#Integrase Enzyme
Explore tagged Tumblr posts
Text
Introducing the top ten stories they chose not to tell you this week.
The Vigilant Fox
Dec 22, 2024
#10 - Drug regulators BUSTED hiding COVID jab risks.
Internal emails reveal that the TGA (Australia’s FDA) KNEW that, yes, foreign DNA from the COVID shots really could integrate into the human genome—despite repeatedly assuring the public it was impossible.
Rebekkah Barnett dropped the bombshell report exposing what TGA staff were saying behind closed doors—and it’s nothing like the rosy picture they painted in public.
In one email, a TGA staffer debunked Dr. Paul Offit’s claims that DNA from the COVID shots couldn’t integrate into the human genome without an enzyme called integrase. The staffer wrote:
“Foreign DNA can integrate into chromosomal DNA in the absence of an integrase in mammalian cells. This comes from the DNA damage/repair literature where breaks in DNA are repaired through processes called non-homologous end joining or homologous recombination.”
This directly contradicted the TGA’s official narrative, which repeatedly denied that such genomic integration was even possible.
Another email revealed that the TGA wasn’t even aware of studies to back up their public denials. A senior staffer admitted:
“I would be uncomfortable with that [statement about plasmid DNA entering the human genome] as I am unaware of studies which have tested this.”
So, behind closed doors, they had serious, unanswered questions about DNA contamination and the potential for genomic integration. But to the public, they said, “Everything is fine.”
This isn’t how things are supposed to work. Regulators like the TGA are meant to protect people, not withhold critical information.
This is yet another shocking example of how the so-called “experts” prioritized controlling the narrative over presenting the facts.
Adding to the alarm, new research from Yale has found spike protein lingering in the blood years after individuals received their last COVID shot, making concerns about integration with human DNA even more plausible.
Senator Gerard Rennick from Australia joins the show to discuss these damning revelations.
Join 100K+ Substack readers and 1.4 million 𝕏 users who follow the work of Vigilant Fox. Subscribe to Vigilant News for exclusive stories you won’t find anywhere else.Subscribe
(See 9 More Revealing Stories Below)
#9 - Joe Biden Targets Pelosi and Coup Leaders in Stunning Act of Revenge
#8 - Michael Cohen Turns Heads on CNN: Trump Is RIGHT About Media Lies
#7 - CNN Reveals "Troubling" Poll, Showing American Trust In Vaccines Is Plummeting
#6 - The Wall Street Journal drops a bombshell report, exposing Biden’s mental decline from the very START of his presidency.
While you’re here, don’t forget to subscribe to this page for more weekly news roundups.Subscribe
#5 - Pennsylvania Woman Charged with Registering Dead and Non-Existent Voters
#4 - Pfizer mRNA ‘Vaccinated’ Children Significantly More Likely to Get COVID-19 Than Unvaccinated Peers – New Study
#3 - "Stop Squinting at me!" Tim Pool BLASTS Liberal Guest on January 6 LIES
#2 - New Study Finds Hydroxychloroquine Safe with No Evidence of Cardiac Complications
#1 - Biden and Harris Rush Back to White House, prompting speculation that something big is coming.
Share
BONUS #1 - BUSTED: ‘The View’ Co-Host May Face Criminal Investigation
BONUS #2 - The Meat Upgrade You Didn’t Know You Needed
BONUS #3 - Will Most Pregnant Women and Babies Who Get Bird Flu Die?
BONUS #4 - How to Get Ivermectin, Z-Pak and More
BONUS #5 - Outrageous: Homeowner Ends Up in Jail After Calling Police to Evict Squatter From Her Own Home
11 notes
·
View notes
Text
SOHM, Inc., a globally recognized pharmaceutical company, has secured a pivotal patent from the Japan Patent Office (JPO) for its innovative ABBIE (A Base Binding Integrase Enzyme) genome editing technology. This advancement is poised to significantly impact India's biotechnology landscape, offering unprecedented precision and safety in gene editing. The ABBIE technology holds promise for transforming treatment approaches for various genetic disorders prevalent in India, including sickle cell anemia, thalassemia, and certain genetic cancers. By enabling more accurate and efficient gene modifications, this technology could lead to groundbreaking therapies, improving the lives of millions affected by these conditions.
One Health Assist is committed to supporting such initiatives by providing resources and assistance to facilitate access to advanced healthcare services for vulnerable populations.
For more details, please visit the full article.
0 notes
Text
Dolutegravir Prices | Pricing | Trend | News | Database | Chart | Forecast
Dolutegravir is an antiretroviral medication primarily used in the treatment of HIV/AIDS. As part of the class of drugs known as integrase inhibitors, Dolutegravir works by blocking the action of an enzyme called integrase, which the HIV virus uses to replicate. Since its approval by the U.S. Food and Drug Administration (FDA) in 2013, Dolutegravir has become a crucial component in many HIV treatment regimens. However, one of the most significant concerns around the world, especially in low-income and middle-income countries, has been the pricing of Dolutegravir. Pricing influences access to this essential medication, and efforts are ongoing to make it more affordable for those who need it most.
The cost of Dolutegravir varies widely depending on geographic location, patent laws, and whether the drug is branded or generic. In high-income countries such as the United States, Dolutegravir is often sold under the brand name Tivicay, manufactured by ViiV Healthcare. The brand-name version of Dolutegravir tends to be expensive, with prices in the U.S. being notably higher than in other regions. For example, a month's supply of Tivicay can cost thousands of dollars. This high cost is driven by research and development expenses, regulatory costs, and the need to recoup the investment in bringing a new drug to market. However, the high cost in wealthier nations often makes it difficult for those without adequate health insurance or government assistance to access the medication.
Get Real Time Prices for Dolutegravir : https://www.chemanalyst.com/Pricing-data/dolutegravir-1534
In contrast, many low- and middle-income countries rely on generic versions of Dolutegravir, which are significantly less expensive. Generic Dolutegravir became available after licensing agreements and patent waivers were negotiated between pharmaceutical companies, non-governmental organizations, and international agencies. These agreements allowed for the production of more affordable versions of the drug, making it accessible to larger populations. In countries such as India, which has a robust generic pharmaceutical industry, the price of Dolutegravir can be as low as a few dollars per month. This drastic reduction in price has been crucial in improving access to HIV treatment, particularly in regions that are heavily burdened by the epidemic, such as sub-Saharan Africa.
Global health organizations like the World Health Organization (WHO) and the Joint United Nations Programme on HIV/AIDS (UNAIDS) have played a pivotal role in advocating for lower prices for HIV medications, including Dolutegravir. The Medicines Patent Pool (MPP) is another initiative that has helped to lower the cost of Dolutegravir. By facilitating voluntary licensing agreements, the MPP enables generic drug manufacturers to produce and sell Dolutegravir at a fraction of the price of the branded version. These efforts have resulted in broader availability of the drug, but challenges remain in ensuring that everyone who needs Dolutegravir can afford it.
The introduction of Dolutegravir as part of first-line HIV treatment regimens in many countries has been a game-changer. Not only is Dolutegravir highly effective in suppressing the HIV virus, but it also has a high barrier to resistance, meaning that patients are less likely to develop resistance to the drug over time. Moreover, Dolutegravir has fewer side effects compared to older antiretroviral medications, making it a preferred option for many patients and healthcare providers. Given its effectiveness, there has been a global push to make Dolutegravir the standard of care in HIV treatment. However, pricing remains a barrier in some parts of the world, where even the generic versions may be out of reach for the most vulnerable populations.
Efforts to reduce the price of Dolutegravir are ongoing, with various international partnerships and coalitions working to negotiate lower costs. For example, the Global Fund to Fight AIDS, Tuberculosis and Malaria has been instrumental in securing lower prices for Dolutegravir through bulk purchasing agreements. These agreements allow for large quantities of the drug to be purchased at a reduced cost, which can then be distributed to countries in need. Additionally, some countries have implemented pricing controls or subsidies to make Dolutegravir more affordable for their populations. These strategies have been successful to varying degrees, depending on the political and economic landscape of each country.
Another factor influencing the price of Dolutegravir is the expiration of patents. As patents on the drug begin to expire in more countries, the opportunity for increased competition among generic manufacturers grows. This competition is expected to drive prices down even further, making Dolutegravir more accessible to a wider range of people. However, patent expirations are staggered across different regions, meaning that some countries may experience price reductions sooner than others. In countries where the patent is still in effect, advocacy efforts are focused on encouraging pharmaceutical companies to voluntarily lower their prices or enter into more licensing agreements that would allow for the production of generics.
In recent years, there has been a growing emphasis on the importance of affordable HIV treatment as part of global health initiatives. The United Nations has set ambitious goals for reducing the number of new HIV infections and ensuring that those living with HIV have access to treatment. Dolutegravir is central to these efforts, but its price remains a critical issue. Without continued pressure on pharmaceutical companies and governments, there is a risk that some populations will continue to be left behind in the fight against HIV/AIDS.
In conclusion, the pricing of Dolutegravir reflects a complex interplay of factors, including patent laws, production costs, and global health policies. While progress has been made in making the drug more affordable, particularly in low- and middle-income countries, there is still work to be done to ensure that everyone who needs Dolutegravir can access it. The future of HIV treatment depends not only on the development of new and effective medications but also on the ability to make these treatments affordable and accessible to all. Through continued international cooperation and advocacy, it is possible to further reduce the price of Dolutegravir and ensure that it reaches those who need it most.
Get Real Time Prices for Dolutegravir : https://www.chemanalyst.com/Pricing-data/dolutegravir-1534
Contact Us:
ChemAnalyst
GmbH - S-01, 2.floor, Subbelrather Straße,
15a Cologne, 50823, Germany
Call: +49-221-6505-8833
Email: [email protected]
Website: https://www.chemanalyst.com
#Dolutegravir#Dolutegravir Price#Dolutegravir Prices#Dolutegravir News#Dolutegravir Market#Dolutegravir Pricing
0 notes
Text
Advances in HIV Treatment

Despite advances in treatment, HIV still poses complex challenges in cure research and epidemic control. This retrovirus embeds itself into host DNA, enabling viral persistence and lifelong infection. Complete eradication remains unlikely with current tools, but recent research suggests new advances in treatment.
A notable advance is with antiretroviral therapy, which suppresses the virus, controlling or stopping the disease’s progression. Where early regimens once necessitated up to two dozen daily doses, novel pharmaceutical formulations now provide an option for less frequent administrations, with augmented immune system support and fewer side effects.
Advances in 2021 brought an effective new option for long-acting HIV management. Researchers created Cabenuva, a combination therapy that combines two drugs. Dosed monthly or bi-monthly, Cabenuva pairs cabotegravir, an inhibitor for the integrase enzyme enabling viral replication, and rilpivirine, which inhibits the enzyme, allowing the virus to copy its genetic material. Cabenuva’s infrequent dosing schedule significantly improves adherence for those struggling to maintain strict medicine dosing routines.
In 2022, research led scientists to develop another injectable medication, lenacapavir, the first in a new class of capsid inhibitors. Unlike existing antiretrovirals that target HIV’s genetic material or enzymes, lenacapavir disrupts the protective casing that encloses the virus’s genes, rendering it unable to replicate and infect host cells. Best of all, its long-lasting activity could significantly decrease the treatment burden on patients and providers alike, as trials thus far indicate biannual dosing may be sufficient to sustain viral suppression.
0 notes
Text
THE DOLUTEGRAVIR OPPORTUNITY
DTG is a relatively new ARV available in generic form in low- and middle-income countries. It is an integrase inhibitor that blocks an HIV enzyme (a protein that starts or increases the speed of a chemical reaction) called integrase. By blocking integrase, integrase inhibitors prevent HIV from multiplying and can reduce the amount of HIV in the body. As a single dose, in conjunction with other drugs to form a regimen, 50mg DTG single has been on the market in developing countries for a couple of years. However, its relatively high price and unavailability in a triple fixed-dose form limited significant uptake. Botswana, however, was an early adopter of the DTG single through a special initiative of the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR).
In August 2017, the US Food and Drug Administration (FDA) provided tentative approval for two Indian generic ARV suppliers – Aurobindo Pharma and Mylan Laboratories Limited – for the fixed-dose combination of tenofovir 300mg /lamivudine 300mg /dolutegravir 50mg (TLD), reducing one barrier for adoption in developing countries.
0 notes
Text
Global Integrase Inhibitors Market Is Estimated To Witness High Growth Owing To Escalating HIV/AIDS Prevalence
The global Integrase Inhibitors Market is estimated to be valued at US$ 29.34 billion in 2023 and is expected to exhibit a CAGR of 3.9% over the forecast period of 2023 to 2030, as highlighted in a new report published by Coherent Market Insights. Market Overview: Integrase inhibitors are a class of antiretroviral drugs used for the treatment of HIV/AIDS. They work by blocking the action of the integrase enzyme, which is responsible for inserting the viral genetic material into the DNA of the host cell. This prevents the replication of the virus and helps in controlling the progression of HIV infection. The need for effective anti-HIV drugs has become crucial due to the rising prevalence of HIV/AIDS worldwide. According to the World Health Organization (WHO), approximately 38 million people were living with HIV/AIDS in 2019. This high prevalence has led to increased demand for integrase inhibitors, as they offer several advantages over other antiretroviral drugs, such as improved efficacy, better tolerability, lower toxicity, and ease of administration. Market Key Trends: One key trend driving the growth of the global integrase inhibitors market is the development of novel and more potent drugs. Pharmaceutical companies are constantly investing in research and development activities to discover and develop new integrase inhibitors with improved therapeutic outcomes. For instance, Gilead Sciences recently received approval from the U.S. Food and Drug Administration (FDA) for a new integrase inhibitor called Biktarvy. This drug is a combination of three active ingredients and offers high efficacy with a low risk of drug resistance. PEST Analysis: Political: The political environment plays a crucial role in shaping the regulations and policies related to HIV/AIDS prevention and treatment. Governments across the globe are increasingly focusing on combating the HIV/AIDS epidemic by implementing awareness campaigns, providing access to affordable healthcare services, and ensuring the availability of antiretroviral drugs, including integrase inhibitors. Economic: The economic factors influencing the integrase inhibitors market include healthcare expenditure, affordability, and reimbursement policies. The high cost of integrase inhibitors can pose a challenge in low-income countries, where access to these drugs is limited due to financial constraints. However, government initiatives and collaborations with pharmaceutical companies are aimed at reducing the cost of these drugs and making them more accessible to all. Social: Social factors such as awareness, stigma, and healthcare infrastructure influence the adoption of integrase inhibitors. Increasing awareness about HIV/AIDS and the importance of early diagnosis and treatment is driving the demand for integrase inhibitors. Efforts are being made to reduce the stigma associated with HIV/AIDS, which can affect patients' willingness to seek treatment and adhere to medication regimens. Technological: Technological advancements in drug delivery systems and diagnostics have improved the efficacy and accessibility of integrase inhibitors. The development of long-acting formulations and point-of-care testing methods has facilitated better disease management and monitoring. Furthermore, advancements in genetic engineering techniques have enabled the discovery and development of more potent integrase inhibitors. Key Takeaways: - The Global Integrase Inhibitors Market Size is expected to witness high growth, exhibiting a CAGR of 3.9% over the forecast period. This growth can be attributed to increasing HIV/AIDS prevalence globally and the demand for effective antiretroviral drugs. - Regionally, North America is expected to dominate the market due to a well-established healthcare infrastructure, high healthcare expenditure, and favorable reimbursement policies.
0 notes
Text
Gene Editing via Integrase Enzyme by Umair Masood* in Open Access Journal of Biogeneric Science and Research

Short Communication
Targeted integrase enzyme is a most powerful tool for mediating genome alteration with high precision. The gene of the interest is directly catalyze nucleophilic attack of the 3 prime hydroxyl group at the end of processed DNA on a pair of phosphodiaster bond in the targeted DNA or genome of the interest. Integrase gene editing method contain two parts gene of the interest with integrase enzyme and desire genome. The integrase enzyme is start integration the gene of the interest into the desire genome or DNA.
Results via Gel electrophoresis
In order to check that whether gene of interest is integrated or not we can perform a gel electrophoresis. The gel contain a two band A and B.A band is a vector of human insulin vector which can show the negative control and B band is a vector of human insulin but the gene have some sequence mutation [1-5]. We can add a gene of the stranded human insulin by using integrase enzyme and gene can be precisely add to the B band that why B band is in 3.0 and A band is 6.0 which mean that A band is high molecular weight than the B band and a Marker must be 1kb and agarose should be 1% [6-9].
More information regarding this Article visit: OAJBGSR
https://biogenericpublishers.com/pdf/JBGSR.MS.ID.00249.pdf https://biogenericpublishers.com/jbgsr-ms-id-00249-text/
0 notes
Text

peaceful morning ☀️ 10.14.21
———————————-
3 ways transposons can move:
1. Cut & paste mechanism
2. Replicative transposition (maintains original transposon site, copy & paste mechanism)
3. Retrotransposon integration (using integrase enzyme among other cell components after reverse transcriptase creates dsDNA copy)
41 notes
·
View notes
Text
HIV(AIDS)
HIV stands for human immunodeficiency virus. It is a virus that attacks cells that help the body fight infection, specifically the white blood cells called CD4 cells. HIV destroys these CD4 cells (T cells), making a person more vulnerable to other infections and diseases such as tuberculosis and some cancers. It is spread by contact with certain bodily fluids of a person with HIV, most often spreads through unprotected sex (without a condom or HIV medicine to prevent or treat HIV) with a person who has HIV. It may also spread by sharing drug needles or through contact with the blood of a person who has HIV. Women can give it to their babies during pregnancy or childbirth. First identified in 1981, HIV is the cause of one of humanity’s deadliest and most persistent epidemics.
The human body can’t get rid of HIV and no effective HIV cure exists. So, once you have HIV, you have it for life. However, by taking HIV medicine (called antiretroviral therapy or ART), people with HIV can live long and healthy lives and prevent transmitting HIV to their sexual partners. In addition, there are effective methods to prevent getting HIV through sex or drug use, including pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP).
Without treatment, the infection might progress to an advanced disease stage where it reduces the number of CD4 cells (T cells) in the body, making the person more likely to get other infections or infection-related cancers. Over time, HIV can destroy so many of these cells that the body can’t fight off infections and disease, this stage is called AIDS (acquired immunodeficiency syndrome). However, modern advances in treatment mean that people living with HIV in countries with good access to healthcare very rarely develop AIDS once they are receiving treatment.
Causes:
Scientists identified a type of chimpanzee in Central Africa as the source of HIV infection in humans. Scientists suspect the simian immunodeficiency virus (SIV) jumped from chimps to humans when people consumed infected chimpanzee meat or when they came into contact with their infected blood. Studies show that HIV may have jumped from apes to humans as far back as the late 1800s. Over decades, the virus slowly spread across Africa over the course of several decades and later into other parts of the world. We know that the virus has existed in the United States since at least the mid to late 1970s.
To become infected with HIV, infected blood, semen or vaginal secretions must enter your body. This can happen by having sex, you may become infected if you have any kind of sex with an infected partner whose blood, semen or vaginal secretions enter your body. By sharing needles, sharing contaminated IV drug paraphernalia (needles and syringes) puts you at high risk of HIV and other infectious diseases, such as hepatitis. The risk of HIV transmitting through blood transfusions is extremely low in countries that have effective screening procedures in place for blood donations. Infected mothers can pass the virus on to their babies during pregnancy or delivery or through breast-feeding. Mothers who are HIV-positive and get treatment for the infection during pregnancy can significantly lower the risk to their babies.
How HIV doesn't spread
You can't become infected with HIV through ordinary contact. That means you can't catch HIV or AIDS by hugging, kissing, dancing or shaking hands with someone who has the infection. HIV doesn't spread through the air, water nor insect bites.
Symptoms:
After the first month or so, HIV enters the clinical latency stage. This stage can last from a few years to a few decades. Some people don’t have any symptoms during this time, while others may experience a flu-like illness within 2 to 4 weeks after infection (Stage 1 HIV infection). But some people may not feel sick during this stage. Flu-like symptoms include fever, chills, rash, night sweats, muscle aches, sore throat, fatigue, swollen lymph nodes, or mouth ulcers. Other symptoms may include dark splotches under the skin or inside the mouth, nose, or eyelids, sores, spots, or lesions of the mouth and tongue, genitals, or anus, bumps, lesions, or rashes of the skin, recurrent or chronic diarrhea, rapid weight loss, neurologic problems such as trouble concentrating, memory loss, and confusion, and anxiety and depression.
HIV symptoms at this stage may come and go, or they may progress rapidly. This progression can be slowed substantially with treatment. With the consistent use of this antiretroviral therapy, chronic HIV can last for decades and will likely not develop into AIDS, if treatment was started early enough.
For the most part, infections by other bacteria, viruses, fungi, or parasites cause the more severe symptoms of HIV. These conditions tend to progress further in people who live with HIV than in individuals with healthy immune systems. A correctly functioning immune system would protect the body against the more advanced effects of infections, and HIV disrupts this process.
As with the early stage, HIV is still infectious during this time even without symptoms and can be transmitted to another person. However, the only way to know for sure whether you have HIV is to get tested. If someone has these symptoms and thinks they may have been exposed to HIV, it’s important that they get tested.
For the most part, symptoms of HIV are similar in men and women. However, symptoms they experience overall may differ based on the different risks men and women face if they have HIV. Both men and women with HIV are at increased risk of sexually transmitted infections (STIs). However, women may be less likely than men to notice small spots or other changes to their genitals. In addition, women with HIV are at increased risk of recurrent vaginal yeast infections, other vaginal infections, including bacterial vaginosis, pelvic inflammatory disease (PID), menstrual cycle changes, and human papillomavirus (HPV), which can cause genital warts and lead to cervical cancer.
Diagnosis:
The only way to know for sure if you have HIV is to get tested. Knowing your status is important because it helps you make healthy decisions to prevent getting or transmitting HIV. Testing is relatively simple. You can ask your health care provider for an HIV test. Many medical clinics, substance abuse programs, community health centers, and hospitals offer them too. You can also buy a home testing kit at a pharmacy or online, however, such results should only be considered as a full diagnosis following review and confirmation by a qualified health worker.
Knowledge of one’s HIV-positive status has two important benefits. People who test positive can take steps to get treatment, care and support before symptoms appear, which can prolong life and prevent health complications for many years. And people who are aware of their status can take precautions to prevent the transmission of HIV to others.
HIV can be diagnosed through blood or saliva testing. Available tests include, antigen/antibody tests. These tests usually involve drawing blood from a vein. Antigens are substances on the HIV virus itself and are usually detectable in the blood within a few weeks after exposure to HIV. Antibody tests. These tests look for antibodies to HIV in blood or saliva. Most rapid HIV tests, including self-tests done at home, are antibody tests. Antibody tests can take three to 12 weeks after you're exposed to become positive. Nucleic acid tests (NATs). These tests look for the actual virus in your blood (viral load). They also involve blood drawn from a vein. If you might have been exposed to HIV within the past few weeks, your doctor may recommend NAT. NAT will be the first test to become positive after exposure to HIV. Talk to your doctor about which HIV test is right for you. If any of these tests are negative, you may still need a follow-up test, weeks to months later to confirm the results.
Treatment:
Currently, there's no cure for HIV/AIDS. Once you have the infection, your body can't get rid of it. However, there are many medications that can control HIV and prevent complications. These medications are called antiretroviral therapy (ART). Everyone diagnosed with HIV should be started on ART, regardless of their stage of infection or complications. A person living with HIV can reduce their viral load to such a degree that it is no longer detectable in a blood test. After assessing a number of large studies, the CDC concluded that individuals who have no detectable viral load “have effectively no risk of sexually transmitting the virus to an HIV-negative partner.” Medical professionals refer to this as undetectable = untransmittable (U=U). The person still has HIV, but the virus is not visible in test results. However, the virus is still in the body. And if that person stops taking antiretroviral therapy, the viral load will increase again and the HIV can again start attacking CD4 cells. People living with HIV generally take a combination of medications called highly active antiretroviral therapy (HAART) or combination antiretroviral therapy (cART). This approach has the best chance of lowering the amount of HIV in the blood. There are many ART options that combine three HIV medications into one pill, taken once daily.
There are a number of subgroups of antiretrovirals, such as:
- Protease inhibitors, protease is an enzyme that HIV needs to replicate. These medications bind to the enzyme and inhibit its action, preventing HIV from making copies of itself. These include:
o atazanavir/cobicistat (Evotaz)
o lopinavir/ritonavir (Kaletra)
o darunavir/cobicistat (Prezcobix)
- Integrase inhibitors: HIV needs integrase, another enzyme, to infect T cells. This drug blocks integrase. These are often the first line of treatment due to their effectiveness and limited side effects for many people.
o elvitegravir (Vitekta)
o dolutegravir (Tivicay)
o raltegravir (Isentress)
o Nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs)
- This class of drugs, also referred to as “nukes,” interfere with HIV as it tries to replicate:
o abacavir (Ziagen)
o lamivudine/zidovudine (Combivir)
o emtricitabine (Emtriva)
o tenofovir disproxil (Viread)
o Non-nucleoside reverse transcriptase inhibitors (NNRTIs) that work in a similar way to NRTIs, making it more difficult for HIV to replicate.
There are also emergency HIV pills, or post-exposure prophylaxis
If an individual believes they have been exposed to the virus within the last 3 days, anti-HIV medications, called post-exposure prophylaxis (PEP), may be able to stop infection. Take PEP as soon as possible after potential contact with the virus. PEP is a treatment lasting a total of 28 days, and physicians will continue to monitor for HIV after the completion of the treatment.
People will often use a combination of these drugs to suppress HIV. A medical team will adapt the exact mix of drugs to each individual. HIV treatment is usually permanent, lifelong, and based on routine dosage. A person living with HIV must take pills on a regular schedule. Each class of ARVs has different side effects, but possible common side effects include:
nausea
fatigue
diarrhea
headache
skin rashes
When people get HIV and don’t receive treatment, they will typically progress through three stages of disease. Treatment can slow or prevent progression from one stage to the next. Also, people with HIV who take HIV medicine as prescribed and get and keep an undetectable viral load have effectively no risk of transmitting HIV to an HIV-negative partner through sex.
Stage 1: Acute HIV infection
Within 2 to 4 weeks after infection with HIV, people may experience a flu-like illness, which may last for a few weeks. This is the body’s natural response to infection. When people have acute HIV infection, they have a large amount of virus in their blood and are very contagious. But people with acute infection are often unaware that they’re infected because they may not feel sick right away or at all. To know whether someone has acute infection, either an antigen/antibody test or a nucleic acid (NAT) test is necessary. If you think you have been exposed to HIV through sex or drug use and you have flu-like symptoms, seek medical care and ask for a test to diagnose acute infection.
Stage 2: Clinical latency (HIV inactivity or dormancy)
This period is sometimes called asymptomatic HIV infection or chronic HIV infection. During this phase, HIV is still active but reproduces at very low levels. People may not have any symptoms or get sick during this time. For people who aren’t taking medicine to treat HIV, this period can last a decade or longer, but some may progress through this phase faster. People who are taking medicine to treat HIV (ART) as prescribed may be in this stage for several decades. It’s important to remember that people can still transmit HIV to others during this phase. However, people who take HIV medicine as prescribed and get and keep an undetectable viral load (or stay virally suppressed) have effectively no risk of transmitting HIV to their HIV-negative sexual partners. At the end of this phase, a person’s viral load starts to go up and the CD4 cell count begins to go down. As this happens, the person may begin to have symptoms as the virus levels increase in the body, and the person moves into Stage 3.
Stage 3: Acquired immunodeficiency syndrome (AIDS)
AIDS is the most severe phase of HIV infection. People with AIDS have such badly damaged immune systems that they get an increasing number of severe illnesses, called opportunistic illnesses.
Source: x x x x x x
6 notes
·
View notes
Photo

Antiretroviral drugs (ARD) prevent HIV entry into the host cell and/or its replication.
Entry inhibitors are co-receptor antagonists block the co-receptor from binding with the HIV virion.
Fusion inhibitors prevent fusion of the HIV virion with the host cell membrane.
Reverse transcriptase inhibitors block the reverse transcription of RNA to dsDNA, aka, the provirus.
Integrase inhibitors prevent integration of the provirus with the host's genome.
Protease inhibitors prevent the synthesis of viral enzymes.
#ditki#medicalscience#medstudyblr#hiv#hivtreatments#antiretroviraldrugs#pharmacology#physiology#pathology#virology#immunology#microbiology#viraldna#retroviruses#fusioninhibition#entryinhibitors
1 note
·
View note
Text
Understanding Medicare coverage for HIV/AIDS

The global impact of HIV is substantial, with a large population affected by the virus. Although a vaccine has yet to be developed, early identification and medical intervention have proven effective in enabling HIV-positive individuals to enjoy extended and improved quality of life.
If you are enrolled in Medicare, you have access to coverage for HIV diagnosis, prevention, and treatment, regardless of whether you are on Original Medicare, Medicare Advantage, or prescription drug plans. This means that if you have HIV, you can receive medical care to manage and treat the disease, as well as preventive services to avoid contracting the virus.
The coverage for HIV under Medicare includes a range of services such as HIV testing, counseling, antiretroviral medications, and other medical treatments. Medicare also covers hospitalization, doctor visits, and lab tests related to HIV, as well as prescription drug coverage for medications that are used to treat HIV and other related conditions.
What Parts of Medicare Include HIV/AIDS Coverage
Medicare provides several different types of coverage for people with HIV. Medicare Part A covers hospital, hospice, and limited skilled nursing facility and home health care, including short-term inpatient hospital stays, medications and therapies, short-term inpatient care at a skilled nursing facility, limited home health care, and end-of-life hospice care.
Medicare Part B covers preventative, diagnostic, and outpatient treatment services, including doctor and specialist appointments, preventative HIV screenings, HIV vaccinations, lab testing, imaging, diagnostic testing, medications administered at an outpatient facility, and mental health counseling.
Medicare Part C, also known as Medicare Advantage, is a private insurance option that covers everything included in original Medicare (Parts A and B), as well as additional coverage for prescription drugs used to treat HIV.
Medicare Advantage Special Needs Plans (SNPs) offer additional benefits to people with chronic health conditions in specific situations like prescription drug coverage, access to specialized healthcare providers, and other medical services tailored to specific or chronic conditions. These are standard features across all SNPs, and those living with HIV may particularly benefit from these offerings.
The Medicare Part D initiative is aimed at offering coverage for prescription medications that are commonly used at home, such as those required for the prevention and treatment of HIV. Medicare beneficiaries who have HIV can enroll in a Part D plan to obtain coverage for antiretroviral drugs, which are used to control the virus and prevent it from progressing to AIDS. These drugs can be expensive, and Medicare Part D helps to alleviate some of the costs associated with HIV treatment. Additionally, people with HIV may be eligible for Medicare's Extra Help program, which provides assistance with out-of-pocket costs for prescription drugs.
Medigap is supplemental coverage for those with Original Medicare and helps cover costs associated with coverage, but does not offer additional Medicare benefits, such as prescription drug coverage.
What Medications And Services to Treat HIV Are Provided Under Medicare?
Medications known as antiretrovirals are frequently prescribed to treat HIV and are covered by all Medicare prescription drug plans. Antiretroviral treatment may include several different types such as integrase inhibitors, which inhibit the HIV enzyme called integrase that is necessary for duplication and spread.
Another type of antiretroviral treatment is nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), which stop the virus from replicating by inhibiting the enzyme known as reverse transcriptase. Examples of NRTIs include Abacavir, Lamivudine, and Zidovudine.
Medications known as non-nucleoside reverse transcriptase inhibitors (NNRTIs) operate in a similar way to NRTIs by blocking the replication of HIV through the inhibition of the enzyme reverse transcriptase. Efavirenz and Nevirapine are examples of NNRTIs.
Inhibiting cytochrome P4503A (CYP3A) is another approach used by antiretroviral treatment. These medications prevent the metabolization of certain medications in the liver, increasing the circulation of HIV drugs. Cobicistat and Ritonavir are examples of medications that inhibit the enzyme cytochrome P4503A, and they are typically used in combination with other antiretroviral drugs.
Protease inhibitors are a class of antiretroviral medications that prevent HIV replication by attaching to protease, an enzyme that the virus uses to copy itself. Examples of PIs include Darunavir and Ritonavir.
There are various types of drugs available for managing HIV, including those that can prevent the entry and replication of the virus. Medicare prescription drug plans typically cover these medications, along with other drugs used to manage HIV symptoms such as pain, anxiety, depression, and appetite. It's important to confirm with your Part C or Part D plan provider to ensure that your necessary medications are covered and to understand their associated costs.
Services for preventing, diagnosing, and treating HIV are typically covered by Medicare Part A or Part B, as well as Medicare Advantage. Coverage may include HIV screenings, preventative care, limited skilled nursing care, and mental health care. Part B covers HIV screenings for qualifying beneficiaries aged 15 to 65 and those at an increased risk, while Part A covers short-term skilled nursing care for those who require daily skilled care. Mental health services are covered under all Medicare Part B plans.
In cases where there are complications from the virus, durable medical equipment such as canes, crutches, hospital beds, infusion supplies, nebulizers, oxygen equipment, walkers, wheelchairs, and scooters may be necessary during treatment. Medicare Part B covers this equipment.
What Is The Difference Between HIV and AIDS?
Human Immunodeficiency Virus (HIV) is a virus that specifically targets the immune system. It infects certain cells of the immune system, such as CD4+ T cells and macrophages, and replicates inside them. Over time, HIV can weaken the immune system by killing or damaging these cells, making it harder for the body to fight off infections and diseases.
AIDS, on the other hand, is a condition that occurs as a result of untreated HIV infection. When HIV severely damages the immune system and reduces the number of CD4+ T cells below a certain level, a person is said to have acquired immunodeficiency syndrome (AIDS). At this point, the immune system is so weakened that the person becomes susceptible to life-threatening infections and cancers, which can ultimately lead to death if left untreated.
However, it's important to note that not everyone with HIV develops AIDS. With proper treatment, people living with HIV can prevent the virus from progressing to AIDS and live long and healthy lives. Antiretroviral therapy (ART) is a medication regimen that can suppress the replication of HIV in the body, allowing the immune system to recover and function normally. When taken consistently and as prescribed, ART can reduce the amount of HIV in the blood to undetectable levels, preventing further damage to the immune system and reducing the risk of transmission to others.
In addition to ART, other strategies such as regular medical check-ups, vaccinations, and healthy lifestyle choices can also help people living with HIV maintain good health and prevent the progression of the disease. It's important for people living with HIV to work closely with their healthcare providers to manage their condition and stay informed about the latest advances in HIV treatment and care.
0 notes
Text
VIROPIL DỰ PHÒNG VÀ ĐIỀU TRỊ ARV
Giá bán: 2.000.000₫
Thuốc Viropil được sử dụng để điều trị nhiễm HIV-1 áp dụng cho người lớn và bệnh nhi có cân nặng ít nhất 40kg
Thành phần:
Dolutegravir 50mg
Tenofovir disoproxil fumarate 300mg
Lamivudine 300mg
Thuốc Viropil là thuốc gì?
Thuốc Viropil là loại thuốc được dùng trong quá trình điều trị bệnh HIV. Nhờ khả năng ức chế và kháng virus HIV hiệu quả, Thuốc Viropil là phương pháp đặc trị HIV được ứng dụng phổ biến tại các phòng khám và cơ sở y tế hàng đầu.
Thuốc Viropil có công dụng hạn chế sự lây lan của HIV trong cơ thể người bệnh, giảm nguy cơ xuất hiện các biến chứng liên quan HIV. Sử dụng Viropil sau một thời gian, hệ miễn dịch của bệnh nhân sẽ được phục hồi và bảo vệ đáng kể, giúp duy trì sức khỏe.
Tuy nhiên, cũng như các loại thuốc HIV khác, Viropil không có khả năng điều trị dứt điểm bệnh HIV. Người bệnh vẫn phải học cách “sống chung” với căn bệnh này.
Thành phần thuốc Viropil
Dolutegravir 50mg
Dolutegravir giúp hạn chế tối đa sự tích hợp của gen integrase HIV. Từ đó ngăn ngừa virus HIV nhân đôi và phát triển trong cơ thể con người. Dolutegravir được nghiên cứu là an toàn cho phụ nữ có thai hoặc đang cho con bú.
Tenofovir disoproxil fumarate 300mg
Thành phần này có tác dụng ngăn cản quá trình tổng hợp DNA của virus HIV. Nó giúp chống nhiễm khuẩn vượt trội, giúp thúc đẩy quá trình kháng virus.
Lamivudine 300mg
Lamivudine là thành phần rất hiệu quả trong việc kháng retrovirus, từ đó giúp ức chế enzym phiên mã ngược trong cơ thể người bệnh. Giúp bảo vệ hệ miễn dịch tối đa khỏi sự tấn công của virus HIV. Ngoài ra, đây là thành phần giúp kháng sự phát triển của virus viêm gan B hiệu quả.
Chỉ định thuốc Viropil
Thuốc Viropil ược sử dụng để điều trị nhiễm HIV-1 áp dụng cho người lớn và bệnh nhi có cân nặng ít nhất 40kg.
Nếu bệnh nhân bị ức chế virus học, sẽ dùng Rilpivirine. Đây là một chế độ hoàn chỉnh để điều trị nhiễm HIV-1 ở người lớn để thay thế các loại thuốc kháng retrovirus hiện tại.
Liều dùng – Cách dùng thuốc Viropil
Người lớn: Nên sử dụng thuốc với liều lượng 1 viên/lần/ngày. Nên uống thuốc trong bữa ăn.
Bệnh nhân dưới 18 tuổi: Không có liều lượng cụ thể, bạn nên tìm đến các y bác sĩ để được kê đơn phù hợp.
Bệnh nhân trên 65 tuổi: Không có liều lượng cụ thể, bạn nên tìm đến các y bác sĩ để được kê đơn phù hợp.
Chống chỉ định Viropil
Viropil chống chỉ định với những bệnh nhân được dùng đồng thời elbasvir & grazoprevir. Phản ứng quá mẫn được tạo ra do bệnh nhân chống chỉ định với thành phần của Viropil.
Sử dụng đồng thời Viropil với dofetilide, dẫn đến tăng nồng độ trong huyết tương của dofetilide dẫn đến độc tính.
Tác dụng phụ thuốc Viropil
Nhiễm axit lactic / gan to nặng với nhiễm mỡ, làm nặng thêm viêm gan B, khởi phát mới hoặc suy thận nặng.
Ở bệnh nhân đồng nhiễm HIV-1 và viêm gan C sẽ bị mất bù gan. Viêm tụy, giảm mật độ xương, giảm miễn dịch.
Chi tiết sản phẩm: https://phongkhamgalant.com/product/thuoc-viropil-du-phong-va-dieu-tri-arv
0 notes
Text
How HIV Develops Resistance to Vital Medicines Identified
How HIV Develops Resistance to Vital Medicines Identified
They all work by binding with one of HIV’s key enzymes, called integrase, to stop it from inserting the virus’ genetic information into DNA of human cells. While initially highly effective, over time HIV can develop resistance to these drugs. Although the drugs are normally very effective at binding and blocking integrase, over time, the virus can weaken this bond and thus enable its key enzyme…

View On WordPress
0 notes
Text
Pharmacology - Chapter 5 - Therapies for Viral Infections and Immunizations

Part One Part Two Part Three Part Five
Transcription under the cut!





HIV/AIDS and Antiretroviral Agents
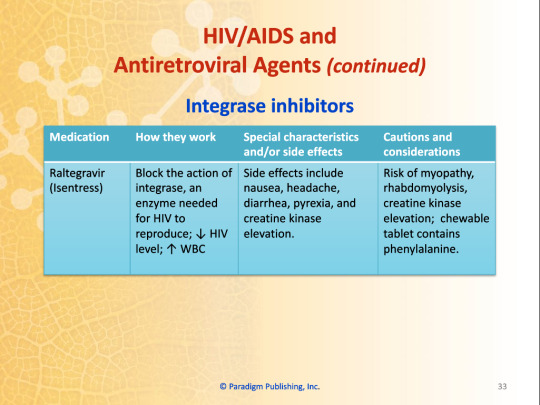
Integrase Inhibitors
Blocks the action of integrase, an enzyme needed for HIV to reproduce; decreased HIV level; increased WBC
Dolutegravir (Tivicay)
Believed to work better than other integrase inhibitors in viral resistance; side effects include elevated serum lipase, insomnia, elevated liver enzymes, hyperglycemia
Risk of fat redistribution and hypersensitivity reaction
Elvitagravir (Vitekta)
Side effects include depression, fatigue, insomnia, headache, diarrhea, abdominal pain
Risk of lactic acidosis, to be avoided in severe liver impairment
Raltegravir (Isentress)
Side effects include nausea, headache, diarrhea, pyrexia, and creatine kinase elevation
Risk of myopathy, rhabdomyolysis, creatine kinase elevation; chewable tablets contain phenylalanine
Work Wise
Raltegravir film-coated tablets and chewable tablets or oral suspensions are not bioequivalent. This means they cannot be substituted on a mg-for-mg basis.
Responding to Exposure to HIV
Healthcare worker risks
Exposure to blood and other bodily fluids
Needlestick injuries
Postexposure Prophylaxis (PEP)
Can decrease the risk of infection by 80%
Should start within 2 hours of exposure
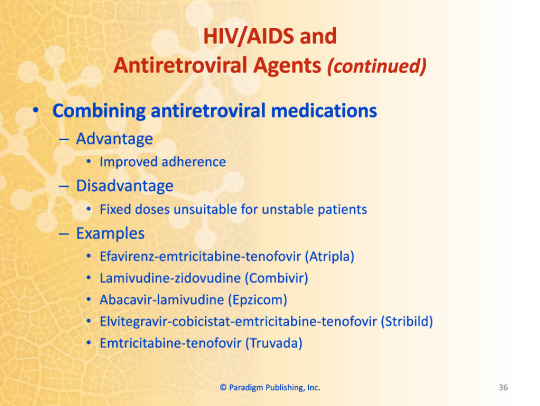
Combining Antiretroviral Medications
Advantage
Improved adherence
Disadvantage
Fixed doses unsuitable for unstable patients
Examples
Efavirenz-emtricitabine-tenofovir (Atripla)
Lamivudine-zidovudine (Combivir)
Abacavir-lamivudine (Epzicom)
Elvitegravir-cobicistat-emtricibine-tenofovir (Stribild)
Emtricitabine-tenofovir (Truvada)
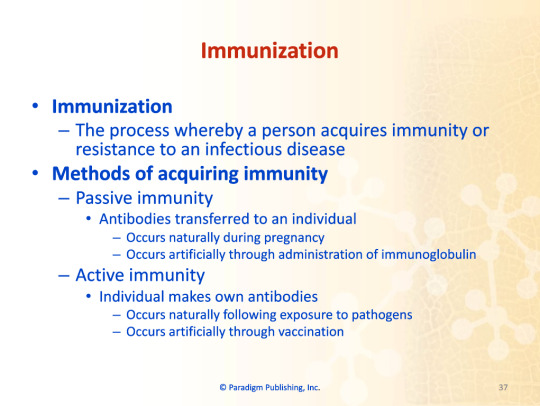
Immunization
Immunization
The process whereby a person acquires immunity or resistance to an infectious disease
Methods of acquiring immunity
Passive immunity
Antibodies transferred to an individual
Occurs naturally during pregnancy
Occurs artificially through administration of immunoglobulin
Active immunity
Individual makes own antibodies
Occurs naturally following exposure to pathogens
Occurs artificially through vaccination
Live attenuated vaccines
Use live but weakened pathogens to induce an immune response
Inactivated vaccines
Use pathogens that have been killed
Pharm Facts
Community immunity describes a situation in which a sufficient proportion of a population has immunity to an infectious disease (usually through vaccination or prior exposure). Community immunity, in theory, makes it less likely for diseases to spread from person-to-person; this means that protection is extended to those within the community that do not have immunity. Another term for community immunity is herd immunity.
Immunization Schedule
Schedule source
Adults and childhood vaccination schedules are published by the CDC
Vaccines for children
Most lead to lifetime immunity
Boosters given for continued protection
Vaccines for healthcare workers
Hepatitis B vaccination and annual influenza vaccine often required by employers
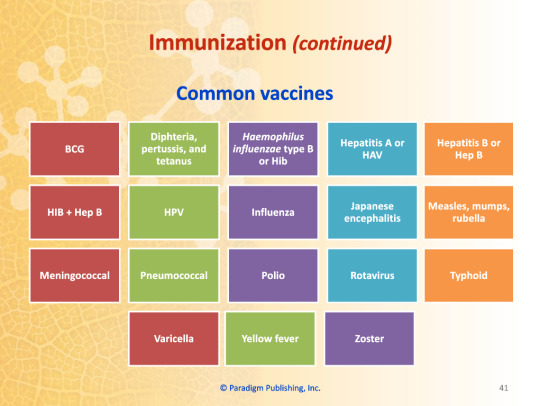
Common Vaccines
Role of pharmacy technicians
Screen patients
Complete necessary forms
Stock and store vaccines
Draw up vaccine for pharmacist to administer
Common Vaccines
BCG
HIB+Hep B
Meningococcal
Varicella
Diphtheria, Pertussis, and Tetanus
HPV
Pneumococcal
Yellow Fever
Haemophilus influenzae type B (HiB)
Influenza
Polio
Zoster
Hepatitis A (HAV)
Japanese encephalitis
Rotavirus
Hepatitis B (Hep B)
Measles, Mumps, and Rubella
Typhoid
0 notes
Text
HIV and AIDS treatment and prevention
According to UNAIDS, around 38 million people across the world were living with HIV in 2019. In the same year, reports suggest about 1.7 million new cases across the globe, a decline of 23% compared to 2010. Although these numbers are staggering, HIV/AIDS is no longer the death sentence that it once was.

HIV or human immunodeficiency virus weakens our immune system by targeting and destroying white blood cells that fight infections. HIV increases the risk of developing other illnesses and can soon progress to AIDS without proper treatment.
AIDS or acquired immunodeficiency syndrome is a chronic and life-threatening condition, the last stage of HIV infection.
Today with the progress in medicine, people diagnosed with HIV can live as long as those without HIV. Similarly, the life expectancy of those living with AIDS has gone up considerably.
HIV/AIDS Treatment
The first step towards treating HIV/AIDS is detecting it. The earlier you diagnose, the better. Let us first take a peek into how you can diagnose HIV/AIDS.
Diagnosing HIV
You cannot depend on symptoms to tell you if you have HIV. The only sure-shot way to know your HIV status is by getting tested.
There are three primary tests for HIV,
Nucleic Acid Test or NATs
Antigen Tests
Antibody Tests
If you doubt having contact with someone with HIV, seek help immediately. The initial test will be an antigen or antibody test like ELISA. These tests check for antigens or antibodies that your body develops to fight HIV.
If you test positive for the initial test, your healthcare provider runs a follow-up test such as a NAT. A nucleic acid test searches for the specific virus and allows them to be sure that the diagnosis is correct.
Treatment
Although there is still no cure for AIDS, modern medicine can now slow the progression of the disease to a large extent. These medications and therapies have considerably reduced the number of deaths due to HIV/AIDS.
Antiretroviral Therapy
The most effective treatment for AIDS today is Antiretroviral Therapy or ART, which is a combination of numerous medicines. ART prevents the patient from developing resistance to any particular drug while slowing the spread of the virus and reducing the amount of virus (viral load) in the body.
Today there are several types of Antiretroviral medications available, including:
Protease Inhibitors: HIV requires an enzyme called protease to multiply. These meds bind to this enzyme and prevent growth.
Integrase Inhibitors: Integrase is another enzyme that is essential for HIV to multiply. Integrase Inhibitors bind to Integrase and prevent replication of the virus. Doctors often suggest these as the first line of treatment for HIV patients due to their effectiveness in stopping the virus.
Nucleoside and nucleotide reverse transcriptase inhibitor: These meds block another enzyme called Nucleoside or Nucleotide reverse transcriptase.
Entry Inhibitors: Entry Inhibitors prevent HIV from entering the cells (T cells in particular), thus prevents replication of the virus.
PEP Emergency Pills
If you suspect you have come in contact with HIV, get in touch with a healthcare provider immediately. Your healthcare provider depending on the situation may prescribe PEP or post-exposure prophylaxis pills.
Although they aren't 100% effective, consuming PEP pills within 72 hours of exposure significantly reduces the risk of getting infected.
These pills fight the infection and stop the spread of the virus in your body.
HIV/AIDS Prevention
To date, we have not discovered a vaccine to prevent an HIV infection. However, there are some practices you can adopt to prevent an HIV infection.
TasP
TasP or Treatment as Prevention refers to medications taken to prevent the sexual transmission of HIV.
HIV patients who effectively take TasP medications as prescribed have a minimal risk of transmitting HIV.
Barrier protection
Using barrier protection such as condoms can considerably reduce the risks of contracting HIV infections. Using a new latex condom every time you have sex and choosing a water-based lubricant, if appropriate, can prevent HIV and other STIs.
PrEP
Persons having high risks of being exposed to HIV can take PrEP or pre-exposure prophylaxis medications.
Other Methods
If you have paid attention in sex-ed classes, you might remember some ways to prevent STIs. These methods are also applicable in the prevention of HIV. Below are some additional preventive measures you can take,
Do not share needles. If you need syringes to inject drugs, use sterile and clean syringes.
Know your partner's HIV status.
Limit exposure to bodily fluids that may carry HIV.
For More Information of Healthcare Products
please contact Following us:
Phone Number: +91-9773529259, +91-7303072644 E-mail: [email protected] [email protected] Visit Website: https://www.mediseller.com/
0 notes
Text
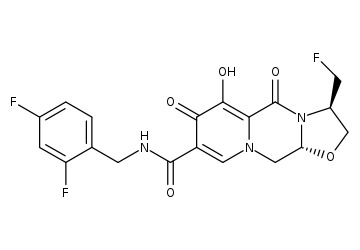
Raltegravir
In this article, we will discuss about Raltegravir (Overview). So, let’s get started. Raltegravir Mechanism of ActionRaltegravir inhibits the catalytic activity of HIV-1 integrase, an HIV-1 encoded enzyme that is required for viral replication. Inhibition of integrase prevents the covalent insertion, or integration of unintegrated linear HIV-1 DNA into the host cell genome preventing the…
View On WordPress
#Antiretroviral Agents#Antiviral Agents#Antiviral Drugs#ISENTRESS®#Medicine#Pharmacology#Physiotherapy#Raltegravir
0 notes