#Veterinary pathology
Explore tagged Tumblr posts
Text

Happy spooky month ft a neoplastic cell who decided it needed to grow cytoplasmic tentacles in order to produce the glob of Mystery Pink Stuff instead of just hugging it like a normal cell
821 notes
·
View notes
Text
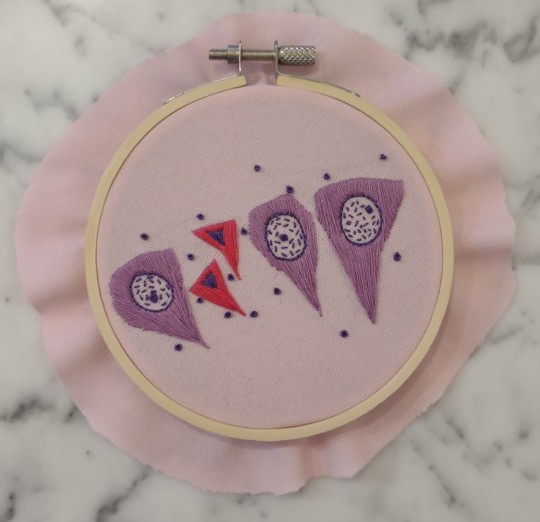
Necrosis of the pyramidal neurons
The classic necrotic neuron is the "dead red". Shrunken, angular, and hypereosinophilic (red) with a pyknotic nucleus, scattered dead neurons can be associated with a wide range of neurologic disease. Necrosis of the pyramidal neurons in the hippocampus is commonly associated with seizure activity, particularly in the CA2 and CA3 regions. Whether this necrosis is the cause or effect of the seizure activity is not always entirely clear, although it is typically attributed to the abnormal electrical activity and hypoxia associated with seizures.
(embroidery by @lizziedoesvetpath)
73 notes
·
View notes
Text

17 Feb., 2024
for weeks ive worked on a personal statement for a summer internship and an introduction email to the faculty member who’s research im interested in. i finally completed my final draft this morning and sent the email…now we wait ⏳ feeling so shaky but very optimistic!
2 notes
·
View notes
Text
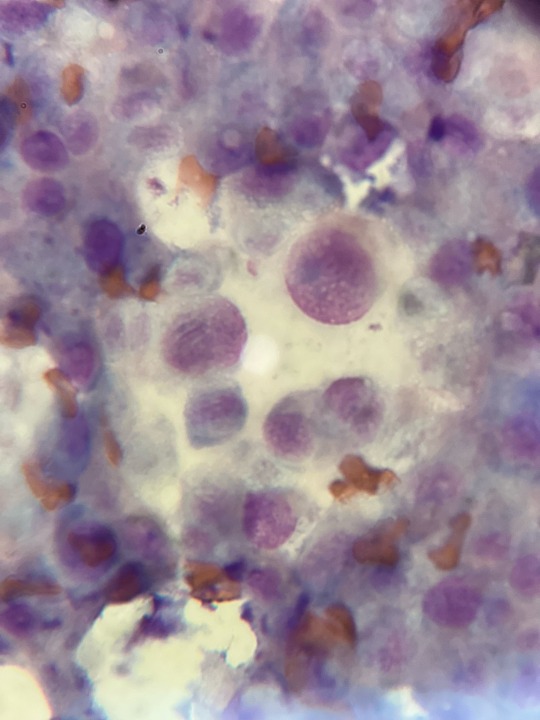

Image 1: Cancerous changes in cells from a mass on the toe. Near the center is an actively dividing cell, you can also see that the cells have nuclei of variable sizes, and a few multinucleate cells. We suspect it is a mast cell due to the findings in image 2.
Image 2: Mass on the left chest that has been growing and shrinking for the last 6 months. Microscopic findings show a field of granules consistent with mast cells, though no mast cells were seen. Not included in this image were cancerous changes of the cells.
Ultrasound of the spleen was inconclusive (mast cells can sometimes have splenic involvement). Owner is electing for surgical removal of both masses.
2 notes
·
View notes
Text
I did it. I sent an email to CRISPR Therapeutics about my final project. I am in fear. I dont know if I cry or if I scream or if I should run around the house in panic.
0 notes
Text

Viroses que comprovadamente causam neoplasia em:
Cães - Papilomavirus -> Carcinoma Oral de Células Escamosas . Entre as lesões hiperplásicas, a PV (PapilomaVirose) está frequentemente associada a verrugas cutâneas e oromucosas. Esta apresentação clínica é típica de cães jovens.
Geralmente surgem como lesões semelhantes a couve-flor em áreas sujeitas a trauma, como pés ou ao redor da face, lábios e orelhas [116, 117, 153,154,155] e geralmente são autolimitadas. Existem muito poucos relatos de verrugas induzidas por CPV -papilomavirus canino - transformadas em CEC - carcinoma de células escamosas [156]. No entanto, existem alguns relatos de lesões que continuaram a aumentar de tamanho, espalhando-se para a pele pilosa [157] ou progredindo para CEC [158].
A excisão cirúrgica ou crioterapia é recomendada se estas lesões se tornarem demasiado grandes e interferirem com a alimentação ou a respiração [159]. Em gatos, entretanto, essa apresentação clínica não é tão frequente: suspeita-se que as verrugas sejam causadas pelo FPV1 - papilomavirus felino tipo 1. Existem poucos relatos sobre esta manifestação aparecendo no plano nasal, na pálpebra ou surgindo em aglomerados na face ventral da língua. Há evidências cada vez mais convincentes de que o papilomavírus canino (CPV) e o papilomavírus felino (FPV) também podem causar câncer em cães e gatos, respectivamente, mas são necessários estudos adicionais para investigar seu papel no desenvolvimento de tumores [117].
Gatos - Doença de Bowen/Carcinoma de Células Escamosas (CCE) na pele, Linfoma (FELV, FIV). Particularmente, foi relatado que gatos infectados por FIV são 5 a 6 vezes (até 80 vezes no caso de coinfecção FeLV/FIV) mais suscetíveis a desenvolver linfoma em comparação com gatos não infectados.
From: Exploring the link between viruses and cancer in companion animals: a comprehensive and comparative analysis.
0 notes
Text
CISTITE HEMORRÁGICA DE ORIGEM NEOPLÁSICA
A cistite hemorrágica é a inflamação do revestimento da bexiga urinária que pode ser uma complicação grave do câncer ou seu tratamento. Agentes alquilantes à base de oxazofosforina, como ciclofosfamida e ifosfamida, são os agentes citotóxicos mais comuns associados a esse distúrbio.
Referência:
Chapter 70 - Supportive Medical Care and Pain Management in Feline Cancer Patients, Editor(s): John R. August. Consultations in Feline Internal Medicine (Fifth Edition), W.B. Saunders, 2006, Pages 665-672, ISBN 9780721604237, https://doi.org/10.1016/B0-72-160423-4/50073-1. (https://www.sciencedirect.com/science/article/pii/B0721604234500731).
#veterinary clinical medicine#veterinary pathology#feline internal medicine#urinary disease#small animal veterinary medicine#veterinary medicine#cat
0 notes
Text
The Effects of Freeze-Thaw Cycles and of Storage Time on the Stability of Proakap4 Polypeptide in Raw Sperm Samples: Implications for Semen Analysis Assessment in Breeding Activities
Abstract
Evaluation of the concentrations of the sperm macromolecule called proAKAP4, has been successfully introduced as a pertinent sperm parameter to assess sperm quality and high concentrations of proAKAP4 was shown to be highly correlated with sperm motility and fertility in large mammals. The sandwich ELISA kits known as Pig 4MID® Kits allowed the artificial insemination stations to monitor sperm quality more accurately with threshold values qualifying each ejaculate and animal. Introducing new methods and procedures are always challenging and sperm frozen collections have been suggested to standardize sperm assessment in daily routine. We thus have designed an experimental study to assess proAKAP4 stability and integrity in neat frozen ejaculates. Following baseline measurements, fresh ejaculates were aliquoted and stored at -20 °C for stability experiments up to 6 months and following up to 10 freeze-thawing cycles. ProAKAP4 concentrations were assayed at each time point using the Pig 4MID® Kit and western blot. Median or mean changes from baseline concentrations were evaluated statistically. We showed that the frozen storage conditions neither modified the total proAKAP4 concentrations nor changed the degradation rates of the proAKAP4 into mature AKAP4, that should in turn ensures signaling, capacitation and motility. This sperm parameter was shown then to be robust for semen quality analysis on fresh and on frozen neat semen. Taken together, proAKAP4 polypeptide can be considered as a highly stable analyte when kept frozen in raw semen up to the semen quality analysis using the Pig 4MID® Kit
Keywords: Boar; Proakap4; 4MID®; Fertility; Stability; Precursor; Freeze-thaw cycle; Storage; Semen processing
Abbrevations: AKAP4: A-kinase Anchor Protein 4; PKA: Protein Kinase A; CASA: Computer Assisted Semen Analysis
Introduction
ProAKAP4 concentrations are considered as a new sperm parameter that have been validated by field studies for sperm analysis assessment in large mammals [1-6]. Measurement of proAKAP4 concentrations was thus reported to generate pertinent information to guide the prognosis of sow fertility and prolificity in highly competitive breeding activities [1,4]. This quantitative approach of semen assessment is based on a sandwich ELISA method that allow to compare up to 87 semen simultaneously and is commercialized under the brand name of the 4MID® Kits (4BioDx, France). The Pig 4MID® Kits provide then a reliable and valuable figure reflecting the amount of proAKAP4 in pig ejaculates, with threshold values that are allowing a follow-up of the sperm quality inside and between pig breeding centers. Structurally, proAKAP4 is a precursor protein and will have to be converted by motile and alive spermatozoa in AKAP4 (A-kinase anchor protein 4) that in turn, coordinate the main transduction signals regulating sperm motility, capacitation and fertility [1,7-11]. ProAKAP4 concentrations has been reported to be correlated with total and progressive motility in stallion, in human and in bull [2,3,6,12,13]. Clearly the proAKAP4 concentrations is a reflect of the sperm motility giving a more objective figure compared to microscopic observations of the spermatozoa that are motile only at the time of analysis. In contrast, with the Pig 4MID® Kit, the more the proAKAP4 concentration is high in the ejaculate, the more the spermatozoa will be motile and efficient to go up to the site of fecundation. They have been evidences that spermatozoa with few or without proAKAP4 will be less motile or immotile and then infertile [14-18]. Therefore, we considered as essential to determine the stability and integrity of the full-length proAKAP4 in frozen storage conditions before the critical step of the sperm quality analysis. Data concerning the effects of freezing, thawing, and long-term storage effect on sperm proAKAP4 concentrations were not yet available in the literature. In this study, we aimed then to examine the analytical stability of proAKAP4 in fresh boar semen. We then assess the variations of proAKAP4 concentrations and proAKAP4 degradation rates in following freeze-thaw cycles and in long-term storage at minus 20 °C, in a final goal to improve operating procedures for semen analysis in swine breeding centers.
Materials and Methods
Sperm Preparation
Fresh boar sperm samples (Large White strain) were obtained from a boar stud and was first checked for total volume. They were then aliquoted into 1.5-mL polypropylene cryovials for the stability experiment. For stability assessment, the sampling of each ejaculate was then composed of 5 aliquots (2 for freezethaw cycle experiment and 3 for long-term storage experiment). Following baseline measurement (T0), they were all maintained frozen until analysis. The remaining boar ejaculates were either processed for the control quality experiment or for proAKAP4 expression controls. Samples stored at -20 °C were kept in a freezer equipped with a temperature recorder.
Freeze thaw Cycles and Long-term Storage Experiments
After 24 hours, 2 frozen sperm aliquots were thawed at room temperature until completely thawed, and then mixed properly with a micropipette before analysis (freeze-thaw 1). Samples were immediately re-frozen at -20 °C. This cycle was repeated for ten consecutive time points (T1, T2, T3, T4, T5, T6, T7, T8, T9, T10) to yield freeze- thaw processing. A group of 3 semen aliquots were stored at -20 °C for up to 1, 3 and 6 months, and then analyzed for stability at three-time intervals (T1M, T3M, T6M). As described below, the concentrations of proAKAP4 were assessed at each time point using the Pig 4MID® Kit (4BioDx, France). In parallel, proAKAP4 expressions and metabolism of the same aliquot were examined by western blotting. The results were compared with those obtained from the initial analysis of fresh samples. Median or mean changes from baseline (T0) concentrations were evaluated statistically.
Spermatozoa and Seminal Plasma Preparation from Boar Semen
A volume of 500μL of the remaining fresh semen was added in a 1.5mL Eppendorf tube and then centrifuged during 10 min at 2000rpm. The supernatant over the spermatozoa pellet was recovered with a 200μL micropipette and corresponded to the seminal plasma fraction. One volume of Tris Buffer (10 mM Tris HCl pH 6.8) with 2% SDS was added to the seminal plasma and sonicated at 22kHz (15 Watts) for 30 seconds. In parallel, 250μL of Tris Buffer with 2% SDS was added to the spermatozoa pellet, mix thoroughly with a vortex and then sonicated for 30 seconds (22 kHz, 15 Watts). Protein concentrations were determined using the Bradford’s method (BioRad, France). Then 50μL of the Tris-SDS sample was added to 1 volume of 2x concentrated NuPAGE LDS Sample Buffer (ThermoFisher, USA) and 10μL of NuPAGE Sample Reducing Agent (ThermoFisher, USA). Samples were vortexed and heated at 80 °C for 10 min.
Analysis of pig ProAKAP4 Expression and Metabolism by Western Blot
Equal protein concentrations of each sperm preparation were loaded on polyacrylamide gel (4-12% NuPage Precast Gels) and then transferred onto 0.45μm nitrocellulose membranes (G&E Healthcare, USA) using the Liquid Transfer System (Life Technologies, USA). Membranes were incubated overnight at 4 °C with the first antibody at a dilution of 1:4000 in 25 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.1% (v/v) Tween 20 (TNT Buffer), either with the clone 7E10, a monoclonal antibody anti-AKAP4 (4BioDx, 4BDX-1602, France), or with the clone 6F12, a monoclonal antibody anti-proAKAP4 (4BioDx, 4BDX-1701, France). After washing 3 times in TNT (10 min), each membrane was incubated with the secondary anti-mouse antibody coupled to horseradish peroxidase at 1:50000 diluted (Vector Laboratories, Burlingame, CA USA) and revealed with the ECL™ chemioluminescence kit (G&E Healthcare, USA). Images were acquired using the Image Quant™ LAS 4000 system (G&E Healthcare, USA).
The Pig 4MID® ProAKAP4 ELISA Assays
Thawed semen samples (respectively 50, 25 and 12μL) were mix with the Pig Lysis Buffer (450, 475 and 488μL) and then proceeded for ELISA quantification using the Pig 4MID® Kit (4VDX-18K2) according to the manufacturer’s instructions (4BioDx, France). Briefly, 100μL of semen lysates was then added to each well of the antibody-coated plate. A solution with conjugated proAKAP4 antibody was then added and after appropriate washing, the complexed sandwich was incubated with a substrate solution. The resulting color intensity was proportional to the amount of proAKAP4 present in each semen sample and could be measured by spectrophotometry at 450nm. A standard curve was determined in parallel for precise concentrations of proAKAP4 in the pig semen sample. Results of proAKAP4 concentrations were always expressed in ng/mL.
Statistical Analysis
Statistical analysis was achieved using Prism 8.2 GraphPad software (GraphPad Software, USA). D’Agostino and Pearson normality tests were performed to determine if the populations were following a Gaussian distribution and Pearson correlation coefficients were determined for each proAKAP4 concentration. The threshold for statistical significance was set to be p<0.05. In normally distributed groups, results were presented as mean ± standard deviation. The significant differences from T0 value were determined by a non-parametric paired samples t-test Mann Whitney U-test. Stabilities of proAKAP4 after freeze thaw cycles and after long term storage were assessed by the percentage change from T0 for paired groups (T0-T1, T0 -T2, etc. and T0 – T1M, T0 -T3M, etc.). Bias was calculated by the formula: [(CX - C1)/C1] × 100%, with C1: the mean or median of the T0 sample; and Cx: the mean or median of the experimented sample. For non- Gaussian groups, median variations from T0 were determined by non-parametric Friedman test and Wilcoxon signed rank test
Results
ProAKAP4 Expression in Boar Raw Semen
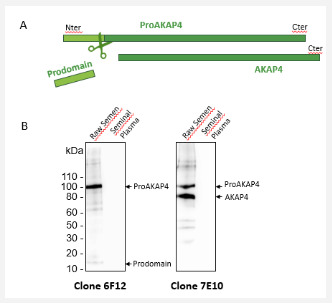
As observed previously from other mammals [1-3] proAKAP4 was only expressed in spermatozoa preparation and not in the seminal liquid as revealed with the monoclonal antibody (clone 6F12) against proAKAP4 (Figure 1). The proAKAP4 was cleaved in AKAP4 mature protein and the prodomain was released (Figure 1A). This cleavage and metabolism of the precursor proAKAP4 can also be followed by western blotting using specific monoclonal antibodies such as the clone 7E10 which recognized the C-terminus of both proAKAP4 and AKAP4 (Figure 1B). Therefore, in this initial T0 experiment, we observed the same amount of proAKAP4 and AKAP4 in the spermatozoa preparation sample of the fresh pig ejaculate. As expected, we confirmed that proAKAP4 is a spermatozoa specific protein expressed in the flagellum of pig spermatozoa
Stability of Boar proAKAP4 during Freeze-Thaw Cycles of the Same Aliquot
The concentration of proAKAP4 was measured in the ejaculate using the Pig 4MID® Kit as T0 value for the stability experiments. The initial mean concentration of ProAKAP4 was of 50.7 ± 1.3ng / mL, reflecting a high-quality semen [1]. After semen aliquots have been frozen and thawed up to ten times, there were no statistically significant differences in proAKAP4 concentrations as quantified using the Pig 4MID® Kit from T0 to T10 (Table 1).
All concentrations were in ng /mL and indicated as a mean± SD and median (interquatile ranges). Clearly, the proAKAP4 concentrations were not modified statistically after ten freezethaw cycles and the global percentage of variations was at 9.54%. Dilutions of the neat semen (half and quarter dilution factor) had no effect on the recovery of proAKAP4 concentrations as shown graphically on Figure 2. These dilutions highlighted the robustness of the Pig 4MID® Kit to quantify accurately the amount of the proAKAP4 polypeptide in neat pig semen. We checked then the expression and metabolism of proAKAP4 by western blotting (Figure 3). None of proAKAP4 and AKAP4 expressions or metabolisms were altered by the freeze-thaw cycles. Neither the integrity of proAKAP4 or AKAP4 was shown to be altered along the 10 freeze-thawing cycles and proAKAP4 was not further converted into AKAP4 showing that proAKAP4 and AKAP4 processing were not modified by freeze thawing cycles. The proAKAP4 was therefore considered as a very stable analyte when kept frozen in raw semen until we performed the Pig 4MID® Kit analysis
Stability of the Frozen proAKAP4 Polypeptide in longterm Storage Conditions
They were no significant variation in proAKAP4 concentrations as measured with the Pig 4MID® Kit for fresh pig sperm when stored until 6 months at -20 °C (Table 2). No variations were obtained when stored at - 80 °C (data not shown). Statistical significances were evaluated as described in the Materials and methods section. Our results showed that total proAKAP4 concentrations were clearly stable up to six months of storage at -20 °C with the variation in proAKAP4 concentration always below 5%. The western-blot analysis displayed no degradation of the sample stored at -20 °C from up to 6 months highlighting the robustness of the protein when kept frozen in raw semen (data not shown).
Intra-assay and Inter-Assay of the Pig 4MID® Kit
We further assess the robustness of the Pig 4MID® Kit by evaluation of the intra-assay and inter-assay CV’s on the Pig 4MID® Kit with neat pig semen as in the design of our study. These intra-assay and inter-assay CV’s were performed with two different ejaculates of the same animal (Table 3). Inter-assay variation was assessed from 10 determinations (with 2 aliquots each day) on ten consecutive study days, and intra-assay variation was calculated from eight sequential determinations obtained from the first day of the study period
Discussion
This study examined the storage effects and repeated freezethaw cycles on pig proAKAP4 sperm protein integrity in preanalytical conditions (meaning before the 4MID® Kit procedures) to evaluate the robustness of this new parameter in daily routine of semen analysis for swine breeding activities. We clearly show that proAKAP4 polypeptide is highly stable when frozen at minus 20 °C, for a long time period (up to 6 months) and will not be altered by multiple freeze-thaw cycles in neat semen. These data are of importance as they highlighted for the first time, that specimens of one ejaculate can be aliquoted and kept at minus 20 °C until their analysis and shipped from AI stations to central laboratories without loss of proAKAP4 integrity.
The reason of this stability could be due to the localization and the inherent functionality of the proAKAP4 itself. As shown on Figure 1, the proAKAP4 is a sperm specific protein that is neither found on the membrane nor released in the seminal plasma. The proAKAP4 polypeptide is inside the spermatozoa, more precisely in the fibrous sheath of the principle piece of the flagellum [19-22] and will need to be released from the fibrous sheath to be further quantified using the 4MID® assay. ProAKAP4 has been shown to be strictly localized to the principal piece of the flagellum and not in other spermatozoa compartments [20-21], tightly anchored to the fibrous sheath, along the longitudinal columns and ribs of the sperm tail [2,3,20-21].
According to the Pig 4MID® assay procedure, the proAKAP4 has then first to be extracted from the spermatozoa. Proteins markers described in sera or in seminal fluids [1,23] are frequently reported to suffer from the shear stress induced in buffered solutions and from long-term storage conditions. In contrast of what we reported with sperm proAKAP4, proteins in buffer solution can be fragile and they may even acquire conformations susceptible to degradation during frozen and post-thawed conditions. Clearly, proAKAP4 concentrations appears to be stable as long as the polypeptide is maintained in neat semen within the spermatozoa flagellum, with the fibrous sheath bringing stability for proAKAP4 integrity. The maintenance of proAKAP4 as a fulllength precursor is then important for the aliquot processed for the initial quality assessment of the ejaculate and at further steps, for the quality control during dose processing in AI stations. High proAKAP4 concentrations in the ejaculate and then in doses, will ensure to have enough motile and functional spermatozoa populations in the hours post the artificial insemination
The total amount of proAKAP4 per spermatozoa is fully synthetized within the testis and before ejaculation. Therefore, an aliquot of the ejaculate could be frozen immediately after semen collection in boar studs as this will represent the exact picture of the long-term motility of the spermatozoa. Freezing of an aliquot of ejaculate at collection point will then facilitate the analysis of semen (related to the proAKAP4 concentration) and favors also transport of such aliquot up to external laboratories. Our results clearly showed that degradation rates of the proAKAP4 were not impacted by frozen storage conditions of the aliquot and are in favor of such collection for delocalized sperm quality assessments. Furthermore, proAKAP4 stability when stored in aliquots in sperm frozen collections, will allow to better take in account technical and logistical constraints such as i) delays in shipping frozen aliquot when in need to analyze hypofertile animal; ii) being less dependent of any power cut or voltage fluctuations of the low-cost freezers; or the use of frost-free freezer that goes through numerous defrost cycles, as may happen in small breeding centers.
In boar stud, the storage of frozen aliquoted samples could also be convenient to process all the semen in the same time to compare ejaculates of different animals at the end of collection time. The dose semen processing will then not be impacted as the 4MID® analysis will be completely run in 2 hours. The amount of proAKAP4 as a read out of sperm quality should add marketing values for AI stations by ensuring high quality semen. In swine industry, there is also a real interest to identify the best male and then to follow up the sperm production during exploitation. Boars are usually kept from 6 to 9 months in the AI stations. That will be of importance to have a stable parameter to follow animal along all his career and keeping a safe measure of the initial quality of the first ejaculate after quarantine. In this context, the storage of frozen aliquoted samples may allow likewise to identify genetical traits of interest in a particular pig strains, such as fertility, death at birth or litter size, that may be related to proAKAP4 levels of expression [1,24-27].
Finally, keeping frozen aliquots of pig semen could allow to reanalyze the same samples stored to confirm previous results or to perform additional analysis, establishing new path for boar sperm preservation investigations. Better understanding proAKAP4 stability allows now to compare ejaculates at different collection points and compared to extended semen which is being shipped and used many days later. The storage capacity of extenders should be then further explored in relation with proAKAP4 consumption and degradation rates, during several days and in chilled conditions, when spermatozoa will stay alive.
Conclusion
One of current challenge of the swine industry is to standardize the semen processing procedures within boar studs. The proAKAP4 parameter have been initially introduced to facilitate the identification of ejaculates of inferior motility and quality, that were not identified by classical sperm parameters, and that could then be withheld before their release into the field. Having a stable sperm parameter such as proAKAP4 that can be kept stable as frozen up to the analysis time should be further interesting for quality check control and to follow up this parameter evolution during all the boar career. Taken together, the proAKAP4 parameter stability present then multiple advantages in favor of harmonizing sperm quality assessment between laboratory and AI centers.
To Know More About Journal of Dairy & Veterinary sciences
Please click on: https://juniperpublishers.com/jdvs/index.php
For more Open Access Journals in Juniper Publishers
please click on: https://juniperpublishers.com/index.php
#veterinary pathology#veterinary pharmacology#Veterinary Toxicology#Veterinary sonography#Juniper Publishers#open access journals
0 notes
Text
"The 'stretchy dog test' might be a better test, rather than sending a biopsy."
- on diagnosing Ehlers-Danlos in dogs
4K notes
·
View notes
Note
Vet school is like this too. I didn't quite reach that "I don't wanna!!11!!1 until into my residency but hooooboy was I there when I was studying for the pathology board exam. Idk if it's still the horrible 3 day marathon academic hazing experience it was in 2013, but that exam ate my life until I had passed out and it was over. I thought I never wanted to see another journal or text book and that's... suboptimal for a pathologist.
But it's true! I like reading about vet path things again now! Maybe it's because some of that high-stakes stress is lifted and I'm not constantly second guessing what I MUST retain to pass a test and can just focus on what's interesting? I love what I'm studying again 💙
And I'm never taking an exam like that ever again.
Hi!!! I need some advice from you or other doctors/med students. I just have a few exams left till the end of med school but I just. Can’t. Study. Anymore. It’s making me want to throw up. I hate it so much. I open the book and I’m just like 😭😭😭. And the worst thing is that after I’m done with med school I will STILL have to study. Till I die. And I currently just don’t want to study ever again. I feel like it’s sucking all the life out of me. So if someone here ever felt like that, I would greatly appreciate some words of wisdom haha
Oh sweetheart, I feel you! And I assure you, most of us had been here, and still succeeded against all odds. I'm sure others will have some advice too :)
To be honest, I'm not entirely sure how I passed my last exams, I was also suffering from a very heavy case of "I can't study"-ness (and apparently also undiagnosed ADHD, not helping my case). What worked for me (kinda), I took breaks pretty often. Might seem counterproductive, but it's better if you do something fun/relaxing/anything else for an hour and then study effectively for half an hour, than suffering through that one and a half hour without getting any studying done... Eat well, do sports, and sleep enough! And then, just take a deep breath, and suck it up, force yourself trough the material. Pure force of will. And my favourite motivational pinterest quote: "the quickest way out is trough". (You can't imagine how many times I repeated this sentence like a mantra in my head, instead of actually studying, naturally)
And it's important to remember, you are at the end of med school. You know way more than you believe. If your school is anything like mine, most of these exams will test you on stuff you've already learned once (or twice or thrice etc). You need to freshen it up, definitely, but you already have most of the knowledge you need to pass.
Believe me, I know the feeling that you think about the future and you want to throw up because of all the studying you will have to do after med school. Hell, I'm still in my "school? never again" phase. (I might have slight PTSD from med school... But yeah, we are talking about you and not me here. Anyway.) First off, you will most probably have some time off after finishing med school and before starting your first doctor job. Hide all the medical books from view! If you have a few months, by the end of it you might even be able to read more than a page of a non-science-related book you actually enjoy! But joking aside, time off after med school really works wonders. It's absolutely necessary for your sanity and survival, and it will help regain some of your motivation.
Second off, studying after med school is much more fun! If anyone told me this, I definitely wouldn't have believed them, but it's true. Now I have to study things I'm interested in, things I care about. I see it put to work in day to day life, hell, I use it in day to day life. Never thought I would say this, but studying now is (mostly) fun. I'm starting to remember that I used to like studying, that I'm curious about the world around me... So to summarize, it gets better. It's still studying, sometimes it's still an "I don't wanna" chore, but when the difference between studying or not can literally and actually make the difference between a patient's life or death, that terror makes for a pretty fucking great motivation. And when you studied something and then use it in your work the next week? Amazing feeling.
So hang in there. You got this far already, you can do this! It's going to hurt all the way, but you are going to be standing there on the other side victorious, so just puff out you chest and keep going until you get there.
Best of luck with your exams!
#vetblr#veterinary#pathology#ACVP exam#vet path#veterinary pathology#academic hazing#I'm still twtichy if anyone mentions Ames Iowa#or Iowa State University
17 notes
·
View notes
Text









#please vote for science#pathology#microbiology#histology#vetblr#pathblr#veterinary pathology#microscopy#dermatology
906 notes
·
View notes
Note
Hello,
I’m a vet student entering into my second year and I’d be lying if I said I haven’t considered leaving vet med for human med since I’ve discovered clinical practice isn’t for me. I’m becoming increasingly interested in pathology and I love the thought of performing necropsy often in my daily life. However, I’m starting to wonder if veterinary pathology is worth the large pay cut compared to human pathology. I would love to hear your thoughts on this topic as I’d appreciate any advice!
Hi there!
There are several layers to this question and I'm going to do my best to address them all, so bear with.
The first factor you're considering here is clinical practice vs pathology. I also realized part way through vet school that I didn't see clinical practice as a good fit for me. That's what I had gone into vet school planning to do, but as the years went by I eventually came to the conclusion that the pace, the day to day tasks, and the overall feel of a clinical practice job didn't suit me. Luckily, I also found pathology during that process. I love histopathology and working at the microscope, I enjoy necropsy and interpreting lesions, and I like the pace of working in pathology. It suits me much better. It sounds like you've already got a feel for that difference, and that's great! If you haven't yet, I would highly recommend looking into this further by reaching out to some veterinary pathologists to talk about what their day to day looks like (make sure it lines up with what you have in your head) and, if that all sounds interesting to you, what steps you need to take during and after vet school to head down that path. The pathologists at your vet school are a great place to start. Other resources that may benefit you are the Davis-Thompson Foundation (veterinary pathology education charity who do lots of seminars and I believe offer a scholarship to support veterinary students doing pathology externships); the American College of Veterinary Pathologists (ACVP); and the Society for Toxicologic Pathologists (STP). The ACVP and STP both hold yearly conferences which are discounted for vet students (and have scholarships) and are another great way to meet pathologists and see what we're up to! (I'll link these organisations at the bottom of this post). The ACVP also has a job board where you can see what options are currently being advertised (although this is not an exhaustive list). There are a lot of different ways to be a veterinary pathologist, including positions in academia, research (I know several veterinary pathologists doing research for the USDA), industry/toxicologic pathology (medical device development, pharma, etc.), zoo and wildlife pathology, aquatic pathology, commercial diagnostics (e.g. IDEXX), and many more.
Next, what may be the biggest question here, should you do human med or vet med? Nobody else can answer this question for you, but I'll try to provide some thinking points to help you figure it out. Firstly, while there are similarities, it's worth noting that the two aren't identical fields just with different species. What kinds of cases you see, how residency and senior positions work, what your day to day looks like; all of these differ somewhat between the two professions. I've never worked in human med, but there are some great pathology residents sharing their experiences online if you want some insight. Think about what you're comfortable with too - I find lesions on people gross, but lesions on animals fascinating. In vet path we also get to work on a huge range of species! Just during my residency so far I've worked on everything from coral, insects, and fish, to bears, elk, and cougars. This inherent comparative nature of vet path is, I think, unique to our field. Or maybe the increased access to specific diagnostic tools and broader testing capabilities of human path appeals to you more. Again, you may want to reach out to people working in human pathology to learn more about what they actually do.
Importantly, you already chose vet school over med school at some point. What was your reasoning? Do those reasons still hold if you don't want to work in clinical practice? You're already in vet school, is the appeal of human pathology worth starting over in med school? Only you can answer those questions.
Finally, the "pay cut". You're not wrong that, as a general rule, you will be paid less to work in vet med than in human med. Despite being equally highly educated, trained, and skilled, that's a given and something we all have to reckon with when deciding which field is for us. But it's also worth noting that there are ways you can work in vet med and still be well paid. And there are a lot of different ways to be a pathologist. For example, the ACVP 2017 Salary Survey reported that the median salary for veterinary pathologists in industry, with 0-2 years post training experience, was between $140,000 USD and $199,999 USD (depending on location). Highly paid and more experienced industry pathologists reported salaries over $280,000 (these numbers are due to be updated soon). Now, depending on your lifestyle, obligations, debts, location etc. what you consider "high paying" or "adequate" is going to differ, so I can't tell you that any of those numbers is "enough". But I would factor into your decision what kind of pay you think you need and want, and how highly "paid well" ranks in what is specifically important to you in picking a career.
Ultimately, while I think the gap should be smaller, I don't see working in vet med instead of human med as taking a "pay cut" because we're not doing the same job, and because vet med is the right place for me. But that is based on how I rank my priorities, and you're in no way wrong if you feel differently.
I hope this helps, and let me know if you have more questions!
Links ACVP: https://www.acvp.org/ STP: https://www.toxpath.org/ Davis-Thompson Foundation: https://davisthompsonfoundation.org/
15 notes
·
View notes
Text

Days like this 🩷
#personal stuff#uni student#university#medicine#morphology#heart#real heart#medicine student#pathology#Pathological Anatomy#doctor#cardiology#Tablet#samsung tablet#study#studying#Lightroom#notebook#veterinary medicine#veterinary medicine student#jar#notes#bucharest#days like this#Autumn#fall#October#exams#laboratory medicine#laboratory
71 notes
·
View notes
Text







I found an old fish pathology book (published in 1975) at a used book store and while it’s information is probably pretty outdated it has some really cool images.
3 notes
·
View notes
Note
A bit morbid, but I'm curious: When a human dies in suspicious circumstances, a forensics expert does an autopsy to look for any evidence of foul play. If an animal dies in suspicious circumstances, are there specialist vets who do the same kinds of checks, or does it fall to general practice vets, or do the human forensics experts handle animals too? (I'm sure the police aren't remotely as interested in investigating animal deaths as they are human deaths, but it must come up occasionally, right?)
Sueanoi here
Veterinary pathology is a thing, and we do Necropsy (like Autopsy but for animals) all the time. It's quite a specialized work. It was a mandatory lesson but I never had performed a necropsy on my own in my career.
I do use their service for Biopsy (same work but the sample came from an animal that is still alive) and other lab works.
Note: it is quite routine for Large animal vets that they would perform necropsy on culled animals so they can evaluate the health of the entire herd.
75 notes
·
View notes
Text



Had the best day!! Of course first pathology in the morning, but in the afternoon a meeting in central Utrecht and as the perfect ending I got 7 chicken nuggets in my 6-nuggies box. Honestly could cry!
#studyspo#studyblr#medblr#medspo#bujo#vetblr#vetmed#veterinary medicine#veterinary science#med school#light academia#academia aesthetic#ipad#goodnotes#studying#uni#thesis#studyblr community#light aesthetic#Utrecht#Utrecht university#pathology#chicken nuggets#mcdonalds
20 notes
·
View notes