#Sulfuric Acid Manufacturers
Explore tagged Tumblr posts
Text
Sulfuric Acid Industry Ecosystem, Growth, Size, Opportunities, Top Manufacturers, Share, Market Analysis, Trends, Segmentations, Regional Graph and Forecast
Sulfuric acid is a highly corrosive mineral acid with the chemical formula H2SO4. It’s a colorless, odorless liquid with a viscous consistency. Sulfuric acid is widely used in various industries, including fertilizer production, chemical synthesis, metal processing, and wastewater treatment. Its primary applications include pH regulation, chemical manufacturing, and as a drying agent. The report…

View On WordPress
#demand for sulfuric acid#Global Sulfuric Acid Market#Global Sulfuric Acid Market#Sulfuric Acid#sulfuric acid commodity price#Sulfuric Acid Companies#Sulfuric Acid Manufacturers#Sulfuric Acid Market#Sulfuric Acid Market Growth#sulfuric acid market report#sulfuric acid market size#sulfuric acid price#sulfuric acid price trend#Sulfuric Acid Applications#Sulfuric Acid Ecosystem#Sulfuric Acid Industry#Sulfuric Acid Industry Forecast#Sulfuric Acid Industry Opportunities#Sulfuric Acid Industry Share#Sulfuric Acid Industry Size#Sulfuric Acid Industry Trends#Sulfuric Acid Manufacturers#Sulfuric Acid Producers#Sulfuric Acid Products#Sulfuric Acid Suppliers#Sulfuric Acid Trends#sulphuric acid market#sulphuric acid spot price#ultra pure sulfuric acid
0 notes
Text
Although many people here would know that sulfuric acid is used in industrial chemicals, apart from this we have given the list of raw materials.
0 notes
Text
instead of doing art the other day i may have updated our artfight profile with almost nothing but digimon ocs
anyone in the digimon fandom thinking about participating this year?
#sky talks#digimon#artfight#artfight 2024#if you wanna add us we're Rukmau on AF#character profiles technically have spoilers for TWALB but only because i haven't talked like at all about it here#i will be adding some more stage references methinks though#just maybe not for a little while because god my hands are killing me this week#(manufacturing and chronic pain mix like hydrogen peroxide and sulfuric acid)
0 notes
Note
Y’all aren’t having your characters write on one of these?

Are you the "I have strong feelings about parchment" Tumblr user? If not, I feel like I saw you might know who they are. I was going to put parchment in a fic, but then I was like "no, I can't do that without asking the Parchment Person first because I don't want to get it Wrong."
...no, but I know the post you're talking about, and I too have strong feelings about parchment, esp because I've worked on it before.
I took an illumination course while getting my illustration degree and did an "H.M." in the style of a medieval manuscript on a small piece of the stuff.
Parchment is not paper. It's cured calf or pig skin.
It's thick, and HARD like non-corrugated cardboard. If it's been stored in a roll, it does not want to unroll. If it's been stored flat, it does not want to roll. It's got the same texture as skin, because it IS skin, and you have to account for that while working on it. It smells like rawhide. It actually takes ink in a really interesting way- there's a half-second to blend of fix something before it actually sinks into the parchment, but it doesn't bleed once it's in there. It also never comes back out. It's not bright white like paper, almost a buff color, and white stands out on it.
Fascinating stuff. Actually pretty fun to work on, but it's definitely a medium for highly polished and important pieces (like illuminated manuscripts), not for casual note-taking (because it's MAD EXPENSIVE to make)
I should go hit up the local art stores and get different paper-and-other-art-media samples to demo for everyone for fanfic purposes because they are VERY different things that have different purposes, prices, origins, and societal connotations, all of which can be used in your writing.
#tbf anything after 1850s could possibly have what would be called “parchment paper HOWEVER#the manufacturing process for parchment paper crucially involves a sulfuric acid bath#making it ENTIRELY unsuitable for archival use i.e. anything you want to write/draw and have it be around in a few years#but it does sound nice when you crinkle a sheet of it and throw it away 😌#not being contradictory towards the main discussion point just bein a lil cheeky <3
4K notes
·
View notes
Text
Satisfactory: The Full Ficsonium-Chain Nuclear Power Plant
Okay.
So.
I did it.

The thing I love doing most in Satisfactory is creating giant power plants that give me way more power than I need so I don't have to worry about my overhead while making other things. It's the thing that really captured and hooked me into a loyal player.
In the 1.0 release, they added a way to fully engage your uranium resources and make plutonium power without unsinkable waste product, by adding a third step called Ficsonium.
And so the gauntlet was thrown down.
Starting out, making this all work looked impossible. But slowly, I whittled away at it, optimized it, until I had a workable plan. And now that I've built it, I'm going to subject you all to the write-up.

93.75 Uranium, 93.75 Sulfur, 56.25 Silica, and 281.25 Quickwire goes into 3 Manufacturers (overclocked to 125%) to make 75 Encased Uranium Cells (using the Infused Uranium Cell alternate recipe).
(All material numbers are in parts-per-minute, by the way, in case that confuses anybody.)

Those E.U. Cells go straight into 3 more Manufacturers (again, overclocked to 125%, and the Cell outputs/inputs are 1:1), along with 7.5 Electromagnetic Control Rods, 2.25 Crystal Oscillators, and 7.5 Rotors to make 2.25 Uranium Fuel Rods (using the Uranium Fuel Unit alternate recipe).

The Uranium Fuel Rods are fed into 6 Nuclear Power Plants (each overclocked to 187.5%, so the equivalent of 11.25 power plants). Each fuel rod Manufacturer splits its outputs to two of the power plants. The power plants are fed with 450 cubic meters of water each from the framework above (except for the nearest one, which is also taking water byproduct from the next stage). Together, the uranium power plants produce 28,125 MW of electrical power and 112.5 Uranium Waste.

The Uranium Waste is quickly conveyed beneath the floor over to three Blenders (overclocked to 150%), along with another 112.5 raw Uranium, 67.5 Nitric Acid, and 112.5 Sulfuric Acid, to make 450 Non-Fissile Uranium (using the Fertile Uranium alternate recipe). This also produces 180 water byproduct, which is fed back into the first uranium power plant.

Each Blender feeds into its own row of 2 Particle Accelerators, for a total of 6. (No overclocking here, for once!) The Non-Fissile Uranium is mixed with 60 total Aluminum Casings to produce 60 Encased Plutonium Cells (using the Instant Plutonium Cell alternate recipe).

The Encased Plutonium Cells are fed into 4 Manufacturers (overclocked to 200% - and we're back), along with 36 Steel Beams, 12 Electromagnetic Control Rods, and 20 Heat Sinks, to make a whole whopping 2 Plutonium Fuel Rods!
(Everything up to this point is considered "Stage One" of the overall nuclear power plant. At this juncture, I left the remaining power plants unconnected and simply destroyed the Plutonium Fuel Rods in the AWESOME Sink while I took a break and worked on other parts with the electrical power the uranium plants alone were giving me. Everything after this is considered "Stage Two," the even more complicated part.)

Once the rest of the chain was ready, I connected the Plutonium Fuel Rod Manufacturer outputs to 8 more nuclear power plants (overclocked to 250%, effectively 20 power plants!), 2 for each line. Each plant gets 600 water from overhead as well. This alone gives me another 50,000 MW of power!
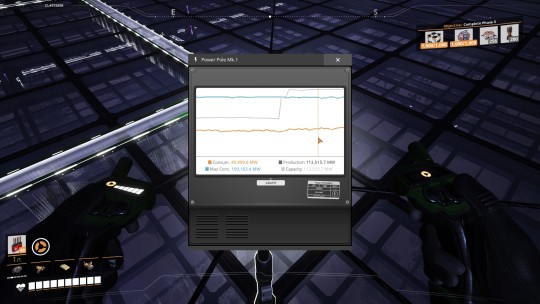
...But it also produces 20 Plutonium Waste, which until 1.0 was completely indestructible and had to be stored away in a dedicated dump indefinitely.
(Sure, people have done the math. With enough space, you can buy yourself literal months of in-game uptime before your dump fills up with waste and the power plants become inoperable, in exchange for writing off that part of the map. But why give yourself any such hassle at all if you don't have to? Sustainability is a part of efficiency!)

The Plutonium Waste is brought over to 2 Particle Accelerators and combined with 20 Singularity Cells and 400 Dark Matter Residue (neither of which are cheap!) to make 20 Ficsonium.
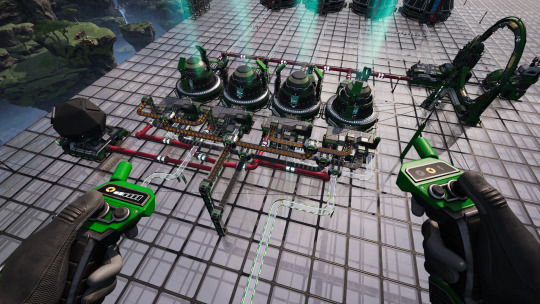
The Ficsonium, 20 Electromagnetic Control Rods (again! so many!), 400 Ficsite Trigons (!!!), and 200 Excited Photonic Matter (dirt simple, actually - phew!) are fed into 4 Quantum Encoders to make 10 Ficsonium Fuel Rods. This also produces 200 Dark Matter Residue as a byproduct, which I put into a Particle Accelerator to make Dark Matter Crystals that I just toss into an AWESOME Sink.
(If you're smart, you could just loop the byproduct here back into the previous step and cut that input in half, but... I just could not be bothered this time. I wanted this plant fully operational as soon as possible.)

And finally, the Ficsonium Fuel Rods are sent directly from the Quantum Encoders to 4 Nuclear Power Plants (overclocked to 250% again, effectively 10 power plants) and mixed with 600 overhead water each to produce another 25,000 MW of power and absolutely zero waste or byproduct.
For a grand total of... 103,125 MW!
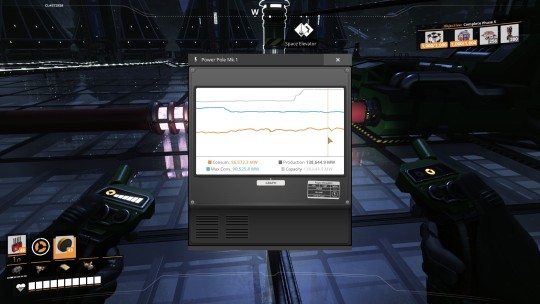
(And that's before adding bonus power from Alien Power Augmenters later!)

This was a massive build that took up the entire swamp region of the map (because to hell with the swamp, all my homies hate the swamp and their eldritch-moving alpha spiders). There's an interim layer of the platform for "spaghetti," or conveying the materials to where they need to go, and then just about everything needed for this process was made from scratch from within the swamp or close-by-ish.
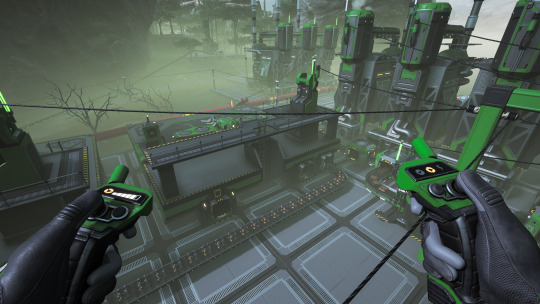
With one exception: I drone'd in Pressure Conversion Cubes that I was making elsewhere for the Nuclear Pasta that's required for the Singularity Cells. Having to make Radio Control Units and Fused Modular Frames from scratch on top of everything else just would've tipped this over the breaking point for me personally.

And that's that. Probably the biggest power plant I'll ever want to make in Satisfactory, at least on my own in single-player. I've taken the gauntlet and thrown it back. And now I have more than enough power to finish the rest of the game.
Thanks for reading! I worked way too hard on this whole project.
(Also, for those who may be looking at this flow and thinking, "Holy crap, you'll need hundreds of Reanimated SAM and thus thousands of SAM!" - here's a pro tip: Somersloops. Overclock the Constructors making your Reanimated SAM to max and then throw in 1 Somersloop. Instantly improves the recipe from 4:1 to 2:1. I'm dead certain this was an intended part of SAM balancing, because holy crap there's just not enough of it on the map otherwise.)
#satisfactory#satisfactory game#satisfactory screenshot#nuclear power#nuclear energy#factory post-mortem write-up#please come look I worked so hard on this#shouting into the void
22 notes
·
View notes
Text



15th February 1817 saw the birth in Glasgow of Robert Angus Smith.
You may not have heard of him, or maybe you read about him in my previous post? Anyway we have all heard of acid rain, defined by National Geographic magazine thus: “Acid rain describes any form of precipitation that contains high levels of nitric and sulfuric acids. It can also occur in the form of snow, fog, and tiny bits of dry material that settle to Earth.”
The words “acid rain” were coined as long ago as 1859 by Angus Smith, who seven years earlier had made the discovery that northern cities across Britain were suffering from rainfall that contained heavy pollutants that were the result of the burning of coal that was rich in sulphur. His research found that the worst-affected city was his home town of Glasgow.
Robert Angus Smith was born in Pollokshaws the seventh son and 12th child of John Smith, originally from Ayrshire, and his wife Janet, daughter of James Thomson who owned a mill at Strathaven in Lanarkshire.
His elder brother John was a big influence on Angus’s life. John eventually became a senior teacher at Perth Academy, and was himself a scientist who would research theories on colour and light. He encouraged his younger brother to read the works of Joseph Priestley, the pioneering English chemist, and Angus Smith was greatly influenced by Priestley’s writings.
He attended Glasgow University from the age of 13, apparently to prepare for a career in the Church of Scotland ministry, but he left without graduating and then became a tutor to families, first in Scotland and then in England. In 1839 he accompanied the Bridgeman family to Germany where he remained to study under the Professor Justus Liebeg, gaining his PhD in 1841.
On returning to England he took a post at Manchester Royal Institution as assistant to Lyon Playfair, an Indian-born Scot and a scientist and politician.
Playfair passed on his own interest in the sanitation of towns and cities to Angus Smith, who left the Institution to set up in business as an analytical chemist. As concern grew about pollution, his services were in demand, and in one famous experiment he waited until a crowded room had emptied then collected the residue on windows to prove that human breath exuded not just carbon dioxide but organic matter dangerous to health.
Smith once graphically described the effects of Manchester’s polluted atmosphere, in a letter to the Manchester Guardian published on November 2, 1844.
He wrote: “Coming in from the country last week on a beautiful morning, when the air was unusually clear and fresh, I was surprised to find Manchester was enjoying the atmosphere of a dark December day… Those who would defend such evils, who would remain careless as long as any probable cause is unremoved, must surely be devoid not only of mercy, but of clear perception and of good taste. The gloominess of uncleanness is everywhere around us.”
In 1851 he began the research that would make him the “father of acid rain” as he is often known. Smith proved that sulphur compounds in the air of towns and cities were the result of burning coal and coke transported in air and rainwater, and even as the industrial revolution was bringing more and more factories into being, Smith was arguing that manufacturers should be held responsible for their pollution.
He investigated poor housing and water quality, and published numerous papers that formed the basis of the developing science of environmental chemistry. One report on the problems of pollution for the Royal Mines Commission was particularly devastating in its scientific indictment of the polluters.
Smith was called as an expert witness in a court case over factory and mine pollution and his testimony was convincing. Consequently when the British Government decided to legislate – in the Alkali Act of 1863 – to try and cut pollution from mining and manufacturing, there was really only one man to turn to as the first chief of the alkali inspectorate and thus Smith spent much of the next two decades transforming attitudes to pollution.
In 1872 Smith published his Air and Rain, the beginnings of a Chemical Climatology, in which he collected the result of his experiments. It proved how ground-breaking his work had been.
With honorary degrees from both Glasgow and Edinburgh University, Angus Smith was honoured in his own lifetime. His health declined badly in his later years and he died at at Colwyn Bay, North Wales, on May 12, 1884, being buried in the churchyard of St Paul’s, Kersal, Manchester.
He was paid a most generous tribute in the first edition of Nature magazine following his death: “For upwards of 40 years he laboured unceasingly to show how chemistry might minister to the material comfort and physical well-being of men — not in the manufacture of new compounds useful in the arts, or in the establishment of new industries – but in raising the general standard of the health of communities by checking or counteracting the evils which have followed in the train of that enormous development of the manufacturing arts which is the boast of this century.
“In his true vocation, as the chemist of sanitary science, Smith worked alone, and we have yet to find the man on whom his mantle has fallen."
12 notes
·
View notes
Note
The following is the list of ingredients listed on the Internet as required for the manufacture of methamphetamine:
Ephedrine (cold and allergy medicine)
Pseudoephedrine (cold and allergy medicine)
Alcohol (Rubbing/gasoline additive)
Toluene (brake cleaner)
Ether (engine starter)
Sulfuric Acid (drain cleaner)
Methanol (gasoline additive)
Lithium (camera batteries)
Trichloroethane (gun scrubber)
Anhydrous Ammonia (farm fertilizer)
Sodium Hydroxied (lye)
Red Phosphorous (matches)
Iodine (Veterinarian products)
Sodium metal (can be made from lye)
Table/Rock salt
Kerosene
Gasoline
Muriatic Acid
Campfire fuel
Paint thinner
Acetone
Red Phosphorous (flammable solid) and iodine are mixed. Crystals produce hydrogen iodine gas (corrosive) and phosphine gas (toxic), which is immediate health and safety hazards. When the process is complete, the reaction solution is then finished with lye and solvents.
Or, hear me out, I can just buy meth.
3 notes
·
View notes
Text
ok i shouldnt say ABANDON but i can foresee my posts beign veeeery very sparse (at least until winter break) . like ive been working on an nsfw alphabet for toby for like 2 months
alr yea gotta b honest think im gunna end up abandoning this blog
#it doesnt help that im the SLOWEST writer ever#i manufacture paragraphs as meticulously as someone handling sulfuric acid#but yes I DO LIKE WRITING i will just get back to it when i am able#lov u friends thnkx 4 sticking around#tobywords
31 notes
·
View notes
Text
This story from Anthropocene Magazine tells us how some of the obscure research projects being conducted in labs around the world can produce some boring but environmentally stunning outcomes that can be beneficial to all of us. So be careful the next time you think "nerds." Excerpt:
A common mineral present just beneath the Earth’s crust could help to negate the carbon footprint of concrete, researchers report in the journal Royal Society Open Science. The study details a way to turn the mineral olivine, which also forms the green gemstone peridot, into an alternative for cement and other construction materials. The research team has launched a startup to commercialize their patented process.
Concrete, the most widely used material in the world, is a mix of cement, water, gravel and sand. The production of cement and concrete results in about 8 percent of the world’s carbon dioxide emissions.
Most of these emissions are generated when limestone is heated at high temperatures to produce powdery cement. The emissions come from burning fossil fuels for heat, but also from the chemical reaction itself.
Some manufacturers are reducing concrete’s emissions by replacing part of the cement with waste material such as fly ash and slag or adding other recycled materials. Studies have shown that this replacement does not reduce the strength of concrete.
Civil and environmental engineers at Imperial College London turned to olivine, a magnesium silicate mineral that is found in the rocks in the Earth’s upper mantle. The mineral naturally reacts with carbon dioxide from the air and turns into magnesium carbonate. But this process works at a very slow geological timescale.
The team wanted to see if they could speed up this carbonate-forming process. They crushed olivine samples and mixed them into sulfuric acid. This separated the silica from the olivine and created magnesium sulfate. When they bubbled carbon dioxide gas through the mixture, it reacted with the sulfate to produce magnesium carbonate, resulting in the sequestration of carbon dioxide.
The silica can be used as a cement substitute in concrete to add strength. And the magnesium carbonate can be used as a binder or filler in other low-carbon construction products such as bricks, blocks and board, the team writes in the paper.
Replacing 35 percent of regular Portland cement in concrete with the silica would give carbon-neutral cement, the researchers write. Replacing more than that could would make concrete carbon negative.
Further, they add that the olivine processing is not energy intensive and could be done electrically using renewable energy.
6 notes
·
View notes
Text
What Is Hemp?
It’s A Trillion Dollar Cash Crop.
youtube
Hemp is a name given to a strain of the cannabis plant.
Hemp is a name given to cultivars of the cannabis plant (Cannabis sativa) that have been selected over many generations for fiber and seed production. Most hemp cultivars contain less than 1.5% THC, a narcotic compound that has the potential for abuse in high concentrations. Cannabis sativa cultivars selected and developed for their drug properties, referred to as marijuana, or dagga, can have a THC content of 3%-25%. Hemp is a bast fiber, producing its fibers in the stalk similar to flax, kenaf, and sun hemp.
Multiple Uses
Hemp fiber and seed are used to produce a wide range of commodities including food and beverage products, fiberboard, insulation, paper, composites, textiles, carpets, animal bedding and feed, cosmetics, body-care products, soaps, paints, fuels, and medicines.
Hemp Seed Food and Beverage Products
Hemp seed contains about 25% protein, 30% carbohydrates, & 15% insoluble fiber. Hemp seed is reported to contain more easily digestible protein than soybeans. Hemp seed contains all 8 amino acids essential to human nutrition. Hemp seed is high in calcium, magnesium, phosphorus, potassium, carotene, sulfur, iron and zinc, as well as Vitamins A, E, C, B1, B2, B3, and B6.
Hemp seed imported into the United States or Canada must be steam sterilized at between 180 degrees F and 212 degrees F for 15 minutes to prevent sprouting. Many US facilities receive imported viable seed under customs bond, steam it, and release it to the consignee or customer with a Certificate of Sterilization.
Hemp food and beverage products include hemp oil and seed, flour, pasta, cheese, tofu, salad dressings, snacks, sweets, hemp protein powders, soft drinks, beer, and wine. Hemp beer can be made from the seed, flowers, sprouts, and seed cake that is a by-product of oil pressing. Hemp beer is produced and sold in Europe and the United States of America.
Hemp Oil
Hemp seed is 25% to 35% oil, and is one of the oils lowest in saturated fats (8%). Hemp seed oil is the richest source of polyunsaturated essential fatty acids (80%). Hemp seed oil is the only common edible seed oil containing Omega-6 Gamma-Linolenic Acid. Hemp seed oil is very fragile and not suitable for cooking.
Pressed hemp seed oil must be bottled immediately under oxygen-free conditions, and must be refrigerated in dark, airtight containers.
Fiberboard
Hemp fiberboard tested by Washington State University Wood Materials and Engineering Laboratory proved to be two and one half times stronger than wood MDF composites, and the hemp composite boards were three times more elastic.
Hemp herds can be used in existing mills without major changes in equipment. Russia, Poland and other Eastern European countries already manufacture composite boards from hemp and other plant materials.
Pulp and Paper
The major use of hemp fiber in Europe is in the production of specialty papers such as cigarette paper, archival paper, tea bags, and currency paper. The average bast fiber pulp and paper mill produces 5,000 tons of paper per year. Most mills process long bast fiber strands, which arrive as bales of cleaned ribbon from per-processing plants located near the cultivation areas.
Composites
Until the 1930’s, hemp-based cellophane, celluloid and other products were common, and Henry Ford used hemp to make car doors and fenders. Today hemp herds can be used to make new plastic and injection-molded products or blended into recycled plastic products. Hemp fibers are introduced into plastics to make them stiffer, stronger and more impact resistant. Hemp plastics can be designed that are hard, dense, and heat resistant, and which can be drilled, ground, milled, and planed.
Hemp plastic products currently made include chairs, boxes, percussion instruments, lampshades, bowls, cups, spectacles, jewelry, skateboards, and snowboards.
Hemp Animal Care
Hemp horse bedding and cat litter are produced and sold in Europe. After oil is extracted from the hemp seed, the remaining seed cake is about 25% protein and makes an excellent feed for chicken, cattle, and fish. Chickens fed hemp seed on a regular basis have been found to produce more eggs, without the added hormones used in most poultry plants.
Fuels
Hemp seed oil can be combined with 15% methanol to create a substitute for diesel fuel which burns 70% cleaner than petroleum diesel. Hemp stalks are rich in fiber and cellulose, making them conducive for conversion into ethanol and methanol fuels that have a higher octane than gasoline and produce less carbon monoxide. These biomass fuels are also free from sulfur, and do not require the addition of lead and benzene used to boost octane and improve engine performance in fossil fuels. Ethanol holds condensation, eliminating oxidation and corrosion, and is reported to reduce carbon dioxide emissions by more than 30%.
Hemp has been studied in Ireland as a biomass fuel to generate electricity. Hemp has been reported to yield 1000 gallons of methanol per acre year. Hemp stalk can be converted to a charcoal-like fuel through a thermochemical process called pyrolysis. Henry Ford operated a biomass pyrolitic plant at Iron Mountain, Michigan in the mid-20th.
Paints and Varnishes and Binders
Until the 1930's, most paints were made from hemp seed oil and flax seed oil. Hemp oil makes a durable, long lasting paint that renders wood water-resistant. Hemp herds have the potential to make glues for composite construction products that are non-toxic and superior to binders currently used. With this technology, industry can produce composite products where all components are derived from hemp.
Markets for Hemp Pulp
Some paper manufacturers already have the equipment to process decorticated hemp fiber into paper. The leading European supplier of non-wood pulp, Celesa, currently produces about 10,000 tons per year of pulp from hemp. The use of hemp pulp in blends with recycled fiber of other non-wood fibers is growing. Tests by several European pulp and paper producers suggest that hemp pulp may replace cotton cost effectively in several specialty paper applications.
Potential Markets for Medical Application of Low-THC Hemp Cultivars
Many cannabis medicines have been produced using cannabis cultivars high in THC, and there has been medical research into cannabis that is low in THC and high in CBD. CBD is a cannabinoid that does not have many of the psychoactive effects associated with THC. CBD has been used to treat the following medical conditions: epilepsy, dystonic movement disorders, inflammatory disorders, pain, chronic insomnia, chorea, cerebral palsy, and Tourette's syndrome. According to a July 1998 report by the National Institute of Health, CBD may hold promise for preventing brain damage in strokes, Alzheimer’s disease, Parkinson’s disease and even heart attacks and has been found to prevent brain cell death in an experimental stroke model.
Please share this with your friends.
youtube
#hemp farming#hemp foods#hemp fuel#industrial hemp#hemp plastic#hemp paper#hemp fibers#hemp seeds#hemp prohibition
4 notes
·
View notes
Text
Horrible Solution, "Piranha Solution"
"Piranha Solution," a menacing chemical cocktail, is a 3:1 mixture of concentrated sulfuric acid (95-98%) and hydrogen peroxide solution (30%). Named for its ferocity, it generates Caro's acid, a superoxidizer, and fiercely reacts with organics, resembling a frenzy of piranhas hunting prey. It finds applications in microelectronics and semiconductor manufacturing.

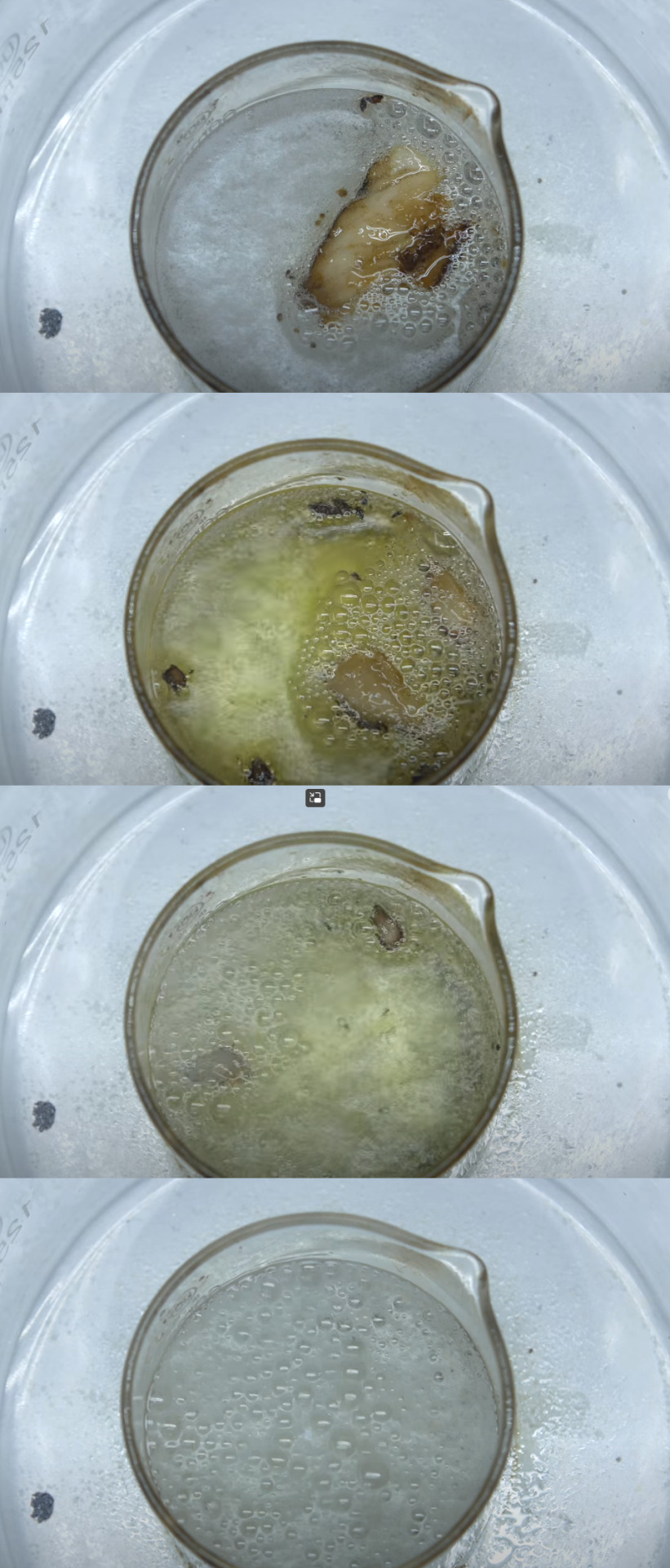
#chemistry class#science#chemical solutions#interesting#organicchemistry#educational#stem studyblr#chemblr#did u know#amazing facts
8 notes
·
View notes
Text
Industrial Applications of Sulfuric Acid: Global Market Insights 2022-2027
Sulfuric acid, often referred to as the “king of chemicals,” is a highly corrosive and strong mineral acid with the chemical formula H2SO4. It is one of the most widely used industrial chemicals globally, with a wide range of applications in various industries. Sulfuric acid is produced through the contact process, where sulfur dioxide gas (SO2) is oxidized to sulfur trioxide (SO3) using a…

View On WordPress
#COVID-19 Impact on Sulfuric Acid Market#demand for sulfuric acid#Global Sulfuric Acid Market#Sulfuric Acid#sulfuric acid commodity price#Sulfuric Acid Manufacturers#Sulfuric Acid Market#Sulfuric Acid Market Growth#sulfuric acid market report#sulfuric acid market size#sulfuric acid price#sulfuric acid price trend#sulphuric acid market#sulphuric acid spot price#ultra pure sulfuric acid
1 note
·
View note
Text
STRAY KIDS OFFICIAL COLOR THEORY




Realgar also known as “ruby sulphur” or “ruby of arsenic”, is an arsenic sulfide mineral with the chemical formula α-As4S4. It is a soft, sectile mineral occurring in monoclinic crystals, or in granular, compact, or powdery form, often in association with the related mineral, orpiment (As2S3). It is orange-red in color, melts at 320 °C, and burns with a bluish flame releasing fumes of arsenic and sulfur. Realgar is soft with a Mohs hardness of 1.5 to 2 and has a specific gravity of 3.5. Its streak is orange colored. It is trimorphous with pararealgar and bonazziite.

Its name comes from the Arabic rahj al-ġār (“powder of the mine”), via Medieval Latin, and its earliest record in English is in the 1390s.

Realgar was used by firework manufacturers to create the color white in fireworks prior to the availability of powdered metals such as aluminium, magnesium and titanium. It is still used in combination with potassium chlorate to make a contact explosive known as “red explosive” for some types of torpedoes and other novelty exploding fireworks branded as “cracker balls”, as well in the cores of some types of crackling stars.
Realgar is toxic. It was sometimes used to kill weeds, insects, and rodents, even though more effective arsenic-based anti-pest agents are available such as cacodylic acid, (CH3)2As(O)OH, an organoarsenic compound used as herbicide and also to kill ants and mice.
Realgar was commonly used in leather manufacturing to remove hair from animal pelts. Because it is a known carcinogen and an arsenic poison, and because substitutes are available, it is rarely used today for this purpose.

The ancient Greeks, who called realgar (sandarákē), understood that it was poisonous. From this, realgar has also historically been known in English as “sandarac”.
Realgar was also used by Ancient Greek apothecaries to make a medicine known as “bull's blood”. The Greek physician Nicander described a death by “bull's blood”, which matches the known effects of arsenic poisoning. Bull's blood is the poison that is said to have been used by Themistocles and Midas for suicide.


After a long period of exposure to light, realgar changes form to a yellow powder known as pararealgar (β-As4S4). It was once thought that this powder was the yellow sulfide orpiment, but is a distinct chemical compound.

In China, the traditional Chinese medicine still plays an important role in the treatment of various diseases. The traditional Chinese medicine Realgar (mainly As4S4, tetraarsenic tetrasulfide) has been used well in the treatment of both internal and external diseases, such as infection, inflammation, fever, mouth ulcers, tongue ulcers, headache, and convulsion, even some skin disease.
Realgar is said to heal emotional trauma and aid in mental health. Promotes positive thinking and provides insight.
Wikipedia
3 notes
·
View notes
Text
How Plastics Are Poisoning Us
They both release and attract toxic chemicals, and appear everywhere from human placentas to chasms thirty-six thousand feet beneath the sea. Will we ever be rid of them?
— By Elizabeth Kolbert | June 26, 2023

Annual production of plastic exceeds eight hundred billion pounds; much of it ends up as microplastics, spreading across the ocean. Illustration by Daniel Liévano
In 1863, when much of the United States was anguishing over the Civil War, an entrepreneur named Michael Phelan was fretting about billiard balls. At the time, the balls were made of ivory, preferably obtained from elephants from Ceylon—now Sri Lanka—whose tusks were thought to possess just the right density. Phelan, who owned a billiard hall and co-owned a billiard-table-manufacturing business, also wrote books about billiards and was a champion billiards player. Owing in good part to his efforts, the game had grown so popular that tusks from Ceylon—and, indeed, elephants more generally—were becoming scarce. He and a partner offered a ten-thousand-dollar reward to anyone who could come up with an ivory substitute.
A young printer from Albany, John Wesley Hyatt, learned about the offer and set to tinkering. In 1865, he patented a ball with a wooden core encased in ivory dust and shellac. Players were unimpressed. Next, Hyatt experimented with nitrocellulose, a material made by combining cotton or wood pulp with a mixture of nitric and sulfuric acids. He found that a certain type of nitrocellulose, when heated with camphor, yielded a shiny, tough material that could be molded into practically any shape. Hyatt’s brother and business partner dubbed the substance “celluloid.” The resulting balls were more popular with players, although, as Hyatt conceded, they, too, had their drawbacks. Nitrocellulose, also known as guncotton, is highly flammable. Two celluloid balls knocking together with sufficient force could set off a small explosion. A saloon owner in Colorado reported to Hyatt that, when this happened, “instantly every man in the room pulled a gun.”
It’s not clear that the Hyatt brothers ever collected from Phelan, but the invention proved to be its own reward. From celluloid billiard balls, the pair branched out into celluloid dentures, combs, brush handles, piano keys, and knickknacks. They touted the new material as a substitute not just for ivory but also for tortoiseshell and jewelry-grade coral. These, too, were running out, owing to slaughter and plunder. Celluloid, one of the Hyatts’ advertising pamphlets promised, would “give the elephant, the tortoise, and the coral insect a respite in their native haunts.”
Hyatt’s invention, often described as the world’s first commercially produced plastic, was followed a few decades later by Bakelite. Bakelite was followed by polyvinyl chloride, which was, in turn, followed by polyethylene, low-density polyethylene, polyester, polypropylene, Styrofoam, Plexiglas, Mylar, Teflon, polyethylene terephthalate (familiarly known as pet)—the list goes on and on. And on. Annual global production of plastic currently runs to more than eight hundred billion pounds. What was a problem of scarcity is now a problem of superabundance.
In the form of empty water bottles, used shopping bags, and tattered snack packages, plastic waste turns up pretty much everywhere today. It has been found at the bottom of the Mariana Trench, thirty-six thousand feet below sea level. It litters the beaches of Svalbard and the shores of the Cocos (Keeling) Islands, in the Indian Ocean, most of which are uninhabited. The Great Pacific Garbage Patch, a collection of floating debris that stretches across six hundred thousand square miles between California and Hawaii, is thought to contain some 1.8 trillion plastic shards. Among the many creatures being done in by all this junk are corals, tortoises, and elephants—in particular, the elephants of Sri Lanka. In recent years, twenty of them have died after ingesting plastic at a landfill near the village of Pallakkadu.
How worried should we be about what’s become known as “the plastic pollution crisis”? And what can be done about it? These questions lie at the heart of several recent books that take up what one author calls “the plastic trap.”
“Without plastic we’d have no modern medicine or gadgets or wire insulation to keep our homes from burning down,” that author, Matt Simon, writes in “A Poison Like No Other: How Microplastics Corrupted Our Planet and Our Bodies.” “But with plastic we’ve contaminated every corner of Earth.”
Simon, a science journalist at Wired, is especially concerned about plastic’s tendency to devolve into microplastics. (Microplastics are usually defined as bits smaller than five millimetres across.) This process is taking place all the time, in many different ways. Plastic bags drift into the ocean, where, after being tossed around by the waves and bombarded with UV radiation, they fall apart. Tires today contain a wide variety of plastics; as they roll along, they abrade, sending clouds of particles spinning into the air. Clothes made with plastics, which now comprise most items for sale, are constantly shedding fibres, much the way dogs shed hairs. A study published a few years ago in the journal Nature Food found that preparing infant formula in a plastic bottle is a good way to degrade the bottle, so what babies end up drinking is a sort of plastic soup. In fact, it is now clear that children are feeding on microplastics even before they can eat. In 2021, researchers from Italy announced that they had found microplastics in human placentas. A few months later, researchers from Germany and Austria announced that they’d found microplastics in meconium—the technical term for an infant’s first poop.
The hazards of ingesting large pieces of plastic are pretty straightforward; they include choking and perforation of the intestinal tract. Animals that fill their guts with plastics eventually starve to death. The risks posed by microplastics are subtler, but not, Simon argues, any less serious. Plastics are made from by-products of oil and gas refining; many of the chemicals involved, such as benzene and vinyl chloride, are carcinogens. In addition to their main ingredients, plastics may contain any number of additives. Many of these—for example, polyfluoroalkyl substances, or PFASs, which confer water resistance—are also suspected carcinogens. Many of the others have never been adequately tested.
As plastics fall apart, the chemicals that went into their manufacture can leak out. These can then combine to form new compounds, which may prove less dangerous than the originals—or more so. A couple of years ago, a team of American scientists subjected disposable shopping bags to several days of simulated sunlight, in order to mimic the conditions that they’d encounter flying or floating loose. The researchers found that a single bag from CVS leached more than thirteen thousand compounds; a bag from Walmart leached more than fifteen thousand. “It is becoming increasingly clear that plastics are not inert in the environment,” the team wrote. Steve Allen, a researcher at Canada’s Ocean Frontier Institute who specializes in microplastics, tells Simon, “If you’ve got an IQ above room temperature, you have to understand that this is not a good material to have in the environment.”
Microplastics, meanwhile, don’t just leach nasty chemicals; they attract them. “Persistent bioaccumulative and toxic substances,” or PBTs, are a hodgepodge of harmful compounds, including DDT and PCBs. Like microplastics, which are often referred to in the scientific literature as MPs, PBTs are everywhere these days. When PBTs encounter MPs, they preferentially adhere to them. “In effect, plastics are like magnets for PBTs” is how the Environmental Protection Agency has put it. Consuming microplastics is thus a good way to swallow old poisons.
Then, there’s the threat posed by the particles themselves. Microplastics—and in particular, it seems, microfibres—can get pulled deep into the lungs. People who work in the synthetic-textile industry, it has long been known, suffer from high rates of lung disease. Are we breathing in enough microfibres that we are all, in effect, becoming synthetic-textile workers? No one can say for sure, but, as Fay Couceiro, a researcher at England’s University of Portsmouth, observes to Simon, “We desperately need to find out.”
Whatever you had for dinner last night, the meal almost certainly left behind plastic in need of disposal. Before tossing your empty sour-cream tub or mostly empty ketchup bottle, you may have searched it for a number, and if you found one, inside a cheerful little triangle, you washed it out and set it aside to be recycled. You might also have imagined that with this effort you were doing your part to stem the global plastic-pollution tide.
The British journalist Oliver Franklin-Wallis used to be a believer. He religiously rinsed his plastics before depositing them in one of the five color-coded rubbish bins that he and his wife kept at their home in Royston, north of London. Then Franklin-Wallis decided to find out what was actually happening to his garbage. Disenchantment followed.
“If a product is seen as recycled, or recyclable, it makes us feel better about buying it,” he writes in “Wasteland: The Secret World of Waste and the Urgent Search for a Cleaner Future.” But all those little numbers inside the triangles “mostly serve to trick consumers.”
Franklin-Wallis became interested in the fate of his detritus just as the old order of Britain’s rubbish was collapsing. Up until 2017, most of the plastic waste collected in Europe and in the United States was shipped to China, as was most of the mixed paper. Then Beijing imposed a new policy, known as National Sword, that prohibited imports of yang laji, or “foreign garbage.” The move left waste haulers from California to Catalonia with millions of mildewy containers they couldn’t get rid of. “plastics pile up as china refuses to take the west’s recycling,” a January, 2018, headline in the Times read. “It’s tough times,” Simon Ellin, the chief executive of Britain’s Recycling Association, told the paper.
Trash, though, finds a way. Not long after China stopped taking in foreign garbage, waste entrepreneurs in other nations—Malaysia, Indonesia, Vietnam, Sri Lanka—started to accept it. Mom-and-pop plastic-recycling businesses sprang up in places where they were regulated laxly, if at all. Franklin-Wallis visited one such informal recycling plant, in New Delhi; the owner allowed him inside on the condition that he not reveal exactly how the business operates or where it is situated. He found workers in a fiendishly hot room feeding junk into a shredder. Workers in another, equally hot room fed the shreds into an extruder, which pumped out little gray pellets known as nurdles. The ventilation system consisted of an open window. “The thick fug of plastic fumes in the air left me dazed,” Franklin-Wallis writes.
Nurdles, which are key to manufacturing plastic products, are small enough to qualify as microplastics. (It’s been estimated that ten trillion nurdles a year leak into the oceans, most from shipping containers that tip overboard.) Usually, nurdles are composed of “virgin” polymers, but, as the New Delhi plant demonstrates, it is also possible to produce them from used plastic. The problem with the process, and with plastic recycling more generally, is that a polymer degrades each time it’s heated. Thus, even under ideal circumstances, plastic can be reused only a couple of times, and in the waste-management business very little is ideal. Franklin-Wallis toured a high-end recycling plant in northern England that handles pet, the material that most water and soda bottles are made from. He learned that nearly half the bales of pet that arrive at the plant can’t be reprocessed because they’re too contaminated, either by other kinds of plastic or by random crap. “Yield is a problem for us,” the plant’s commercial director concedes.
Franklin-Wallis comes to see plastic recycling as so much (potentially toxic) smoke and mirrors. Over the years, he writes, “a kind of playbook” has emerged. Under public pressure, a company like Coca-Cola or Nestlé pledges to insure that the packaging for its products gets recycled. When the pressure eases, it quietly abandons its pledge. Meanwhile, it lobbies against any kind of legislation that would restrict the sale of single-use plastics. Franklin-Wallis quotes Larry Thomas, the former president of the Society of the Plastics Industry, who once said, “If the public thinks recycling is working, then they are not going to be as concerned about the environment.”
Right around the time that Franklin-Wallis started tracking his trash, Eve O. Schaub decided to spend a year not producing any. Schaub, who has been described as a “stunt memoirist,” had previously spent a year avoiding sugar and forcing her family to do the same, an exercise she chronicled in a book titled “Year of No Sugar.” The year of no sugar was followed by “Year of No Clutter.” When she proposes a trash-free annum to her husband, he says he doubts it is possible. Her younger daughter begs her to wait until she goes away to college. Schaub plunges ahead anyway.
“As the beginning of the new year loomed, I was feeling pretty good about our chances,” she recalls in “Year of No Garbage.” “I mean, really. How hard could it be?”
What Schaub means by “no garbage” is not exactly no garbage. Under her scheme, refuse that can be composted or recycled is allowed, so her family can keep tossing out old cans and empty wine bottles along with food scraps. What turns out to be hard—really, really hard—is dealing with plastic.
At first, Schaub divides plastic waste into two varieties. There’s the kind with the little numbers, which her trash hauler accepts as part of its “single stream” recycling program and so, by her definition, doesn’t count as trash. Then, there’s the kind with no numbers, which isn’t supposed to go in the recycling bin and therefore does count. Schaub finds that even when she purchases something in a numbered container—guacamole, say—there’s usually a thin sheet of plastic under the lid that’s numberless. A lot of her time goes into rinsing off these sheets and other stray plastic bits and trying to figure out what to do with them. She is excited to find a company called TerraCycle, which promises—for a price—to “recycle the unrecyclable.” For a hundred and thirty-four dollars, she purchases a box that can be returned to TerraCycle filled with plastic packaging, and for an additional forty-two dollars she buys another box that can be filled with “oral care waste,” such as used toothpaste tubes. “I sent my TerraCycle Plastic Packaging box as densely packed with plastic as any box could be,” she writes.
Eventually, though, like Franklin-Wallis, Schaub comes to see that she’s been living a lie. Midway through her experiment, she signs up for an online course called Beyond Plastic Pollution, offered by Judith Enck, a former regional administrator for the E.P.A. Only containers labelled No. 1 (pet) and No. 2 (high-density polyethylene) get melted down with any regularity, Schaub learns, and to refashion the resulting nurdles into anything useful usually requires the addition of lots of new material. “No matter what your garbage service provider is telling you, numbers 3, 4, 6 and 7 are not getting recycled,” Schaub writes. (The italics are hers.) “Number 5 is a veeeery dubious maybe.”
TerraCycle, too, proves a disappointment. It gets sued for deceptive labelling and settles out of court. A documentary-film crew finds that dozens of bales of waste sent to the company for recycling have instead been shipped off to be burned at a cement kiln in Bulgaria. (According to the company’s founder, this is the result of an unfortunate mistake.)
“I had wanted so badly to believe that TerraCycle and Santa Claus and the Easter bunny were real, that I had been willing to overlook the fact that Santa’s handwriting looks suspiciously like Mom’s,” Schaub writes. Toward the end of the year, she concludes that pretty much all plastic waste—numbered, unnumbered, or shipped off in boxes—falls under her definition of garbage. She also concludes that, “in this day, age and culture,” such waste is pretty much impossible to avoid.
A few months ago, the E.P.A. issued a “draft national strategy to prevent plastic pollution.” Americans, the report noted, produce more plastic waste each year than the residents of any other country—almost five hundred pounds per person, nearly twice as much as the average European and sixteen times as much as the average Indian. The E.P.A. declared the “business-as-usual approach” to managing this waste to be “unsustainable.” At the top of its list of recommendations was “reduce the production and consumption” of single-use plastics.
Just about everyone who contemplates the “plastic pollution crisis” arrives at the same conclusion. Once a plastic bottle (or bag or takeout container) has been tossed, the odds of its ending up in landfill, on a faraway beach, or as tiny fragments drifting around in the ocean are high. The best way to alter these odds is not to create the bottle (or bag or container) in the first place.
“So long as we’re churning out single-use plastic . . . we’re trying to drain the tub without turning off the tap,” Simon writes. “We’ve got to cut it out.”
“We can’t rely on half-measures,” Schaub says. “We have to go to the source.” Her own local supermarket, in southern Vermont, stopped handing out plastic bags in late 2020, she notes. “Do you know what happened? Nothing. One day we were poisoning the environment with plastic bags in the name of ultra-convenience and the next? We weren’t.”
“We now know that we can’t start to reduce plastic pollution without a reduction of production,” Imari Walker-Franklin and Jenna Jambeck, both environmental engineers, observe in “Plastics,” forthcoming from M.I.T. Press. “Upstream and systemic change is needed.”
Of course, it’s a lot easier to talk about “turning off the tap” and changing the system than it is to actually do so. First, there are the political obstacles. For all intents and purposes, the plastics industry is a subsidiary of the fossil-fuel industry. ExxonMobil, for instance, is the world’s fourth-largest oil company and also its largest producer of virgin polymers. The connection means that any effort to reduce plastic consumption is bound to be resisted, either openly or surreptitiously, not just by companies such as Coca-Cola and Nestlé but also by corporations like Exxon and Shell. In March, 2022, diplomats from a hundred and seventy-five nations agreed to try to fashion a global treaty to “end plastic pollution.” At the first negotiating session, held later that year in Uruguay, the self-described High Ambition Coalition, which includes the members of the European Union as well as Ghana and Switzerland, insisted that the treaty include mandatory measures that apply to all countries. This idea was opposed by major oil-producing nations, including the U.S., which has called for a “country-driven” approach. According to the environmental group Greenpeace, lobbyists for the “major fossil fuel companies were out in force” at the session.
There are also practical hurdles. Precisely because plastic is now ubiquitous, it’s difficult to imagine how to replace all of it, or even much of it. Even in cases where substitutes are available, it’s not always clear that they’re preferable. Franklin-Wallis cites a 2018 study by the Danish Environmental Protection Agency which analyzed how different kinds of shopping bags compare in terms of life-cycle impacts. The study found that, to have a lower environmental impact than a plastic bag, a paper bag would have to be used forty-three times and a cotton tote would have to be used an astonishing seventy-one hundred times. “How many of those bags will last that long?” Franklin-Wallis asks. Walker-Franklin and Jambeck also note that exchanging plastic for other materials may involve “tradeoffs,” including “energy and water use and carbon emissions.” When Schaub’s supermarket stopped handing out plastic shopping bags, it may have reduced one problem only to exacerbate others—deforestation, say, or pesticide use.
“In the grand scheme of human existence, it wasn’t that long ago that we got along just fine without plastic,” Simon points out. This is true. It also wasn’t all that long ago that we got along just fine without Coca-Cola or packaged guacamole or six-ounce bottles of water or takeout everything. To make a significant dent in plastic waste—and certainly to “end plastic pollution”—will probably require not just substitution but elimination. If much of contemporary life is wrapped up in plastic, and the result of this is that we are poisoning our kids, ourselves, and our ecosystems, then contemporary life may need to be rethought. The question is what matters to us, and whether we’re willing to ask ourselves that question. ♦
— Published in the print edition of the July 3, 2023, The New Yorker Issue, with the headline “A Trillion Little Pieces.”
#Plastic Poisoning#Toxic Chemicals#On Earth and Under Seas#Microplastics#Plastic Pollution#PFASs#PBTs#MPs DDT & PCBs#E.P.A.#MIT Press#Coca Cola | Nestlé | Exxon | Shell#Greenpeace
3 notes
·
View notes
Text
PTFE Lined Sight Flow Indicators: Understanding their Functionality and Applications
In many industrial processes, it is important to monitor the flow of liquids or gases through a pipeline or system. This is where sight flow indicators come in, providing a visual way to monitor the flow of fluids. Sight flow indicators can come in a range of materials and designs, but in this blog, we will focus on PTFE lined sight flow indicators manufacturers in India and their functionality and applications.
PTFE (Polytetrafluoroethylene) is a highly non-reactive and versatile material that is resistant to many corrosive chemicals and has a high temperature tolerance. When PTFE is lined on the inside of a sight flow indicator, it provides excellent corrosion resistance and makes it suitable for use in highly corrosive environments.
Functionality of PTFE Lined Sight Flow Indicators:
PTFE Lined Sight Flow Indicators are used to visually monitor the flow of liquids or gases through a pipeline. They consist of a transparent window, usually made of glass or acrylic, and a PTFE lined body that allows the fluid to flow through. The window is typically circular in shape and allows operators to see the flow of the fluid.
The PTFE lining helps protect the sight flow indicator from corrosive substances and ensures the fluid does not come into contact with any metal parts. This lining also helps to reduce friction between the fluid and the sight flow indicator, making it easier for the fluid to flow through.
Applications of PTFE Lined Sight Flow Indicators:
PTFE Lined Sight Flow Indicators have a wide range of applications across various industries due to their excellent resistance to corrosive substances. Some common applications Include:
Chemical Processing: PTFE Lined Sight Flow Indicators in Vadodara are used in the chemical industry to monitor the flow of corrosive chemicals such as acids, alkalis, and Solvents.
Pharmaceutical Industry: In the pharmaceutical industry, PTFE Lined Sight Flow Indicators are used to monitor the flow of high-purity liquids such as purified water, pharmaceutical solutions, and sterile liquids.
Food and Beverage Industry: PTFE Lined Sight Flow Indicators are used in the food and beverage industry to monitor the flow of liquids such as fruit juices, milk, and beer.
Petrochemical Industry: In the petrochemical industry, PTFE Lined Sight Flow Indicators are used to monitor the flow of corrosive liquids such as hydrochloric acid, sulfuric acid, and other hazardous substances.
Manufacturers of PTFE Lined Sight Flow Indicators:
There are many manufacturers of PTFE Lined Sight Flow Indicators manufacturers in India around the world. These manufacturers offer a range of designs and materials to suit various applications. Some of the top manufacturers of PTFE Lined Sight Flow Indicators include:
- L.J. Star Incorporated
- Kenco Engineering Company
- Techniquip Corporation
- Mettler-Toledo International Inc.
- Gems Sensors & Controls
Conclusion:
PTFE Lined Sight Flow Indicators are an essential component in many industrial processes, providing a visual way to monitor the flow of fluids through a pipeline or system. Their excellent resistance to corrosive substances and high temperature tolerance make them ideal for use in a range of applications across various industries. With the increasing demand for PTFE Lined Sight Flow Indicators, manufacturers are constantly improving their designs and materials to meet the needs of their customers.
#PTFE lined sight flow indicators manufacturers in India#PTFE Lined Sight Flow Indicators in Vadodara#manufacturers#india#vadodara
2 notes
·
View notes
Text
MONEL 400 ROUND BARS IN AEROSPACE APPLICATIONS: A DEEP DIVE
Monel 400, an alloy composed primarily of nickel and copper, is renowned for its exceptional resistance to corrosion, especially in high-stress environments. It plays a pivotal role in various industries, including aerospace, due to its remarkable strength, durability, and resistance to seawater and acidic environments. At SMM Industries, we specialize in providing high-quality Monel 400 round bars, which are extensively used in aerospace applications, making them ideal for critical components in aircraft and space vehicles.
The aerospace industry demands materials that can withstand extreme conditions, including high temperatures, pressure, and corrosive environments. Monel 400 round bars meet these requirements with outstanding performance, offering high tensile strength and good weldability. This makes them suitable for manufacturing components such as valves, pumps, and high-performance fasteners used in aircraft engines and structural parts. The alloy’s resistance to saltwater corrosion also makes it a preferred choice for aerospace applications in coastal or marine environments.
Another key attribute of Monel 400 round bars is their ability to perform under extreme temperatures. The alloy can endure both cryogenic and high temperatures without compromising its structural integrity, ensuring reliability and safety in critical aerospace components. Additionally, its resistance to oxidation and scaling at elevated temperatures ensures that the material maintains its mechanical properties even in harsh conditions.
At SMM Industries, our Monel 400 round bars are meticulously crafted to meet the stringent standards of the aerospace industry. We ensure that our products undergo thorough testing for quality control, ensuring they are free from defects and meet the precise specifications required for aerospace applications. Whether it’s for turbine components, pressure vessels, or specialized aircraft parts, our Monel 400 round bars are the trusted solution for manufacturers and engineers looking for superior materials.
The use of Monel 400 in aerospace is not just limited to its mechanical properties. The alloy’s ability to withstand corrosive gases, including sulfur and carbon dioxide, ensures longevity and performance in aircraft systems exposed to the atmosphere. This makes Monel 400 round bars an indispensable part of the aerospace supply chain, particularly in parts exposed to the harsh conditions of flight.
In conclusion, Monel 400 round bars continue to be an essential material in aerospace applications, offering unmatched corrosion resistance, mechanical strength, and performance under extreme conditions. SMM Industries is proud to supply these high-quality round bars, providing aerospace engineers and manufacturers with the material needed for precision-engineered components that meet the most demanding specifications.
For more information on our Monel 400 round bars and their applications in aerospace, feel free to contact SMM Industries today!
To Know More https://www.smmindustriesllp.com/monel-400-round-bar/

0 notes