#What is Acetic Acid?
Explore tagged Tumblr posts
Text
Acetic Acid Market Size and Share Analysis: Key Growth Trends and Projections
Acetic Acid Market Strategies: Taking Advantage of Trends to Drive Growth in 2032
The Acetic Acid Market Report provides essential insights for business strategists, offering a comprehensive overview of industry trends and growth projections. It includes detailed historical and future data on costs, revenues, supply, and demand, where applicable. The report features an in-depth analysis of the value chain and distributor networks.
According to Straits Research, the global Acetic Acid Market market size was valued at USD 21.10 Billion in 2022. It is projected to reach from USD XX Billion in 2024 to USD 31.37 Billion by 2031, growing at a CAGR of 4.5% during the forecast period (2024–2031).
Get Free Request Sample Report @ https://straitsresearch.com/report/acetic-acid-market/request-sample
TOP Key Industry Players of the Acetic Acid Market
British Petroleum Plc
Celanese Corporation
Daicel Corporation
DuPont de Nemours, Inc.
Eastman Chemical Company
LyondellBasell Industries N.V
GNFC Limited
HELM AG
Mitsubishi Chemical Corporation
PetroChina
SABIC
Showa Denko K.K.
Sinopec
SvenskEtanolkemi AB (SEKAB)
Wacker Chemie AG
Global Acetic Acid Market: Segmentation
As a result of the Acetic Acid market segmentation, the market is divided into sub-segments based on product type, application, as well as regional and country-level forecasts.
By Manufacturing Process
Synthetic
Natural
By Application
Vinyl Acetate Monomer
Cellulose Acetate
Acetate Esters
Acetic Anhydride
Chloroacetic Acid
Terephthalic Acid
By End User
Adhesives & Paints
Pharmaceutical
Food & Beverages
Browse Full Report and TOC @ https://straitsresearch.com/report/acetic-acid-market/request-sample
Reasons for Buying This Report:
Provides an analysis of the evolving competitive landscape of the Automatic Rising Arm Barriers market.
Offers analytical insights and strategic planning guidance to support informed business decisions.
Highlights key market dynamics, including drivers, restraints, emerging trends, developments, and opportunities.
Includes market estimates by region and profiles of various industry stakeholders.
Aids in understanding critical market segments.
Delivers extensive data on trends that could impact market growth.
Research Methodology:
Utilizes a robust methodology involving data triangulation with top-down and bottom-up approaches.
Validates market estimates through primary research with key stakeholders.
Estimates market size and forecasts for different segments at global, regional, and country levels using reliable published sources and stakeholder interviews.
About Straits Research
Straits Research is dedicated to providing businesses with the highest quality market research services. With a team of experienced researchers and analysts, we strive to deliver insightful and actionable data that helps our clients make informed decisions about their industry and market. Our customized approach allows us to tailor our research to each client's specific needs and goals, ensuring that they receive the most relevant and valuable insights.
Contact Us
Email: [email protected]
Address: 825 3rd Avenue, New York, NY, USA, 10022
Tel: UK: +44 203 695 0070, USA: +1 646 905 0080
#Acetic Acid Market#Acetic Acid Market Share#Acetic Acid Market Size#Acetic Acid Market Research#Acetic Acid Industry#What is Acetic Acid?
0 notes
Text
To add injury to insult I am apparently allergic to the adhesive on the envelope that was included in the extra medical bill I was sent for my MRI I am okay but oh my gosh
Edit: in a common sense idea that should have occurred to me, oh, about fifteen minutes ago, if you are sensitive to adhesives enough that bandaids leave welts maybe don't lick the adhesive on envelopes. Learn from my mistakes lol
#the person behind the yarn#paying for the opportunity to wheeze I guess#I have stopped wheezing but now my lips feel like they are burning#ahhhhhhhh#I don't even know what in the adhesive I'm allergic to!#is this my general adhesive sensitivity I should have thought of before I licked the damn thing?#is it coconut?????#is it acetic acid??????????#I just don't know but you know saying it out loud#uh. I suspect it's the adhesive sensitivity#like. if bandaids leave welts presumably it's a bad idea to lick adhesive#well. this morning has been a series of unwise decisions for me
183 notes
·
View notes
Text

My knowledge in microbiology and biology informs my decisions
#Knowing how extensive#mold#is#and what#snails#carry#nooooo thanks#also I just hate the smell#texture and taste of overripe food#that#acetic acid#butyric acid#food#food disgust
47K notes
·
View notes
Text
Once is a mistake twice is peak dumbassery
#me about forgetting acetals are a thing for two exams in a row#there's a shitton of reactions WHAT ARE THE CHANCES OF GETTING THE SAME ONE TWICE#me last week: if they told me to make this compound from aldehyde instead of alcohol it'd have clicked for me that they want acetal reaction#me today: aldehyde and alcohol under acidic conditions... hmm what could that be#I KNOW THE FUCKING REACTION#istg#ema rambles
1 note
·
View note
Note
What's vinegar syndrome?? (Please infodump at us (if you want))
Happily! So, film (both photo/movie film and microfilm that's used as a preservation format) is on a film base that serves as a support layer under the emulsion. Infamously, silver nitrate was once used as a film base, but it was phased out due to being highly flammable. (Once it catches fire, it's almost impossible to put the fire out until it runs out of fuel.) In the 50s, silver nitrate was replaced by cellulose acetate, which was referred to as "safety film" because it did not catch fire. Unfortunately, acetate comes with its own problems, which we could describe as a metaphorical slow burn rather than a literal quick one. Over time, the acetate goes through a chemical reaction that makes the film base shrink and become brittle. As that happens, the emulsion is distorted, which has some pretty neat effects tbh.

This is called channeling and is most visually interesting on photographs.
One of the products of this chemical reaction is acetic acid, aka vinegar, and film that's far enough along in its degradation smells strongly, hence the name. Also, the reaction is auto-catalytic, meaning it feeds on itself. Once the reaction starts, you want to remove the reels impacted before they make the surrounding reels worse. There's also no cure, so all you can do is freeze the reels (which slows or halts the process) or copy/digitize the content before the film becomes unreadable.
Alas, the hope was microfilm as a preservation format would last hundreds of years, but a lot of acetate film is now hitting its expiration date and decaying. We switched to a poly base in the early 1980s, though, so the real sweet spot for vinegar syndrome is 1950-1980. That being said, a lot of home film is still acetate, assuming anyone records on film these days.
37 notes
·
View notes
Note
In your crack beans recipe, you mentioned that using apple cider vinegar was important. Is it because apple cider vinegar sweeter than other vinegars? Or just not as bitter?
Do you have any opinions about the different types of vinegars out there and what they’re best suited for?
(Also I tried your crack beans recipe and omfg it’s soooo good. Very simple to make and so tasty.)
Vinegar is made by allowing yeast to consume sugar in fruit or grains, producing alcohol. (fermentation!)
Then you introduce bacteria that eats the alcohol, and spits out acetic acid: vinegar.
It's basically a process of making beer, wine, or spirits, and then letting bacteria turn it into vinegar.
Each of those alcohols have different flavors mixed into them, based on what ingredients were used in the original fermentation, and how much it was allowed to ferment.
Apple Cider vinegar has a particular smell and taste. Yes, it's a bit sweeter. It's made with apple cider fermentation.
White vinegar is made with grain alcohol.
Red Wine vinegar is made with grape wine.
There's also Rice vinegar, Malt vinegar (barley malt!), White Wine vinegar, and much more~
I grew up using Apple Cider vinegar with this recipe, so that's what I'm familiar with, and how I think it should taste.
If you want to try it with other vinegars, feel free! A Red Wine or Malt vinegar actually sounds pretty good!
It'll just taste different =)
53 notes
·
View notes
Note
Hi, hope you're doing well 👋
I'm in my mid 20s, AFAB and have PCOS. I recently had penetrative sex for the first time. It was unprotected but I don't think he ejaculated inside (he said he didn't and I didn't feel like he did, but I wouldn't really know how that feels either).
I took a morning-after pill the next day (less than 12 hours after having intercourse), it contained ulipristal acetate.
Ever since then I've been really paranoid that I'm gonna get pregnant, I've been to my gynecologist since then and she said I should just wait for my next period and try to relax because the stress might delay my period even more (I'm not regular because of my PCOS, the most regular my periods are is usually like 40~ days apart but sometimes I skip a whole month entirely)
I'm not currently taking any medication for my PCOS (just folic acid), my gynecologist said after my next period ends she's gonna start me on birth control since I plan on becoming more sexually active and it will also help manage some of my PCOS symptoms.
I'm so scared of getting pregnant I've been having nightmares about it, pregnancy is one of my biggest fears (also I live in a place where abortion isn't legalized). I don't want to know the exact statistics because unless it's a flat 0% I don't think it would reassure me at all.
So I guess I just wanted to get some reassurance, someone to tell me it's very unlikely that I will get pregnant from this experience (there's no one in my real life I can go to for this). One of my worries is that since I have PCOS it somehow made the morning-after pill not work or something like that, I don't know, is that possible?
Sorry for the lengthy ask, thanks in advance for answering and have a nice day ❤️
Hi! Thank you, you too! 💕
Just so that you're aware, someone does not have to ejaculate inside you to cause pregnancy! Pre-ejaculate can also contain sperm, which is what can get you pregnant. It's rarer but can still happen.
You also don't necessarily have to feel it to tell if someone ejaculated inside of you. An easy way to tell is feeling inside of you with a finger and checking if there's cum, though if it was just pre-ejaculate or even just a very small amount of ejaculate, its much harder to tell.
I understand being scared of getting pregnant. It's unfortunately a common worry, especially in places without [free/stable] abortion access. Getting on birth control for that and your PCOS sounds like a good idea, I'm glad your doctor is being helpful!
Your PCOS isn't going to negate the morning-after-pill. Unless someone wants to jump on here to correct me, I've never read anything about PCOS messing with that. Actually, PCOS often makes it harder for people to get pregnant, Anon!
No apologies needed, thank you for trusting me to answer! I hope this helps a little, let me know if you have any other questions! <3
40 notes
·
View notes
Text
Share to slowly dissolve your followers
176 notes
·
View notes
Note
my mother has asked me to ask “that weaver friend of yours” lol — do you have experience dyeing linen? what does the process look like for natural vs synthetic dyes?
happy to be that weaver friend of yours 🥰❤️ dyeing linen is basically the same as dyeing cotton or any other cellulose fiber, so any synthetic dye that works for cotton will also work for linen. a professional grade fiber reactive dye like procion mx or dharma's procion (here) dyes cellulose fiber without heat, and the process is quick and painless. it just involves a large bucket, water, the dye powder and the cloth you wish to dye. i have little experience with rit dye so i'm not sure if you'd need heat for that, but procion dye is higher quality, comes in a lot more colours than rit, and a 2oz container is like $2 usd and goes a long way
the natural dye process for linen takes a lot longer than the procion dye process and requires several steps. cellulose fibers really don't like to take dyes so you basically have to do a bunch of alchemy to convince it to do what you want (compared to protein fibers like wool and silk which love dyes and only need some gentle nudges)
naturally dyeing linen depends on the dye you'd wish to use, but the process is essentially: scouring, mordanting, and dyeing. it's really important that you scour linen especially because it contains a lot of pectins that prevent dye from penetrating the fiber, so a harsh scouring is best (ie. washing it with hot water and ph neutral soap, even to the point of boiling the cloth. linen can take a lot of heat and is better for it, cotton is more sensitive) you'll probably have to do this before dyeing it with the synthetic dye too for best results
most natural dyes require that you mordant the cloth before dyeing. some dyes don't require a mordant (indigo is the big one, but if you're working with onion skins or other materials that contain tannins this is also true. however mordanting the cloth before dyeing with tannins or even mordanting with tannins is still recommended for better colour performance long-term unless you're working with indigo in which case using a mordant can actually cause problems) but if you're unsure, assume that you need to apply a mordant. you essentially have to simmer the cloth in a hot pot with either a material that contains tannins (tannic acid), a natural bio-accumulator of aluminum (symplocos), or use a metal salt (alum acetate is best for cellulose, but iron and copper salts can also be used. the metal salts route requires more safety precautions esp if you use copper salt, you can't dump that down the drain) your choice of mordant impacts the final colour with different mordants shifting the chemical reaction that happens in the cloth when you dye it
with cotton and linen, after you use the mordant you need to use either a chalk or wheat bran bath to remove excess mordant from the cloth, esp if you use alum acetate, otherwise it can leave a whitish cast over the cloth and also impede dyeing lol. wheat bran baths tend to cause a warmer tone to the final dyed cloth, chalk baths cause a cooler tone. i only use wheat bran baths bc i prefer the warmth and i get the bran cheaply at my local punjabi grocer
only then can you dye the cloth, again unless you're working with a dye like coffee or tea or onion skins OR indigo. linen really doesn't like to take natural dyes unless you do all the above steps, it's stubborn. the dye process itself depends on what dye you use and you can do stuff like solar dyeing if you don't want to simmer it in a pot on a stove. if you plan to go the natural dye route lmk and i can send you some scans of a book i have that contains precise instructions for preparing linen for dyeing
#fun fact the word for mordant in bahasa (both msia and indonesia) is mordan lol#i love when stuff like that happens. like computer in bahasa msia being komputer#as a caveat these are the western steps for natural dyeing there are other approaches that include the same chemical processes#but dif techniques like ex. in malaysia/indonesia mordants and dye baths are fermented for several weeks to months#and the fermentation makes it so they can be used cold and you only have to dip them in the baths rather than simmer#but that takes even longer than the western methods and is less documented in an instructional sense so the western approach is easier
45 notes
·
View notes
Text
Acetic Acid Market Size and Share Analysis: Key Growth Trends and Projections

Global Acetic Acid Market Report
The Acetic Acid Market research report offers an in-depth analysis of market dynamics, competitive landscapes, and regional growth patterns. This comprehensive report provides businesses with the strategic insights necessary to identify growth opportunities, manage risks, and develop effective competitive strategies in an ever-evolving market.
According to Straits Research, the global Acetic Acid Market market size was valued at USD 21.10 Billion in 2022. It is projected to reach from USD XX Billion in 2024 to USD 31.37 Billion by 2031, growing at a CAGR of 4.5% during the forecast period (2024–2031).
Request a Sample Report Today @ https://straitsresearch.com/report/acetic-acid-market/request-sample
Global Acetic Acid Market Segmental Analysis
As a result of the Acetic Acid market segmentation, the market is divided into sub-segments based on product type, application, as well as regional and country-level forecasts.
By Manufacturing Process
Synthetic
Natural
By Application
Vinyl Acetate Monomer
Cellulose Acetate
Acetate Esters
Acetic Anhydride
Chloroacetic Acid
Terephthalic Acid
By End User
Adhesives & Paints
Pharmaceutical
Food & Beverages
You can check In-depth Segmentation from here:
Why Invest in this Report?
Leverage Data for Strategic Decision-Making: Utilize detailed market data to make informed business decisions and uncover new opportunities for growth and innovation.
Craft Expansion Strategies for Diverse Markets: Develop effective expansion strategies tailored to various market segments, ensuring comprehensive coverage and targeted growth.
Conduct Comprehensive Competitor Analysis: Perform in-depth analyses of competitors to understand their market positioning, strategies, and operational strengths and weaknesses.
Gain Insight into Competitors' Financial Metrics: Acquire detailed insights into competitors' financial performance, including sales, revenue, and profitability metrics.
Benchmark Against Key Competitors: Use benchmarking to compare your business's performance against leading competitors, identifying areas for improvement and potential competitive advantages.
Formulate Region-Specific Growth Strategies: Develop geographically tailored strategies to capitalize on local market conditions and consumer preferences, driving targeted business growth in key regions.
List of Top Leading Players of the Acetic Acid Market -
British Petroleum Plc
Celanese Corporation
Daicel Corporation
DuPont de Nemours, Inc.
Eastman Chemical Company
LyondellBasell Industries N.V
GNFC Limited
HELM AG
Mitsubishi Chemical Corporation
PetroChina
SABIC
Showa Denko K.K.
Sinopec
SvenskEtanolkemi AB (SEKAB)
Wacker Chemie AG
Reasons to Purchase This Report:
Access to Comprehensive Information: Gain access to an extensive collection of analysis, research, and data that would be challenging to acquire independently. This report offers valuable insights, saving you considerable time and effort.
Enhanced Decision-Making: Equip yourself with detailed insights into market trends, consumer behavior, and key industry factors. This report provides essential information for strategic planning, including decisions on investments, product development, and marketing strategies.
Achieving Competitive Advantage: Stay ahead in your industry by understanding market dynamics and competitor strategies. This report delivers deep insights into competitor performance and market trends, enabling you to craft effective business strategies and maintain a competitive edge.
Credibility and Reliability: Trust in the expertise of industry professionals and the accuracy of thoroughly researched data. Authored by experts and grounded in rigorous research and analysis, this report enhances credibility and reliability.
Cost-Effective Research: Reduce research expenses by investing in this comprehensive report instead of conducting independent research. It provides a cost-effective means of accessing detailed analysis and insights on a specific topic without requiring extensive resources.
Regional Analysis Acetic Acid Market
The regional analysis section of the report offers a thorough examination of the global Acetic Acid market, detailing the sales growth of various regional and country-level markets. It includes precise volume analysis by country and market size analysis by region for both past and future periods. The report provides an in-depth evaluation of the growth trends and other factors impacting the Acetic Acid market in key countries, such as the United States, Canada, Mexico, Germany, France, the United Kingdom, Russia, Italy, China, Japan, Korea, India, Southeast Asia, Australia, Brazil, and Saudi Arabia. Moreover, it explores the progress of significant regional markets, including North America, Europe, Asia-Pacific, South America, and the Middle East & Africa.
To Understand How Covid-19 Impact Is Covered in This Report - https://straitsresearch.com/buy-now/acetic-acid-market
About Straits Research
Straits Research is dedicated to providing businesses with the highest quality market research services. With a team of experienced researchers and analysts, we strive to deliver insightful and actionable data that helps our clients make informed decisions about their industry and market. Our customized approach allows us to tailor our research to each client's specific needs and goals, ensuring that they receive the most relevant and valuable insights.
Contact Us
Email: [email protected]
Address: 825 3rd Avenue, New York, NY, USA, 10022
Tel: UK: +44 203 695 0070, USA: +1 646 905 0080
#Acetic Acid Market#Acetic Acid Market Share#Acetic Acid Market Size#Acetic Acid Market Research#Acetic Acid Industry#What is Acetic Acid?
0 notes
Text
Lately I'd been wondering if maybe my asthma was doing so much better since I stopped living in a place with fire season that I could talk to my doc about stopping my asthma meds, but uh. Accidentally tested that? My maintenance inhaler was empty for at least a day, possibly more, before I realized so I was using it but getting no meds and it turns out I was improved because my maintenance meds were working, so I started coughing again. Not super bad, but like if I had a nickel for every time I've accidentally ran a double-blind trial* on myself I'd have two nickels, and that's weird, right? *the other time was when I figured out I'm allergic to acetic acid, because I'd checked the ingredients of the individual serving of a pre-made meal from a store and it was fine, but I reacted to the family meal portion of it (after not checking it, assuming the ingredients would be the same), and the only thing different was the acetic acid.
#the person behind the yarn#medical mention#food mention#allergy mention#arguably I've actually done it three times#because the moderna covid vax has acetic acid in it#not much! and for most people it's not a big deal! but it turns out if you inject something you are allergic to into you#it sucks! it's terrible. feels real bad. thought that was just how I react to vaccinations but no it was the allergy#switched brands and it was still unpleasant but not 'require a wheelchair because my bp is too low' unpleasant#hey in retrospect that was bad? that was probably bad enough I should have gone to a doctor when I say it out loud#huh. well. always good to contextualize memories I suppose. I did look unwell enough when I got the first shot#that when I got the second shot the nurse (who had been giving a LOT of people shots)#remembered me and commented on how much better I was looking (I'd taken benadryl preemptively for the second one)#I also had to get the first two shots from a nurse and not a pharmacy because I'd had an allergic reaction from the flu shot#still don't know what that was about but another fun fact: when they say redness at the injection site is a possible effect#they do NOT mean a handprint-sized hive and that is. in fact. a thing you should tell your doctor if it happens#I only told my doc because I happened to have an appointment like a week later and they asked about the giant bruise#and then were like no!! that's not supposed to happen!! you are not allowed to have the flu shot anymore
14 notes
·
View notes
Text
Burrows end observation
So in the latest episode in the Last Stand we hear descriptions of pipes that painted to match the concrete, but also some in red, orange and blue so I looked up what those pipe colors mean
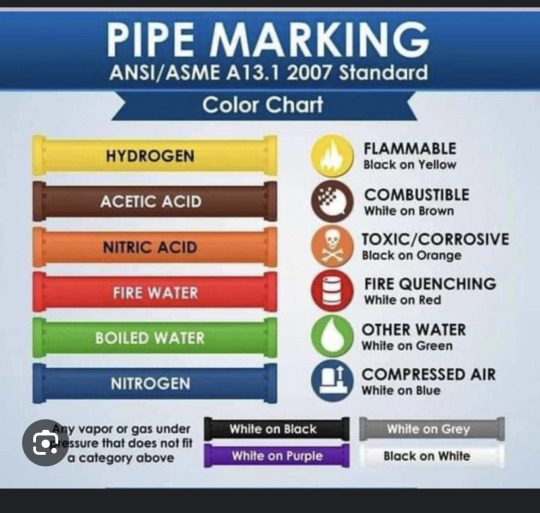
a graphic that contains the meaning of pipe colors with Yellow meaning Hydrogen Brown meaning Acetic acid Orange meaning Nitric Acid Red meaning fire water green meaning boiled water and Blue meaning Nitrogen
with the mentions of red orange and blue pipes this base or factory was at least working with Nitric Acid, Fire Water, and Nitrogen.
If anyone knows what this means please share with the class I am no expert on this and so will not attempt to make a random end guess
#dimension 20#aabria iyengar#burrows end#d20#dropout#siobhan thompson#brennan lee mulligan#jasper william cartwright#rashawn scott#izzy roland#erika ishii#burrows end spoilers#burrow’s end speculation
71 notes
·
View notes
Text
Apple Cider Vinegar and Your Metabolism: A Natural Way to Boost Your Energy
Apple cider vinegar (ACV) has gained significant popularity as a natural health booster. From aiding digestion to supporting weight management, this versatile condiment is packed with benefits. Among its many advantages, its impact on metabolism stands out, making it a go-to choice for health-conscious individuals. If you are looking to understand how apple cider vinegar can enhance your energy levels, you are in the right place. As a leading apple vinegar manufacturer in India, Modern Food Products brings you insights into the power of this golden elixir.

The Science Behind Apple Cider Vinegar and Metabolism
Metabolism refers to the chemical processes your body uses to convert food into energy. An efficient metabolism helps maintain energy levels, supports weight management, and keeps your body functioning optimally. Apple cider vinegar contains acetic acid, which plays a key role in boosting metabolic processes.
1. Improved Blood Sugar Regulation
One of the primary ways apple cider vinegar influences metabolism is by improving blood sugar regulation. It helps slow down the absorption of carbohydrates, preventing sudden spikes and crashes in blood sugar levels. This stabilizes energy levels throughout the day.
2. Enhanced Fat Burning
Studies suggest that apple cider vinegar can promote fat burning by activating specific enzymes that break down fats. This makes it an excellent addition to your diet if you are aiming to manage weight while staying energetic.
3. Support for Digestive Health
Healthy digestion is critical for efficient metabolism. Apple cider vinegar boosts the production of stomach acids, aiding in the breakdown of food and improving nutrient absorption. As a key product offered by food processing companies in Gujarat, ACV is increasingly valued for its digestive benefits.
How to Incorporate Apple Cider Vinegar into Your Diet
Integrating apple cider vinegar into your daily routine is easy and versatile. Here are some popular ways to enjoy its benefits:
Morning Detox Drink: Mix one tablespoon of apple cider vinegar with a glass of warm water and a dash of honey.
Salad Dressings: Use ACV as a tangy ingredient in your salad dressings for a flavorful and healthy boost.
Cooking: Add it to marinades, soups, and sauces to enhance flavors while reaping its benefits.
As an apple cider vinegar supplier in UAE, Modern Food Products offers high-quality ACV that is perfect for culinary and health applications.
Private Labeling Opportunities in Vadodara
With the growing demand for apple cider vinegar, businesses are exploring opportunities to introduce their own branded products. At Modern Food Products, we offer private labeling services in Vadodara, enabling businesses to market premium-quality apple cider vinegar under their brand names. Our expertise in the food processing industry in Vadodara ensures that every product meets stringent quality standards.
Why Choose Modern Food Products?
As one of the best food product companies in Vadodara, Modern Food Products stands out for its commitment to quality, innovation, and customer satisfaction. Here is what sets us apart:
State-of-the-Art Facilities: Our advanced manufacturing unit in Vadodara enables us to produce high-quality apple cider vinegar efficiently.
Custom Solutions: From private labelling to bulk supply, we cater to diverse business needs.
Global Reach: As a trusted apple cider vinegar exporter in United Arab Emirates, we ensure timely delivery and exceptional service for our international clients.
The Role of Food Processing in Enhancing Apple Cider Vinegar’s Benefits
The food processing industry in Vadodara plays a crucial role in maximizing the health benefits of apple cider vinegar. By employing advanced techniques, Modern Food Products ensures that our ACV retains its natural properties while meeting global quality standards. This dedication makes us one of the leading food processing companies in Gujarat and a preferred condiments manufacturer in India.
Health Benefits of Apple Cider Vinegar Beyond Metabolism
While boosting metabolism is a key benefit, apple cider vinegar offers a range of additional health advantages:
1. Supports Weight Management
ACV helps curb appetite and promotes a feeling of fullness, aiding in weight management efforts.
2. Promotes Heart Health
Regular consumption of ACV can improve cholesterol levels and support heart health.
3. Boosts Immunity
Rich in antioxidants and antimicrobial properties, apple cider vinegar strengthens the immune system.
4. Enhances Skin Health
When used topically, ACV can help balance skin pH and reduce acne.
The Future of Apple Cider Vinegar in the Health Industry
The demand for natural health products like apple cider vinegar is growing rapidly. As a top food manufacturing company in Vadodara, we are committed to meeting this demand with innovative and high-quality offerings. Our position as a trusted food company in Gujarat reflects our dedication to excellence in the food processing companies in India.
Partnering with Modern Food Products
Whether you are a retailer looking for a reliable apple cider vinegar supplier in UAE or a brand exploring private labeling services in Vadodara, Modern Food Products is your go-to partner. With our expertise as a leading apple vinegar manufacturer in India, we deliver products that align with your business goals and customer expectations.
Conclusion
Apple cider vinegar is more than just a condiment; it is a powerhouse of health benefits that can transform your metabolism and energy levels. By choosing high-quality apple cider vinegar from a trusted condiments manufacturer in India like Modern Food Products, you can ensure optimal results for your health and business needs.
Modern Food Products’ commitment to quality, innovation, and customer satisfaction makes us the preferred choice for clients worldwide. Whether you need a reliable apple cider vinegar exporter in United Arab Emirates or are looking to leverage private labeling services in Vadodara, we are here to help.
Boost your metabolism naturally with premium apple cider vinegar from Modern Food Products. Contact us today to learn more about our offerings and how we can support your journey to health and success.
#Apple cider vinegar supplier in UAE#Apple cider vinegar exporter in United Arab Emirates#Food processing companies in India#India#Gujarat#Vadodara#Food manufacturing companies in Vadodara#Apple vinegar manufacturer in India#United Arab Emirates#Best food product company in Vadodara#Food processing industry in Vadodara#Food processing companies in Gujarat#Food companies in Gujarat#Private labelling services in Vadodara#Condiments manufacturer in India
8 notes
·
View notes
Text
An international team of astrophysicists, astronomers and chemists has found evidence of carbonic acid (HOCOOH) in interstellar space, marking the first time it has been detected in such a setting. In their paper published in The Astrophysical Journal, the group describes their discovery, where it was found, and what it might mean for research into the origins of life. Prior research has led to the discovery of acetic and formic acid in interstellar space; both are carboxylic acids, as is carbonic acid. All three are believed to be building blocks of life. Finding them in such distant places gives credence to theories that suggest that they were delivered to Earth via comets or meteorites. In this new effort, the researchers were studying the molecular cloud G+0.693-0.027 near the center of the Milky Way when they found evidence of HOCOOH.
Continue Reading.
91 notes
·
View notes
Note
i agree with you on the dio thing. please please please tell me your other music opinions. also what kind of punishments will be dealt towards men that are snobbish about music, ask women to name 3 albums if they claim to like a band, and/or are generally yeasty little pissholes about music.
Beatings with a cheese grater and acetic acid. My only other music opinion you should consider is that the third men at work album isn't actually as bad as people thought it was and overall that band was a tragedy
10 notes
·
View notes
Text

Coca-Cola’s VitaminWater is being marketed as a healthy, hydrating drink. The company claims that the drinks prevent chronic diseases, reduce the risks of eye diseases, promotes healthy joints, and supports optimal immune function. However, nothing could be further from the truth.
This is what John Robbins, Esq., PhD., M.D. says in his Mat Hoffman Post article:
The product is basically sugar-water, to which about a penny’s worth of synthetic vitamins have been added. And the amount of sugar is not trivial. A bottle of vitaminwater contains 33 grams of sugar, making it more akin to a soft drink than to a healthy beverage.
The ingredients of “orange-orange”-flavored vitaminwater:
Reverse osmosis water, crystalline fructose, cane sugar, less than 0.5% of: citric acid, magnesiumlactate and calcium lactate and potassium phosphate (electrolyte sources), natural flavors, vitamin C (ascorbic acid), gum acacia, vitamin B3 (niacinamide), vitamin E (alpha-tocopheryl acetate), vitamin B5 (calcium pantothenate), glycerol ester of rosin, vitamin B6 (pyridoxine hydrochloride), vitamin B12, beta-carotene, modified food starch, sorbitol.
VitaminWater’s sugar levels are very very high. One 500ml bottle contains 27 grams of sugar, that’s about 8 teaspoons of sugar!
Crystalline Fructose
Crystalline fructose is produced by allowing the fructose to crystallize from a fructose-enriched corn syrup. So basically, it is made from corn syrup, and not only corn syrup, but “fructose enriched” corn syrup. Crystalline Fructose contains 99.5% minimum of fructose assay, which is a greater higher percentage of fructose than what makes up high fructose corn syrup. Crystalline fructose may be contaminated with arsenic, lead, chloride and heavy metals. This type of fructose leads to increased belly fat, insulin resistance, and metabolic syndrome. Large amount of fructose can create a fatty liver and cirrhosis as it can not be processed completely in the liver. The fructose interferes and alters the metabolic process in our cells, which causes oxidative damage.
14 notes
·
View notes