream Bioprocessing Market look like?
QWhat does the value chain of the Upstream Bioprocessing Market look like?
Q What is the regulatory framework within the Upstream Bioprocessing Market ?
Q What is the patent analysis trend based on country and year in the Upstream Bioprocessing Market ?
Q How has the COVID-19 outbreak affected the future trajectory of the Upstream Bioprocessing Market ?
Q What are the next frontiers in the Upstream Bioprocessing Market ?
Conclusion
Upstream bioprocessing is a critical and foundational phase in the production of biopharmaceuticals, playing a vital role in the cultivation and optimization of cells or microorganisms to produce high-quality biological products.
As the biopharmaceutical industry continues to evolve, upstream bioprocessing will remain a cornerstone, driving the development of new and innovative therapies that improve patient outcomes and address unmet medical needs.
0 notes
Text
Reference archived on our website
I know some people will celebrate, but the West has been dragging behind in covid vaccine development, and the issue with these vaccines is they only help provide herd immunity if people actually get them. Two struggles to overcome before we can party.
BACKGROUND. The level of nasal spike-specific secretory IgA (sIgA) is inversely correlated with the risk of SARS-CoV-2 Omicron infection. This study aimed to evaluate the safety and immunogenicity of intranasal vaccination using Ad5-S-Omicron (NB2155), a replication-incompetent human type 5 adenovirus carrying Omicron BA.1 spike.
METHODS. An open-label, single-center, investigator-initiated trial was carried out on 128 health care workers who had never been infected with SARS-CoV-2 and had previously received 2 or 3 injections of inactivated whole-virus vaccines, with the last dose given 3–19 months previously (median 387 days, IQR 333–404 days). Participants received 2 intranasal sprays of NB2155 at 28-day intervals between November 30 and December 30, 2022. Safety was evaluated by solicited adverse events and laboratory tests. The elevation of nasal mucosal spike-specific sIgA and serum neutralizing activities were assessed. All participants were monitored for infection by antigen tests, disease symptoms, and the elevation of nucleocapsid-specific sIgA in the nasal passage.
RESULTS. The vaccine-related solicited adverse events were mild. Nasal spike-specific sIgA against 10 strains had a mean geometric mean fold increase of 4.5 after the first dose, but it increased much higher to 51.5 after the second dose. Serum neutralizing titers also increased modestly to 128.1 (95% CI 74.4–220.4) against authentic BA.1 and 76.9 (95% CI 45.4–130.2) against BA.5 at 14 days after the second dose. Due to the lifting of the zero-COVID policy in China on December 7, 2022, 57.3% of participants were infected with BA.5 between days 15 and 28 after the first dose, whereas no participants reported having any symptomatic infections between day 3 and day 90 after the second dose. The elevation of nasal nucleocapsid-specific sIgA on days 0, 14, 42, and 118 after the first dose was assessed to verify that these 2-dose participants had no asymptomatic infections.
CONCLUSION. A 2-dose intranasal vaccination regimen using NB2155 was safe, was well tolerated, and could dramatically induce broad-spectrum spike-specific sIgA in the nasal passage. Preliminary data suggested that the intranasal vaccination may establish an effective mucosal immune barrier against infection and warranted further clinical studies.
#covid#mask up#pandemic#covid 19#wear a mask#public health#coronavirus#sars cov 2#still coviding#wear a respirator
2 notes
·
View notes
Text
Scientists Unravel Blot Clotting Disorder Caused By Covid 19 Vaccines
dangerous PF4 antibodies involved in vaccine-induced thrombosis (VITT) and similar disorders from common cold infections share identical molecular structures, highlighting implications for future vaccine development and disease management.
New research conducted by Flinders University and global specialists is deepening our knowledge of vaccine-induced immune thrombocytopenia and thrombosis (VITT). During the peak of the COVID-19 pandemic in 2021, VITT was recognized as a new condition linked to adenovirus vector-based vaccines, particularly the Oxford-AstraZeneca vaccine.
VITT was found to be caused by an unusually dangerous blood autoantibody directed against a protein termed platelet factor 4 (or PF4). In separate research in 2023, researchers from Canada, North America, Germany, and Italy described a virtually identical disorder with the same PF4 antibody that was fatal in some cases after natural adenovirus (common cold) infection.
0 notes