#absorption spectra
Explore tagged Tumblr posts
Text

The Science Research Manuscripts of S. Sunkavally. Page 212.
#inorganic cations#absorption spectra#superoxide dismutase#catalase#sodium chloride#ionization#absorption lines#pressure broadening#flame photometry#atomic sodium#transition metal ions#gas chromatography#body size#longevity#satyendra sunkavally#theoretical biology#manuscripts
1 note
·
View note
Text
Beta Lyrae - Sheliak
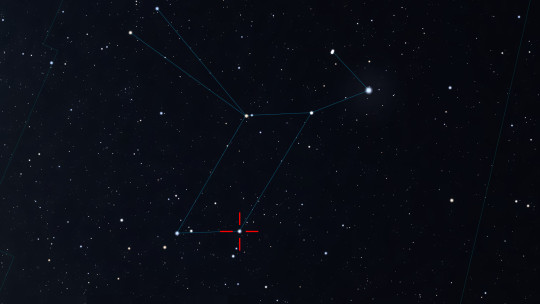
The small constellation of Lyra is not only very beautiful in the configuration of the stars that make up it, but also contains among them absolutely unique universal diamonds. The star Sheliak - Beta Lyrae is one of such jewels.
Translated from Arabic, Sheliak means a turtle shell (or even the turtle itself), but the same word is used to name an ancient musical instrument similar to a harp or, in fact, a Lyre, because a resonator body was made from a turtle shell, which was necessary for a more melodious sound of the strings. A musical culture on Earth was born somewhat cruelly, singing, among other things, the heavenly distances in which man saw the Worlds of his future - incomparably happier than the one in which he lived then. But man evolved, and at some point, wood began to be used more and more often for making musical instruments - Kifaras and Lyras with a wooden body sounded even better than turtle ones. But the dim star in the constellation Lyra remained called the Turtle.

What exactly this star attracted the attention of ancient astronomers is unknown now, but it was clearly not deprived of attention. It is simply that much of the scientific information from that historical period was lost. And in the 18th century it was discovered that about once a week the star slightly "winks" - fading for a short time, becoming dimmer by about two times. This could easily have been noticed in ancient times, and certainly was noticed (as astronomers of all developed countries of the ancient era noticed similar behavior of the star Algol in the constellation Perseus), but they just could not explain it.
The variability of Beta Lyrae (as well as the variability of Algol) was explained by a young amateur astronomer, John Goodricke. He had no special education, and was also deaf and dumb, but a very keen-sighted and insightful person. John's passion was observing stars, and by the age of 20 he had already examined many of them for brightness variations - simply with the naked eye, since there were no other methods at the end of the 18th century.

Only intuition can explain John Goodricke's guess that the apparent brightness of some stars most likely changes because in reality it is not one star, but two, which, rotating around the center of mass of the system, periodically obscure each other for an observer on Earth. This bold assumption was confirmed a hundred years after the death of the amazing seer.
John Goodricke died very young - at 22, succumbing to pneumonia - two years after discovering and explaining the variability of Beta Lyrae, and just four days after being elected a Fellow of the Royal Society of London.
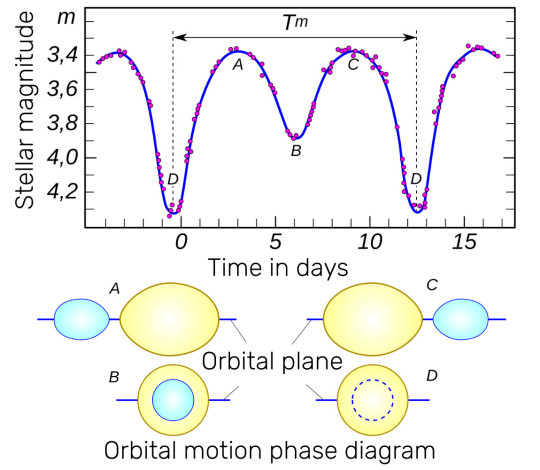
At the turn of the 19th and 20th centuries, astronomers learned to perform detailed spectral analysis of stars and track the behavior of absorption lines in their spectrograms. In the spectra of Sheliak (Beta Lyrae) and Beta Persei (Algol), the lines behaved similarly - slightly swaying around the places assigned to them by the chemistry of the star - synchronously with the dynamics of brightness changes. This clearly indicated the presence of two luminaries in the system and their orbital motion. This was irrefutable evidence, but indirect. A century later - already in our days - a direct image of the Beta Lyrae system was obtained using the CHARA infrared interferometer. And astronomers saw with their own eyes two stars orbiting the center of mass of the system, alternately eclipsing each other.

It was also discovered that the shapes of these stars are distorted by tidal interactions and resemble oblong melons. It was even discovered that matter flows from one star to another, which changes the proportion of masses in the system and slows down the rotation, increasing the period by 19 seconds per year. At the same time, part of the matter forms an extended accretion disk around the system, which partially dissipates into interstellar space. And in addition to the exchange of mass in this system, there is a significant loss of it.
The period of brightness variation in the Beta Lyrae system is 13 days. But since eclipses occur twice during the entire period (the stars eclipse each other in turn, as if changing places), brightness minima occur approximately once a week. But they are slightly different in depth - 0.9 and 0.5 stellar magnitudes.
Nowadays, the horizons of astronomy have expanded somewhat, thanks to advanced technologies for studying the Universe. And it is already believed that Beta Lyrae is a relatively close system to us. But still, 900 light years is not that close. To see separately stars that are twice as close to each other as the Sun and Mercury is a great achievement for optical interferometry.
The distance separating the components of this system is estimated at 40 million km. Somewhere at this distance from the Sun, in the era of the famous Le Verrier, astronomers were looking for the planet Vulcan. And they found nothing. But now they see a system of two giant stars with similar orbits.
Both components of this system are quite massive — 3 and 13 times the mass of the Sun. And in luminosity — 26 thousand and 6 thousand times, respectively. It is interesting that during the existence of the system — about 20 million years — these stars seemed to have swapped roles. The one that is now massive was skinny, developed more slowly, and the neighboring star — having quickly swelled to the size of a giant — began to share matter with the smaller star. The neighbor was not modest, and took over as much as was given. And now the star that had less mass has gained it in excess. And continues to do so.
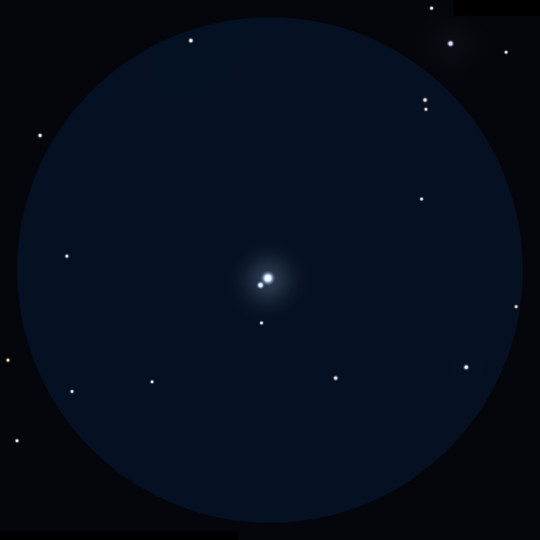
Somewhat removed from this close and amazing pair of stars, another component of the system, the so-called Beta Lyrae B, slowly drifts along a huge orbit. What we talked about before was Beta Lyrae A. But there are many more stars in this system.
Beta Lyrae B is clearly visible even in binoculars - it is a 7th magnitude star, separated from the eclipsing variable pair by 46 arc seconds (this is the apparent size of Jupiter). In a small telescope, this double star looks very nice and is an easy object for beginner astronomy enthusiasts. Now it is known that it is also a double system, though a spectral one - not divisible into components in any telescope (they are very close to each other, or maybe it just has not deserve such close attention, and some super-powerful interferometer has not yet been deployed on it).

Another star was found 64 arc seconds away from the main pair of stars, though rather faint for amateur optics - Beta Lyrae C - 13th stellar magnitude. And further away from the aforementioned luminaries, astronomers are now checking for involvement in the system of several more stars - D, E and F. Moreover, in relation to Beta Lyrae F, even convincing evidence of a gravitational connection has been found.
Once again, we started with one star and ended up with a whole star cluster. However, this situation is not uncommon in our Universe. A rare type of star in it are stars like our Sun - stable and completely alone. But maybe that is why we now have the opportunity to study the entire diversity of other cosmic luminaries.

26 notes
·
View notes
Text
Steve Saga Rainbow Factory AU Lore
Just a quick heads up. If you're a kid reading this, please don't. I know it says Steve Saga and that's a show for a younger audience, but this AU definitely isn't. It contains mature topics such as mental issues, violence, death and the likes. So please, when reading stuff on the internet: Be safe, not brave. You have been warned.
________________________________________
It all started with an incredibly traumatic event for Rainbow Steve. Essentially he had to watch his best friend Sabre die during some gruesome fight.
Yep. Sabre’s dead.
Rainbow snapped after that. This event is also where he got the scars on his face and the blind eye from. (The post with his design is Here.)
Rainbow became so sick and tired of being a useless, weak idiot, that he decided to switch to drastic measures in order to finally become strong. That day he swore to himself, to become the strongest Steve to have ever existed and quench all “evil” and “weakness” under his heel.
But, oh, the price he’d have to pay.
Rainbow founded and built the Rainbow Factory. Initially he started to experiment on power sources like crystals and power stone, but his efforts were quickly proven fruitless. So he switched to Steves relatively soon.
Experiments quickly became ugly and terrifying, he didn’t fully know what he was doing at first.
Eventually he discovered that a colorful substance could be extracted from Steves. Some sort of liquid energy.
Rainbow named this substance “Spectra”. He also discovered that Spectra could be injected back into Steves and had different effects on them, based on the colors used.
Did the Spectra and Steve match colorwise, then the Steve absorbed it no problem and became stronger.
This was a great success and Rainbow started injecting himself, quickly becoming addicted to the initial thrill of Spectra absorption.
Did the Spectra and Steve not match colorwise, then the effects would be negative to varying degrees. The further the two colors were apart from each other, the more different they were, the more horrific the effects and subsequent suffering would be.
But Rainbow didn’t stop there… Doing this all by himself was tiresome after all. He opted to build himself a team. He wanted to make more Steves like himself.
Experiments continued. All of his attempts to fuse different colors failed however. Except for one. It was more of an accident, but somehow Rainbow managed to unite all colors within one Steve, albeit in the wrong order.
Rainbow named his experiment Reverse Steve, who immediately became his favorite experiment. A bit of “improvements” and training later, Reverse was allowed to work alongside Rainbow.
Together they started a campaign to brainwash more people into joining their cause. Plague Steve stepped up, voicing his interest. So he also works for the factory now.
Some experiments got either broken or manipulated so badly by Rainbow, that he can let them work for his factory freely as well.
So, basically, Rainbow became worse than Nightmare. He became the very thing he’d sworn to destroy. But he doesn’t care anymore. He’s done with the world’s bullshit.
He turned from the prey to the hunter, now actively searching for Nightmare and his crew. Rainbow plans on turning Dark and Shadow into experiments as well, eager to find out how exactly their dark powers work and if they had Spectra in them as well.
For Nightmare the plan is a lot worse. Because Nightmare absorbed so many Steves during his lifetime, he is a giant battery for lots - and I mean LOTS - of Spectra of all kinds. Giving him the “Extraction Treatment” once, simply won’t be enough. In Rainbow’s ideal world, Nightmare will have to endure the Pegasus Device multiple times, so he can take that sweet, sweet Spectra all for himself.
You’re wondering what Lucas and Light are doing? As soon as Rainbow went downhill mentally, Lucas grabbed Light and went into hiding with him, hoping to protect the innocent Steve from any harm.
#steve saga#favremysabre#the steve saga#steve saga au#rainbow steve#rainbow factory au#RF! Rainbow Steve
20 notes
·
View notes
Text

Absorption Spectra Of Solutions - Plates. 1910.
Internet Archive
129 notes
·
View notes
Text
Study on the storage stability of phycocyanin from Spirulina obtusususiae
Abstract: The effects of temperature, sunlight and different additives on the stability of aqueous solutions of phycocyanin were studied. It was concluded that phycocyanin should be stored at 40 ℃ and protected from light, and should be stored under neutral conditions; glucose, sodium chloride and sorbitol could effectively improve the stability of phycocyanin, and the pigment preservation rate of phycocyanin increased from 50.90% to 78.10%, 67.02% and 69.08% after 72 h at room temperature, respectively; the stabilizers of phycocyanin were compounded with glucose, sodium chloride and sorbitol in the mass ratio of 1 : 1 : 0.3 and left at 4 ℃ for 14 days. After adding glucose, sodium chloride and sorbitol as stabilizers in the mass ratio of 1:1:0.3, the pigment retention rate of the alginate was increased by 54.4% compared with that of the unadded alginate after being placed at 4 ℃ for 14 d. The pigment retention rate of the alginate added with the additive was increased by 16.1% compared with that of the unadded one after being placed at 25 ℃.

Spirulina (English name spirulina), also known as "spirulina", belongs to the family of Cyanobacteria, Chlamydomonas; at present, there are three types of large-scale cultivation at home and abroad, namely, Spirulina major, Spirulina obtususus and Spirulina indica. Spirulina obtususus is a blue-green seaweed (cyanobacteria) belonging to the Candida family.
It is a non-branched, multicellular spiral mycelium with a length of about 200 μm~300 μm and a width of about 5 μm~10 μm [1]. The amino acid composition of the proteins contained in Spirulina obtusususiformis is very uniform and reasonable, which suggests that it can be used as a potential health food for human beings [2].
Phycocyanin is one of the photosynthesizing proteins in the phycobilins, which are chromophore polypeptides consisting of α and β subunits with a molecular weight of about 20,000 daltons [3]. The phycobilisome in the cyanobacterium Spirulina obtususus is composed of an alpha and beta subunit in the center and a phycocyanin in the periphery. Phycocyanin is the most important bile protein in Spirulina, accounting for about 20 % of the dry weight [4-6]. It has a blue color in aqueous solution and fluoresces in purple. The UV-Vis spectra of phycocyanin in Spirulina obtusususiformis show characteristic absorption peaks at 278, 360 and 620 nm [7]. It has also been shown that the maximum absorption peak of L. obtususus is at 620 nm and its fluorescence emission peak at room temperature is at 645 nm [8].
Natural pigments are very rich in variety and are classified according to a variety of bases. According to solubility can be divided into fat-soluble pigments, water-soluble pigments; according to the source can be divided into animal pigments, microbial pigments and phytochromes; in order to classify the different chemical structures for anthocyanins, carotenoids and other five categories [9-10].
Alginin is a natural blue pigment with high application value. It has been shown to be anticancer[11-12] and can be used as a health food for patients with enteritis[13] . It is highly water-soluble and can be easily extracted from Spirulina. In the process of extraction and purification, the control of pH value and ionic strength is very crucial for the stability of algal blue protein. The discoloration and denaturation of phycocyanin is determined by the grade of protein polymers, and its polymer form is mainly affected by light intensity, light time, temperature, pH value, irradiation and protein concentration [14-17].
It has been studied that the higher concentration of sodium chloride can protect the stability of alginate, and the appropriate amount of sodium benzoate can protect the color and preservation of alginate to a certain extent [18-19], but the stability of alginate is still low. Therefore, on the basis of previous studies, this experiment was carried out to investigate the effects of different food-grade additives as well as glucose, sodium chloride and sorbitol additives on the stability of alginate.
1 Materials and Methods
1.1 Materials and Main Instruments
Spirulina obtususifolia powder: Inner Mongolia Wuxingzhao Ecological Industry Development Co.
FD-10 Freeze Dryer: Beijing DTY Technology Development Co., Ltd; 756PC UV Spectrophotometer: Tianjin Prius Instrument Co., Ltd; DK-98-II Electric Thermostatic Water Bath: Tianjin Taiste Instruments Co.
1.2 Extraction and purification of algal blue protein
1.2.1 Extraction of algal blue proteins[19]
Appropriate amount of spirulina powder was dissolved in distilled water according to the material-liquid ratio of 1:40 (mass ratio), and then stirred with a stirring rotor at a speed of 1,000 r/min for 1.5 h. It was frozen at -18 ℃, and then thawed rapidly in a 37 ℃ water bath for 24 h. After repeating this procedure for four times, it was centrifuged at a high speed for 10 min at 10,000 r/min, and the absorbance at 620 and 280 nm was measured after taking the supernatant and diluting it with appropriate multiplicity.
1.2.2 Purification of algal blue proteins[17]
Take the crude extract of algal blue protein with the concentration of 5 mg/mL, slowly add ammonium sulfate solid to the saturation degree of 40%, and at the same time, carry out magnetic stirring until complete dissolution, stand at 4 ℃ for 2 h, then centrifuged at 10 000 r/min for 15 min, collect the precipitate, dissolve it in an appropriate amount of distilled water, and then freeze-dried after dialysis and set aside.
1.3 Research on storage stability of algal blue protein
1.3.1 Effect of temperature on the stability of phycocyanin[19]
30 mg of alginate was dissolved in 30 mL of citrate phosphate buffer at pH 5.0, 6.0 and 7.0, and incubated in 6 temperature gradients (20, 30, 40, 50, 60 and 70 ℃) for 30 min. The absorbance was measured at 620 nm after appropriate dilution, and the pigment retention rate was calculated. The pigment retention rate was calculated according to equation (1):
Pigment retention rate/% = ×100 Equation (1)
1.3.2 Effect of daylight illumination on the stability of phycocyanin [19]
Two groups of 1 mg/mL aqueous phycocyanin solution were taken, one group was irradiated under a single light source (sunlight) and the other group was stored away from light, and then diluted appropriately after 12, 24, 36, 48, 60, and 72 hours, respectively, and the absorbance value was measured at 620 nm to compare the changes in the retention rate of phycocyanin pigments.
1.3.3 Effect of pH on the stability of algal blue protein
Take 0.1 g of alginate powder and dissolve it in 100 mL of citrate phosphate buffer with pH value of 5.0, 5.5, 6.0, 6.5 and 7.0 respectively, there are 5 groups in total, and take samples at 30 min intervals to dilute appropriately, and measure the absorbance value at the wavelength of 620 nm, and then compare the changes of the preservation rate of the alginate pigment.
1.3.4 Effect of food additives on the stability of algal cyanoproteins [20-21]
Take 100 mL of algal blue protein solution with a concentration of 1 mg/mL, and add the following additives in order according to the maximum additive amount of food additives stipulated in GB 2760-2011 Standard for the Use of Food Additives: glucose (5 g), sucrose (5 g), sodium chloride (5 g), sorbitol (0.003 g), sodium benzoate (0.000 2 g), ascorbic acid (0.002 g), and sodium benzoate (0.000 2 g), and the following additives are added to the solution. 0.002 g). After 24, 48 and 72 hours of exposure to sunlight at room temperature and appropriate dilution, the absorbance value at 620 nm was measured to compare the changes in the retention rate of phycocyanin pigments. The effects of different concentrations of glucose and sodium chloride on the stability of algal blue protein were measured according to the above method. Select appropriate concentrations of glucose, sodium chloride and sorbitol and add them into the aqueous solution of phaeocyanin, and carry out the test according to the above method to observe the change of pigment retention rate.
2 Results and analysis
2.1 Effect of temperature on the stability of phycocyanin
The effect of temperature on the stability of phycocyanin is shown in Fig. 1.
As can be seen from Fig. 1, the pigment retention rate of algal blue protein decreased with the increase of temperature when it was placed at different temperatures for 30 min. When the temperature was 20 ℃
The pigment retention rate of alginate stored at 40 ℃ was almost unchanged; the pigment retention rate of alginate stored at 50 ℃ and 60 ℃ decreased by 11.68% and 20.71%, respectively, compared with that of the initial one after 30 min, and the pigment retention rate of alginate stored at 70 ℃ showed the greatest decrease, which was 58.58% lower than that of the initial one.
High temperature will destroy the structure of algal blue protein and cause its denaturation, resulting in a decrease in the pigment retention rate of algal blue protein. It can be seen from the results that phycocyanin has the highest and most stable pigment retention rate between 20 ℃ and 40 ℃. Therefore, high temperature storage should be avoided below 40 ℃.
2.2 The effect of light on the stability of phycocyanin
The effect of sunlight illumination on the stability of phycocyanin is shown in Fig. 2.
As can be seen from Fig. 2, under the irradiation of room temperature and single sunlight source, the pigment retention rate of the algal blue protein solution decreased greatly from 48 h. At the same time, the color fading was obvious, and the color gradually changed from sapphire blue to light blue from 48 h, and became almost colorless and transparent at 60 h. The pigment retention rate decreased by 59.31% compared with that at 0 h, and the rate of the pigment retention rate was only 29.26% of the initial one at 72 h. The color retention rate of the solution decreased from 0 h to 60 h, and the color retention rate of the solution was only 29.26% of the original one at 72 h. After 72 h, the pigment retention rate was only 29.26%. The pigment retention rate of phycocyanin stored at room temperature under the condition of light protection was higher than that of sunlight, but the effect was not great, and the pigment retention rate of phycocyanin at 72 h was 13.51% higher than that of sunlight. It can be concluded that the sensitivity of phycocyanin to heat is greater than that to light, but light also has a certain effect on the pigment stability of phycocyanin. Therefore, phycocyanin should be stored under light-proof conditions.
2.3 Effect of pH value on the stability of algal blue protein
The effect of pH on the stability of phycocyanin is shown in Fig. 3.
Figure 3 shows that the pigment retention rate of phycocyanin solution at pH 5.0, 5.5, 6.0, 6.5 was small, and the pigment retention rate was kept in the range of 95.49%~102.19%; and it can be seen that the phycocyanin was the most stable and the highest pigment retention rate was found at pH 6.0. At pH 7.0, the pigment retention rate decreased greatly, from 100 % to 87.46 % gradually. This may be due to the fact that the alkaline condition damaged the structure of phycocyanin, so it should be preserved in neutral condition instead of alkaline condition.
2.4 Effect of additives on the stability of algal blue proteins
The effect of food additives on the stability of algal blue proteins is shown in Fig. 4.
Additive type
Fig. 4 Effect of food additives on the stability of algal blue proteins
Fig.4 The influence of food additives on stability of phycocyanin
Figure 4 shows that the retention rate of phycocyanin pigments in phycocyanin solutions with different additives increased and then decreased during 72 h of storage at room temperature under sunlight. This may be due to the incomplete dissolution of phycocyanin at the beginning. The highest pigment retention was observed in the alginate with glucose, sorbitol and ascorbic acid, which decreased from the initial 100 % to 78.10 %, 69.08 % and 67.24 %, respectively, which was significantly higher than that of the blank group (50.90 %). This may be attributed to the fact that the additives can protect the color of the algal blue protein and increase its pigment retention rate. However, the solution of phycocyanin with ascorbic acid produced a large amount of precipitation. Therefore, glucose, sodium chloride and sorbitol were selected for further study.
2.5 Effect of glucose concentration on the stability of algal blue proteins
The effect of glucose concentration on the stability of phycocyanin is shown in Fig. 5.
As shown in Fig. 5, the color retention rate of glucose-added phaeocyanin increased after 24 h, and then decreased with time. This may be due to the color protection effect of glucose on phycocyanin. The pigment retention rate of the alginate without glucose did not change much after 24 h at room temperature. When the concentration of glucose was 10 mg/mL, the absorbance value of phycocyanin increased greatly after 24 h, and the pigment retention rate of phycocyanin increased by 16.15%, which was 12.62% higher than that of phycocyanin without added glucose; the pigment retention rate of phycocyanin with added glucose at 10 mg/mL reached 78.09%, which was 27.19% higher than that of phycocyanin without glucose. After 72 h, the color retention rate of the solution with 10 mg/mL glucose reached 78.09%, which was 27.19% higher than that of the solution without glucose, and then the retention rate of alginate color tended to slow down as the concentration of glucose solution increased. Therefore, for the purpose of cost saving, 10 mg/mL of glucose was chosen for the next study.
2.6 Effect of sodium chloride concentration on the stability of algal blue proteins
The effect of NaCl concentration on the stability of algal blue protein is shown in Fig. 6.
Fig. 6 Effect of sodium chloride concentration on the stability of algal blue protein
As can be seen from Fig. 6, the pigment retention rate of the alginate without NaCl remained almost unchanged after 24 h, while the absorbance values of the alginate with NaCl increased, which was attributed to the protective effect of NaCl on the color of the alginate to inhibit the denaturation of the alginate. The color retention rate of the solution with 10 mg/mL NaCl was significantly higher than that of the blank group after 72 h, reaching 75.90%, and then leveled off. Therefore, in order to save the cost of the experiment, 10 mg/mL NaCl was chosen for the next study.
2.7 Effects of sorbitol, sodium chloride and glucose on the stability of phycocyanin
The effects of sorbitol, NaCl and glucose on the stability of phycocyanin are shown in Figure 7.
Figure 7 shows the complex color protection effect of the three additives on phycocyanin. The pigment retention rate of the alginate solutions increased to different degrees after 24 h at room temperature under sunlight, which was attributed to the color protection effect of the additives. In the blank group, the pigment content of the alginate solution remained almost unchanged after 24 h, and then decreased rapidly; the absorbance value of the alginate solution with the addition of sorbitol, dextrose and sodium chloride increased the most obviously, which was 41.29% higher than that at 0 h, and 38.38% higher than that of the alginate solution without the addition of the additives; and the color preservation was 23.01% higher than that of the blank group at 72 h. The effect of color preservation was very obvious. After 72 h, the color preservation rate was higher than that of the blank control group by 23.01%, and the color preservation effect was obvious. The stability of sorbitol-added phycocyanin was second, and its pigment preservation rate was 19.09% higher than that of the blank control group after 72 h at room temperature under sunlight. This is due to the compound effect of sorbitol, glucose and sodium chloride on alginate to play a good role in color protection and preservation, which is better than several other combinations of additives. Therefore, sorbitol, dextrose and sodium chloride can be added as compound stabilizers in alginate at a mass ratio of 1 : 1 : 0.3.
2.8 Effect of three additives on the stability of algal blue proteins
The initial pictures of phycocyanin (without additive) and phycocyanin (with additive) at (4±5)°C and (25±5)°C are shown in Fig. 8, and the pictures of phycocyanin (without additive) and phycocyanin (with additive) at (4±5)°C and (25±5)°C after 14 d are shown in Fig. 9, and the effects of three additives on the stability of phycocyanin are shown in Fig. 10.
Figures 8, 9 and 10 show the changes in pigment content of phycocyanin after the addition of glucose, sodium chloride and sorbitol as stabilizers for 14 d. The pigment retention rate of phycocyanin solutions decreased with the increase of storage days and varied under different conditions. The pigment retention of phycocyanin solutions decreased with the increase of storage days, and the pigment retention varied under different storage conditions. The most suitable storage condition for phycocyanin solution was 4 ℃ with preservative, and its pigment retention rate only decreased by 30.21% after 14 d of storage, which was 54.5% higher than that of phycocyanin stored at 4 ℃ without additive. However, the pigment retention rate of the unadditive alginate solution was almost zero after 14 d of storage at 25 ℃, with almost total loss of phycocyanin, and the pigment retention rate of the additive solution was 16.1% higher than that of the unadditive one. The pigment retention rate of the additive solution was significantly higher than that of the unadditive one at 25 ℃ and 4 ℃, which was attributed to the excellent color protection and anticorrosive effect of the three additives on the phycocyanin. This is due to the fact that the combination of the three additives has a good effect on the color protection and preservation of phycocyanin. Therefore, alginate is suitable for storage at low temperature with additives.
3 Conclusion
Differences in temperature, sunlight and pH all affect the storage stability of phycocyanin, with temperature having the most pronounced effect on the stability of phycocyanin and sunlight having a lesser effect on the stability of phycocyanin.
Appropriate concentrations of sorbitol, dextrose and sodium chloride can obviously protect the color of alginate and preserve it, and do not affect its properties. In this experiment, the three additives were added into the aqueous solution of phycocyanin, and it was found that they had obvious improvement effects on the storage stability of phycocyanin pigments. The compound additives added to phycocyanin can be widely used in food, cosmetics and other fields, and has high application value.
References:
[1] Hedenskog G, Hofsten A V. The Ultrastructure of Spirulina platensis -A New Source of Microbial Protein[J].Physiologia Plantarum, 1970, 23(1):209- 216
[2] Belay A, Ota Y, Miyakawa K, et al. Current knowledge on potential health benefits of Spirulina[J]. Journal of Applied Phycology, 1993, 5(2):235-241
[3] Serena Benedetti, Sara Rinalducci, Francesca Benvenuti, et al. Pu - rification and characterization of phycocyanin from the blue-green alga Aphanizomenon flos-aquae [J]. Journal of Chromatography B, 2006, 833(1):12-8
[4] Jaouen P, Lépine B, Rossignol N, et al. Clarification and concentra- tion with membrane technology of a phycocyanin solution extracted from Spirulina platensis[J]. Biochemical Society Transactions, 1999, 13(12):877-881
[5] Cohen Z. Spirulina platensis (Arthrospira), Physiology, Cell-Biology and Biotechnology [J]. Quarterly Review of Biology, 1997 (3):353 - 354
[6] Jespersen L, Stromdahl L D, Olsen K, et al. Heat and light stability of three natural blue colorants for use in confectionery and bever- ages[J]. European Food Research & Technology, 2005, 220 (3/4): 261-266
[7] Yin Gang, Li Chen. Separation and purification of algal bile proteins and polysaccharides from Spirulina and product characterization [J]. Fine Chemical Industry, 1999, 16(2):10-13
[8] PENG Weimin, SHANG Shutian, FU Youlan, et al. Studies on the nature of bile protein in Spirulina obtususus[J]. Journal of China Agricultural University, 1999, 4(C00):35-38
[9] Hui Qiusha. Research overview of natural pigments[J]. Northern Pharmacology, 2011, 8(5):3-4
[10] GUO Fenghua,WANG Hui . Research on the extraction and application of natural pigments[J]. Shandong Food Fermentation , 2007, 36(4):36-38
[11] Ch R,González R,Ledón N,et al. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects[J]. Cur- rent Protein & Peptide Science, 2003, 4(3):207-216
[12] Eriksen N T. Production of phycocyanin--a pigment with applica - tions in biology, biotechnology, foods and medicine[J]. Applied Mi- crobiology & Biotechnology, 2008, 80(1):1-14
[13] Fretland D J, Djuric S W, Gaginella T S. Eicosanoids and inflamma DOI: 10.3969/j.issn.1005-6521.2017.12.008
#phycocyanin #Spirulinaobtusususiae #phycocyaninpowder
9 notes
·
View notes
Text



oh. zepp tours motif is wind and dust/ashes in a empty world like timoris's talk about the lack of wind and dust in a world where time has stopped in ave mujica's 1st live.

the secondary motif is related to zodiacal light which is diffused sunlight reflecting off interplanetary dust.


& the rings are the ecliptic plane.

it's also on tomori's bag for the wego collab, plus some other recent things like the autumn classic fair tomori acrylic stand/keychain & the comiket 103 mygo goods.





polaris, the north star is the central symbol of mygo. the ring surrounding the mygo logo also works to convey a compass.
"Because Polaris lies nearly in a direct line with the Earth's rotational axis "above" the North Pole—the north celestial pole—Polaris stands almost motionless in the sky, and all the stars of the northern sky appear to rotate around it. Therefore, it makes an excellent fixed point from which to draw measurements for celestial navigation and for astrometry. The elevation of the star above the horizon gives the approximate latitude of the observer."

sirius is the brightest star visible from earth. sirius is also called the morning star because it appears in the sky just before sunrise from early july to mid-september.
"The brightest star seen from Earth, Sirius is recorded in some of the earliest astronomical records. Its displacement from the ecliptic causes its heliacal rising to be remarkably regular compared to other stars."
"Sirius appears bright because of its intrinsic luminosity and its proximity to the Solar System. At a distance of 2.64 parsecs, the Sirius system is one of Earth's nearest neighbours. Sirius is gradually moving closer to the Solar System; it is expected to increase in brightness slightly over the next 60,000 years to reach a peak magnitude of −1.68. Coincidentally, at about the same time, Sirius will take its turn as the southern Pole Star, around the year 66,270 AD. In that year, Sirius will come to within 1.6 degrees of the south celestial pole."
"After that time, its distance will begin to increase, and it will become fainter, but it will continue to be the brightest star in the Earth's night sky for approximately the next 210,000 years, at which point Vega, another A-type star that is intrinsically more luminous than Sirius, becomes the brightest star."
"Sirius A is about twice as massive as the Sun and has an absolute visual magnitude of +1.43. It is 25 times as luminous as the Sun"

vega is one of the stars making up the summer triangle, that also includes altair, & deneb.
"At present the pole star is Polaris, but around 12,000 BCE the pole was pointed only five degrees away from Vega. Through precession, the pole will again pass near Vega around 14,000 CE. Vega is the brightest of the successive northern pole stars."
"Astrophotography, the photography of celestial objects, began in 1840 when John William Draper took an image of the Moon using the daguerreotype process. On 17 July 1850, Vega became the first star (other than the Sun) to be photographed,"
"In August 1872, Henry Draper took a photograph of Vega's spectrum, the first photograph of a star's spectrum showing absorption lines. Similar lines had already been identified in the spectrum of the Sun. In 1879, William Huggins used photographs of the spectra of Vega and similar stars to identify a set of twelve "very strong lines" that were common to this stellar category. These were later identified as lines from the Hydrogen Balmer series. Since 1943, the spectrum of this star has served as one of the stable anchor points by which other stars are classified."
"The distance to Vega can be determined by measuring its parallax shift against the background stars as the Earth orbits the Sun."


this all connects back to the beat of the stars felt by kasumi that already seems to connect to uika/sakiko, as well as the poppin' party song star beat.
many of mygo's motifs are already inspired by star beat's cd insert art, along with various other motifs used by popipa from around the time of the first season of the anime.



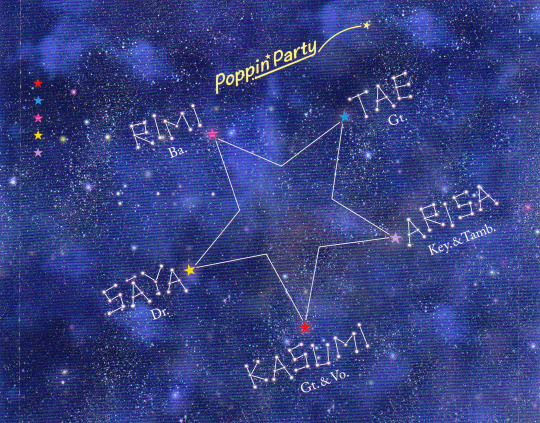





mygo's 'a small moment' live already used the motif of zodiacal light.

in the interlude sakiko's footsteps are marked with stars, and the empty spotlight represents the place sakiko used to occupy.
is the rotation path on the ecliptic plane an additional meaning to mygo's compass imagery?


the missing piece of avemuji's gear is the sun, the moon is forced to orbit the sun & earth due to tidal locking but cosmic dust & stars aren't necessary tied to a set ecliptic path.
both gears and compass move in a set direction so to be lost/stop moving is to be removed/break your ties with the path set out for you.
because of tidal locking the dark side of the moon exists preventing it from ever being seen from all sides from earth. the lunar lacus oblivionis is the only one of ave mujica's on the far side where it can't be perceived.
moonlight is a reflection of sunlight so what does it mean for ave mujica after perdere omnia, to create their own moonlight?
#i'm just thinking this out as it comes to mind so don't take it too seriously even if i'm wrong about this i had fun looking into it#either way fun ideas for fan art#also considering whether the poynting–robertson effect works with mygo#bandori#bang dream#ave mujica#mygo!!!!!#tomori takamatsu#sakiko togawa#bang dream it's my go
23 notes
·
View notes
Text

European X-ray laser explores a poorly understood state of matter
The properties of warm dense matter have until recently been little known. Now, thanks to the use of X-ray lasers, physicists are gaining more and more information about this important but still mysterious state of matter. The first comprehensive observations of ionisation processes in warm dense matter, carried out at the European X-ray Free-Electron Laser (European XFEL), have just been presented in one of the most prestigious physics journals.
State of matter with a temperature of a few thousand degrees and a high density, close to that of a solid, can be found, among others, in the interiors of brown dwarfs or gaseous planets. Although common in the Universe, it is very difficult to be produced and analysed in the laboratory. A new era in experimental research of this so-called warm dense matter (WDM) state began just a dozen years ago, when physicists launched the first free-electron X-ray lasers. At the forefront of this type of device is the nearly 3.5 km-long European XFEL laser. A series of experiments recently carried out there made it possible to observe for the first time how quickly a metal transforms into the exotic state of ionised WDM to become transparent (non-absorbing) to X-rays at the end of the process. The achievement of the international team of scientists – including those from the Institute of Nuclear Physics of the Polish Academy of Sciences (IFJ PAN) in Cracow – is discussed in a paper published in the journal Nature Physics.
X-Ray Free-Electron Lasers (XFELs) are used to generate high-intensity X-ray pulses lasting single femtoseconds, i.e. millionths of a billionth of a second. These can be used to study the structure of matter at atomic length scales and to track phenomena on extremely short time scales. One of only a dozen such devices in the world is the European XFEL in Hamburg, built in cooperation with the DESY research centre.
“In our experiment at the European XFEL, we illuminated copper samples with X-ray pulses lasting 15 femtoseconds, using different, gradually increasing intensities”, Prof. Beata Ziaja-Motyka (IFJ PAN, DESY) introduces the experiment. The first author of the paper in question, Dr. Laurent Mercadier from the European XFEL, adds some physical details: “When a single X-ray laser pulse reached the material, it caused strong ionisation. The electrons released in the process were characterised by high temperatures. Under these extreme conditions, the copper was transformed into a state of warm dense matter. We meticulously recorded how much radiation passed through the matter and from this inferred the ionisation changes in the observed system.”
Simulations carried out using the BOLTZMANN SOLVER software, developed since 2004 at DESY by Prof. Ziaja-Motyka, were particularly helpful in interpreting the measurement results. This tool was used to simulate changes in the electronic occupancy of individual energy levels in WDM depending on the intensity of the incident laser radiation.
By confronting experimental data with simulations, it was established that when the X-ray intensity becomes sufficiently high, atoms of WDM become strongly ionised. As a result of this phenomenon, new energy levels appear which can be occupied by excited electrons – making WDM opaque for photons resonant with transitions to these new energy levels. These states had already been observed previously with optical lasers, however, the lasers’ energy limitations did not allow them to be studied in more detail. Now, thanks to the European X-ray laser XFEL, it is possible to characterise them accurately also in response to various intensities of X-ray pulses. In accordance with theoretical predictions for X-ray absorption spectra, prepared by Dr. Joshua Kas (University of Washington, USA) and Dr. Andrei Benediktovitch (DESY, Hamburg), it was further observed that with increasing the laser intensity the warm dense matter becomes first opaque and then – at highest intensities – transparent to the laser pulse.
“The appearance of ‘transparency’ – i.e. lack of absorption – in WDM is a consequence of the high ionisation of WDM atoms occurring at sufficiently high X-ray pulse intensities. The energy of the X-ray photons available in the experiment then becomes too small to excite further electrons. As a result, these photons cannot be absorbed by the warm dense matter at all,” explains Prof. Ziaja-Motyka.
Knowledge of the properties of warm dense matter and the processes taking place within it is not only of astrophysical, but also of practical, engineering importance. Matter in this state plays an important role in certain types of controlled nuclear fusion (ICF – Inertial Confinement Fusion), and also appears during the ablation of metallic heat shields of spacecraft returning from orbit to Earth.
The team of physicists at the European X-ray XFEL laser, led by Prof. Nina Rohringer (DESY, Universität Hamburg), intends to continue research into the electron and ionisation processes occurring in WDM and their dynamics. On the Polish side, the work is co-financed by the Institute of Nuclear Physics of the Polish Academy of Sciences.
IMAGE: Warm dense matter occurs inside Jupiter-type giant planets (where it surrounds the rocky core as a metallic liquid at a temperature of many thousands of kelvin) and in the interiors of small stars – brown dwarfs. Credit Source: IFJ PAN / NASA
#science#space#astronomy#physics#news#nasa#astrophysics#esa#spacetimewithstuartgary#starstuff#spacetime
7 notes
·
View notes
Text
Bodies taking their colour from:
molecular absorption: look around you
molecular emission: i thought this one would be hard because most molecules fall apart before you excite them enough to glow, but there are also chemiluminescent, sonoluminescent and electroluminescent effects, so this is probably also look around you
atomic emission: slightly harder but you're probably not very far from a tube with some tenuous gas in it which would present an atomic spectrum to you if provoked using the apparatus provided. you can also put some salts in a flame.
atomic absorption: this seems like the hard one. I don't think there would be any cases where you could see this because atomic absorption lines are very thin and sparse. obvious exception is if you use monochromatic light, but even then the only instance of this I have heard of is if you illuminate a sodium fire (free Na+ in the corona of the flame) with a brighter sodium lamp -- the flame is black.
Related question: what are the spectra of salts like generally? Presumably the greater the ionic nature of their bonding, the more atomic their spectra look, but how far does that go?
20 notes
·
View notes
Text

#Archovember Day 18 - Cascocauda rong
The Anurognathids of the Jurassic and Cretaceous were highly derived pterosaurs that filled a similar niche to bats. They were nocturnal or crepuscular, insectivorous (though some larger species may have eaten fish as well), arboreal, and had short tails. However, Cascocauda rong of Middle - Late Jurassic China was an exception to the short tail rule. It’s tail was longer than other known anurognathids, earning it a name meaning “fluffy ancient tail.” But more importantly, Cascocauda provides us evidence of the complexity of pycnofibers in pterosaurs. Long thought to be simple and furlike, pterosaur pycnofibers were thought to be unique structures that evolved independently from feathers. However, Cascocauda had an array of different pycnofiber shapes and structures, one of them being similar to downy feathers with frayed ends. This further strengthens the hypothesis that feathers evolved before dinosaurs and pterosaurs even split into two different clades. Also, infrared spectral analysis was used on these pycnofibers, showing they had a similar absorption spectra to red human hair, making Cascocauda one of the only pterosaurs for which we know its coloration!

Found in the Tiaojishan Formation, Cascocauda rong would have lived with other anurognathids like Jeholopterus, Sinomacrops, and the tiny Luopterus. A wealth of other types of pterosaurs existed here as well, such as Darwinopterus, Kunpengopterus, Archaeoistiodactylus, Pterorhynchus, Wukongopterus, Daohugoupterus, Douzhanopterus, Fenghuangopterus, Jianchangnathus, Jianchangopterus, Qinglongopterus, and Liaodactylus. Dinosaurs lived here too, including the famously colored Anchiornis and other Anchiornithids like Aurornis, Caihong, Eosinopteryx, Pedopenna, Serikornis, and Xiaotingia, as well as the bizarrely bat-winged Scansoriopterygids Epidexipteryx, Scansoriopteryx, and Yi, and the quilled heterodontosaur Tianyulong. Arboreal cynodonts like Agilodocodon, Juramaia, Maiopatagium, Arboroharamiya, Volaticotherium, Vilevolodon, and Xianshou would have shared the trees with Cascocauda rong, adding to the busy, fluffy, feathery nature of this ancient forest.
#my art#SaritaDrawsPalaeo#Cascocauda rong#Cascocauda#anurognathids#pterosaurs#archosaurs#archosauromorphs#reptiles#Archovember#Archovember2023
27 notes
·
View notes
Text
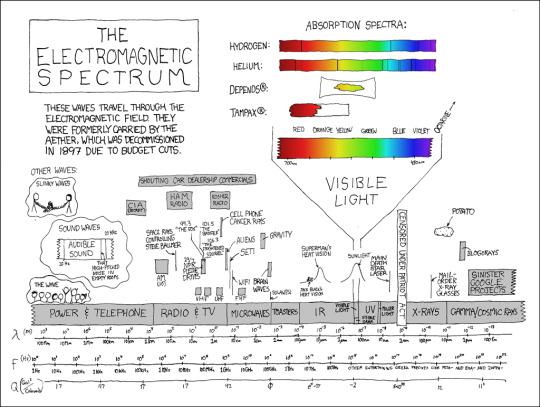
Sometimes I try to picture what everything would look like if the whole spectrum were compressed into the visible spectrum. Also sometimes I try to picture your sister naked.
Electromagnetic Spectrum [Explained]
Transcript
The Electromagnetic Spectrum These waves travel through the electromagnetic field. They were formerly carried by the aether, which was decommissioned in 1897 due to budget cuts. Other waves:
Slinky waves [Cueball and Megan hold the ends of a tangled slinky.]
Sound waves [There is a snippet of a frequency band. Between 20 Hz and 20 KHz is labeled "Audible Sound." Towards the top is a line labeled "That high-pitched noise in empty rooms."]
The wave [A row of people does a wave.]
[Three parallel scales are across the bottom. The first is lambda (m), ranging from 100Mm to 100fm; second is f (Hz), which starts at 1 Hz and reaches 100 THz about 2/3 of the way along, after which the labels read "other entertaining greek prefixes like peta- exa- and zappa-;" last is Q (Gal^2/Coloumb), whose labels are 17, 117, pi, 17, 42, phi, e^pi-pi, -2, 540^50, and 11^2. Above the scales and lined up accurately with the first two are the following:]
Power & Telephone (100Mm to 1km)
Radio & TV (1km to somewhere between 1m and 10cm); above that are many boxes showing subranges (AM, VHF, UHF, 24/7 NPR pledge drives, a very thin band for the space rays controlling Steve Ballmer, 99.3 "The Fox," 101.5 "The Badger," 106.3 "The Frightened Squirrel," cell phone cancer rays, CIA, ham radio, kosher radio, shouting car dealership commercials.)
Microwaves (a bit more than 10cm to a bit more than 1mm); it also has subranges (aliens, just below SETI, wifi, FHF, brain waves, sulawesi, gravity)
Toasters (about 1mm to about 100 micrometers)
IR (about 100 micrometers to somewhere between 1 micrometer and 1 nm); above that is a bell graph labeled "Superman"s heat vision," with a motorcycle driving up the left side labeled "Jack Black's Heat Vision."
Visible light (and, under it, visible dark); above that is a bell graph labeled "sunlight." There's a breakout chart above it showing the visible spectrum from 700nm (red) to 450nm (violet). There's an arrow pointing to where octarine would be, somewhere off to the side. Above that are bars showing the absorption spectra for hydrogen, helium, Depends(R) (yellow only), and Tampax(R) (red only).
UV (about 100nm to about 10nm)
Miller Light (a thin bar around 10nm)
An unlabeled section with a thin line above it showing the frequency of the main death star laser
A blocked-off portion labeled "Censored Under Patriot Act."
X-rays (from about 1nm to about 10pm); a line above shows the frequency of mail-order x-ray glasses. Somewhere vaguely above the 10pm mark is a potato.
Gamma/cosmic rays (10pm and smaller); above that is a bar marked Sinister Google Projects that also trails off into higher frequencies, and blogorays, which are slightly lower.
18 notes
·
View notes
Text

The Scientific Research Diaries of S. Sunkavally. Page 211.
#metal ions#absorption spectra#ultraviolet#dinoflagellates#algal blooms#satyendra sunkavally#phosphate#lysine#arginine#histones#toxicity#hydra#budding#meristem#viral infection of plants#gum#function#frictional drag#virus#iodine#antiseptic#lycophytes#inbreeding depression#light pressure#chlorophyll#light absorption
0 notes
Note
I need tips on how to study/revise for chemistry,especially physical chemistry.
Dear anon, I don't know whether you meant boards or competitive exams, so I covered for both since it is based on common ground anyway.
So👇
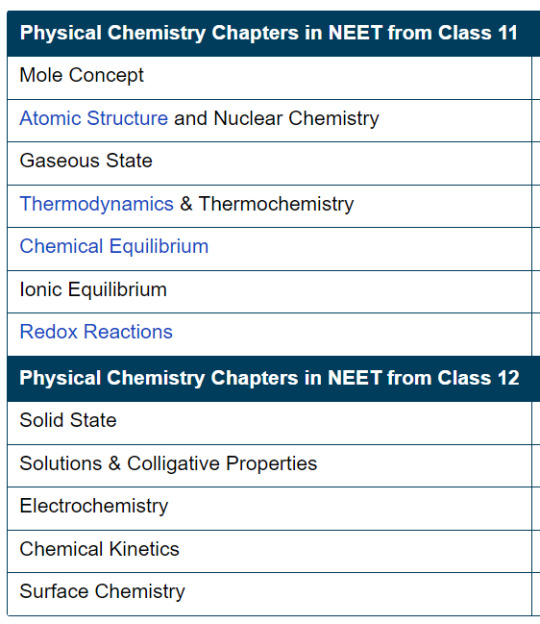
There is no other way that we could do well in physical chemistry except practice. A LOT.
What I understand about this dilemma (that I too have at this point, not fully solved) is that by the time you do at least 15 questions on a topic, you can get a grasp on the important formulae/which topics the questions hail from.
Mole concept : limiting reagent, M, m, w/w or w/v% (this particular option is not that frequent), reactions with stoichiometry coinciding with chemical kinetics, electrochemistry and metallurgy
Atomic Structure : which spectral series is in which region of the spectrum, sums with ratio of wavelengths (largest, smallest, comparison of different series), emission and absorption spectra, parts correlating to the physics part of energy levels and radius of hydrogen-like species
Gaseous state : GRAHAM'S LAW OF DIFFUSION (I cannot stress this enough, do it), the Cv and Cp values for Mono, Di and Polyatomic gases which connects thermo in Chem as well as Physics, mean free path proportionalities
Thermo (unit) : everything. All the laws, equations and graphs. adiabatic, isothermal, isochoric, my head and my tongue. Do every numerical in thermo. It's a weak point for a lot of us and we, right now, have the time to make it... Well, a not weak point.
Equilibrium : learn all the formulae and before you learn the formulae visualise/logically understand how something is happening. Log tables, roots, figure out some way to make decimal operations easier. A lot of sums from this one tpo because it isn't that connected to physics like thermo or electrochemistry
Redox : Make a trick for recognising which one is oxidation and which one is reduction. Balancing reactions must be practiced.
Solid state : Repetitive revision of the lattice examples is the only way we can remember them. Muscle memory can serve us well here. Make charts or stick it up on your wall to look at it every few days if that works for you. The rest are formulae and 4-5 numbers to be remembered. Density sums, chemical formula sums, voids sums <- practice
Solutions : formulae, how you get van't hoff factor for a compound, association and dissociation which is linked with electrochemistry molar conductivity part
Electrochemistry : formulae, graphs, molar conductivity sums, kohlrausch's law sums, electrolyte difference/spotting (will help in equilibrium), the cathode and anode of cells (this rarely comes)
Chemical Kinetics : some zero and first order reaction examples (will connect to radioactivity in nuclei chapter), half life formulae, and the 75% and 99% concentration formulae too (these 2 are not there in the tbk but it makes life easier in both phy and chem), all the graphs (should be able to read them even if they are messed around with or changed a bit)
Surface Chemistry : gold number sums, coagulation power and value orders in sols, recognising positive and negative sols, purification methods, electrophoresis definition (you'd be surprised how many times this came), helm holtz double layer theory, tindal's effect (connects a bit to optics, but vaguely), micelles (connects to bot biomolecules and cell unit). This chapter is very theoretical so keep revising stuff you don't get at first glance
Now briefly about Inorganic and Organic Chemistry:
Inorganic : write all the orders and the logics behind it. Some trends are weird so remember them with some trick (keep the tricks to the minimum in inorganic btw, it messes with your brain otherwise). That's as far as I've gotten with inorganic myself, but we can still work on it. If you have any advice regarding this, please do share.
Organic : understand the mechanism behind any reactions. Not just the way it's given in the textbook, but try to connect all organic chapters to each other. Practice a lot of questions, the direct ones as well as the weird ones. Organic does not have any tricks, it just requires practice and that can be done if we understand how each reaction goes about and why we do it.
Hope this helps you with Physical Chemistry and the like🤗 Thank you for approaching me with this so I could think out loud
Have a nice productive day!
#academiawho#physical chemistry#neet 2023#cbse boards#cbse 11#studyblr#chapter summary#cbse#ncert#cbse 2023#organic chemistry
55 notes
·
View notes
Text




On December 11th 1781 David Brewster was born.
Born in Jedburgh, Roxburghshire in 1781, to James Brewster, the rector of Jedburgh Grammar School and Margaret Key, he was educated at the University of Edinburgh, graduating MA in 1800. Although he was originally intending to be a Minister. Though a successful preacher in the Church of Scotland, his phobia of public speaking curtailed his involvement in the church. However, his faith continued to play an important role in his life, and his support for the Free Church of Scotland in 1843 nearly led to his dismissal from the University of Edinburgh.
Concentrating on the science of optics, Brewster made significant discoveries about polarisation of light and absorption spectra, invented the kaleidoscope and developed the stereoscope. His religious faith is also thought to have played a part in his scepticism about the wave theory of light.
In his lifetime, he published an impressive 315 scientific papers and articles, mostly on his pet subject of optics. His experiments in polarisation, which represent the bulk of his work, investigated in particular the different forms of polarisation that occur when light is reflected or refracted by various materials. Brewster's investigations of spectra included analysis of Newton's theory of colours and experiments on the absorption spectra shown in light shone through gases, and the similar lines seen in the sun's spectrum. He never abandoned the emission theory of light, and never accepted as true the theory that light was a wave - he preferred to see this as a convenient tool for calculation.
Brewster's invention of the kaleidoscope, popular as the instrument was, brought him little pecuniary benefit because of a botched patent application that left the design unprotected from unauthorised reproduction. He had a keen interest in photography from its infancy, and though he wrote about it and received a medal from the Photographic Society of Paris in 1865, he never found time to experiment properly with the chemistry of light. Brewster also promoted the use of the Fresnel lens in lighthouses, and oversaw a series of successful experiments with these in 1833. Brewster's finances were improved in his later life by his appointment as Principal of the United Colleges of St. Salvator and St. Leonard in St. Andrews in 1837, and his subsequent election by Edinburgh University as Principal in 1859, a post he held until his death in 1868. He was the first layman to occupy this post since Patrick Sands in 1622. Brewster was also a founder member of the British Association for the Advancement of Science and a keen, active participant in it.
David Brewster contracted pneumonia at the age of 87. His life soon to pass, he simply stated, "I shall see Jesus and that will be grand. I shall see Him who made the worlds". On February 10th, 1868, at Allerby, Melrose Brewster did just that.
9 notes
·
View notes
Text

Plate 97. The Absorption Spectra of Solutions. 1910.
Internet Archive
138 notes
·
View notes
Link
If alien technological civilizations exist, they almost certainly use solar energy. Along with wind, it’s the cleanest, most accessible form of energy, at least here on Earth. Driven by technological advances and mass production, solar energy on Earth is expanding rapidly. It seems likely that ETIs (Extraterrestrial Intelligence) using widespread solar energy on their planet could make their presence known to us. If other ETIs exist, they could easily be ahead of us technologically. Silicon solar panels could be widely used on their planetary surfaces. Could their mass implementation constitute a detectable technosignature? The authors of a new paper examine that question. The paper is “Detectability of Solar Panels as a Technosignature,” and it’ll be published in The Astrophysical Journal. The lead author is Ravi Kopparapu from NASA’s Goddard Space Flight Center. In their paper, the authors assess the detectability of silicon-based solar panels on an Earth-like habitable zone planet. “Silicon-based photovoltaic cells have high reflectance in the UV-VIS and in the near-IR, within the wavelength range of a space-based flagship mission concept like the Habitable Worlds Observatory (HWO),” the authors write. The HWO would search for and image Earth-like worlds in habitable zones. There’s no timeline for the mission, but the 2020 Decadal Survey recommended the telescope be built. This research looks ahead to the mission or one like it sometime in the future. Naturally, the authors make a number of assumptions about a hypothetical ETI using solar power. They assume that an ETI is using large-scale photovoltaics (PVs) based on silicon and that their planet orbits a Sun-like star. Silicon PVs are cost-effective to produce, and they are well-suited to harness the energy from a Sun-like star. Kopparapu and his co-authors aren’t the first to suggest that silicon PVs could constitute a technosignature. In a 2017 paper, Avi Loeb and Manasvi Lingam from the Harvard-Smithsonian Center for Astrophysics wrote that silicon-based PVs create an artificial edge in their spectra. This edge is similar to the ‘red edge‘ detectable in Earth’s vegetation when viewed from space but shifted to shorter wavelengths. “Future observations of reflected light from exoplanets would be able to detect both natural and artificial edges photometrically if a significant fraction of the planet’s surface is covered by vegetation or photovoltaic arrays, respectively,” Lingam and Loeb wrote. “The “edge” refers to the noticeable increase in the reflectance of the material under consideration when a reflected light spectrum is taken of the planet,” the authors of the new research explain. Satellites monitor the red edge on Earth to observe agricultural crops, and the same could apply to sensing PVs on other worlds. This figure shows the reflection spectrum of a deciduous leaf (data from Clark et al. 1993). The large sharp rise (between 700 and 800 nm) is known as the red edge and is due to the contrast between the strong absorption of chlorophyll and the otherwise reflective leaf. Image Credit: Seager et al. 2005. While Lingam and Loeb suggested the possibility, Kopparapu and his co-authors dug deeper. They point out that we could generate enough energy for our needs (as of 2022) if only 2.4% of the Earth’s surface was covered in silicon-based PVs. The 2.4% number is only accurate if the chosen location is optimized. For Earth, that means the Sahara Desert, and something similar may be true on an alien world. The authors explain, “This region is both close to the equator, where a comparatively greater amount of solar energy would be available throughout the year, and has minimal cloud coverage.” The authors also work with a 23% land coverage number. This number reflects previous research showing that for a projected maximum human population of 10 billion people, 23% land coverage would provide a high standard of living for everyone. They also use it as an upper limit because anything beyond that seems highly unlikely and would have negative consequences. On Earth, the entire continent of Africa is about 23% of the surface. The authors’ calculations show that an 8-meter telescope similar to the HWO would not detect an Earth-like exoplanet with 2.4% of its surface covered with PVs. If an ETI covered 23% of its surface with energy-harvesting PVs, would that be detectable? It would be difficult to untangle the planet’s light from the star’s light and would require hundreds of hours of observation time to reach an acceptable Signal-to-Noise (S/N) ratio. “Because we have chosen the 0.34 ?m–0.52?m range to calculate the impact of silicon panels on the reflectance spectra, the difference between a planet with and without silicon is not markedly different, even with 23% land cover,” the authors explain. Technological progress adds another wrinkle to these numbers. As PV technology advances, an ETI would cover less of its planet’s surface area to generate the same amount of energy, making detection even more difficult. This figure from the research shows the planet-star contrast ratio as a function of wavelength for2.4 % land coverage with PVs (blue solid), 23 % PVs (red solid) and 0% (green dashed) land coverage of solar panels. “This suggests that the artificial silicon edge suggested by Lingam & Loeb (2017) may not be detectable,” the authors write. Image Credit: Kopparapu et al. 2024. Solar energy is expanding rapidly on Earth. Each year, more individual homes, businesses, and institutions implement solar arrays. Those might not constitute technosignatures, but individual installations aren’t the only thing growing. China built a vast solar power plant called the Gonghe Photovoltaic Project in its sparsely populated Qinghai Province. It generates 3182 MW. India has the Bhadla Solar Park (2,245 MW) in the Thar Desert. Saudi Arabia has built several new solar plants and intends to build more. Other innovative solar projects are announced regularly. But will we realistically ever cover 2.4% of our planet in solar arrays? Will we need to? There are many questions. Generating solar power in the heat of the Sahara Desert is challenging. The extreme heat reduces efficiency. Building the infrastructure required to deliver the energy to population centres is also another challenge. Then consider that silicon-based PVs may not be the end point in solar panel development. Perovskite-based PVs hold a lot of promise to outperform silicon. They’re more efficient than silicon, and researchers frequently break energy records with them (in laboratories.) Would perovskite PVs create the same “edge” in a planet’s spectra? The authors didn’t consider specific technological advances like perovskite because it’s beyond the scope of their paper. The bottom line is that silicon-based solar arrays on a planetary surface are unlikely to create an easily detectable technosignature. “Assuming an 8-meter HWO-like telescope, focusing on the reflection edge in the UV-VIS, and considering varying land coverage of solar panels on an Earth-like exoplanet that match the present and projected energy needs, we estimate that several hundreds of hours of observation time is needed to reach a SNR of ~5 for a high land coverage of ~23%,” the authors write. The Bhadla Solar Park is a large PV installation that aims to generate over 2,000 MW of solar energy. Image Credit: (Left) Google Earth. (Right) Contains modified Copernicus Sentinel data 2020, Attribution, https://commons.wikimedia.org/w/index.php?curid=90537462 The authors also wonder what this means for the Kardashev Scale and things like Dyson Spheres. In that paradigm, ETIs require more and more energy and eventually build a mega engineering project that harvests all of the energy available from their star. A Dyson Sphere would create a powerful technosignature, and astronomers are already looking for them. But if the numbers in this research are correct, we may never see one because they’re not needed. “We find that, even with significant population growth, the energy needs of human civilization would be several orders of magnitude below the energy threshold for a Kardashev Type I civilization or a Dyson sphere/swarm which harnesses the energy of a star,” they conclude. “This line of inquiry reexamines the utility of such concepts and potentially addresses one crucial aspect of the Fermi paradox: We have not discovered any large-scale engineering yet, conceivably because advanced technologies may not need them.” The post Could Alien Solar Panels Be Technosignatures? appeared first on Universe Today.
2 notes
·
View notes
Photo

Scientists discover semi-metallization and novel photoelectric behavior in lead iodide under high pressure
According to a study published in Advanced Optical Materials, Prof. Ding Junfeng and his team at the Hefei Institutes of Physical Science of the Chinese Academy of Sciences showed that the semiconductor lead iodide (PbI2) undergoes a transition to a semi-metallic state when subjected to pressure. This transition is accompanied by an improvement in photoelectric properties and an extension of the spectral response range into the infrared band.
PbI2 is a versatile semiconductor with applications in X-ray and gamma ray detection and in the development of perovskite solar cells. By compressing the lattice constants and inducing structural transitions, the application of hydrostatic pressure can be used to modify structural and electronic properties. Therefore, pressure can be an effective method to enhance the photoelectric performance of PbI2.
In this study, the high-pressure absorption spectra of PbI2 suggested that the electrical band was closed at the transition point, while the charge transport indicated that the sample remained non-metallic. The non-metallic transport was well explained by the development of a semi-metallic phase at high pressure, as determined by first-principles calculations of the photocurrent spurt and infrared band response.
Read more.
9 notes
·
View notes