#Tumor necrosis factor-alpha (TNF-alpha)
Explore tagged Tumblr posts
Link
1 note
·
View note
Text
https://bundas24.com/read-blog/118482_tumor-necrosis-factor-tnf-alpha-inhibitors-market-size-overview-share-and-foreca.html
The Tumor Necrosis Factor (TNF) Alpha Inhibitors Market in 2023 is US$ 41.88 billion, and is expected to reach US$ 44.35 billion by 2031 at a CAGR of 0.7%.
#Tumor Necrosis Factor (TNF) Alpha Inhibitors Market#Tumor Necrosis Factor (TNF) Alpha Inhibitors Market Analysis#Tumor Necrosis Factor (TNF) Alpha Inhibitors Market Overview
0 notes
Text
Progress in the Study of the Protective Effect and Mechanism of C-phycocyanin on Liver Injury
Abstract: C-phycocyanin (C-phycocyanin) is a pigment-containing protein from marine algae that has shown promising results in the treatment of many inflammatory diseases and tumors. C-alpha-cyanobilin is a pigment-containing protein from marine algae that has been shown to be effective in the treatment of various inflammatory diseases and tumors. C-alpha-cyanobilin has a protective effect on various liver diseases, such as drug-induced or toxic substance-induced liver damage, non-alcoholic fatty liver disease, hepatic fibrosis, and hepatic ischemia-reperfusion injury. The protective effect of C-alginin on liver injury is mainly realized through the regulation of signaling pathways such as nuclear factor (NF)-κB, phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) and AMP-dependent protein kinase (AMPK), and the inhibition of oxidative stress, etc., and is not toxic to normal cells. Therefore, C-alginin has a broad application prospect as a potential natural hepatoprotective marine active substance. In recent years, the research progress of the protective effect of C-alginin on liver injury and its mechanism is summarized.

C-phycocyanin (C-phycocyanin) is a complex protein of cyanobacteria and a natural food protein pigment with pharmacological effects such as antioxidant, anti-inflammatory and anti-tumor effects, as well as fast-acting and low-toxicity, it can be used as a functional food [1-2]. C-Alginin can also enhance immunity and is safe, without causing acute and subacute toxic reactions [3]. Selenium-enriched PC has been shown to have stronger pharmacological effects [4]. Therefore, C-alginate has important research value both as a drug and a functional food, and has become a hot spot in the field of pharmaceutical research [5]. In this paper, we summarize the progress of research on the application and mechanism of C-alginin in liver diseases.
1 Ameliorative effect of C-phycocyanin on liver injury caused by drugs and toxic substances
The liver is the metabolic center of drugs and exogenous toxic substances, and metabolites are prone to liver injury. C-PC can inhibit the synthesis and release of inflammatory factors such as tumor necrosis factor (TNF)-α and interferon-γ, and increase the activities of catalase and superoxide dismutase (SOD), which can inhibit hepatic inflammation and alleviate hepatic injury [3]. It has been found that C-PC can significantly prevent thioacetamide-induced liver injury, significantly reduce the levels of alanine aminotransferase (ALT) and aliquot aminotransferase (AST), shorten the prothrombin time and reduce the hepatic histopathological damage, and improve the survival rate of rats with fulminant hepatic failure [6]. C-alginin also has a good effect on thioacetamide-induced hepatic encephalopathy, which can be seen in the reduction of tryptophan and lipid peroxidation indexes in different regions of the brain, and the enhancement of catalase and glutathione peroxidase activities in rats with fulminant hepatic failure [6].
Another study found that C-alginin not only attenuates the oxidative stress induced by 2-acetylaminofluorene and reduces the generation of reactive oxygen species (ROS) radicals, but also inhibits the phosphorylation of protein kinase B (Akt) and the nuclear translocation of nuclear factor (NF)-κB induced by 2-acetylaminofluorene, thus inhibiting the expression of multidrug resistance genes [7]. Osman et al. [8] also showed that C-alginin could normalize the levels of ALT, AST, catalase, urea, creatinine, SOD and glutathione-s-transferase in the livers of rats poisoned with carbon tetrachloride (CCl4). This result was also verified in human liver cell line (L02) [9]. C-phycocyanin can effectively scavenge ROS and inhibit CCl4-induced lipid peroxidation in rat liver [10], and C-PC can improve the antioxidant defense system and restore the structure of hepatocytes and hepatic enzymes in the liver of gibberellic acid-poisoned albino rats [11]. As a PC chromophore, phycocyanin can also significantly inhibit ROS generation and improve liver injury induced by a variety of drugs and toxic substances [10]. Liu et al. [12] found that phycocyanin showed strong anti-inflammatory effects in a CCl4-induced hepatic injury model in mice, which could significantly reduce the levels of ALT, AST, the expression of TNF-α and cytochrome C, increase the levels of albumin and SOD, and proliferate cytosolic nuclei. It can significantly reduce ALT and AST levels and the expression of TNF-α and cytochrome C, increase albumin levels and the expression of SOD and proliferating cell nuclear antigen, promote hepatocyte regeneration and improve the survival rate of mice with acute liver failure.
Gammoudi et al [13] used response surface method to optimize the extraction process of C-phycocyanin, and obtained high extraction recovery. C-phycocyanin extracted by the optimized method has the ability of scavenging hydroxyl, superoxide anion and nitric oxide radicals as well as the ability of metal chelating, and it has stronger antioxidant effect; C-PC significantly increased the activity of SOD and inhibited the increase of ALT, AST, and bilirubin in cadmium-poisoned rats. C-PC significantly increased the activity of SOD and inhibited the increase of ALT, AST and bilirubin in rats with cadmium poisoning. The above studies show that C-phycocyanin can effectively protect liver injury caused by drugs and toxic substances, and has the efficacy as the basis for drug development.
2 Preventive effect of C-alginin on hepatic fibrosis
Liver fibrosis is an inevitable process in the development of various chronic liver diseases and may be reversed with early and timely treatment. The key to liver fibrosis is the activation of hepatic stellate cells. Previous studies have found that low-dose C-alginin combined with soy isoflavones can inhibit hepatic stellate cell activation by inhibiting the activity of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase[14], but it is not clear whether C-alginin alone can inhibit the activity of NADPH oxidase. Therefore, the combination of C-algin and soy isoflavones at appropriate doses may have a preventive effect on liver fibrosis in high-risk groups. C-alginin may inhibit the progression of NADPH by suppressing oxidative damage, thereby inhibiting the development of hepatic fibrosis [15].
Epithelial mesenchymal transition (EMT) is one of the key mechanisms contributing to the development of fibrotic diseases. C-alginin inhibits transforming growth factor β1 (TGF-β1)-induced human EMT [16]. Although the effect of C-alginin on EMT in hepatic fibrosis has not been reported, it has been found that C-alginin can reduce pulmonary fibrosis by inhibiting epithelial mesenchymal transition [17]. Another study found that C-alginin could reduce the expression of α-smooth muscle actin (α-SMA) and connective tissue growth factor (CTGF) mRNA in human dermal fibroblasts and alleviate fibrous contracture [18]. The results of these studies also have significance for the inhibition of hepatic fibrosis, and provide a theoretical basis for the further study of C-PC as a potential antifibrotic drug.
3 Protective effect of C-alginin on hepatic ischemia-reperfusion injury
Liver ischemia/reperfusion injury is an important clinicopathophysiological phenomenon. It was found that the addition of two different doses (0.1 g/L and 0.2 g/L) of C-alginin to the Krebs Henseleit preservation solution significantly decreased hepatic ALT, AST and alkaline phosphatase activities, and reduced the rate of lipid peroxidation and malondialdehyde content in an isolated perfused rat liver model, and increased the activities of hepatic glutathione-s-transferase and glutathione peroxidase, as well as sulfhydryl groups in hepatic tissue. On the other hand, it can increase the activities of hepatic glutathione-s-transferase and glutathione peroxidase and the content of sulfhydryl groups in liver tissues, therefore, C-alginin can significantly reduce hepatic ischemia/reperfusion injury as an antioxidant [19]. In isolated perfused mouse livers, it was found that C-alginin significantly reduced the phagocytosis and respiratory burst activity of hepatic macrophages (Kupffer cells), attenuated cytotoxicity and inflammation induced by highly active Kupffer cells, and dose-dependently inhibited carbon phagocytosis and carbon-induced oxygen uptake by perfused livers, and then inhibited the increase of hepatic nitric oxide synthase activity induced by gonadotropins [20]. and thus inhibit the thyroid hormone-induced elevation of hepatic nitric oxide synthase activity [20].
However, C-alginin has a very short half-life in vivo, which limits its application in vivo. It was found that the use of polyethylene glycol-b-(polyglutamic acid-g-polyethyleneimine), a macromolecular material with good drug-carrying capacity and slow-release properties, as a nanocarrier of C-alginin could solve this problem, and the release of C-alginin could be delayed by subcutaneous injection into the abdominal region of rats, which could attenuate islet damage caused by hepatic ischemia/reperfusion and enhance the function of the islets [21]. This study broadens the scope of application of C-alginin in vivo and improves the therapeutic effect of C-alginin.
4 Inhibitory effect of C-alginin on hepatocellular carcinoma
It was found that C-alginin significantly reduced the expression of matrix metalloproteinase (MMP)-2 and MMP-9 and the expression of tissue inhibitor of metalloproteinase 2 (TIMP2) mRNA in human hepatocellular carcinoma cells (HepG2 cells) [22]. C-alginin is a natural photosensitizer, and photodynamic therapy (PDT) mediated by alginin microcystin induced a large accumulation of ROS in HepG2 cells, which promoted mitochondrial damage and cytochrome C release, and led to apoptosis of hepatocellular carcinoma cells [23].
Liu et al. [24] used nanoscale C-alginate particles prepared by lactobionic acid grafting and adriamycin loading to enhance the growth inhibition of HepG2 cells when combined with chemo-PDT, and the C-alginate particles could effectively accumulate and diffuse in tumor multicellular spheres. In vitro and in vivo studies on the effects of selenium-enriched PCs on PDT in hepatocellular carcinoma showed that selenium-enriched PCs could migrate from lysosomes to mitochondria in a time-dependent manner, and that selenium-enriched PCs could induce the death of tumor cells through the generation of free radicals in vivo, increase the activities of antioxidant enzymes in vivo, induce mitochondria-mediated apoptosis, and inhibit autophagy, thus offering a relatively safe pathway to tumor treatment and showing new development perspectives [4]. It can provide a relatively safe way to treat tumors and shows a new development prospect [4].
Lin et al. [25] combined C-phycocyanin with single-walled carbon nanohorns and prepared phycocyanin-functionalized single-walled carbon nanohorn hybrids, which enhanced the photostability of C-phycocyanin and protected the single-walled carbon nanohorns from adsorption of plasma proteins, and synergistically used with PDT and photothermal therapy (PTT) to treat tumors. C-phycocyanin covalently coupled with biosilica and PDT or non-covalently coupled with indocyanine green and PTT on tumor-associated macrophages can also increase the apoptosis rate of tumor cells [26-27]. The development of PDT and PTT synergistic methods for the treatment of cancer has broadened the application of C-PC and enhanced its value in the treatment of hepatocellular carcinoma.
In addition, C-phycocyanin can inhibit the expression of multidrug-resistant genes in HepG2 cells through NF-κB and activated protein-1 (AP-1)-mediated pathways, and C-phycocyanin increases the accumulation of adriamycin in HepG2 cells in a dose-dependent manner, which results in a 5-fold increase in the susceptibility of cells to adriamycin [28]. Even in adriamycin-resistant HepG2 cells, C-PC induced the activation of apoptotic pathways such as cytochrome C and caspase-3 [29], and the results of Prabakaran et al. [30] also confirmed the inhibitory effect of C-PC on the proliferation of HepG2 cells, mediated by the inactivation of BCR-ABL signaling and the downstream PI3K/Akt pathway. mediated by BCR-ABL signaling and inactivation of downstream PI3K/Akt pathway. In addition, C-phycocyanin modifies the mitochondrial membrane potential and promotes apoptosis in cancer cells [30]. Currently, C-phycocyanin is a synergistic molecule with other drugs that have been widely used in the treatment of cancer [31]. The above studies demonstrate that C-phycocyanin has good therapeutic potential in the field of hepatocellular carcinoma.
5 Amelioration of metabolic syndrome and non-alcoholic fatty liver disease by C-phycocyanin
It has been found that C-alginin can reduce ALT and AST levels, decrease ROS production and NF-κB activation, and attenuate hepatic fibrosis in rats induced by high-fat choline-deficient diets, and thus C-alginin has a protective effect on NAFLD rats through anti-inflammatory and antioxidant mechanisms [15].
Another study on the effects of aqueous extract of Spirulina (mainly C-alginin) on NAFLD induced by a high-calorie/high-fat Western diet in C57Bl/6J mice showed that aqueous extract of Spirulina significantly improved glucose tolerance, lowered plasma cholesterol, and increased ursodeoxycholic acid in bile in mice [32]. Kaspi-Chadli et al. Kasbi-Chadli et al. [33] showed that aqueous extract of Spirulina could reduce cholesterol and sphingolipid levels in the liver and aortic cholesterol levels in hamsters fed a high-fat diet by significantly decreasing the expression of hydroxy-3-methylglutaryl-coenzyme A reductase (HMG CoA) gene, a limiting enzyme for cholesterol synthesis, and TGF-β1 gene, and that ursodeoxycholic acid levels in the feces of hamsters fed high-fat diets were increased in the high Spirulina aqueous extract treatment group.
A daily dose of C-alginin-enriched Spirulina can reduce the harmful effects of oxidative stress induced by a diet rich in lipid peroxides [34]. Ma et al. [35] found that C-alginin promoted the phosphorylation of hepatocyte AMP-dependent protein kinase (AMPK) in vivo and ex vivo, and increased the phosphorylation of acetyl coenzyme A carboxylase. In the treatment of NAFLD in mice, C-alginin can improve liver inflammation by up-regulating the expression of phosphorylated AMPK and AMPK-regulated transcription factor peroxisome proliferator-activated receptor α (PPAR-α) and its target gene, CPT1, and by down-regulating the expression of pro-inflammatory factors such as TNF-α and CD36 [35]. This suggests that C-phycocyanin can also improve lipid deposition in the liver through the AMPK pathway.
Endothelial dysfunction is associated with hypertension, atherosclerosis and metabolic syndrome. Studies in animal models of spontaneous hypertension have shown that long-term administration of C-alginin may improve systemic blood pressure in rats by increasing aortic endothelial nitric oxide synthase levels, with a dose-dependent decrease in blood pressure, and thus C-alginin may be useful in preventing endothelial dysfunction-related diseases in the metabolic syndrome [36]. In the offspring of ApoE-deficient mice fed C-alginate during gestation and lactation, male littermates had an elevated hepatic reduced/oxidized glutathione ratio and significantly lower hepatic SOD and glutathione peroxidase gene expression.
C-PC is effective in preventing atherosclerosis in adult hereditary hypercholesterolemic mice [37]. In vitro, C-phycocyanin also improved glucose production and expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-Pase) in high-glucose-induced insulin-resistant HepG2 cells [38]. C-alginin also increases glucose uptake in high glucose-induced insulin-resistant HepG2 cells through the insulin receptor substrate (IRS)/PI3K/Akt and Sirtuin-1 (SIRT1)/liver kinase B1 (LKB1)/AMPK signaling pathways, activates glycogen synthase, and increases the amount of glycogen [38]. C-phycocyanin can improve blood glucose and fasting serum insulin levels in tetracycline-induced diabetic mice [39]. Therefore, C-phycocyanin can maintain cellular glucose homeostasis by improving insulin resistance in hepatocytes.
6 Hepatoprotective role of C-phycocyanin in other liver diseases
Studies have shown that C-alginin can inhibit total serum cholesterol, triacylglycerol, LDL, ALT, AST, and malondialdehyde levels in mice modeled with alcoholic liver injury, significantly increase SOD levels in the liver, and promote the activation and proliferation of CD4+ T cells, which can have an ameliorative effect on alcoholic liver injury [40]. In addition, C-phycocyanin may enhance the intestinal barrier function, regulate the intestinal flora, reduce the translocation of bacteria and metabolites to the liver, and inhibit the activity of the Toll-like receptor 4 (TLR4)/NF-κB pathway, which may reduce the inflammation of the liver and prevent the occurrence of hepatic fibrosis in mice [41]. In mice with X-ray radiation-induced liver injury, C-phycocyanin can reduce radiation-induced DNA damage and oxidative stress injury by up-regulating the expression of nuclear factor (NF)-E2-related factor 2 (Nrf2) and downstream genes, such as HO-1, and play a hepatoprotective role by enhancing the activities of SOD and glutathione peroxidase [42].
7 Outlook
Liver fibrosis is the common final process of chronic liver diseases, and there is no effective therapeutic drug at present. Although some research progress has been made in the field of traditional Chinese medicine (TCM) on the reversal of liver fibrosis [43], its toxicological effects have not yet been clarified. Although the incidence of viral hepatitis has gradually decreased with the development and popularization of vaccines and antiviral drugs, the incidence of drug-induced liver injury (DILI) and liver diseases such as NAFLD has been increasing year by year with the improvement of people's living conditions [44]. Therefore, there is an urgent need to find drugs or nutrients that can help maintain normal hepatocyte function and effectively inhibit liver inflammation and fibrosis. C-alginin, with its anti-inflammatory, antioxidant, and antitumor effects, as well as good food coloring, has a wide range of applications in both the pharmaceutical and food industries.
References:
[ 1 ] LIU Q, HUANG Y, ZHANG R, et al. Medical aapplication of spirulina platensis derived C-phycocyanin [J]. Evid Based Complement Alternat Med, 2016, 2016: 7803846. doi: 10. 1155/ 2016/7803846.
[2] BRAUNE S , KRÜGER-GENGE A , KAMMERER S , et al. Phycocyanin from Arthrospira platensis as potential anti-cancer drug: review of in vitro and in vivo studies[J]. studies[J]. Life (Basel), 2021, 11 (2): 91. doi:10.3390/life11020091.
[3] GROVER P, BHATNAGAR A, KUMARI N, et al. C-phycocyanin- a novel protein from spirulina platensis- in vivo toxicity, antioxidant and immunomodulatory studies[J]. Saudi J Biol Sci, 2021, 28(3):1853-1859. doi: 10. 1016/j.sjbs.2020.12.037.
[4] LIU Z, FU X, HUANG W, et al. Photodynamic effect and mechanism study of selenium-enriched phycocyanin from spirulina platensis against liver tumours. [J Photochem Photobiol B] J Photochem Photobiol B, 2018, 180: 89-97. doi: 10. 1016/j.jphotobiol.2017.12.020.
[5] JIANG L, WANG Y, YIN Q, et al. Phycocyanin: a potential drug for cancer treatment[J]. J Cancer, 2017, 8 (17): 3416-3429. doi: 10.7150/jca.21058.
[6] SATHYASAIKUMAR K V, SWAPNA I, REDDY P V, et al. Co- administration of C-phycocyanin ameliorates thioacetamide- induced hepatic encephalopathy in Wistar rats[J]. J Neurol Sci, 2007, 252(1):67-75. doi: 10. 1016/j.jns.2006.10.014.
[7] ROY K R , NISHANTH R P , SREKANTH D , et al. C- phycocyanin ameliorates 2-acetylaminofluorene induced oxidative stress and MDR1 expression in the liver of albino mice [J]. of albino mice[J]. Hepatol Res, 2008, 38(5): 511-520. doi: 10.1111/j. 1872-034X. 2007. 00290.x.
[8] OSMAN A, SALAMA A, EMAM MAHMOUD K, et al. Alleviation of carbon tetrachloride-induced hepatocellular damage and oxidative stress in rats by anabaena oryzae phycocyanin[J]. J Food Biochem, 2021, 45(1):e13562. doi:10.1111/jfbc.13562.
[9] OU Y, ZHENG S, LIN L, et al. Protective effect of C-phycocyanin against carbon tetrachloride-induced hepatocyte damage in vitro and in vivo[J]. Chem Biol Interact, 2010, 185(2): 94-100. doi: 10. 1016/j.cbi.2010.03.013.
[10] BHAT V B, MADYASTHA K M. C-phycocyanin: a potent peroxyl radical scavenger in vivo and in vitro [J]. Biochem Biophys Res Commun, 2000, 275(1): 20-25. doi: 10. 1006/bbrc.2000.3270.
[ 11] HUSSEIN M M, ALI H A, AHMED M M. Ameliorative effects of phycocyanin against gibberellic acid induced hepatotoxicity[J]. Pestic Biochem Physiol, 2015, 119: 28-32. doi: 10. 1016/j. pestbp. 2015.02.010.
[ 12]LIU J, ZHANG Q Y, YU L M, et al. Phycocyanobilin accelerates liver regeneration and reduces mortality rate in carbon tetrachloride-induced liver injury mice[J]. World J Gastroenterol, 2015, 21(18):5465-5472. doi:10.3748/wjg.v21.i18.5465.
[13] GAMMOUDI S, ATHMOUNI K, NASRI A, et al. Optimization, isolation, characterization and hepatoprotective effect of a novel pigment-protein complex (phycocyanin) producing microalga: phormidium versicolorNCC-466 using response surface methodology [J]. versicolorNCC-466 using response surface methodology [J]. Int J Biol Macromol, 2019, 137: 647-656. doi: 10. 1016/j. ijbiomac.2019.06.237.
[14] MCCARTY M F , BARROSO-ARANDA J , CONTRERAS F. Genistein and phycocyanobilin may prevent hepatic fibrosis by suppressing proliferation and activation of hepatic stellate cells[J]. Med Hypotheses, 2009, 72(3):330-332. doi: 10. 1016/j.mehy.2008. 07.045.
[15]PAK W, TAKAYAMA F, MINE M, et al. Anti-oxidative and anti- inflammatory effects of spirulina on rat model of non-alcoholic steatohepatitis[J]. J Clin Biochem Nutr, 2012, 51(3):227-234. doi:10.3164/jcbn.12-18.
[16] PATTARAYAN D, RAJARAJAN D, SIVANANTHAM A, et al. C- phycocyanin suppresses transforming growth factor- β 1-induced epithelial mesenchymal transition in human epithelial cells [J]. Pharmacol Rep, 2017, 69(3): 426-431. doi: 10. 1016/j. pharep. 2016.12.013.
[ 17]LI C, YU Y, LI W, et al. Phycocyanin attenuates pulmonary fibrosis via the TLR2-MyD88-NF- κB signaling pathway[J]. Sci Rep, 2017, 7 (1): 5843. doi: 10. 1038/s41598-017-06021-5.
[18] AN E, PARK H, LEE A C. Inhibition of fibrotic contraction by C- phycocyanin through modulation of connective tissue growth factor and α-smooth muscle actin expression[J]. Tissue Eng Regen Med, 2016, 13(4):388-395. doi: 10. 1007/s13770-015-0104-5.
[19] GDARA N B, BELGACEM A, KHEMIRI I, et al. Protective effects of phycocyanin on ischemia/reperfusion liver injuries [J]. Biomed Pharmacother, 2018, 102: 196-202. doi: 10. 1016/j. biopha. 2018. 03.025.
[20] REMIREZ D, FERNÁNDEZ V, TAPIA G, et al. Influence of C- phycocyanin on hepatocellular parameters related to liver oxidative stress and kupffer cell functioning[J]. Inflamm Res, 2002, 51(7): 351-356. doi: 10. 1007/pl00000314.
[21] TONG F, TANG X, LIU D. Phycocyanin/PEG-b-(PG-g-PEI) attenuated hepatic ischemia/reperfusion-induced pancreatic islet injury and enlarged islet functionality [J]. Int J Nanomedicine, 2019, 14: 339-351. doi: 10.2147/IJN.S190938.
[22]KUNTE M, DESAI K. The inhibitory effect of C-phycocyanin containing protein extract on human matrix metalloproteinases (MMP-2) and MMP-9 in hepatocellular cancer cell line (HepG2)[J]. and MMP-9) in hepatocellular cancer cell line (HepG2) [J]. Protein J, 2017, 36(3): 186-195. doi: 10. 1007/s10930-017-9707-0.
[23]WANG C Y, WANG X, WANG Y, et al. Photosensitization of phycocyanin extracted from microcystis in human hepatocellular carcinoma cells: implication of mitochondria-dependent apoptosis [J]. J Photochem Photobiol B, 2012, 117: 70-79. doi: 10. 1016/j. jphotobiol.2012.09.001.
[24]LIU X, DU J, XIE Z, et al. Lactobionic acid-modified phycocyanin nanoparticles loaded with doxorubicin for synergistic chemo- photodynamic therapy[J]. therapy[J]. Int J Biol Macromol, 2021, 186: 206- 217. doi: 10. 1016/j.ijbiomac.2021.07.047.
[25]LIN Z, JIANG B P, LIANG J, et al. Phycocyanin functionalized single-walled carbon nanohorns hybrid for near-infrared light- mediated cancer phototheranostics [J]. Carbon, 2019, 143: 814- 827. doi: 10. 1016/j.carbon.2018.12.011.
[26] PU Y, WEI M, WITKOWSKI A, et al. A hybrid biomaterial of biosilica and C-phycocyanin for enhanced photodynamic effect towards tumor cells[J]. Biochem Biophys Res Commun, 2020, 533 (3): 573-579. doi: 10. 1016/j.bbrc.2020.09.049.
[27] WAN D H, MA X Y, LIN C, et al. Noncovalent indocyanine green conjugate of C-phycocyanin: preparation and tumor-associated macrophages-targeted photothermal therapeutics[J]. Bioconjug Chem, 2020, 31(5): 1438-1448. doi: 10. 1021/acs. bioconjchem. 0c00139.
[28]NISHANTH R P, RAMAKRISHNA B S, JYOTSNA R G, et al. C- phycocyanin inhibits MDR1 through reactive oxygen species and cyclooxygenase-2 mediated pathways in human hepatocellular carcinoma cell line[J]. Eur J Pharmacol, 2010, 649(1/3):74-83. doi: 10. 1016/j.ejphar.2010.09.011.
[29] ROY K R, ARUNASREE K M, REDDY N P, et al. Alteration of mitochondrial membrane potential by spirulina platensis C- phycocyanin induces apoptosis in the doxorubicinresistant human hepatocellular-carcinoma cell line HepG2[J]. Biotechnol Appl Biochem, 2007, 47 (Pt 3): 159-167. doi: 10. 1042/BA20060206.
[30] PRABAKARAN G, SAMPATHKUMAR P, KAVISRI M, et al. Extraction and characterization of phycocyanin from spirulina platensis and evaluation of its anticancer , antidiabetic and antiinflammatory effect[J]. Int J Biol Macromol, 2020, 153: 256- 263. doi: 10. 1016/j.ijbiomac.2020.03.009.
[31] SILVA M R O B D, M DA SILVA G, SILVA A L F D, et al. Bioactive compounds of Arthrospira spp. (spirulina) with potential anticancer activities: a systematic review[J]. ACS Chem Biol, 2021, 16 (11): 2057-2067. doi: 10. 1021/acschembio.1c00568.
[32] COUÉ M, TESSE A, FALEWÉE J, et al. Spirulina liquid extract protects against fibrosis related to non-alcoholic steatohepatitis and increases ursodeoxycholic acid [J]. Nutrients, 2019, 11 (1): 194. doi:10.3390/nu11010194.
[33] KASBI-CHADLI F, COUÉ M, AGUESSE A, et al. Spirulina liquid extract prevents metabolic disturbances and improves liver sphingolipids profile in hamster fed a high-fat diet[J]. Eur J Nutr, 2021, 60(8):4483-4494. doi: 10. 1007/s00394-021-02589-x.
[34] OULD AMARA-LEFFAD L, RAMDANE H, NEKHOUL K, et al. Spirulina effect on modulation of toxins provided by food, impact on hepatic and renal functions [J] . . Arch Physiol Biochem, 2019, 125 (2): 184-194. doi: 10. 1080/13813455.2018.1444059.
[35] MA P, HUANG R, JIANG J, et al. Potential use of C-phycocyanin in non-alcoholic fatty liver disease [J]. Biochem Biophys Res Commun, 2020, 526(4):906-912. doi: 10. 1016/j.bbrc.2020.04.001.
[36]ICHIMURA M, KATO S, TSUNEYAMA K, et al. Phycocyanin prevents hypertension and low serum adiponectin level in a rat model of metabolic syndrome[J]. Nutr Res, 2013, 33(5): 397-405. doi: 10. 1016/j.nutres.2013.03.006.
[37] COUÉ M, CROYAL M, HABIB M, et al. Perinatal administration of C-phycocyanin protects against atherosclerosis in apoE-deficient mice by modulating cholesterol and trimethylamine-N-oxide metabolisms[J]. Arterioscler Thromb Vasc Biol, 2021, 41(12): e512-e523. doi: 10. 1161/ATVBAHA.121.316848.
[38]REN Z, XIE Z, CAO D, et al. C-phycocyanin inhibits hepatic gluconeogenesis and increases glycogen synthesis via activating Akt and AMPK in insulin resistant hepatocytes [J]. Food Funct, 2018, 9(5): 2829-2839. doi: 10. 1039/c8fo00257f.
[39]OU Y, REN Z, WANG J, et al. Phycocyanin ameliorates alloxan- induced diabetes mellitus in mice :involved in insulin signaling pathway and GK expression [J]. Chem Biol Interact, 2016, 247: 49- 54. doi: 10. 1016/j.cbi.2016.01.018.
[40] XIA D, LIU B, XIN W, et al. Protective effects of C-phycocyanin on alcohol-induced subacute liver injury in mice [J]. Journal of Applied Phycology, 2015, 28(2):765-772. doi: 10. 1007/s10811- 015-0677-3.
[41] XIE Y, LI W, ZHU L, et al. Effects of phycocyanin in modulating the intestinal microbiota of mice [J]. Microbiologyopen, 2019, 8 (9): e00825. doi: 10. 1002/mbo3.825.
[42]LIU Q, LI W, QIN S. Therapeutic effect of phycocyanin on acute liver oxidative damage caused by X-ray[J]. Biomed Pharmacother, 2020, 130: 110553. doi: 10. 1016/j.biopha.2020.110553.
[43]SONG Y N, CHEN J, CAI F F, et al. A metabolic mechanism analysis of fuzheng-huayu formula for improving liver cirrhosis with traditional chinese medicine syndromes [J]. Acta Pharmacol Sin, 2018, 39(6): 942-951. doi: 10. 1038/aps.2017.101.
[44]XIAO J, WANG F, WONG N K, et al. Global liver disease burdens and research trends : analysis from a chinese perspective[J]. J Hepatol, 2019, 71(1):212-221. doi: 10. 1016/j.jhep.2019.03.004.
#phycocyanin #cphycocyanin #phycocyaninspirulina
3 notes
·
View notes
Text
The Importance of the Amazing Darbepoetin Alfa Injection
Autoimmune and chronic illnesses take more than the typical treatment. Targeted injections and biologic drugs have revolutionized how patients are able to live with long-term illness, offering patients a more targeted and positive effect. Some of the most commonly used injectable medications in this category are Adalimumab and Darbepoetin Alfa injections. Though they are used for very distinct diseases, both are essential for patient quality of life improvement and disease management. Are you someone who wants to gather more facts about the Adalimumab injection, Darbepoetin Alfa injection? If Yes. This is the best place where people can gather more facts about the Adalimumab injection, Darbepoetin Alfa injection.
The Darbepoetin Alfa injection
Adalimumab Injection is a biologic drug that is commonly used in the management of autoimmune diseases like rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, and plaque psoriasis. Adalimumab acts by binding to and blocking a particular protein in the body called tumor necrosis factor-alpha (TNF-α) that leads to inflammation. Blocking TNF-α, Adalimumab decreases swelling, pain, and persistent joint or tissue damage resulting from the uncontrolled immune system.

Darbepoetin Alfa Injection is, nonetheless, utilized mostly for the treatment of anemia, especially in patients with chronic kidney disease (CKD) or patients with cancer on chemotherapy. It triggers bone marrow to produce red blood cells to a greater degree and thus relieves fatigue, weakness, and other anemic symptoms. With a rise in hemoglobin levels, it supports enhanced oxygenation to various body structures, which is vital for energy and health.
Given by intravenous or subcutaneous injection, Darbepoetin Alfa minimizes blood transfusions and enhances patient endurance and quality of life. The dose is titrated on an individual hemoglobin level and degree of response to treatment based on ongoing blood tests.
Adalimumab and Darbepoetin Alfa, together, are a new class of targeted therapy for chronic disease management. They not only cure but also alter the disease course so that patients can lead more active, fuller lives with proper guidance from their healthcare provider.
0 notes
Text
Clinical Bioanalysis in Immunology: Monitoring Immune Responses to New Treatments

In recent years, the field of immunology has witnessed a transformative evolution, driven by the rise of biologics, cell and gene therapies, monoclonal antibodies, and personalized immunotherapies. These advancements offer powerful tools in the treatment of cancer, autoimmune disorders, infectious diseases, and allergies. However, they also demand rigorous and precise evaluation of immune responses during clinical development. This is where clinical bioanalysis plays a critical role.
Clinical bioanalysis in immunology enables the quantification and characterization of drug concentrations, biomarkers, and immunogenicity-related factors in biological matrices. It helps researchers and clinicians monitor how the immune system responds to new treatments, ensuring both efficacy and safety.
Understanding Clinical Bioanalysis in the Context of Immunology
Clinical bioanalysis refers to the scientific discipline focused on the measurement of drugs, biological molecules, and biomarkers in human samples. In immunology, the focus expands to include:
Cytokines and chemokines (signaling proteins)
Antibody levels (therapeutic and anti-drug antibodies)
T-cell and B-cell markers
Immune complexes
Inflammatory mediators
Vaccine titers and immune memory markers
The goal is to obtain a comprehensive profile of how the immune system is activated, suppressed, or altered in response to therapeutic intervention.
Key Applications of Clinical Bioanalysis in Immunological Studies
1. Monitoring Therapeutic Antibody Levels
Monoclonal antibodies (mAbs) are increasingly used in autoimmune diseases and cancer immunotherapy. Clinical bioanalysis quantifies circulating mAb concentrations to ensure that therapeutic levels are achieved and maintained.
LC-MS/MS and ligand-binding assays (LBAs) such as ELISA or electrochemiluminescence (ECL) are commonly used to quantify antibodies in serum or plasma.
Accurate measurement guides dose adjustments and supports pharmacokinetic (PK) and pharmacodynamic (PD) analyses.
2. Immunogenicity Assessment
One of the biggest challenges in immunological treatments is the potential for the body to generate anti-drug antibodies (ADAs), which can reduce drug efficacy or cause adverse effects.
Clinical bioanalysis helps detect binding and neutralizing ADAs using multi-tiered testing approaches.
Screening assays identify the presence, confirmatory assays verify specificity, and neutralization assays assess functional impact.
Immunogenicity profiling is critical for regulatory approval of biologics and biosimilars.
3. Biomarker Monitoring for Treatment Efficacy
Biomarkers such as interleukins (e.g., IL-6, IL-10), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) are indicators of immune activation or suppression.
Elevated cytokine levels may suggest an inflammatory response, cytokine release syndrome, or efficacy in activating immune cells.
Clinical bioanalysis enables multiplex detection of biomarkers to evaluate the immune landscape in real time.
4. Cellular Immune Response Analysis
Clinical bioanalysis also extends to flow cytometry and immunophenotyping methods to track immune cell populations:
T-cell and B-cell profiling helps assess vaccine responses or immune checkpoint therapy effectiveness.
Natural Killer (NK) cell activity, regulatory T cells (Tregs), and dendritic cells are often monitored in immuno-oncology studies.
These insights allow researchers to tailor immunotherapies for better patient outcomes.
5. Vaccine Development and Evaluation
Vaccines rely on generating strong and lasting immune responses. Clinical bioanalysis evaluates:
Antibody titers post-vaccination (e.g., IgG, IgM)
Neutralization assays to assess protection
Cell-mediated responses, including T-cell activation and memory formation
This data is vital for selecting appropriate formulations, adjuvants, and dosing schedules.
Analytical Techniques Used in Immunological Bioanalysis
Ligand-Binding Assays (ELISA, MSD, Gyrolab): For quantification of proteins, cytokines, and antibodies.
Flow Cytometry: For immune cell enumeration and phenotyping.
Luminex Multiplexing: For the simultaneous detection of multiple cytokines or biomarkers in one sample.
qPCR and RT-PCR: For measuring gene expression related to immune pathways.
Surface Plasmon Resonance (SPR): For binding kinetics and affinity analysis of antibodies.
Each technique is selected based on sensitivity, specificity, throughput needs, and nature of the therapeutic being analyzed.
Regulatory and Compliance Considerations
Regulatory agencies such as the FDA, EMA, and ICH have issued clear guidance on clinical bioanalysis, especially regarding biologics and immunogenicity assessment:
FDA’s Immunogenicity Testing Guidance (2019) outlines strategies for ADA testing, cut point determination, and assay validation.
Bioanalytical method validation must adhere to GLP (Good Laboratory Practice) and ensure parameters such as accuracy, precision, specificity, sensitivity, and reproducibility are met.
Proper documentation and data integrity are essential for regulatory submissions and clinical trial approvals.
Challenges in Clinical Bioanalysis for Immunology
Despite technological advancements, several challenges persist:
High Biological Variability: Immune responses vary widely between individuals, complicating biomarker interpretation.
Matrix Interference: Biological samples such as serum or plasma can interfere with assay specificity, especially in multiplex setups.
Assay Sensitivity: Detecting low concentrations of cytokines or ADAs requires ultra-sensitive platforms, which must be rigorously validated.
Stability Issues: Immune proteins may degrade over time, necessitating careful sample handling and storage.
Addressing these challenges requires robust assay design, quality control measures, and continuous method optimization.
The Future of Clinical Bioanalysis in Immunology
As immunology becomes more central to precision medicine, the demand for high-quality bioanalytical data is increasing. Future developments include:
Automation and AI-driven analytics for faster, more accurate data interpretation.
Next-generation sequencing (NGS) integrated with immune profiling.
Digital biomarker platforms for remote patient monitoring and real-time immune surveillance.
Personalized immuno-monitoring kits for at-home use in chronic immunotherapies.
These innovations promise to enhance the granularity and accessibility of immune response data in clinical research.
Conclusion
In the era of precision immunotherapy and advanced biologics, clinical bioanalysis is not just a supporting discipline—it is a cornerstone of immunological research and therapeutic development. By enabling the accurate measurement of immune responses, it allows scientists and clinicians to understand treatment effects, ensure safety, optimize dosing, and demonstrate efficacy.
As the immunology landscape continues to evolve, clinical bioanalysis will remain vital in ensuring that new treatments are not only effective but also immunologically safe and tailored to individual patient needs.
1 note
·
View note
Text
Adalimumab Injection: Empowering Your Journey to Wellness with Medlama
Living with chronic inflammatory conditions can be challenging, but advancements in medical science have introduced effective treatments like adalimumab injection. At Medlama, we are dedicated to providing accurate and comprehensive information to help you understand your treatment options.

Understanding Adalimumab Injection
Adalimumab is a biologic medication classified as a tumor necrosis factor (TNF) inhibitor. It is used to treat various autoimmune and inflammatory conditions by targeting and neutralizing TNF-alpha, a substance in the body that causes inflammation. By inhibiting TNF-alpha, adalimumab helps reduce inflammation and halt disease progression.
Medical Conditions Treated with Adalimumab
Adalimumab is approved for the treatment of several conditions, including:
Rheumatoid Arthritis (RA): An autoimmune disorder causing joint inflammation and pain.
Psoriatic Arthritis: A form of arthritis affecting some people with psoriasis.
Ankylosing Spondylitis: A type of arthritis that affects the spine.
Crohn’s Disease and Ulcerative Colitis: Inflammatory bowel diseases causing digestive tract inflammation.
Plaque Psoriasis: A skin condition characterized by red, scaly patches.
Hidradenitis Suppurativa: A chronic skin condition featuring lumps under the skin.
Uveitis: Inflammation of the middle layer of the eye.
By addressing the underlying inflammation, adalimumab helps alleviate symptoms and improve quality of life for individuals with these conditions.
Administration of Adalimumab
Adalimumab is administered via subcutaneous injection, typically once every other week. It is available in prefilled syringes or pen devices for ease of use. Patients are usually trained by healthcare professionals on proper injection techniques to ensure safety and efficacy.
Potential Side Effects
While adalimumab is effective in managing inflammatory conditions, it may cause side effects. Common side effects include:
Injection Site Reactions: Redness, swelling, or pain at the injection site.
Upper Respiratory Infections: Symptoms include a runny nose or sore throat.
Headache: Mild to moderate headaches may occur.
Nausea: Some patients may experience stomach discomfort.
Serious side effects are rare but can include severe infections, allergic reactions, and blood disorders. It's crucial to monitor for any unusual symptoms and consult a healthcare provider promptly if they occur.
Precautions and Considerations
Before starting adalimumab, inform your healthcare provider about:
Existing Infections: Adalimumab can increase susceptibility to infections.
Tuberculosis (TB) Exposure: A TB test is often required before initiating treatment.
Vaccination History: Live vaccines should be avoided during treatment.
Pregnancy and Breastfeeding: Consult with your doctor to discuss the potential risks and benefits.
Regular monitoring and open communication with your healthcare provider are essential to ensure the safe use of adalimumab.
Tips for Managing Injections
To minimize discomfort during injections:
Warm the Medication: Allow the syringe or pen to reach room temperature before use.
Rotate Injection Sites: Avoid injecting in the same spot repeatedly.
Use Proper Technique: Follow the injection instructions provided by your healthcare provider.
Apply Ice: Using a cold pack before and after injection can reduce pain and swelling.
Implementing these strategies can enhance comfort and adherence to the treatment regimen.
Conclusion
Adalimumab injection represents a significant advancement in the treatment of various inflammatory and autoimmune conditions. By understanding its uses, administration, and potential side effects, patients can make informed decisions about their health. At Medlama, we are committed to providing reliable information to support your healthcare journey.
0 notes
Text
Adalimumab: A Medication for Autoimmune Diseases
Adalimumab: A Medication for Autoimmune Diseases Adalimumab is a medication used to treat autoimmune diseases. It belongs to a class of drugs called TNF-alpha inhibitors. These diseases include rheumatoid arthritis, psoriatic arthritis, and Crohn’s disease. How Adalimumab Works Adalimumab blocks a protein called tumor necrosis factor-alpha (TNF-alpha). TNF-alpha causes inflammation and damage…
0 notes
Text
The Role of Inflammation in Blood Disorders: Exploring the Link Between Chronic Diseases and Hematology
Introduction
Inflammation, a complex biological response to injury or infection, plays a far more intricate role than initially understood. While crucial for tissue repair and pathogen elimination, chronic or dysregulated inflammation is increasingly recognized as a major contributor to numerous diseases, significantly impacting the field of hematology?the study of blood and blood-forming tissues. This intricate interplay between inflammation and blood disorders underscores the interconnectedness of seemingly disparate chronic diseases and highlights the need for a holistic approach to diagnosis and treatment. Understanding this relationship is critical for advancing our understanding and management of various hematological conditions.
This article will delve into the multifaceted relationship between inflammation and several key blood disorders, illustrating how chronic inflammation contributes to their pathogenesis and progression. We will examine the underlying mechanisms, exploring how inflammatory mediators impact the production, function, and lifespan of blood cells.
1. Anemia and Inflammatory Mediators
Anemia, characterized by a deficiency in red blood cells or hemoglobin, is frequently associated with
chronic inflammatory conditions. The inflammatory process itself can directly contribute to the development of anemia through several pathways. Cytokines, such as tumor necrosis factor-alpha (TNF-?) and interleukin-6 (IL-6), released during inflammation, suppress erythropoietin production, a hormone essential for red blood cell formation in the bone marrow. This suppression leads to decreased red blood cell production, resulting in anemia of chronic disease (ACD).
Furthermore, inflammation can also accelerate the destruction of red blood cells. Inflammatory mediators can damage red blood cell membranes, leading to premature destruction in the spleen. Additionally, chronic inflammation often leads to iron sequestration, meaning iron, crucial for hemoglobin synthesis, becomes less available for red blood cell production. This iron deficiency further exacerbates the anemia, compounding the effects of reduced erythropoietin production.
2. Inflammatory Bowel Disease and Hematological Manifestations
Inflammatory bowel disease (IBD), encompassing Crohn's disease and ulcerative colitis, is characterized by chronic inflammation of the gastrointestinal tract. This chronic inflammation has significant implications for hematological health. Patients with IBD frequently experience anemia, often due to malabsorption of nutrients, including iron and vitamin B12, crucial for red blood cell production. The constant inflammation can also lead to increased bleeding, contributing to iron deficiency anemia.
Beyond anemia, IBD patients are also at increased risk of developing other hematological complications. The chronic inflammation can trigger thrombosis, the formation of blood clots, increasing the risk of potentially life-threatening conditions like deep vein thrombosis and pulmonary embolism. Furthermore, the constant immune activation associated with IBD can lead to an elevated risk of certain types of blood cancers, particularly lymphoma.
3. Leukemia and the Inflammatory Microenvironment
Leukemia, a cancer of the blood-forming cells in the bone marrow, is also intricately linked to inflammation. The bone marrow microenvironment plays a crucial role in the development and progression of leukemia. Chronic inflammation within this microenvironment can create a supportive niche for leukemia stem cells, promoting their survival, proliferation, and resistance to therapy. Inflammatory mediators can also influence the expression of genes involved in leukemia development, further fueling disease progression.
Moreover, certain types of leukemia are associated with specific inflammatory conditions. For instance, some forms of acute myeloid leukemia (AML) have been linked to prior exposure to infections or inflammatory diseases. Understanding the precise role of inflammation in leukemia development is crucial for the development of targeted therapeutic strategies aimed at modifying the inflammatory microenvironment and improving treatment outcomes.
4. Thrombocytopenia and Immune Thrombocytopenic Purpura (ITP)
Thrombocytopenia, a condition characterized by a low platelet count, can be a consequence of various inflammatory processes. Immune thrombocytopenic purpura (ITP), an autoimmune disorder, exemplifies the direct link between inflammation and platelet dysfunction. In ITP, the immune system mistakenly attacks platelets, leading to their destruction and a subsequent decrease in platelet count. This autoimmune response is driven by inflammatory mediators, highlighting the role of dysregulated inflammation in the pathogenesis of this disorder.
Treatment strategies for ITP often target the underlying inflammatory mechanisms. Corticosteroids, for instance, are frequently used to suppress the immune response and reduce inflammation. Understanding the specific inflammatory pathways involved in ITP is essential for developing more targeted and effective therapies aimed at restoring platelet counts and mitigating the risk of bleeding complications.
5. The Therapeutic Implications of Targeting Inflammation
Given the prominent role of inflammation in various blood disorders, targeting inflammatory pathways has emerged as a promising therapeutic strategy. Anti-inflammatory medications, such as corticosteroids and biologics targeting specific cytokines, are already used to treat certain hematological conditions. However, research continues to explore more targeted approaches aimed at specifically inhibiting inflammatory pathways relevant to each particular disorder.
Future research will likely focus on identifying specific inflammatory biomarkers that can be used to predict disease severity and response to treatment. This personalized approach will help optimize treatment strategies and minimize the risk of adverse effects. The potential for combining anti-inflammatory therapies with conventional treatments represents a significant advancement in the management of numerous blood disorders.
Conclusion
The intricate relationship between inflammation and blood disorders is increasingly being recognized as a pivotal factor in disease pathogenesis and progression. Understanding the specific mechanisms by which inflammation contributes to various hematological conditions is crucial for
developing innovative therapeutic strategies. Focusing on targeting inflammatory pathways, alongside conventional treatments, presents a promising avenue for improving patient outcomes and enhancing the management of these complex disorders. Further research focusing on personalized medicine approaches and the development of novel anti-inflammatory therapies will be essential for advancing the field of hematology and improving the lives of those affected by these conditions.
1 note
·
View note
Text
Top Blood Tests to Detect Chronic Inflammation
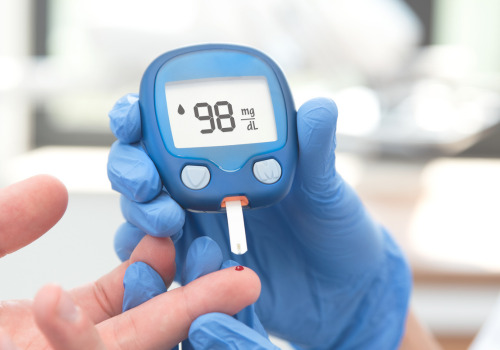
Chronic inflammation is a hidden driver behind many health conditions, including autoimmune diseases, heart disease, diabetes, and even cancer. Unlike acute inflammation, which is a short-term response to injury or infection, chronic inflammation persists over time, often going unnoticed until significant damage has occurred. Identifying chronic inflammation early through blood tests can help individuals take proactive steps to improve their health. Here are the top blood tests used to detect chronic inflammation:
1. C-Reactive Protein (CRP) Test
C-Reactive Protein (CRP) is a protein produced by the liver in response to inflammation. High levels of CRP in the blood indicate the presence of inflammation, which can be linked to conditions such as cardiovascular disease, autoimmune disorders, and infections. A high-sensitivity CRP (hs-CRP) test is particularly useful for assessing low-grade inflammation associated with heart disease.
2. Erythrocyte Sedimentation Rate (ESR) Test
The Erythrocyte Sedimentation Rate (ESR) test measures how quickly red blood cells settle at the bottom of a test tube. A higher-than-normal rate indicates inflammation in the body. While ESR is a general marker and does not pinpoint the exact cause of inflammation, it is often used alongside other tests to help diagnose inflammatory conditions such as rheumatoid arthritis or lupus.
3. Fibrinogen Test
Fibrinogen is a protein involved in blood clotting and is also an acute-phase reactant, meaning its levels rise in response to inflammation. Elevated fibrinogen levels can indicate chronic inflammation and an increased risk of cardiovascular disease. Since fibrinogen levels can be influenced by various factors, including infection and stress, this test is typically used in conjunction with other markers.
4. Interleukin-6 (IL-6) Test
Interleukin-6 (IL-6) is a cytokine, a type of protein involved in the body's immune response. While IL-6 plays a crucial role in fighting infections, persistently elevated levels are associated with chronic inflammatory conditions such as arthritis, metabolic syndrome, and even depression. Measuring IL-6 levels can provide insight into the severity of systemic inflammation.
5. Tumor Necrosis Factor-Alpha (TNF-α) Test
TNF-α is another cytokine involved in inflammation and immune system regulation. Excess TNF-α is linked to autoimmune diseases such as rheumatoid arthritis, Crohn’s disease, and psoriasis. Testing for TNF-α can help assess the level of inflammation in the body and guide treatment options for inflammatory conditions.
6. Homocysteine Test
Homocysteine is an amino acid that, when present at high levels, is associated with inflammation and an increased risk of cardiovascular disease. Elevated homocysteine levels can indicate underlying chronic inflammation and oxidative stress, which contribute to various chronic diseases.
7. Ferritin Test
Ferritin is a protein that stores iron in the body. While low ferritin levels indicate iron deficiency, elevated ferritin levels can signal chronic inflammation, liver disease, or certain infections. Measuring ferritin can provide insight into hidden inflammatory processes in the body.
Conclusion
Chronic inflammation is a silent but significant health threat. Fortunately, various blood tests can help detect inflammation early, allowing individuals to make lifestyle changes and seek medical interventions if necessary. If you suspect chronic inflammation, consult with your healthcare provider to determine which tests are appropriate for you. By identifying and addressing inflammation, you can take proactive steps toward better health and disease prevention.
0 notes
Text
Adalimumab Biosimilars: Market Size, Share, and Forecast Analysis

The Adalimumab Biosimilars Market is witnessing significant growth as biosimilar versions of Humira (adalimumab) gain traction globally. Adalimumab, a tumor necrosis factor-alpha (TNF-α) inhibitor, has been widely used to treat autoimmune diseases such as rheumatoid arthritis, psoriasis, Crohn’s disease, and ulcerative colitis. With the expiration of Humira’s patent, numerous Adalimumab Biosimilar Companies have entered the market, offering cost-effective alternatives and increasing patient access to these life-changing therapies.
Market Growth and Key Drivers
The expansion of the Adalimumab biosimilar market is driven by several factors:
Patent Expiry and Market Competition – The loss of exclusivity for Humira has paved the way for multiple biosimilars, intensifying competition and driving down treatment costs.
Regulatory Approvals and Expanding Market Access – Regulatory agencies like the FDA and EMA have approved multiple adalimumab biosimilars, promoting broader adoption.
Cost-Effectiveness and Healthcare Savings – Biosimilars offer a lower-cost alternative to biologics, helping healthcare systems reduce spending while maintaining treatment efficacy.
Increased Prevalence of Autoimmune Diseases – The rising incidence of conditions like rheumatoid arthritis and inflammatory bowel disease is fueling demand for adalimumab biosimilars.
Key Adalimumab Biosimilar Companies and Market Players
Several pharmaceutical and biotech firms are actively competing in the Adalimumab Biosimilars Market. Some of the key Adalimumab Biosimilar Companies include:
Amgen (Amjevita/Bijeljira) – One of the first biosimilars approved for Humira, launched in multiple markets.
Boehringer Ingelheim (Cyltezo) – The first FDA-approved interchangeable adalimumab biosimilar, providing greater market penetration.
Samsung Bioepis (Hadlima) – Developed in collaboration with Biogen, expanding access to biosimilars globally.
Sandoz (Hyrimoz) – A major player in the biosimilar space, offering competitive pricing and accessibility.
Viatris & Biocon Biologics (Hulio) – A strong competitor in both U.S. and European markets.
Future Outlook and Market Expansion
The Adalimumab Biosimilars Market is poised for substantial growth as more biosimilars gain regulatory approval and manufacturers expand their geographic reach. Increased physician and patient confidence in biosimilars, coupled with pricing advantages, will further drive adoption. As competition intensifies, innovation in formulation, delivery methods, and interchangeability designations will shape the future of the market.
Overall, the rise of Adalimumab Biosimilar Companies marks a transformative shift in the autoimmune disease treatment landscape, providing cost-effective and widely accessible therapeutic options for patients worldwide.
Latest Reports Offered By DelveInsight:
papular warts | chronic illness app | aln-app | how do you treat epi | bronchial spasms | biogen postpartum depression | synox therapeutics | whim disease | is gepotidacin available | accutar biotech | outlast amber | osteoarthritis epidemiology | pde3 inhibitors | sarco penia | technological advances in healthcare | copd newsletter | elastomeric pump | apellis pipeline | tarpeyo iga nephropathy | mugard cost | otc deficiency treatment | adagrasib cetuximab | pers emergency response system | dupixent alternatives | progressive pipeline | cefepime-taniborbactam in complicated urinary tract infection
#Global Adalimumab Biosimilar#Global Adalimumab Biosimilar Market#Global Adalimumab Biosimilar Forecast#Global Adalimumab Biosimilar Companies#Global Adalimumab Biosimilar Therapies#Global Adalimumab Biosimilar Epidemiology#Global Adalimumab Biosimilar Pipeline#Global Adalimumab Biosimilar Market Size#Global Adalimumab Biosimilar Market Trends
0 notes
Text
The Tumor Necrosis Factor (TNF) Alpha Inhibitors Market in 2023 is US$ 41.88 billion, and is expected to reach US$ 44.35 billion by 2031 at a CAGR of 0.7%.
#Tumor Necrosis Factor (TNF) Alpha Inhibitors Market#Tumor Necrosis Factor (TNF) Alpha Inhibitors Market Forecast#Tumor Necrosis Factor (TNF) Alpha Inhibitors Market Overview
0 notes
Text
All About Kineret Injection
Kineret 100 mg Injection 0.67 ml is an immunosuppressive agent. It is widely used for the treatment of rheumatoid arthritis (RA), deficiency of interleukin-1 receptor antagonist (DIRA), and neonatal-onset multisystem inflammatory disease (NOMID). Kineret 100 mg/0.67 ml price in India can vary depending on certain factors. Its active ingredient, Anakinra, is an interleukin-1 receptor antagonist. This medicine is designed to work by blocking interleukin, which is a protein responsible for inflammation in the body. This unique mechanism helps alleviate symptoms of inflammatory disorders, improving the quality of life for many patients.
How does Kineret work? The active component in Kineret, Anakinra, plays a critical role in suppressing inflammation. It does this by inhibiting interleukin-1, a protein linked to inflammatory responses and immune system activation. By shielding the body from harmful cytokines, this medicine effectively reduces swelling, redness, pain, and other symptoms associated with autoimmune and inflammatory diseases.
Uses and Benefits:
Kineret 100 mg Injection 0.67 ml is a preferred treatment option for:
Rheumatoid arthritis (RA): A chronic autoimmune condition affecting joints.
Deficiency of interleukin-1 receptor antagonist (DIRA): An ultra-rare autoinflammatory disease.
NOMID: A rare genetic inflammatory disease.
COVID-19: It is used (off-label) as part of treatment for severe inflammatory responses.
To ensure the authenticity and affordability of this life-changing medication, many patients seek reliable options for the Kineret price in India.
Common Side Effects: Although this therapeutic drug is highly effective, it may cause some side effects such as:
headache
redness or swelling at the injection site
increased cholesterol levels These side effects are typically mild and resolve on their own. However, consult your doctor if they persist or worsen.
Important Precautions:
Before using Kineret, inform your doctor about any allergies, especially to its components or E. coli, as the medication is produced using this bacteria. Patients with HIV, asthma, liver or kidney problems, epilepsy, or ongoing cancer treatments must exercise caution. A thorough medical and vaccination history is essential before starting treatment.
Kineret is not recommended for use alongside tumor necrosis factor (TNF-alpha) inhibitors like etanercept or with blood thinners such as warfarin without medical advice. Women of childbearing potential should use effective contraception during treatment, as its safety in pregnancy is not fully established.
Kineret Price in India:
For patients, hospitals, or healthcare professionals looking to know about the Kineret price in India. The price varies depending on so many factors. Indian Pharma Network (IPN) is a trusted facilitator and can ensure the legal and reliable supply of Kineret 100 mg Injection 0.67 ml. With strong ties to reputable manufacturers from all over the world, TIP makes it easier for patients to access this medicine at a very competitive price.

Conclusion:
Kineret 100 mg Injection 0.67 ml is an important treatment for autoimmune and inflammatory diseases. By effectively targeting inflammation, it provides significant relief to patients struggling with chronic conditions. If you are looking to buy Kineret, connect with The Indian Pharma (TIP) for authentic supply. For more details about pricing and availability, consult us today.
What is Kineret 100 mg/0.67 ml Injection used for? Kineret 100 mg/0.67 ml Injection is an immunosuppressive medicine used to treat rheumatoid arthritis, deficiency of interleukin-1 receptor antagonist (DIRA), and neonatal-onset multisystem inflammatory disease (NOMID), and COVID-19.
How does Kineret 100 mg/0.67 ml Injection work? Kineret blocks the action of a protein called interleukin, which causes inflammation. By doing so, it protects the body from the harmful effects of inflammatory proteins called cytokines.
Does Kineret 100 mg/0.67 ml Injection cause neutropenia? Yes, Kineret may cause neutropenia, which is a low level of white blood cells. This condition can increase the risk of infections. It is important to monitor your white blood cell count before starting treatment, every three months during treatment, and every four months for up to a year after treatment. If you notice any signs of infection, consult your doctor immediately.
Can Kineret 100 mg/0.67 ml affect my ability to get pregnant? There isn’t enough information to confirm how Kineret affects fertility. However, it should be avoided during pregnancy unless absolutely necessary. Women who can become pregnant should use effective contraception while taking Kineret. Always consult your doctor if you have concerns.
Where can I find Kineret 100 mg/0.67 ml injections in India? If you are looking for reliable suppliers or need information about the Kineret 100 mg/0.67 ml price in India, consult a trusted provider like Indian Pharma Network (IPN) to ensure access to genuine medicine. Always prioritize legal and safe channels for purchasing medications.
Is it safe to take Kineret 100 mg/0.67 ml with etanercept? No, Kineret should not be taken with etanercept, a TNF inhibitor used for rheumatoid arthritis (RA). Combining these medicines can increase the risk of infections.
Can I take Kineret 100 mg/0.67 ml with warfarin or phenytoin? You should talk to your doctor before using Kineret with medicines like warfarin or phenytoin. Your doctor may need to adjust the dosage or monitor you closely.
Can I buy Kineret if it is not available or approved in my country? Yes, you can buy Kineret 100 mg/0.67 ml injection if it is not available in your country. Simply contact Indian Pharma Network (IPN) via Call/WhatsApp at +91 9310090915 or TOLL-FREE: 1800-889-1064 to facilitate access to Kineret 100 mg/0.67 ml injection through legal channels.
1 note
·
View note
Text
What makes the amazing Adalimumab injection famous?
In the new medicines of the modern age that accompanies globalization, specialty drugs such as Cresp 40 and Adalimumab are central to treatment of life-changing and chronic diseases. Bangalore, with its massive need for innovative healthcare interventions, requires guaranteed access to such life-changing drugs through authentic distributors. Are you someone who wants to gather more facts about the Cresp 40 injection, Adalimumab injection? If Yes. This is the best place where people can gather more facts about the Cresp 40 injection, Adalimumab injection.

Adalimumab injection
Cresp 40 Injection, which is bought along with Darbepoetin Alfa, is prescribed for the management of anemia—chronic kidney disease or chemotherapy condition alone. The drug stimulates the bone marrow to create more red blood cells, hence giving greater energy and lowering the need for blood transfusion. Cresp 40 is a standard part of the treatment process under Bangalore hospitals and dialysis centers, and being easily accessible can immensely help patient experience. There are many facts about the Adalimumab injection that you must know. You should gather all the important information about the Adalimumab injection.
Meanwhile, Adalimumab Injection is a biological drug that most frequently occurs with autoimmune disease conditions like rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and Crohn's disease. Adalimumab follows the mechanism of tumor necrosis factor-alpha (TNF-α), which is a molecule in the human body that generates inflammation. Because it is highly specific and powerful, Adalimumab significantly changed the therapy process for inflammatory chronic diseases.
As greater and better treatment options are within easy reach in India's large health cities such as Bangalore, medicines such as injections Cresp 40 and Adalimumab are enabling patients to live healthier and better lives despite their chronic condition. In intricate medical interactions where patients receive complicated problems, the choice of the right supplier is secondary to that of having the right medicine.
0 notes
Text
What are inflammatory biomarkers?
What are inflammatory biomarkers? Inflammatory biomarkers are molecules found in blood or other body fluids that indicate the presence and intensity of inflammation in the body.
What are the common inflammatory biomarkers? Common biomarkers include C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), interleukins (e.g., IL-6), tumor necrosis factor-alpha (TNF-α), and procalcitonin.
What conditions can inflammatory biomarkers detect? They can indicate infections, autoimmune diseases (e.g., rheumatoid arthritis), cardiovascular diseases, and some cancers.
What does a high CRP level indicate? High CRP levels suggest acute or chronic inflammation, often due to infection, autoimmune diseases, or tissue injury.
What is ESR, and what does it measure? ESR measures the rate at which red blood cells settle in a test tube, with faster rates indicating inflammation.
0 notes
Text
The Connection Between Damaged Mitochondria and Arthritis

Mitochondria are integral organelles responsible for various critical cellular functions, primarily energy production through oxidative phosphorylation. They are involved in maintaining cellular homeostasis, regulating metabolism, modulating calcium levels, and controlling apoptosis. Emerging evidence has highlighted mitochondrial dysfunction as a key contributor to a variety of diseases, including arthritis. This formal overview aims to explore the complex relationship between damaged mitochondria and arthritis, focusing on the molecular mechanisms that link mitochondrial dysfunction to the pathogenesis of inflammatory joint diseases, particularly rheumatoid arthritis (RA) and osteoarthritis (OA).
Mitochondrial Structure and Function
Mitochondria are double-membraned organelles found in eukaryotic cells, and they are crucial for cellular energy metabolism. Their primary role is the production of adenosine triphosphate (ATP) via oxidative phosphorylation, a process that takes place in the inner mitochondrial membrane. During this process, the electron transport chain (ETC) generates a proton gradient across the inner membrane, which drives ATP synthesis through ATP synthase. However, this process also generates reactive oxygen species (ROS) as byproducts, primarily from complexes I and III of the ETC. Under normal physiological conditions, ROS are neutralized by antioxidants, including superoxide dismutase (SOD), catalase, and glutathione. However, under pathological conditions, excessive ROS production can lead to oxidative stress, contributing to cellular damage and dysfunction.
In addition to ATP production, mitochondria have essential roles in calcium buffering, apoptosis regulation, and the maintenance of cellular integrity. Damage to these organelles disrupts these functions, contributing to various diseases, including arthritis.
Mitochondrial Dysfunction in Arthritis
Arthritis is a group of diseases characterized by inflammation and degeneration of the joints. It includes conditions like rheumatoid arthritis (RA), an autoimmune disease, and osteoarthritis (OA), a degenerative disease. In both types of arthritis, mitochondrial dysfunction has been identified as a critical factor that exacerbates disease progression through several mechanisms, including increased oxidative stress, immune activation, and tissue damage.
1. Oxidative Stress and Mitochondrial Damage
Oxidative stress is a hallmark of both RA and OA, and mitochondria are central to its production. In these conditions, mitochondrial dysfunction results in an increase in ROS production, overwhelming the cell’s antioxidant defenses. This oxidative stress leads to the modification of cellular structures, including proteins, lipids, and DNA, causing further mitochondrial damage. In RA, pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) stimulate immune cells like macrophages and neutrophils to release large amounts of ROS. These ROS contribute to the local inflammatory environment and accelerate joint destruction by damaging mitochondria and amplifying oxidative stress.
Mitochondrial damage results in a feedback loop where impaired mitochondrial function generates more ROS, further promoting inflammation. For instance, in RA, markers of oxidative damage such as 8-hydroxy-2'-deoxyguanosine (8-OHdG) and malondialdehyde (MDA) have been found to correlate with disease activity, suggesting a direct relationship between mitochondrial dysfunction and disease severity.
2. Mitochondrial DNA Damage and Inflammatory Signaling
Mitochondrial DNA (mtDNA) is particularly vulnerable to oxidative damage due to its proximity to the ETC, where ROS are produced during ATP synthesis. Unlike nuclear DNA, mtDNA is not protected by histones and has limited repair mechanisms, making it prone to mutations. Damage to mtDNA impairs mitochondrial function and can lead to the release of mtDNA fragments into the cytoplasm or extracellular space.
In the context of arthritis, mtDNA damage has been implicated in immune activation. When damaged mtDNA is released into the cytoplasm, it is recognized by pattern recognition receptors (PRRs), such as toll-like receptors (TLRs), on immune cells. TLRs, particularly TLR9, activate downstream inflammatory signaling pathways that lead to the production of pro-inflammatory cytokines such as TNF-α and IL-6. This further exacerbates the inflammatory response in joints and contributes to the progression of arthritis. Studies have shown that the presence of mtDNA fragments in the serum of RA patients correlates with disease activity, indicating the role of mtDNA in driving inflammation.
3. Mitochondrial Dynamics and Arthritis Pathogenesis
Mitochondrial dynamics refer to the continuous processes of mitochondrial fission (division) and fusion (joining), which maintain mitochondrial function and integrity. Fission allows for the removal of damaged mitochondria, while fusion helps to integrate mitochondrial contents and maintain a healthy mitochondrial pool. Imbalance between fission and fusion is associated with several diseases, including arthritis.
In the case of RA, excessive mitochondrial fission and reduced fusion have been observed. This imbalance results in mitochondrial fragmentation, which impairs mitochondrial function, increases ROS production, and contributes to cellular stress. Fission is regulated by proteins such as dynamin-related protein 1 (Drp1) and fission 1 protein (Fis1), while fusion is controlled by mitofusins (Mfn1 and Mfn2) and optic atrophy 1 (OPA1). Dysregulation of these proteins in RA leads to a fragmented mitochondrial network, which exacerbates oxidative stress and inflammation in synovial tissues.
4. Mitochondrial-Dependent Cell Death
Mitochondria are also central regulators of programmed cell death, particularly apoptosis. In the pathogenesis of arthritis, excessive or dysregulated apoptosis contributes to joint destruction. Mitochondrial dysfunction plays a critical role in the intrinsic apoptotic pathway by releasing pro-apoptotic factors such as cytochrome c and apoptosis-inducing factor (AIF). These factors activate caspase-dependent and caspase-independent pathways, leading to the death of synovial cells and cartilage cells, which contributes to the progressive tissue damage observed in both RA and OA.
Furthermore, mitochondrial permeability transition pore (mPTP) opening, which is induced by oxidative stress, can lead to necrosis, a form of uncontrolled cell death. Necrotic cell death in the joints increases inflammation and tissue degradation, particularly in OA, where cartilage breakdown is a hallmark feature.
Therapeutic Approaches Targeting Mitochondrial Dysfunction in Arthritis
Given the significant role of mitochondrial dysfunction in the pathogenesis of arthritis, various therapeutic strategies aimed at improving mitochondrial function are under investigation.
1. Mitochondrial Antioxidants
Mitochondrial-targeted antioxidants, such as MitoQ and MitoTEMPO, have been developed to selectively accumulate in mitochondria, where they can neutralize ROS and reduce oxidative stress. These compounds have shown promise in preclinical models of arthritis, where they help to reduce inflammation, protect mitochondrial function, and limit joint damage. The use of mitochondrial antioxidants could be an effective strategy to mitigate oxidative stress in arthritic conditions.
2. Mitochondrial Biogenesis Enhancement
Another potential therapeutic approach is the activation of mitochondrial biogenesis, the process by which new mitochondria are formed to compensate for damaged mitochondria. Agents that activate peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), a key regulator of mitochondrial biogenesis, could help restore mitochondrial function in arthritic tissues. Compounds such as resveratrol and NAD+ precursors are under investigation for their ability to promote mitochondrial biogenesis and improve cellular metabolism in arthritis.
3. Mitochondrial Dynamics Modulation
Restoring the balance between mitochondrial fission and fusion is another therapeutic strategy. Inhibiting excessive mitochondrial fission or promoting mitochondrial fusion may help maintain mitochondrial integrity and reduce inflammation in arthritis. Drugs targeting Drp1 or enhancing Mfn1/Mfn2 activity are potential candidates for modulating mitochondrial dynamics in arthritic diseases.
4. Mitophagy Enhancement
Mitophagy, the selective autophagic degradation of damaged mitochondria, is essential for maintaining mitochondrial quality. Enhancing mitophagy through the use of compounds like spermidine or activators of the PINK1/PARK2 pathway could help eliminate dysfunctional mitochondria and reduce inflammation, making it a promising therapeutic approach in arthritis.
Conclusion
Mitochondrial dysfunction plays a critical role in the pathogenesis of arthritis, contributing to oxidative stress, inflammation, and joint damage. The intricate relationship between damaged mitochondria and immune activation highlights the importance of targeting mitochondrial health in the treatment of arthritis. Emerging therapeutic strategies aimed at restoring mitochondrial function, reducing oxidative stress, and modulating mitochondrial dynamics hold promise for improving the management of arthritis and preventing joint destruction. Further research into mitochondrial biology and its role in arthritis is essential for the development of more effective, targeted therapies for these debilitating conditions.
#Mitochondrial dysfunction#Autoimmune disorders#Oxidative stress#Reactive oxygen species (ROS)#Mitochondrial dynamics#Mitochondrial fission#Mitochondrial fusion#Mitophagy#Apoptosis#Mitochondrial DNA (mtDNA)#Damage-associated molecular patterns (DAMPs)#Immune cell activation#Systemic lupus erythematosus (SLE)#Rheumatoid arthritis (RA)#Multiple sclerosis (MS)#Pattern recognition receptors (PRRs)#Toll-like receptors (TLRs)#Pro-inflammatory cytokines#Cytochrome c#NF-κB signaling#MitoQ#MitoTEMPO#Spermidine#PINK1/PARK2 pathway#Mitochondrial-targeted antioxidants#Immune dysregulation#Chronic inflammation#Mitochondrial fragmentation#Mitochondrial permeability transition pore (mPTP)#Autoantibodies
0 notes