#RNA PCR Test
Explore tagged Tumblr posts
Text

Book RNA PCR Test For HIV 2 By Drsafehands . Get more info RNA PCR Test For HIV Please Call / WhatsApp : 9013161616 .
#Book RNA PCR Test For HIV 2#RNA PCR Test For HIV 2#RNA PCR Test For HIV#RNA PCR Test#RNA PCR Test For HIV 2 Delhi#RNA PCR Test For HIV Pune
0 notes
Text
2 notes
·
View notes
Text

On #World_AIDS_Day, we're proud to introduce the #HIV-Q Real-Time RT-PCR #Kit. This advanced test detects and quantifies HIV-1 #RNA with high sensitivity and specificity, ensuring precise diagnostics for healthcare professionals. Compatible with most real-time #PCR instruments, it offers reliable, cross-reactivity-free results to support better patient outcomes.
Let’s join hands in the fight against HIV with innovative #solutions!
#WorldAIDSDay2024 #HIVTesting #DiagnosticsInnovation #RealTimePCR #HealthcareInnovation #Diagnostics #Genes2Me #g2m #awareness
#g2m#genes2me#world aids day#world aids day 2024#hiv#rna#kits#rt pcr#rt pcr kit#pcr#hiv testing#hiv testing kit
0 notes
Text
Reference saved on our archive
By Amanda Blum
PCR tests are far superior to rapid antigen tests—and now you can get them for home use.
Last week, I was about to go on a date, and because I'm severely immunocompromised, we agreed he would take a COVID test using one of my rapid home PCR tests. It was a courtesy—he felt perfectly fine— but he tested positive. By the next day, he was sick as a dog. And, by the way, the rapid antigen test he took when he got home that night was negative.
Regardless of how you much of a health risk you see in COVID, it is still, at best, an inconvenience that costs you days off work. A simple home PCR test saved me from that inconvenience (and worse), and if I'd relied on the common rapid antigen test or done nothing at all, I would probably be sick right now.
While the world has desperately attempted to move on from COVID, this summer saw the highest case loads since 2022, with a winter surge just around the corner. Almost 300,000 people died from COVID in the US over the last three months alone, so while the pandemic has transitioned into endemic, according to the CDC, there are still risks to be aware of. Around 400 million people worldwide have long COVID, where symptoms can range from annoying to absolutely debilitating, regardless of your age, pre-COVID health, or fitness levels. Cases of long COVID are crushing our medical system, too. The two best tools to avoid getting COVID continue to be masking and testing. Unfortunately, the PCR testing centers that used to be available in each city have long closed, and obtaining a PCR has become expensive and hard to locate. This is why home testing kits are so important.
While you may be used to thinking of COVID tests as interchangeable, there’s a big difference between the standard at-home antigen test and a PCR (molecular) test. Almost five years in, it’s important to understand why PCR tests are the ones you want when accurate testing is important.
The difference between a PCR and a Rapid Antigen Test What you normally think of as a home COVID test—like the kind you can order for free from the government—is a rapid antigen test. When these at-home COVID tests became available, they were a powerful tool to help people know they were positive so they could isolate themselves from others. Almost all at-home tests were lateral flow tests, also known as rapid antigen tests (RATs). They measure for proteins on the outside of SARS-C0V-2, but they have a major flaw: They can only detect active virus. If you’re asymptomatic or don’t have a high viral load yet, the RAT may show negative results while you have an active and contagious infection.
This is why, if you already have symptoms, a negative antigen test isn't conclusive. You may need to test a number of times to confirm you have COVID. When you first get sick, you may go a number of days (as many as five) without enough virus to set off a positive RAT test. RATs were designed to be taken multiple times in sequence.
A PCR, also known as a NAAT or molecular test, measures RNA and can detect even small amounts of the virus. This is why it has always been considered the “gold standard” of COVID testing. These tests are generally considered accurate starting one to three days before you experience symptoms. Until last year, you needed to get a PCR from a testing center, but home tests have evolved and there are now four rapid, at-home molecular COVID tests, meaning you test and get a result within 30 minutes.
Why we still need COVID testing The world is now divided into people who view COVID as part of regular life and those who, due to chronic illness, immune issues, previous infections, or age, cannot afford to get infected. For a long time, we viewed COVID testing as something you do for your own health, but home PCR testing represents a way you can easily protect those vulnerable people in your life without cutting them off from society.
But even if you're not concerned about others, you should still care about protecting yourself from multiple infections. While the likelihood you will die of COVID has gone down dramatically due to vaccines, medical interventions, and natural immunity from infection, the news has not done a great job talking about long COVID. As people get infected two, three, four, and more times, they are playing against the odds. It’s estimated that one in 10—or even as many as one in five—infections leads to long COVID, and to explain how much it’s not “just the flu,” COVID is now considered to be a vascular illness. That means it affects the blood vessels in your body, which go everywhere. Thinking of COVID as a vascular illness helps explain why long COVID is everything from extreme fatigue to migraines to numbness in your extremities, loss of smell and taste, extreme fatigue, and neurological and cardiovascular conditions.
While lots of people no longer even test to see if they have COVID, there are a few reasons to get a definitive answer. First, you can only get the intervention Paxlovid within the first five days of symptoms. Anti-virals like Paxlovid knock down your viral load, one of the things we think helps prevent long COVID. Second, no one knows who will get long COVID, and you might need proof of that positive test in the future for insurance or benefits or even to justify sick days.
Lastly, you need to get tested because it is hard to know when you have COVID. Symptoms of COVID include headache, body ache, fever, sniffles, congestion, fatigue, sore throat, vomiting, diarrhea, and loss of smell or taste. In other words, absolutely anything out of the ordinary. While a RAT is unreliable for safe socializing with people for the reasons explained above, a molecular test can pretty reliably clear someone to come in your house that day, or be in close proximity. In that way, these molecular tests can be a tool to help immunocompromised people back into the world and make multigenerational celebrations safer.
How to get a molecular/PCR test Outside of your home, your main options now are urgent care clinics and places that do testing for travel. In both cases, they’ll be expensive. In the case of urgent care, they’ll put you in the same space as all the sick people, who are now no longer required to mask in healthcare settings, so if you don't already have COVID, you might pick it up there. Fortunately, there are molecular (PCR quality) tests you can take at home.
Rapid molecular tests require a similar effort on your part as a RAT test. You’ll swab yourself and then insert that swab into a machine that gives you a result. There are currently just four brands of these tests available: Lucira, Metrix, 3EO, and PlusLife. Unlike RAT tests, you have to order them, although Metrix and Lucira tests are available on Amazon, and Walgreens stocks Lucira tests in select stores. For a long time, they were just too expensive for most people, so they were relegated to the likes of movie sets, law firms, and Google employees. Prices have gone down, so now they’re more accessible—as low as $10 a test. Here are your options.
Follow the link to see the full review with relevant links!
#mask up#covid#wear a mask#pandemic#public health#covid 19#wear a respirator#still coviding#coronavirus#sars cov 2#covid test#covid testing
97 notes
·
View notes
Text
This is no small question. It’s huge.
COVID proved that. On the basis of the test alone, millions of people were falsely diagnosed with the disease.
Let me back up. 99.99999999999999 of medical professionals believe the fairy tale called VIRUSES is real. They’ll always believe it, all the evidence to the contrary notwithstanding.
So this article operates within that insane context, because that’s where the pros live and breathe and work.
The PCR test searches for a piece of RNA which is part of a virus. When it finds that piece, it blows it up to a relatively enormous size. A size that can be observed.
Because in its original size, it was far too tiny to detect.
That’s called a clue. If the piece of RNA was so “tiny and alone,” why would anyone think it could cause a disease?
Traditional medical research asserts you need a whole crowd, a whole large mob of a particular virus to create disease.
So the PCR test is mortally flawed at the outset.
On top of this, the test can be adjusted to become even more sensitive. Meaning it will find not just a tiny, tiny remote RNA fragment, but a much, much, much TINIER fragment.
Such an adjustment is made by most testing labs. The PCR is tuned up to become more sensitive. Therefore, the useless test becomes even more useless.
4 notes
·
View notes
Text
Retrospective epidemiologic and genomic surveillance of arboviruses in 2023 in Brazil reveals high co-circulation of chikungunya and dengue viruses
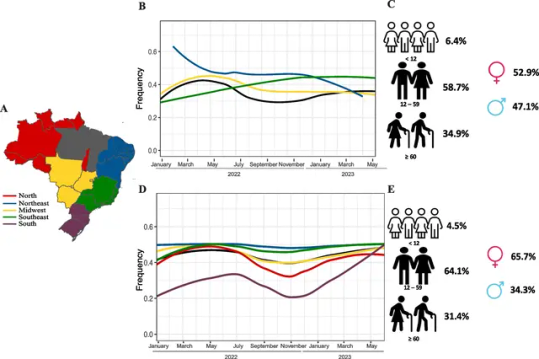
Background
The rapid spread and increase of chikungunya (CHIKV) and dengue (DENV) cases in Brazilian regions in 2023 has raised concerns about the impact of arboviruses on public health. Epidemiological and genomic surveillance was performed to estimate the introduction and spread of CHIKV and DENV in Brazil.
Methods
This study obtained results from the Hermes Pardini (HP), a private medical laboratory, and the Health Department of Minas Gerais state (SES-MG). We investigated the positivity rates of CHIKV and DENV by analyzing the results of 139,457 samples tested for CHIKV (44,029 in 2022 and 95,428 in 2023) and 491,528 samples tested for DENV (163,674 in 2022 and 327,854 in 2023) across the five representative geographical regions of Brazil. Genome sequencing was performed on 80 CHIKV and 153 DENV samples that had been positive for RT-PCR tests.
Results
In our sampling, the data from CHIKV tests indicated that the Northeast region had the highest regional positivity rate in 2022 (58.1%). However, in 2023, the Southeast region recorded the highest positivity rate (40.5%). With regard to DENV, the South region exhibited the highest regional positivity rate in both 2022 (40.8%) and 2023 (22.7%), followed by the Southeast region in both years (34.8% in 2022; 21.4% in 2023). During the first 30 epidemiological weeks of 2023 in the state of Minas Gerais (MG), there was a 5.8-fold increase in CHIKV cases and a 3.5-fold increase in DENV compared to the same period in 2022. Analysis of 151 new DENV-1 and 80 CHIKV genomes revealed the presence of three main clusters of CHIKV and circulation of several DENV lineages in MG. All CHIKV clades are closely related to genomes from previous Brazilian outbreaks in the Northeast, suggesting importation events from this region to MG. We detected the RNA of both viruses in approximately 12.75% of the confirmed positive cases, suggesting an increase of co-infection with DENV and CHIKV during the period of analysis.
Conclusions
These high rates of re-emergence and co-infection with both arboviruses provide useful data for implementing control measures of Aedes vectors and the urgent implementation of public health politics to reduce the numbers of CHIKV and DENV cases in the country.
Read the paper.
#brazil#brazilian politics#politics#science#biology#epidemiology#dengue#chikungunya#image description in alt#mod nise da silveira
2 notes
·
View notes
Text
to do list
- review first manuscript for editor meeting tomorrow
- read papers about RQC/surveillance
- bsu rna prep (gDNA / rRNA)
- J&J library prep (cup pcr & excision / gup test)
4 notes
·
View notes
Text
Oligonucleotide Synthesis Market Outlook, Size, Growth Factors, and Forecast 2025-2032

Oligonucleotide synthesis market is experiencing rapid expansion, driven by increasing applications in genetic research, diagnostics, therapeutics, and drug discovery. As advancements in synthetic biology and molecular diagnostics continue to evolve, the demand for high-quality oligonucleotides is soaring. According to SkyQuest’s latest report on the Oligonucleotide Synthesis Market, Oligonucleotide Synthesis Market size is poised to grow at a CAGR of 17.4% by 2032, driven by technological innovations and rising research investments.
The oligonucleotide synthesis market plays a crucial role in genomics, molecular biology, and biotechnology. It encompasses the development of short DNA and RNA sequences that serve as essential tools for PCR, gene editing, and targeted therapeutics. With the increasing adoption of oligonucleotides in clinical applications, the market is projected to experience significant expansion in the coming years.
Request a sample of the report here: https://www.skyquestt.com/sample-request/oligonucleotide-synthesis-market
Key Market Drivers Shaping Oligonucleotide Synthesis Growth
Growing Demand for Personalized Medicine
The rise of precision medicine has fueled the demand for custom oligonucleotides. Researchers and pharmaceutical companies are increasingly leveraging oligonucleotides for targeted therapies, particularly in cancer treatment and rare genetic disorders.
Advancements in Gene Editing Technologies
Innovations in CRISPR, RNA interference (RNAi), and antisense oligonucleotides are expanding the scope of oligonucleotide-based therapies. These breakthroughs are transforming genetic research, enabling more precise and effective treatments.
Expanding Applications in Diagnostics
Oligonucleotide probes and primers are widely used in molecular diagnostics, particularly in PCR-based testing, next-generation sequencing (NGS), and microarrays. The increasing prevalence of infectious diseases and genetic disorders has driven the demand for oligonucleotide-based diagnostic solutions.
Increased Investment in Biotechnology Research
Pharmaceutical and biotech companies are investing heavily in oligonucleotide research, aiming to develop novel therapeutics and drug delivery mechanisms. Governments and private organizations are also providing funding to accelerate genetic research.
Speak with an analyst for in-depth market insights: https://www.skyquestt.com/speak-with-analyst/oligonucleotide-synthesis-market
Oligonucleotide Synthesis Market Segmentation:
By Product Type
Synthesized Oligonucleotides – Custom sequences used in research, diagnostics, and therapeutics
Reagents and Consumables – Essential materials for synthesis processes
Equipment – Automated synthesizers and analytical tools for high-throughput oligonucleotide production
By Application
Research & Development – Genomic studies, drug discovery, and synthetic biology
Diagnostics – PCR, NGS, and DNA microarrays
Therapeutics – Antisense oligonucleotides, siRNA, and mRNA-based therapies
By End-User
Biotechnology & Pharmaceutical Companies – Focused on drug development and clinical applications
Academic & Research Institutions – Conducting genomics and molecular biology studies
Contract Research Organizations (CROs) – Supporting large-scale oligonucleotide synthesis and testing
Oligonucleotide Synthesis Market Regional Insights
North America: Leading the Market with Strong Research Infrastructure
The United States and Canada dominate the oligonucleotide synthesis market, driven by strong research capabilities, robust funding, and a high concentration of biotechnology companies. The presence of key industry players and increasing clinical trials further contribute to regional growth.
Europe: Rising Investments in Genetic Research
Countries like Germany, the UK, and France are expanding their biotechnology sectors, investing in advanced gene therapy and diagnostic solutions. The European Union’s support for genomic research is fostering innovation in oligonucleotide applications.
Asia-Pacific: Fastest-Growing Market with Expanding Biotech Industry
China, Japan, and India are witnessing rapid market expansion due to increasing investments in genetic research, a growing pharmaceutical industry, and government support for biotechnological advancements. The demand for oligonucleotide-based diagnostics and therapies is significantly increasing in the region.
Latin America & Middle East: Emerging Markets with High Growth Potential
Countries in Latin America and the Middle East are gradually adopting oligonucleotide synthesis technologies, primarily in medical research and infectious disease diagnostics. Increasing healthcare investments are expected to drive market growth in these regions.
Buy the full report for comprehensive market analysis: https://www.skyquestt.com/buy-now/oligonucleotide-synthesis-market
Key Players in the Oligonucleotide Synthesis Market
Several major players dominate the oligonucleotide synthesis market, focusing on innovation, product development, and strategic collaborations. Key companies include:
Thermo Fisher Scientific
Agilent Technologies
Merck KGaA
Integrated DNA Technologies (IDT)
LGC Biosearch Technologies
Eurofins Genomics
GenScript Biotech Corporation
TriLink BioTechnologies
These companies are expanding their production capacities and investing in new technologies to meet the rising demand for synthetic oligonucleotides.
Emerging Trends and Technological Innovations
Automated High-Throughput Synthesis
The adoption of automated systems is improving efficiency, scalability, and precision in oligonucleotide production. Advanced synthesis platforms are enabling rapid turnaround times for research and clinical applications.
Expansion of RNA-Based Therapeutics
RNA-based drugs, including mRNA vaccines and RNAi therapies, are gaining significant traction. This trend is expected to drive the demand for oligonucleotide synthesis in pharmaceutical and biotech industries.
Sustainable and Cost-Effective Synthesis Methods
Researchers are developing green synthesis approaches to minimize environmental impact and reduce production costs, making oligonucleotide synthesis more sustainable.
The Future of the Oligonucleotide Synthesis Market
The oligonucleotide synthesis market is on an upward trajectory, driven by advancements in gene editing, diagnostics, and therapeutics. As personalized medicine gains momentum and biotechnology continues to evolve, the demand for high-quality oligonucleotides will continue to rise. Companies investing in automation, innovative research, and sustainable production methods are well-positioned for success in this rapidly growing industry.
For a detailed market analysis and strategic insights, explore the full SkyQuest report: https://www.skyquestt.com/report/oligonucleotide-synthesis-market
#Asia Oligonucleotide Synthesis Market#Europe Oligonucleotide Synthesis Market#Middle East Oligonucleotide Synthesis Market Size#North America Oligonucleotide Synthesis Market
0 notes
Text
GAT-B 2025 Syllabus: A Comprehensive Guide for Aspiring Biotechnology Professionals
If you are gearing up for the Graduate Aptitude Test in Biotechnology (GAT-B) 2025, understanding the syllabus is your first step towards success. This national-level entrance exam, conducted annually by the Department of Biotechnology, Government of India, opens doors to postgraduate programs in Biotechnology and allied fields at top institutions. Here, we provide an in-depth overview of the GAT-B 2025 syllabus, with a particular focus on the GAT-B Biotechnology syllabus and key tips for preparation.
Overview of GAT-B 2025
The GAT-B exam evaluates candidates' proficiency in Biotechnology and related disciplines. It features multiple-choice questions designed to test both fundamental and advanced knowledge. Knowing the GAT-B 2025 syllabus is crucial to structuring your preparation effectively.
Key Sections of GAT-B 2025 Syllabus
The GAT-B 2025 syllabus can be broadly divided into two sections:
General Aptitude and Analytical Ability
Logical reasoning
Numerical ability
Data interpretation
Verbal reasoning and comprehension
Subject-Specific Knowledge
This section focuses on core topics related to Biotechnology and allied fields. Below is a detailed breakdown of the GAT-B Biotechnology syllabus:
GAT-B Biotechnology Syllabus
Molecular Biology and Genetics
Structure and function of nucleic acids (DNA and RNA)
Gene expression and regulation
Mendelian genetics and genetic engineering
Techniques in molecular biology: PCR, gel electrophoresis, blotting methods
Cell Biology
Cell structure and organelles
Cell cycle and cell signaling
Membrane biology and transport mechanisms
Biochemistry
Biomolecules: Proteins, lipids, carbohydrates, nucleotides
Enzyme kinetics and regulation
Metabolic pathways and energy production
Immunology
Immune system components
Antigens, antibodies, and immune responses
Vaccines and immunological techniques
Microbiology
Microbial classification and diversity
Growth, reproduction, and control of microbes
Pathogenic microorganisms and their mechanisms
Bioinformatics
Sequence analysis and alignment
Genomics and proteomics
Computational tools for data analysis
Biotechnology Applications
Industrial biotechnology
Environmental biotechnology
Agricultural biotechnology
Biostatistics
Probability and distributions
Hypothesis testing
Statistical tools for data interpretation
Importance of GAT-B 2025 Syllabus for Exam Preparation
Understanding the syllabus enables you to:
Identify high-priority topics.
Allocate time effectively for each subject.
Practice with topic-specific mock tests and previous year’s question papers.
Tips to Ace GAT-B 2025
Master the Fundamentals: Focus on building a strong foundation in core subjects like Molecular Biology, Biochemistry, and Cell Biology.
Time Management: Allocate study hours based on the weightage of topics in the GAT-B 2025 syllabus.
Use Reliable Resources: Refer to standard textbooks, online tutorials, and study material tailored for GAT-B.
Practice Regularly: Solve mock tests and previous year’s question papers to familiarize yourself with the exam pattern.
Join Online Coaching: Enroll in online classes for GAT-B preparation to get expert guidance and structured learning.
Upcoming GAT-B 2025 Exam Details
The official notification for GAT-B 2025 is expected to be released soon. Here are the tentative details:
Exam Date: March/April 2025
Application Start Date: January 2025
Mode of Exam: Online (Computer-Based Test)
Stay updated on the official website for exact dates and application details.
Final Thoughts
Success in the GAT-B 2025 exam begins with a thorough understanding of the GAT-B syllabus, particularly the GAT-B Biotechnology syllabus. With dedicated preparation, time management, and the right resources, you can secure a top rank and take a step closer to achieving your academic and professional goals.
Prepare smart, stay consistent, and make the most of your preparation journey!
0 notes
Text
Human Metapneumovirus (HMPV)
Human Metapneumovirus (HMPV) is a respiratory virus that has recently garnered attention due to a surge in cases, particularly in East Asia. Despite its increased prevalence, health experts emphasize that HMPV is a known virus and does not pose an unusual threat.
What is HMPV?
HMPV is a virus that typically causes respiratory symptoms similar to the common cold, including cough, fever, runny or stuffy nose, sore throat, wheezing and shortness of breath. Most individuals experience mild illness, but young children, older adults and those with weakened immune systems may develop more severe conditions such as bronchiolitis or pneumonia.

How Does HMPV Spread?
HMPV spreads through close contact with infected individuals, primarily via respiratory droplets released during coughing or sneezing. The virus can also survive on surfaces for short periods, making hand hygiene crucial in preventing transmission.
HMPV Symptoms
The symptoms of HMPV are often similar to those of the common cold and may include:
Cough
Fever
Runny or stuffy nose
Sore throat
Wheezing
Shortness of breath
Rash
These symptoms typically appear 3 to 6 days after exposure and usually last about 2 to 5 days. In most cases, individuals recover without complications.
When to Worry About HMPV
While HMPV generally causes mild illness, certain groups should be more cautious:
Infants and young children: They are at higher risk for severe respiratory issues like bronchiolitis and pneumonia.
Older adults: Especially those over 65, who may experience more severe symptoms.
Individuals with weakened immune systems: They are more susceptible to complications.
If symptoms worsen or persist beyond a week, or if there is difficulty breathing, it's important to seek medical attention promptly.
Diagnostic Techniques for HMPV
Diagnosing HMPV involves several methods:
Real-time Polymerase Chain Reaction (RT-qPCR): This sensitive test detects viral RNA and is considered the gold standard for HMPV diagnosis.
Reverse Transcription Polymerase Chain Reaction (RT-PCR): Used for detecting HMPV-specific cDNA, though less sensitive than RT-qPCR.
Loop-Mediated Isothermal Amplification (LAMP): An isothermal method that allows for rapid detection without the need for complex equipment.
In some cases, chest X-rays or bronchoscopy may be utilized to assess the extent of respiratory involvement.

Preventing HMPV Infection
Preventing the spread of HMPV involves several key measures:
Hand hygiene: Regularly wash hands with soap and water for at least 20 seconds.
Avoid close contact: Stay away from individuals who are sick and if you're ill, limit contact with others.
Disinfect surfaces: Clean and disinfect frequently touched objects and surfaces.
Respiratory etiquette: Cover your mouth and nose with a tissue or your elbow when coughing or sneezing.
Currently, there is no vaccine for HMPV, making these preventive measures essential.
Protecting Your Family from HMPV
To safeguard your family:
Educate: Ensure all family members understand the importance of hygiene practices.
Monitor health: Keep an eye on symptoms, especially in vulnerable individuals like young children and the elderly.
Seek medical advice: Consult healthcare providers if symptoms escalate or if there's a concern about exposure to HMPV.
By staying informed and adhering to preventive strategies, you can effectively protect your family from HMPV and contribute to reducing its spread within the community.
0 notes
Text
Accumax Screw Cap Tubes: Your Partner in Safe and Reliable Sample Storage
In the ever-evolving world of lab research and testing, Accumax has launched Screw Cap Tubes. These well-made tubes meet the many needs of the lab setting new bars for reliability, safety, and how well they work. Let’s look closer at why these tubes are a must-have for your lab.
Why Pick Accumax Screw Cap Tubes?
Accumax Screw Cap Tubes give you the best of new design and top-notch use. Made from medical-grade polypropylene (USP Class VI), these tubes make sure you get the highest levels of safety and performance making them perfect to handle and store important samples.
Features That Show Excellence
1. Strong Design and Build
Made to hold up in the toughest lab conditions, these tubes are built to last:
These tubes can withstand centrifugal forces up to 20,000g
They stay stable during freeze-thaw cycles from -80°C to room temperature without losing their structural integrity.
You can autoclave them
They’re Gamma Sterile to a SAL of 10⁻⁶
Their design includes a knurled surface to give you a firm grip
These tubes deliver top-notch results, whether you’re spinning samples at high speeds or storing them for a long time.
2. Superior Protection Against Contamination
Made in a Class 100K clean room, these tubes have no risk of contamination. The production doesn’t involve any secondary assembly or any manual handling in between for O ring infusion in caps, which ensures a clean environment. These tubes have been certified as RNase-free, DNase-free, Endotoxin-free, Human DNA-free, and PCR Inhibitor-free. Tests also check for leachable and extractable substances.
3. Advanced Sealability with Infused O-Ring
Regular O-ring designs often struggle to create a perfect seal. However, Accumax screw cap tubes made from USP Class VI US FDA approved material, come with an infused O-ring. This design:
Involves seamless molding during production, which lowers the risk of contamination due to minimal manual handling.
Offers better sealing and leak protection than standard O-ring designs that often become loose with extended use.
Stops your valuable reagents from evaporating.
4. Flexible Choices for All Uses
These tubes come in 0.5 ml, 1.5 ml, and 2 ml sizes with different colored cap options suitable for many lab uses. Pick from:
Conical bottom to collect and separate samples.
Skirted (self-standing) bottom for easy and stable storage.
Low Retention Choices are also on offer.
Uses of Screw Cap Tubes
The flexibility of Accumax screw cap tubes makes them a good pick for many lab uses:
1. To store enzymes and buffers
These tubes have a sealed design and can handle big temperature changes making them great to store delicate chemicals.
The flexibility of Accumax screw cap tubes makes them a good pick for many lab uses:
2. To store enzymes and buffers
These tubes have a sealed design and can handle big temperature changes making them great to store delicate chemicals.
High-Speed Centrifugation
These tubes can take spinning forces up to 20,000 g so they’re perfect for tough separation and cleaning processes.
3. Long-Term Sample Storage
They’re super strong, made in a clean way, and certified to be free from RNase, DNase, Endotoxin, Human DNA, and PCR Inhibitors. This means they’re just right to keep biological samples, DNA/RNA extracts, and other things safe.
4. Sample Transport
Tight-fitting, leak-free caps make sure valuable samples travel between labs and facilities. Accumax screw cap tubes undergo thorough leak tests that meet IATA rules to ensure they’re dependable during shipping.

Plastic Tubes vs. Glass Tubes: Why Plastic is the Better Option
When comparing plastic and glass tubes plastic tubes—such as Accumax Screw Cap Tubes—have several important benefits:
1. Toughness and Ability to Withstand Impacts
Plastic screw cap tubes last much longer than glass tubes and are less prone to breaking even when hit hard. This helps a lot in busy labs where people might drop things by accident. Because plastic resists impacts so well, it lowers the chance of expensive and dangerous breaks keeping important samples safe.
2. Light and Easy to Handle
Plastic screw cap tubes weigh less than glass, which makes them simple to handle, move, and keep. This matters a lot when you’re working with many samples or moving them between different places. The light weight of plastic also helps cut down on tiredness during long lab work.
3. Cheap and Throwaway
Plastic screw cap tubes cost less than glass ones. You can throw them away after using them, which means you don’t need to clean and sterilize them like you do with glass tubes. This saves time and cuts down on running costs, making plastic tubes cheaper to use over time.
Why Accumax?
Accumax has a solid history of coming up with new ideas that work, which keeps changing how labs operate worldwide. Our Screw Cap Tubes show how much we care about being precise, safe, and making things that customers want. These tubes from Accumax can change how your lab gets things done. Check out our website to find out more about these tubes that are changing the game, and put them in your order. When you use Accumax, you’ll see how well new ideas and dependability go together!
Read More:- Accumax Screw Cap Tubes: Your Partner in Safe and Reliable Sample Storage.
0 notes
Text
https://drsafehands.com/hiv-profile
DrSafeHands offers HIV 1 & 2 tests, HIV NAT tests, HIV RNA PCR tests, and HIV PCR tests.Testing at DrSafehands is highly affordable as our HIV tests are reasonably priced . affordable as our HIV tests are reasonably priced .
#Book HIV Profile Tests with counseling | DrSafeHands#HIV Profile Tests#HIV Tests#HIV 1 & 2 tests#HIV 1#HIV Testing#HIV RNA PCR tests
0 notes
Text
Next-Generation Diagnostics: Unveiling 3B BlackBio Biotech's RSV, Flu Panel, and EBV PCR Tests

In the field of healthcare, precise and timely diagnostics are pivotal in ensuring effective treatment. Recognizing this need, 3B BlackBio Biotech has developed advanced molecular diagnostic tools to meet the growing demand for accurate disease detection. Among our premier offerings are the RSV PCR Test, Flu Panel PCR Test, and EBV PCR Test, each meticulously crafted to enhance diagnostic accuracy and streamline patient care.
RSV PCR Test: A Breakthrough in Respiratory Diagnostics
Respiratory Syncytial Virus (RSV) remains a significant cause of respiratory illnesses, particularly in infants, older adults, and immunocompromised individuals. Early and precise detection is crucial to managing the disease and mitigating severe complications. The RSV PCR Test employs cutting-edge molecular techniques to identify RSV RNA with unparalleled accuracy.
This test offers rapid and reliable results, enabling clinicians to take timely actions that can prevent the escalation of symptoms. By streamlining the diagnostic process, the RSV PCR Test reduces the burden on healthcare systems and ensures better outcomes for patients during RSV outbreaks.
Flu Panel PCR Test: Comprehensive Solutions for Seasonal Illnesses
Seasonal flu can often involve multiple viral agents, making it challenging for healthcare providers to pinpoint the exact cause. The Flu Panel PCR Test addresses this issue with its ability to detect Influenza A, Influenza B, and RSV in a single assay. This multiplex diagnostic tool not only saves time but also increases diagnostic efficiency by providing precise results in one go.
By utilizing the Flu Panel PCR Test, clinicians can:
Identify the specific viral agent causing the illness.
Provide targeted treatments, reducing unnecessary medication use.
Minimize the spread of infections through prompt intervention.
This test is an essential asset for laboratories and healthcare providers during flu seasons, ensuring swift and accurate disease management.
EBV PCR Test: Precision in Epstein-Barr Virus Detection
The Epstein-Barr Virus (EBV) is linked to a range of medical conditions, from infectious mononucleosis to serious malignancies like Hodgkin's lymphoma. Accurate detection and monitoring are vital for effective disease management. The EBV PCR Test offers high sensitivity and specificity, making it a reliable choice for clinicians.
This test facilitates the detection and quantification of EBV DNA, enabling:
Monitoring of viral load in patients with chronic conditions.
Early detection of reactivations or primary infections.
Improved treatment strategies tailored to the patient’s needs.
The EBV PCR Test is a cornerstone in the diagnosis and management of EBV-related diseases, offering clinicians the tools they need to provide optimal care.
3B BlackBio Biotech: Setting New Standards in Diagnostics
At 3B BlackBio Biotech, innovation and quality are at the heart of our mission. Our RSV PCR Test, Flu Panel PCR Test, and EBV PCR Test are designed to empower healthcare professionals with reliable diagnostic solutions that prioritize patient care. Through rigorous research and development, we aim to redefine molecular diagnostics, ensuring that clinicians have access to the best tools available.
For more information about our diagnostic kits, visit at https://3bblackbio.com/. Choose 3B BlackBio Biotech for diagnostics that deliver precision, efficiency, and confidence in every result.
0 notes
Text

#Genes2Me's Blood #DNA Purification Kits allow #rapid and efficient #purification of genomic DNA from small volumes of human blood. #Kits are available in both #spin-column and #magnetic bead based formats. The purified genomic DNA is suitable for use in downstream applications including #PCR, restriction #enzyme digestion, and Southern blotting.
Visit our #website for more information. https://www.genes2me.com/nucleic-acid-extraction-solutions
For more details, Call us at +91-8800821778 or drop us an email at [email protected]
#forensic #rna #blood #hair #extraction #nucleicacid #ivd #madeinindia #molecular #diagnostics #g2m #diseases #moleculardiagnostics #moleculardiagnosticslab #risk #testing #testingsolutions
#genes2me#g2m#dna#rna#pcr#enzyme#magnetic#kits#purification#rapid#extraction#genetic testing#diseases#made in india#molecular#testing#risk
0 notes
Text
Reference archived on our website
Published in 2023. Proof positive that just breathing spreads covid over a large area. Mask up. Ventilate. Clean the air.
Summary Background Effectively implementing strategies to curb SARS-CoV-2 transmission requires understanding who is contagious and when. Although viral load on upper respiratory swabs has commonly been used to infer contagiousness, measuring viral emissions might be more accurate to indicate the chance of onward transmission and identify likely routes. We aimed to correlate viral emissions, viral load in the upper respiratory tract, and symptoms, longitudinally, in participants who were experimentally infected with SARS-CoV-2.
Methods In this phase 1, open label, first-in-human SARS-CoV-2 experimental infection study at quarantine unit at the Royal Free London NHS Foundation Trust, London, UK, healthy adults aged 18–30 years who were unvaccinated for SARS-CoV-2, not previously known to have been infected with SARS-CoV-2, and seronegative at screening were recruited. Participants were inoculated with 10 50% tissue culture infectious dose of pre-alpha wild-type SARS-CoV-2 (Asp614Gly) by intranasal drops and remained in individual negative pressure rooms for a minimum of 14 days. Nose and throat swabs were collected daily. Emissions were collected daily from the air (using a Coriolis μ air sampler and directly into facemasks) and the surrounding environment (via surface and hand swabs). All samples were collected by researchers, and tested by using PCR, plaque assay, or lateral flow antigen test. Symptom scores were collected using self-reported symptom diaries three times daily. The study is registered with ClinicalTrials.gov, NCT04865237.
Findings Between March 6 and July 8, 2021, 36 participants (ten female and 26 male) were recruited and 18 (53%) of 34 participants became infected, resulting in protracted high viral loads in the nose and throat following a short incubation period, with mild-to-moderate symptoms. Two participants were excluded from the per-protocol analysis owing to seroconversion between screening and inoculation, identified post hoc. Viral RNA was detected in 63 (25%) of 252 Coriolis air samples from 16 participants, 109 (43%) of 252 mask samples from 17 participants, 67 (27%) of 252 hand swabs from 16 participants, and 371 (29%) of 1260 surface swabs from 18 participants. Viable SARS-CoV-2 was collected from breath captured in 16 masks and from 13 surfaces, including four small frequently touched surfaces and nine larger surfaces where airborne virus could deposit. Viral emissions correlated more strongly with viral load in nasal swabs than throat swabs. Two individuals emitted 86% of airborne virus, and the majority of airborne virus collected was released on 3 days. Individuals who reported the highest total symptom scores were not those who emitted most virus. Very few emissions occurred before the first reported symptom (7%) and hardly any before the first positive lateral flow antigen test (2%).
Interpretation After controlled experimental inoculation, the timing, extent, and routes of viral emissions was heterogeneous. We observed that a minority of participants were high airborne virus emitters, giving support to the notion of superspreading individuals or events. Our data implicates the nose as the most important source of emissions. Frequent self-testing coupled with isolation upon awareness of first symptoms could reduce onward transmissions.
#mask up#covid#pandemic#covid 19#wear a mask#public health#coronavirus#sars cov 2#still coviding#wear a respirator
47 notes
·
View notes
Text
Schweineinfluenza
!ZOONOSE!
Erreger: Influenza A Virus; RNA-Virus, Orthomyxoviridae Verschiedene Subtypen H1N1: Aviärer Typ, "Schweinegrippe" H3N2: Humaner Typ H1N2: Aviärer/humaner Typ Kreuzimmunität zw. Subtypen sehr schwach -> Schwein kann in kurzer Zeit mehrmals an Influenza erkranken
Schwein ist perfektes "mixing vessel" (zur Rekombination v. Influenzaviren), weil auf Schweinerespirationstrakt Rezeptoren f. menschliche u. aviäre Influenza A Viren sind
Übertragung:
aerogen
oronasal (Tröpfcheninfektion)
direkter Kontakt
!Tierarzt sollte sich Impfen um Eintrag zu verhindern!
Pathogenese:
Absorbtion an Zilien u. Membranen d. Respirationstraktes
Vermehrung in Epithel v. nasaler Mukosa, Tonsillen, Trachea, Bronchien, Bronchioli u. Alveolen
Maximale Virusmenge nach 24 Std., Replikation für 6-7 Tage
Symptome: gehäuftes Auftreten in Frühjahr u. Herbst betrifft alle Altersgruppen Verschlimmerung d. Symptome d. Sekundärerreger (meistens kommt Influenza gemeinsam mit PRRSV u. Mycoplasma hyopneumoniae vor)
bei Neuinfektion:
explosionsartiger Ausbruch (bis 100% betroffen), Mortalität aber gering
hohes Fieber
trockener Husten
Allgemeinverhalten vermindert (Apathie, vermehrt Liegen, hundesitzige Stellung)
Inappetenz
Anorexie
Nasenausfluss
Konjunktivitis
Aborte (wg. Fieber)
Endemischer Verlauf:
meist subklinisch
bei pandemischen Subtypen Symptome oft unspezifisch
Diagnose
Sektion:
Interstitielle Pneumonie Bronchiolitis -> später nekrotisierende Bronchitis
Direkter Erregernachweis:
mittels PCR aus Nasen-/Tonsillentupfer od. Lungengewebe !nur max. 5 Tage p.i. (=Akutphase) möglich!
Antikörpernachweis:
mittels Hämagglutinations-Inhibitions-Test paarige Beprobung (1. in Akutphase, 2. 3-4 Wo. später) -> 4-facher Titeranstieg ist beweisend mittels ELISA (nach 14-28 Tagen möglich)
Prophylaxe
Stallklima optimieren (Lüftungsanpassung an aktuelle Witterung)
Bekämpfung v. Sekundärerregern
Zukauf aus möglichst wenigen verschiedenen Betrieben
Abschirmung gegen Kontakt mit Vögeln
Rein-Raus-Verfahren
korrekte Reingung u. Desinfektion
Stress minimieren (Belegdichte, Transport, Fressplatzangebot)
Impfen: trivalent (H1N1, H3N2,H1N2) od. monovalent (H1N1) ab 56. Lebenstag Jungsauengrundimmunisierung (2x Abstand 2-4 Wo.) + 2-3x jährlich Auffrischen (am besten in Säugezeit)
Kolostrumaufnahme optimieren
korrekte Jungsaueneingliederung
Therapie
Symptomatisch
NSAIDs
Wasser- u. Wärmeversorgung sicherstellen
Antibiose gegen Sekundärerreger
0 notes