#Epithelial and Endothelial Difference in the Position
Explore tagged Tumblr posts
Text
Epithelial and Endothelial Differences in the Function
Endothelial cells can regulate blood flow in the blood vessels, by releasing NO (vasodilator) to promote blood circulation and blood pressure regulation. Endothelial cells can also secrete a variety of proteins involved in hemostatic function.
Read more:- https://kosheeka.com/epithelial-and-endothelial-cells-how-do-they-differ/
#Epithelial and Endothelial Cells#Epithelial and Endothelial Difference in the Position#Epithelial and Endothelial Differences in the Function#Epithelial and Endothelial Differences in the Structure#Epithelial and Endothelial Other Differences#Cell culture#customized primary cells#primary cells#biotech company#stem cells#exosomes#stem cell research center#regenerative medicine#bioengineering
0 notes
Text
Metformin Reduces the Extent of Varicocele-Induced Damage in Testicular Tissue
Authored by: Erkan Erdem*
Introduction
Varicocele is an abnormal vascular dilatation of pampiniform plexus, commonly developing at puberty. Although underlying mechanisms remain poorly understood genetic background, anatomical aberrations, incompetence of venous valves, difference between the drainage of left and right testicular veins were suggested in the etiology [1]. As left spermatic vein being longer than the right vein, it is more commonly incurred to increased hydrostatic pressure and dilatation. Compression of the left renal vein between the aorta and the superior mesenteric artery may also contribute to the disturbed intravenous pressure [2].
The prevalence of varicocele varies between 15-20 % in general population and 30-40% in infertile men, and 11-19% of adolescents [3-6]. It was reported that varicocele is a progressive disease and early diagnosis and treatment in youth may enhance fertility potential [7]. Several contributing factors in the pathophysiology of varicocele have been proposed such as higher temperature of testis, the disorder of neuroendocrine system, autoimmunity, accumulation of renal and adrenal metabolites, genetic and epigenetic factors, hypoxia and oxidative stress [8-10].
Varicocele represents a chronic process within the testicle, which is linked to increased reactive oxygen species (ROS) beyond physiologic limits and, subsequently, disrupting sperm membrane fluidity, causing DNA damage and necrosis [11]. Moreover, superoxide dismutase 1, glutathione S-transferase M1 and T1 which are counteracting free superoxide radicals in cells have been reported to be decreased in men with varicocele, that may be important on disturbed sperm parameters [12]. Apoptosis of germ cells was also demonstrated in the pathogenesis of varicocele-related infertility [13]. Clinical findings suggest that surgical repair of varicocele may decrease seminal oxidative stress levels and sperm DNA fragmentation and, thus, may improve sperm quality [14]. Therefore, surgical intervention seems to be a reliable option in the treatment of varicocele-related male infertility, although some controversial reports exist.
Additionally, anti-oxidant medications such as kallikrein, L-carnitine with L-acetyl carnitine, pentoxifylline, coenzyme Q10 have been used to improve the milieu in the testis in men with varicocele [15]. Metformin is a major therapeutic agent in the treatment of type 2 diabetes mellitus as an insulin sensitizer, which decreases hepatic glucose output and increases peripheral glucose uptake. Although its action was not fully elucidated, metformin attenuated intracellular reactive oxygen species and apoptosis in aortic endothelial cells, myocardium, renal tubular cells and testicular cells [16-20].
Aim
Potential effects of metformin on varicocele-induced testicular damage have not been studied in neither humans nor in animal models. Thus, we investigated the impact of metformin on spermatogenesis, testicular integrity, and apoptotic activity in the testis of adolescent rats with experimentally-induced varicocele.
Materials and Methods
Thirty-six male adolescent Wistar rats (6-week-old) were randomly and equally divided into six experimental groups. Surgical procedures were carried out under anesthesia with intraperitoneal injection of ketamine (50 mg/kg). The experimental groups were as follows:
• (C) Control group; no surgical procedure was performed, and testis was examined after removal.
• (S) Sham group, a midline incision was performed, and testis was examined 8 weeks later.
• (V) Varicocele - only group: Experimental varicocele was induced by partial ligation of left renal vein with
Silk suture at the area medial to the insertion of the adrenal and spermatic vein into renal vein as described previously [21].
• (V+M) Varicocele + metformin group: All rats were treated with metformin (300 mg/kg per day by oral gavages) for 8 weeks following induced varicocele.
• (V/E) Varicocele + varicocelectomy group: Varicocelectomy was performed 4 weeks and the examination of the testis 8 weeks after the induction of varicocele. No medication was used.
• (V/E+M) Varicocele + varicocelectomy + metformin group: Varicocelectomy was performed 4 weeks after the induced varicocele. Metformin treatment (300 mg/kg per day by oral gavages) was initiated after the induction of varicocele and continued for 8 weeks. Left testes were examined 8 weeks after the induction of varicocele in all varicocele - induced groups. As maximum apoptotic activity initiates approximately 28 days after the induction of varicocele the procedure of varicocelectomy was performed 4 weeks after the formation of varicocele [22].
Histologic preparation and evaluation
The testicular tissue was fixed in Bouin’s solution (75% picric acid, 5% glacial acetic acid, and 25% formaldehyde) and embedded in paraffin blocks. Sections (5 μm) were formed, deparaffinized, and stained with hematoxylin and eosin. Spermatogenesis was examined in each group using Johnsen’s score (a score of 1-10 was assigned to each tubule regarding epithelial maturation) as described previously [23]. Sections were examined in a random order under a standard light microscope with 10x and 40x magnification by a blinded histologist; unaware of which group each rat belonged to. Histological grading was done by examining approximately 80 randomly selected seminiferous tubules per rat. Thus, a total of approximately 480 seminiferous tubules were scored for each group.
Histomorphometry analysis
A total of 103 randomly selected seminiferous tubules stained with hematoxylin-eosin were analyzed in each group. The presence of round spermatid stage (RSS) and primary spermatocyte stages (PSS) were assessed as described previously and compared among the groups [24].
Immunohistochemical staining for cleaved caspase-3 and ImageJ analysis
Cleaved caspase-3 was used for immunohistochemical staining. Testicular tissue samples were immediately fixed in 10% neutral-buffered formalin, embedded in paraffin, and sectioned (5 μm). Sections were deparaffinized and blocked for endogenous peroxidase activity with methanol containing 3% H2O2 for 10 m. Ultra V Block (Lab vision, Freemont, CA) for 7 m at room temperature. Cleaved Caspase-3 (#9664, Cell Signaling, U.S.) was applied at a dilution of 1: 500 and incubated overnight at +4 °C in a humidified chamber for nonspecific binding. The sections were washed in phosphate-buffered saline (PBS) and incubated with biotinylated horse anti-rabbit IgG (3 mg/mL; Vector, Burlingame, CA) at a 1: 500 dilution for 1 h at room temperature.
Antibodies were detected using a VECTASTAIN avidinbiotin complex (Vector PK 4000) for 30 m at room temperature. Antibody complexes were visualized after incubation with 3,3’-diaminobenzidine tetrahydrochloride (DAB, Bio-Genex, San Ramon, CA.) and were mounted under glass coverslips in Entellan (Merck) and then evaluated under a light microscope. Immunohistochemical staining for cleaved caspase-3 was analyzed by counting 100 seminiferous tubule cross-sections in each group and expressed as the apoptotic index. In each photomicrograph, the following parameter was measured with ImageJ software: expression levels of cleaved-caspase-3 in both groups at round spermatid stage (RSS) of testes. Each of this parameter was measured 3 times for each image and the average of the 9 measurements of each sample was used for the statistical analysis. Histopathological features examined in rats with normal testis and with sham, varicocele, varicocele+ metformin in a subjective scoring (0 - not present; 1 - low grade; 2 - moderate grade; 3 - high grade; 4 - very high grade).
Statistical analysis
Histopathological findings (Johnsen’s score) were assessed by nonparametric Kruskal-Wallis test, and the mean Johnsen’s score was used in the comparison of the groups. Multiple comparisons were made using Tukey’s procedure. p<0.05 was considered statistically significant. Analysis of variance was used for statistical analysis of the apoptotic index among the groups.
Results
Assessment of spermatogenesis
Johnsen’s score was significantly lower in V group (4.14±1.25) compared to C group (9.1±0.3) or S group (9.0 ± 0.2) groups (p<0.05). V+M group had significantly higher score (6.9±0.6) than V group (p<0.05). V/E group and V/E+M group had similar Johnsen scores (8.9 ± 1.02 and 9.2 ± 0.6). These findings suggest that the administration of metformin resulted in 40.6% of improvement in spermatogenesis in rats with varicocele. However, this favorable effect was not observed when metformin was used along with varicocelectomy.
Histological and morphological changes in seminiferous tubules
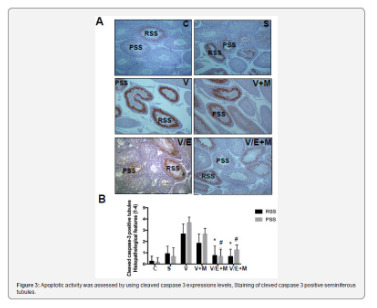
Histological and morphological changes in the testes of rats were compared via hematoxylin and eosin staining and degenerated tubules (DT) were only detected in V and V+ M groups, not in C, S, V/E and V/E+M groups (Figure 1). Visual assessment of the disorganized seminiferous tubules further supported these findings as seen in Figure 2. Seminiferous tubule degeneration scores were used for quantification of data (Figure 2b). V group had significantly higher scores of RSS and PSS compared to C and S group (2.6±0.8 and 3.7±0.4; 0.2±0.4 and 0.2±0.4; 0.9±0.6 and 0.6±0.7, respectively) (p<0.05). V/E group had significantly lower RSS (0.7±0.8) and PSS (0.8±0.7) scores than V group (p<0.05). V+M group had significantly lower RSS and PSS scores (1.8±0.7 and 2.6±0.7, p<0.05) in comparison to V group, implicating beneficial effects of metformin in rats with varicocele. When compared to V/E group, V/E+M group did not exhibit any difference in RSS (0.6±0.6) and PSS (1.4±0.5) scores, suggesting the absence of additive positive effect of metformin in varicocelectomies rats.
Apoptotic activity
Apoptotic activity was assessed by using cleaved caspase 3 expressions levels, staining of cleved caspase 3 positive seminiferous tubules were shown in Figure 3a. Cleaved caspase 3 expressions were significantly higher in V group (3.5 ± 0.5) compared to C (0) and S (0.2 ± 0.4) groups. V+M group had significantly lower cleaved caspase 3 level (3.0 ± 0.7) than V group. V/E group had lower cleaved caspase-3 expression levels (1.0 ± 0.7) compared to V group. Treatment of varicoceleectomy rats with metformin (V/E+M) did not further reduce apoptotic activity in the seminiferous tubules (1.75 ± 0.43) when compared to the varicocelectomy group (V/E) (Figure 3b).
Discussion and Conclusion
The present study demonstrates that metformin can reduce the extent of testicular damage in rats with varicocele, although having no effect in rats following varicocelectomy Spermatogenesis, seminiferous tubule integrity and the degree of apoptosis were improved using metformin in the presence of varicocele although it was not as remarkable as what was obtained through varicocelectomy. A review of the literature revealed that the impact of metformin on varicocele was not investigated in humans or animal models until now.
Although it is a commonly identified abnormality not all men with varicocele present with infertility. Some intrinsic factors may render some men to become susceptible to varicocele, thus, the best candidates who benefit from varicocelectomy yet to be clarified. Since oxidative stress was shown to be important in the pathophysiology of varicocele some agents have been used to improve the milieu in the testis [1]. A number of anti-oxidant medications have been studied to relieve detrimental effects of varicocele in the testis [25]. These agents have been used either alone or as an adjuvant therapy with surgery. However, surgery remains the treatment of choice and there exists insufficient data to recommend medical therapy in men with varicocele. Barekat et al. [26] reported that administration of an antioxidant agent N-acetyl cysytein as an adjunct therapy improved semen quality following varicocelectomy [26]. Tek et al. [21] demonstrated that vascular endothelial growth factor decreased apoptosis in varicoceleinduced rats as evidenced by diminished caspase-3 positive cells [21]. Both studies showed the benefit of these as adjunct therapy following varicocelectomy. However, in the present study metformin did not enhance the effect of varicocelectomy.
Minutoli et al. [13] demonstrated that neuronal apoptosis inhibin factor and surviving expressions were significantly reduced following varicocele induction and polydeoxyribonucleotide, an agonist of adenosine A2A receptor, administration restored testicular function [13]. Several other studies detected increased germ cell apoptosis in rats with varicocele [21,22,27]. However, in another study, apoptosis was found to be decreased in germ cells in the testes of infertile men with varicocele as compared with normal men [28]. It was speculated that the fixation of testis in formaldehyde might have played a role in the different result. In the present study, cleaved caspase 3 expression was used to assess apoptotic activity and it was found that metformin reduced apoptotic activity in rats with varicocele, whereas no additional effect was observed when metformin was administered after varicocelectomy.
Metformin is commonly used in type 2 diabetes mellitus and polycystic ovarian disease as an insulin sensitizer [29]. Also, it is present in various tissues including myocardium, liver, pancreas, thyroid, adipose tissue, hypothalamus, pituitary, and male and female gonads [19,30,31]. It has been reported that metformin is mainly transported into cells by organic cation transporters as passive diffusion is limited [32]. Although the mechanism of action is not yet fully elucidated recent studies suggested that metformin acts through AMP-activated protein kinase (AMPK) pathway, inhibits the activity of the respiratory electron transport chain in mitochondria, induces epigenetic modifications which in part may explain long term effects and decreases oxidative stress and apoptotic activity [16,19, 33-35].
Male reproductive system utilizes all these metabolic pathways and is prone to be affected by metformin administration [20,36,37]. Metformin was found to stimulate lactate production which is important in the development of germ cells and show an anti-apoptotic effect in rat Sertoli cells [38]. It was also reported that metformin reduced the apoptotic cells and caspase-3 level in rat testis [20]. The findings of the present study are consistent with previous studies that metformin reduced apoptosis in testis with varicocele. Yan et al. [37] reported that metformin improved the semen parameters related to its effects on weight loss, increased testicular weight and reduced testicular cell apoptosis [37]. On the other hand, Tartarin et al. [36] reported metformin at concentration 10 times higher than therapeutic levels decreased testosterone secretion and the number of Sertoli cells in rats when it was administered during pregnancy [36]. Faure et al. [39] reduction in testicular weight and testosterone level were observed in 6-week-old chickens treated with metformin for 3 weeks [39].
Several groups demonstrated that post-operative administration of metformin can exert protective effects in male reproductive function in rat models [40]. Bosman et al. [41] demonstrated that infertile hyperinsulinaemic men could benefit from metformin treatment in combination with an enriched antioxidant diet [41]. Besides, metformin was reported to act as a protective compound when used in the media for cryopreservation of spermatozoa [30]. In conclusion, metformin reduces detrimental effects of varicocele, although no additional benefit is expected following varicocelectomy. Further studies are required to apply metformin for this indication in humans.
#open access journals#peer review journals#Juniper Publishers PubMed Indexed Articles#reproductive medicine#GJORM in juniper publishers
1 note
·
View note
Text
Secrets of Mammary Stem Cells Secretome-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF DAIRY & VETERINARY SCIENCES
Abstract
The role of mammary stem/progenitor cells and its secreted proteins in therapeutic application has not been evaluated yet. Here, author reviewed information pertaining to secreted proteins of mammary epithelial cells and mammary stem/progenitor cells and supported their ideas that secretome could potentially be explored for natural antimicrobials against supportive therapy of bovine mastitis.
Keywords: Mammary stem cell; Secretome; Mastitis; Bovine; Spectrophometer; Proteins; Laboratory; Lactoferrin; Receptors; Biomarkers; Pacific; Population; Antibiotics; Progenitor cells
Abbrevations: HMEC: Human Mammary Epithelial Cells; CM: Conditioned Medium; EGFR: Epidermal Growth Factor Receptor; AFDC: Adherent Fraction Derived Cells; MDC: Mammosphere Derived Cells; TGFβ: Transforming Growth Factor Beta; MDBK: Madin Darby Bovine Kidney Epithelial; LPS: Lipopolysaccharides
Opinion
Secretome are the cell secreted proteins released in the cell extracellular space. Groups of proteins present in the secretome are involved in signalling and cell communications. Study of such proteins, thus would be helpful in understanding the niche of the cell. Researchers of Pacific Northwest National Laboratory, Washington probably was the first group which demonstrated secretome of human mammary epithelial cells (HMEC) in 2008 [1]. They identified secretome of human mammary epithelial cells (HMEC)-conditioned medium (CM) cell lysates and showed regulation of matrix metalloproteases through epidermal growth factor receptor (EGFR). In 2009, Simpson and co-investigators have profiled three different secretome of basal MaSC, luminal progenitors and mature luminal cell lines. In this study, in addition to enriched expression of ephrin receptors and integrins, the activity of Wnt signalling pathway was uniquely detected in the MaSC [2]. In a recent publication of Scientific Reports journal, researchers from Cornell University, Ithaca, NY has defined secretome of mammosphere derived MaSC and opened up new doors for the possible treatment of bovine mammary gland infections [3].
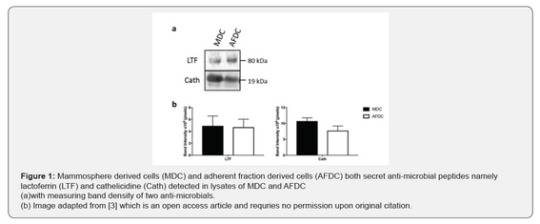
For their studies, the team isolated cultured two different populations of bovine mammary cells- adherent fraction- derived cells (AFDC) and suspension mammosphere-derived cells (MDC). These two cell population were immunopheno typically different as analyzed by various cell surface markers. However, the expression of CD44 and CD49f was higher in MDC CM than the AFDC CM. Level of CD29 was high in CM of both these cells. Proteome analyses of AFDC and MDC CM, using mass spectrophometer, revealed 347 and 537 matched proteins and peptides functionally related to defense and immunity and tissue regeneration like angiogenesis and cell migration. Furthermore, two antimicrobial proteins namely lactoferrin and cathelicidine have been validated and quantified using Western blot (Figure 1) and observed differential expression of the proteins in CM of MDC versus AFDC. Two angiogenesis factors namely, angiopoetin 1 and vascular endothelial growth factor alpha, angiopoetin 1 showed high expression level in MDC CM. Likewise, levels of proteins involved in cell migration (transforming growth factor beta (TGFβ), Insulin-like growth factor-1 and hepatocyte growth factor) were estimated in the CM and found that the concentration of TGFβ was high in CM of MDC. TGFβ induces epithelial cell to mesenchymal type and promotes cell migration. Taken together, this study showed CM of AFDC and MDC contains various proteins which have roles in tissue regeneration and immune defence of the host.
Interestingly, proteins of AFMC and MDC also contained factors which protects bacterial toxin-induced MEC death. Loss of MEC damage is an important consequence of mastitis and it is induced by the toxins of Gram-positive bacteria and lipopolysaccharides (LPS) of Gram-negative bacteria. A significant reduction in growth of bacteria (measured by optical density) was observed when Madin-Darby Bovine Kidney Epithelial (MDBK) cells were grown in presence of Klebsiella (a Gram-negative bacteria) and Staphylococcus aureus (a Grampositive bacteria) in presence of MDC CM. The researchers also evaluated shelf-life of MDC CM for one week and found that frozen CM is biologically effective on MDBK cell migration. The storing of CM for long time would be advantageous for future clinical use in mastitis management even at remote places.
In summary, these studies providing rationale for the potential use of MDC secretome as supportive or adjunct therapy in bovine mastitis. Secretome could be an ideal biological source for naturally occurring anti-microbial and potentially beallogenically safe like antibiotics. In future, an important follow-on studies should include evaluation of novel proteins as biomarkers and identification of newly identified proteins with no to little annotations. In vivo application of MDC secretome in management of naturally occurring bovine mastitis, also warranted future investigations.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/open-access.php
For more articles in
Open Access Journal of Dairy & Veterinary sciences
please click on:
https://juniperpublishers.com/jdvs/index.php
0 notes
Text
Lupine Publishers | Pachychoroid Neovasculopathy within the Spectrum of Pachychoroid Disease

Lupine Publishers | Trends in Ophthalmology Open Access Journal
Abstract
Pachychoroid neovasculopathy is a relatively new retinal disease, characterized by the presence of Type 1 choroidal neovascularization associated with signs of increased choroidal thickening and hyperpermeability. The latter features are distinctive of pachychoroid spectrum disease, which also includes pachychoroid pigment epitheliopathy, central serous chorioretinopathy and polypoidal choroidal vasculopathy. These pathologies share common features, such as choroidal vascular disfunction, abnormalities of the retinal pigment epithelium without showing typical characteristics of age related macular degeneration and, occasionally, choroidal new vessels. Many recent works have tried to analyze the main aspects of pachychoroid neovasculopathy with the aim to better understand the natural course and the proper management of the disease. Multimodal imaging modality, with the recent advent of the optical coherence tomography angiography, plays a fundamental role to help the physicians to distinguish choroidal neovascularization in pachychoroid disease from neovascular age related macular degeneration. In this review, we summarize the latest updates in the pathogenesis, clinical features, and advances in imaging multimodalities in order to delineate a clearer description of the pachychoroid neovasculopathy. Finally, we propose recommendation guidelines for the diagnosis and management of this relatively new clinical entity. Nevertheless, larger studies and clinical trials are needed to achieve standardized diagnostic criteria and treatment modalities.
Keywords: Pachychoroid neovasculopathy; Pachychoroid disease; Neovascular age related macular degeneration; Neovascularization; Anti-VEGF
Abbreviation: EDI: Enhanced Depth Imaging; SSOCT: Swept Source Optical Coherence Tomography; CSC: Central Serous Chorioretinopathy; ICGA: Indo Cyanine Green Angiography; PEDs: pigment epithelial detachments; PPE: Pachychoroid Pigment Epitheliopathy; CNV: Choroidal Neo Vascularization; PCV: Polypoidal Choroidal Vasculopathy; PVN: Pachychoroid Neovasculopathy; BM: Bruch’s Membrane; OCTA: Optical Coherence Tomography Angiography; FAF: Fundus Auto Fluorescence; Anti-VEGF: Anti- Vascular Endothelial Growth Factors.
Introduction
Definition
The recent great progress in the multimodal imaging of the retina has provided new comprehensions into the understanding of various chorioretinal diseases. Mostly, the development of new OCT softwares, such as the enhanced depth imaging (EDI) [1] and the swept source optical coherence tomography (SS-OCT) [2], has contributed to the significant improvement in the analysis of the morphology and physiology of the choroid. Warrow et al. [3], firstly in 2013, observed the presence of EPR abnormalities in the controlateral eye of patients affected by unilateral acute central serous chorioretinopathy (CSC), including increased choroidal thickness and dilated choroidal vessels on EDI OCT, and choroidal hyperpermeability on indocyanine green angiography (ICGA). The presence of the previous findings with an overlying area of EPR abnormalities, such as drusenoid RPE changes and or small pigment epithelial detachments (PEDs), was considered by the same author “a forme frust” of CSC, giving it the denomination of pachychoroid pigment epitheliopathy (PPE). In the last years, the presence of choroidal neovascularization (CNV) in chronic CSC was well documented [4]. Moreover, an association between CSC and polypoidal choroidal vasculopathy (PCV) has been demonstrated [5]. The findings of the important role played by the thick choroid in PCV has driven the researchers to believe that PCV falls within the pachychoroid spectrum instead of AMD [6]. Therefore, recognizing and precisely diagnosing PPE could play a crucial role to distinguish the possible etiology in those cases, in which the origin of the neovascularization is not clear.
Pang et al. [7] termed as “pachychoroid neovasculopathy” (PVN) the presence of Type 1 neovascularization that occurs overlying an area of focal choroidal thickening, in order to differentiate the “pachychoroid” origin of the pathophysiological mechanism from the other sources of Type 1 neovascularization. PVN represents a new clinical entity of CNV, characterized by thick choroid, RPE alterations and choroidal hyperpermeability. It has been accepted to include it within the spectrum of the pachychoroid disease, together with PPE, CSC and PCV. PVN shows the typical characteristics of Type 1 neovascularization, without the presence of AMD features or degenerative changes. Therefore, the most relevant aspect is the possibility that PVN could masquerade as neovascular AMD, leading to misdiagnosis [8]. The aim of this review is to summarize the main aspects of pachychoroid neovasculopathy within the whole spectrum of pachychoroid disease, from the pathogenetic mechanisms to the management and treatment options.
Pathogenesis
Choroid is a tissue characterized by vascularized and pigmented cells. Five different layers compose the choroid, from the innermost, represented by the Bruch’s membrane (BM), to the outermost, namely suprachoroid lamina. The tree central layers carry out the main functions regarding the pathophysiology of the pachychoroid disease. The choriocapillaris is located right after the BM and consists of the fenestrated veins that supply the outer layer of the retina. In the middle is positioned the Sattler’s layer, formed small oval vascular patterns. Haller’s layer is composed of large outer vascular patterns [9].
The single entities included in the spectrum of pachychoroid disease (PPE, CSC, PVN and PCV) share 3 main common features:
a) Increasing in choroidal thickness.
b) The presence of pathological venous dilation at the level of the Haller’s layer.
c) Thinned choroid in Sattler’s and choriocapillaris layers [10].
Starting from these alterations in the morphology and physiology of the choroid, it seems to exist like a “common thread” in the evolution of the pachychoroid disease, leading to the development of a Type 1 neovascularization and, ultimately, to the formation of polyps. It has been demonstrated that PPE could be caused by the mechanical compression of the choriocapillaris and Sattler’s layer by the dilation of the underlying large outer choroidal vessels located in the Haller’s layer [11]. Choroidal hyperpermeability and congestion play a fundamental role in the pathogenesis of CSC, generating the presence of subretinal fluid [12]. The exact mechanism of CNV formation within PNV is still not well understood, but it could be important to analyze it as a dynamic process in which the growth of new vessels is strictly linked with choroidal flow changements. In the recent literature, was established the association between long-standing CSC and Type 1 CNV [13]. Chronic RPE changes and long standing PED allow the ingrowth of sub RPE endothelial cells [14]. Because PPE and CSC share similar pathophysiological mechanisms, it could be possible that eyes with long standing PPE have a predisposition to develop Type 1 CNV, without the typical manifestations of AMD, such as drusen or degenerative changes [7].
Moreover, Miyake et al. [8] have found that patients affected by PVN have a different genetic susceptibility to AMD compared with patients affected by neovascular AMD, suggesting a different etiology of the two conditions. The recent introduction of the Optical Coherence Tomography Angiography (OCTA) has allowed an additional step forwards to better understand the formation of new vessels within the PVN. Azar et al. [15] have proposed that the ingrowth of new vessels may represent an effort to reform the choriocapillaris in response to the dilation of the outer choroidal layer. This could, in part, explains why the new vessels are localized adjacent to the border of the dilated choroidal vessels.
Diagnosis
Although there are no recommended diagnostic criteria for PVN, there are numerous reports describing the principle features of the single entities within the spectrum of pachychoroid disease [16]. Because PVN represents a neovascular late complication of long standing PPE and chronic CSC [7,17], understanding the clinical characteristics of these 2 pathologies allows to the physicians to make a proper diagnosis. PPE is characterized by the absence of normal tessellation by fundus examination; the presence of thick choroid and large choroidal vessels underlying an area with small elevations of the RPE and, occasionally, small PED, observed by OCT; ICGA findings of choroidal hyperpermeability corresponding to the RPE abnormality areas; hyperautofluorescence recognized with the use of the fundus autofluorescence (FAF). Pang [7] described as “PVN” the findings of Type 1 neovascularization in association with choroidal thickening and dilated choroidal vessels, in the absence of AMD or other degenerative changes, which could eventually progress to PCV. Miyake et al. [8] diagnosed PVN when all of the following criteria were met:
a) CNV in either eye.
b) Subfoveal choroidal thickness > 200 μm in both eyes.
c) No drusen or only non extensive (total area, ≤ 125 μm circle) hard drusen (≤ 63 μm) in both eyes (AREDS category) 1, no AMD.
d) Characteristics of PPE or CSC.
Therefore, it can be affirmed that PVN can be differentiated from neovascular AMD by the absence of soft drusen, the presence of a thick choroid with pachyvessels and younger age at onset of neovascularization.
In the last years the introduction of OCTA has enabled the comprehension of the pachyvessels, mostly regarding the finding of neovascularization below a PED [18,19,15]. Pigment epithelial detachments in eyes affected by pachychoroid disease often shows a shallow, flat and irregular morphology, that closely resembles the “double layer sign”, firstly described by Sato in patients with PCV [20]. Dansignani et al. [18] showed, with the aim of OCTA, that 95% of the shallow irregular PEDs harbored Type 1 CNV, in the form of tangled vascular networks. The previous OCTA findings were confirmed by Azar et al. [15], which described the presence of tangled filamentous new vessels overlying a focal area of thickened choroid in patients with typical characteristics of PPE. Further reports regarding the fundamental role of OCTA in detecting CNV within the pachychoroid spectrum were given by the study of Demirel et al. [19]. Certainly, they demonstrated a greater sensitivity of OCTA, compared to conventional dye angiography (FA and ICGA), in detecting the presence of new vessels in case of shallow and irregular flat PED within pachychoroid disease. It can be assert that the presence of shallow, flat and irregular PED detected by EDI OCT and the OCTA sign of tangled filamentous new vessels in a patient with pachychoroid features, could lead to the diagnosis of pachychoroid neovasculapathy.
Management
Neovascular AMD has been managed with various treatment modalities, comprising laser photocoagulation, surgical removal of fibrovascular tissues, photodynamic therapy (PDT), and intravitreal injections of anti-vascular endothelial growth factors (anti-VEGF). Anti-VEGF injections are currently the standard therapy for neovascular AMD in accordance to the two prospective investigations (the Marina [21] and Anchor [22] studies), demonstrating the improvement and the stabilization of visual acuity. Ranibizumab and Aflibercept have been given regulatory approval for ophthalmological applications, while bevacizumab is used only off-label. The effects of all anti-VEGF agents effect via the inhibition of VEGF-A, while only Aflibercept inhibits also VEGF-B and placental growth factor [23]. Various treatment modalities are available: monthly injections [21,22]; pro re natal treatment [24] and treat and extend modality [25]. Although the management of neovascular AMD and pachychoroid neovasculopathy remains the same, requiring anti-VEGF agents as first line therapy, the genotypical and phenotypical difference between these 2 entities may influence the natural course and the prognosis of the disease. Miyake et al. [8] demonstrated that patients with PVN has a significantly longer retreatment period than those with neovascular AMD after a loading dose of anti-VEGF, in this case Ranibizumab. Hata et al. [26] analyzed the concentration of VEGF into the acqueous humor reporting a significant lower concentration of VEGF in patients affected by PVN in comparison to VEGF concentration in eyes with neovascular AMD. They also found a significant correlation between VEGF concentration and the response to 3 monthly anti-VEGF loading dose treatment, suggesting that high VEGF concentrations at baseline may predict a poor response to anti-VEGF therapy in PVN.
Matsumoto et al. [27] have investigated the efficacy of intravitreal aflibercept treatment using treat and extend regimen in PVN and Type 1 neovascular AMD. They found that treat and extend regimen of intravitreal aflibercept injection may be equally effective in terms of BCVA improvement and exudative changes both in PVN and neovascular AMD. At the same time, it has been observed that: central choroidal thickness decreasing was greater in PVN group; eyes affected by PVN required fewer injections, especially for eyes with signs of polypoidal lesion compared to PVN eyes without polyps. Moreover, Padròn Perez et al. [28] have examined changes in choroidal thickness in patients with PVN treated with intravitreal injections of anti-VEGF. A significant choroidal thickness reduction was detected by the authors after 12 months of anti-VEGF treatment. Furthermore they observed a strict correlation between the number of injections and choroidal thickness, suggesting that more intravitreal injections may lead to a greater choroidal thickness reduction.
Conclusion
Pachychoroid disease spectrum includes 4 different retinal pathologies, once considered as separated single entities, such as PPE, CSC, PVN and PCV. They share common pathogenetic and morphological characteristics, arising from the alteration of choroidal vascular physiology.
Pachychoroid neovasculopathy consists of the following main features:
a) younger age of presentation compared to neovascular AMD.
b) absence of normal fundus tessellation and degenerative changes of AMD, like soft drusen.
c) EDI OCT signs of increased choroidal thickness and veins dilation just below an area of RPE abnormalities.
d) choroidal hyperpermeability detected by ICGA, corresponding to the RPE alterations.
e) shallow irregular PED overlying the area with increased choroidal thickness and dilation of choroidal vein.
f) OCTA finding of tangled filamentous vascular networks.
https://lupinepublishers.com/ophthalmology-journal/pdf/TOOAJ.MS.ID.000111.pdf
https://lupinepublishers.com/ophthalmology-journal/fulltext/pachychoroid-neovasculopathy-within-the-spectrum-of-pachychoroid-disease.ID.000111.php
For more Lupine Publishers Open Access Journals Please visit our website: https://lupinepublishersgroup.com/
For more Trends in Ophthalmology Please Click
Here: https://lupinepublishers.com/ophthalmology-journal/
To Know more Open Access Publishers Click on Lupine Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers Follow on Twitter : https://twitter.com/lupine_online
0 notes
Text
NEGATIVE PRESSURE WOUND THERAPY (NPWT)
Negative Pressure Wound Therapy (NPWT) is also known as topical NPWT, Vacuum sealing technique, sealed surface wound suction, Vacuum Assisted Closure (VAC) or Vacuum pack technique. This essentially involves the application of sub atmospheric pressure to a healing wound with the help of a porous dressing and a drain attached to a vacuum device and covered with a polyurethane film to make an air tight seal.
Since its introduction in the early 1990’s NPWT has become widely used in the management of complex wounds of various etiologies in in- patient as well as out- patient settings with significant success. It has now become gold standard of care for wounds like open abdominal wounds and dehisced sternal wounds following cardiac surgery. It is also now being applied in home care settings.
Principles and Mechanism of action of NPWT21
- Functional Principle
The principle of NPWT involves extending the usually narrowly defined suction effect of drainage across the entire area of the wound cavity or surface using an open-pore filler that has been fitted to the contours of the wound.
To prevent air from being sucked in from the external environment, the wound and the filler that rests inside or upon the wound are sealed with an airtight adhesive polyurethane drape that is permeable to water vapor, transparent, and bacteria proof. A connection pad is then applied over a small hole that has been made in the drape and connected to a vacuum source by means of a tube.

MECHANISM OF ACTION
NPWT acts in different ways to promote wound healing. The wound is subject to suction pressure that is propagated through the wound filler to the wound bed. This suction drains exudate from the wound and creates a mechanical force in the wound edges that result in an altered tissue perfusion, angiogenesis and the formation of granulation tissue.
The following effects of NPWT on the wound healing and the affected tissue are considered to be the clinically significant mechanisms of action for this therapy.
- Creating A Moist Wound Environment
A moist environment is vital in wound healing as it facilitates the re- epithelialisation. However, in an overly moist wound, exudate may cause infection and maceration, leading to damage to the wound edge. Removal of exudate is important to prevent the accumulation of necrotic tissue and slough that tend to continually accumulate in wounds
and alter the biochemical and cellular environment. The accumulation of necrotic tissue or slough in a wound promotes bacterial colonisation and hinders repair of the wound. NPWT balances these effects, providing a moist wound environment while removing excess fluid.
- Removal Of Edema
Edema causes increased pressure on the wound tissue, which in turn compromises the microvascular blood flow, reducing the inflow of nutrients and oxygen. This reduces resistance to infections and inhibits healing.
NPWT causes compression of the tissue closest to the surface of the wound, which is believed to reduce interstitial edema
- Mechanical Effects On Wound Edges
NPWT mechanically stimulates the wound bed and produces a suction pressure on the wound edges that push onto the wound and contract it. These mechanical effects lead to tissue remodeling that may facilitate wound closure. It has also has been found that the wound tissue and the filler material interact on a microscopic level to micro deform the tissue. These mechanical deformations affect the cytoskeleton of the cells and initiate a cascade of biological reactions that may accelerate the formation of granulation tissue and subsequent wound healing by stimulating the expression of angiogenic growth factors and receptors, such as vascular endothelial growth factor (VEGF), VEGF receptors and the angiopoietin system receptors.
- Change in perfusion
NPWT has been shown to improve perfusion in a few studies.
NPWT exerts compression on the tissue leading to an anti- edematous effect that might promote perfusion. It has also been speculated that the negative pressure causes a force in the tissue that opens up the capillaries, increasing flow.
- Change in bacterial count, bacterial clearance and immunological effects
NPWT offers a closed system for wound healing, as the adhesive drape provides a barrier against secondary infection from an external source and has been suggested to reduce the bacterial load in the wound. However it must be emphasized that the degree of bacterial colonisation is unrelated to the success or failure of NPWT. It is exceedingly important to perform proper debridement between dressing changes to mechanically remove the microorganisms.
- Molecular mechanisms in wound healing
It is hypothesized that the NPWT device induces the production of pro-angiogenic factors and promotes the formation of granulation tissue and healing.
NPWT is also associated with an up-regulation of basic fibroblast growth factor (bFGF) and extracellular signal-regulated kinase (ERK) 1/2 signaling, which may be involved in promoting the NPWT-mediated wound healing response.
NPWT also influences the local expression of pro inflammatory cytokines in tissue or fluid from acute infected soft-tissue.

Advantages of NPWT
- Handling
o Hygienic wound closure—bacteria proof wound dressing for sealing the wound so no external bacteria can enter the wound and the patient’s own wound bacteria are not spread. This is particularly important in the event of contamination with problematic bacteria, as in patients with methicillin-resistant Staphylococcus
aureus (MRSA)-infected wounds. Thus, it also reduces the risk of cross-infections and development of resistance within the hospital
o Transparent dressing permits continuous clinical monitoring of the surrounding
skin through the film with which the wound has been sealed.
o Odorless and hygienic dressing technique; constant seeping through the dressing onto the patient’s clothing and bedding can be avoided, reducing demands on the nursing staff
o Reduction in the number of required dressing changes (only necessary every two to three days), which reduces nursing time requirements, particularly in patients with exudating wounds.
o Compared with other forms of wound dressing, NPWT is easier to tailor and maintain in position. Almost every configuration of wound, including circumferential extremity wounds and wounds located in proximity to orthopedic fixation frames, can be managed with relative ease.
- Patient comfort
o Easy and early patient mobilization: Accelerated wound healing with NPWT significantly reduces the time to wound closure in diabetic patients, returning these patients to baseline more quickly and improving quality of life.
o Visually appealing dressing method due to clean, exudate-free dressing conditions, even during mobilisation.
Treatment Goals with NPWT22
- Provide a temporary wound cover
- Manage wound fluid and edema
- Accelerate patient mobility
- Improve pain management
- Prevent wound progression
- Increase dermal and wound perfusion
- Stimulate formation of granulation tissue
- Enhance wound bed epithelialization
- Improve matrix material availability
- Reduce bacterial load
- Provide moist wound environment
- Influence expression of genes involved in wound healing
Treatment variables & Precautions
There are a number of different treatment variables related to NPWT that might affect wound healing. The level of negative pressure, the wound filler material (foam or gauze), the presence of wound contact layers, the pressure application mode (continuous, intermittent, or variable), or instillation of fluid may be chosen according to patient needs, disease, wound type and shape.
- Pressure level/ suction strength
Low pressures may be ineffective in draining the exudates while high pressures may be painful and have a negative effect on the microcirculation. The preferred range of pressure is between – 50 to -150 mm Hg. However there is no significant evidence to suggest an ideal clinical range and it is suggested that the negative pressure may be adjusted according to the circumstances. For example in patients with pain it can be appropriate to choose a suction setting less than −125 mmHg. Pressures as low as −40 mmHg may be used for the treatment of sensitive, poorly perfused tissue where there is a risk for ischemia, for example in the case of circumferential dressings, vascular disease, diabetic foot ulcers (DFUs) and thin skin transplants.
High output wounds will require high pressures for initial few days which may be lowered once the exudate lessens.
- Vacuum Source
Traditional NPWT systems use an electrically powered pump to generate negative pressure at the wound bed. Recently portable devices that deliver NPWT without the use of an electrically powered pump have been introduced. These smaller light-weight devices are either battery powered or mechanically powered, and generate continuous sub-atmospheric pressure level to the wound bed between −75 and −125 mmHg. These devices allow the patient to be mobile and independent from hospital’s wall-suction on the ward and to be treated by NPWT in the home care setting.
Some battery-powered NPWT units use an electronically controlled feedback system that ensures the maintenance of the selected pressure level even in the presence of small air leaks, guaranteeing the effectiveness of NPWT. Additionally, audiovisual alarms alert the staff and the patients to large air leaks (loss of seal), blockage of the tubing and full canisters. These therapy units are designed to reduce complication and allow faults to be promptly recognized.
- Intermittent or continuous mode
NPWT can be applied in an intermittent or continuous or variable mode. The most common mode used currently is the continuous mode. Intermittent mode involves repeatedly switching on and off (usually 5 minutes on to 2 minutes off), while variable NPWT provides a smooth cycling between two different levels of negative pressure.
NPWT with intermittent suction has been shown to be of benefit for wound healing. This mode produces a mechanical stimulation of the wound bed (a massaging effect) and a greater circulatory stimuli, oxygenation and angiogenesis, and presumably a lower risk of occurrence of ischemic damage. Intermittent mode is however known to cause more pain. New pressure cycles, without going to 0 mmHg suction, but only lowering the suction, to
50 % for example, should be able to maintain the highest degree of blood vessel
formation and also a significant decrease in pain compared with the traditional intermittent group.
Under special wound conditions, when the wound involves structures such as the peritoneum, between toes, in tunneling injuries, in sternotomies, in the presence of high levels of exudate and when using NPWT on grafts or skin flaps, the continuous mode is the option of choice.
- Wound fillers
The choice of wound filler has been shown to have significant effect on wound healing. The commonly available material for this include foams and gauges with different pore sizes, stability etc.
The pressure distribution is similar for gauze and foam in dry wounds and the differences in performance are related to the structure of the material and its mechanical effects in the wound. The degrees of micro- and macrodeformation of the wound bed are similar after NPWT regardless of whether foam or gauze is used as wound filler. The increase in blood flow following NPWT is also similar with all wound fillers.
Wound contraction is more pronounced with foam than with gauze. Wound fluid retention is lower in foam, while more fluid is retained in the wound when using bacteria and fungus-binding mesh.
Thus NPWT may be tailored to the individual wound type to optimise the effects and minimize the complications by choosing different wound fillers. The choice of filler may be made with regard to the morphology of the wound, the wound characteristics, the patient feedback, possible infection and scar tissue formation.
o Wound morphology
Different wounds have different shapes and depths and thus require different fillers.
Foam may fit better into a wound with a uniform shape, while gauze may be easier to apply in wounds that have an irregular shape, or with undermining edge since it can be better manipulated to the shape of the wounds. Different wound fillers can also be combined. Over a thin graft or a wound sleeve, the gauze will give a more appropriate cover. Foam may be advantageous for ‘bridging therapy’ since the foam compresses to a greater extent and thereby contracts the wound and speeds up the closure.
o Exuding wounds
In heavily exuding wounds, foam at a higher pressure (−120 mmHg) may be useful, Since foam is less dense than gauze and a higher level of negative pressure drains the wound quicker.
o Wounds at risk of ischemia
In circumstances where there is a risk of ischemia, a lower pressure (−40 to −80 mmHg) and using gauze may be considered.
o Infected wounds
Various wound fillers designed for infected wounds include foam with silver, gauze that is impregnated with PHMB, gauze that is impregnated with silver, bacteria and fungus binding mesh.
Instillation techniques allow the irrigation of the wound with antiseptic solutions.
o Tendency to form excessive granulation tissue
Areas such as joints, where movement of the skin and the underlying tissue occur, may benefit from the use of gauze. Foam allows rapid growth of granulation tissue and may be a better choice in wounds where large amounts of granulation tissue is desirable, for example, postsurgical wounds such as sternotomy wounds.
- Wound contact layers
Removal of foam used as fillers in NPWT can lead to pain and trauma. When the clinician anticipates such complications, a non- adherent wound contact layer such as paraffin or silicon may be placed over the wound bed beneath the wound filler. A wound contact layer also may be placed over vulnerable structures such as blood vessels or nerves. This wound contact layer may reduce the pain during dressing changes.
However keeping the quality of wound healing in mind, it is important to use wound contact layers only when there are structures to protect, in order not to slow healing.
- Protection of tissues and organs
NPWT can at times be the only option available to the treating clinician for managing highly infected and large wounds. However of late there have been reports of serious associated complications of NPWT.
The risk of right ventricular rupture and bypass graft bleeding following NPWT of mediastinitis is estimated to be between 4–7 % of all cases treated. Severe bleeding of large blood vessels such as the aorta has also been reported in several patients receiving NPWT. The incidence of NPWT-related bleeding in patients with exposed blood vessels or vascular grafts (such as femoral and femoral-popliteal grafts) in groin wounds were relevant in some studies. Severe bleeding has also been reported in patients receiving NPWT for burn wounds.
FDA has thus stated that NPWT is contraindicated in certain types of wounds: those with necrotic tissue with eschar, in non- enteric and unexplored fistulas, where malignancy is present, in wounds with exposed vasculature, anastomotic sites, exposed nerves, exposed organs and untreated osteomyelitis.
It has been suggested that exposed sensitive structures need to be protected either through the interposition of autologous tissue (muscle flaps) or with heterologous material (dermal substitutes) or a number of wound contact layers.
- Pain treatment
Pain is a significant issue associated with NPWT and may affect patient adherence.
In patients that are neuropathic or paraplegic, where the pain is not of a significant nature, the filler can be used efficiently. In patients with low adherence, especially children and the elderly, and on painful lesions (such as pyoderma gangrenosum, burns, PUs and infected wounds), gauze, tends to be better tolerated.
Another option to reduce pain due to NPWT is to prepare the patient by infiltration of the wound filler with saline solution or local anaesthetics before dressing change.
Clinical Applications
- Acute wounds
Acute wounds are often traumatic but can also be due to surgical debridement of infected or necrotic tissue. Wounds requiring extensive surgical debridement often present a wound dressing challenge due to anatomic location (eg, Fournier's gangrene), the size of the tissue defect, or the patient's body habitus.
- NPWT dressings can be applied immediately following operative debridement, which simplifies postoperative wound care. The ability of the foam and adhesive dressing to conform to almost any wound contour, shape, or size contributes to the success of NPWT in these complex wounds.
- NPWT can also be used in conjunction with skin grafts or flaps, which are frequently needed to cover tissue defects. NPWT has been used instead of traditional bolstering
methods to provide skin graft fixation. The NPWT dressing distributes negative pressure
uniformly over the surface of the fresh graft, immobilizing the graft with less chance of shearing leading to improved qualitative skin graft take and quantitative improvements in skin graft success.
- For acute open wounds, NPWT is associated with a reduced time to wound closure. NPWT has also been used to manage acute wounds resulting from lower extremity fasciotomy, degloving injury, open amputation, and complex traumatic wounds with exposed tendon, bone, or orthopedic hardware.
- NPWT may also have a particular role in the treatment of burn wounds.
- Chronic wounds
- NPWT may improve the healing of some types of chronic wounds/ulceration, such as diabetic foot ulcers, pressure ulcers, and open abdomen, provided that the wounds are well vascularized
- Post-sternotomy mediastinitis is an uncommon but devastating complication of cardiac surgery which may require the use of NPWT to manage the open wound while awaiting sternal closure.
- Prophylactic use
Evidence suggests that there may be a prophylactic role for NPWT in reducing the rates of surgical site infections.
Indications of NPWT
Trauma & Orthopedic Surgery
- Acute trauma & dermatofasciotomy wounds
NPWT is a tool in the treatment of traumatic wounds and high-risk incisions after surgery in
- contaminated acute wounds (open fractures, penetrating injuries) and wounds with tissue defects requiring a step wise procedure.
- amputation stump resulting from a guillotine-like marginal zone amputation.
- management of motorcycle spoke heel injury
- perineal trauma-related wounds.
- Periprosthetic infections of the hip and knee joint
NPWT is a useful option in the management of early or delayed infections following implantation of an endoprosthesis.
- Osteomyelitis and surgical site infection
- Exposed tendon, bone and hardware
NPWT is the treatment of choice in such conditions where plastic surgery procedures cannot be used to provide immediate cover to exposed structures.
Acute burns and scalds
The management of burns with their associated high-fluid exudate following burn excision and skin grafting has always posed a challenge in burn wound care. The ideal dressing should protect the wound from physical damage and microorganisms; be comfortable and durable; allow high humidity at the wound; and be able to allow maximal activity for wound healing without retarding or inhibiting any stage of the process. NPWT fulfils all these criteria.
Plastic and reconstructive surgery
NPWT has produced a change of paradigms within the treatment algorithms in traditional reconstructive surgery.
NPWT may represent a valid alternative to immediate reconstruction in selected cases of acute complex traumas of the lower limb.
NPWT is a valid tool for reliable fixation of skin substitutes, such as tissue-engineered skin
substitute and split skin grafts in all severe traumatised wounds and is associated with improved graft survival.
Abdominal Surgery
NPWT is one of the techniques used for temporary abdominal closure (TAC) in laparotomies with packing, ACS or severe septic intra-abdominal complications.
Cardiovacscular Surgery
While NPWT is the method of choice for management of poststernotomy mediastinitis, its use is also being advocated over closed sternal incisions to reduce the incidence of deep sternal wound infection.
Vascular surgery
- Infected blood vessels and vascular grafts
For high-risk surgical patients with a fully exposed infected prosthetic vascular graft, NPWT along with aggressive debridement and antibiotic therapy is an effective alternative to current management strategies.
- Lymphocutaneous fistulas
NPWT can be used for the management of lymphocutaneous fistulae resulting from axillary and inguinal lymph node dissections as well as surgery of the infra-inguinal vessels.
Non-healing wounds
- Leg ulcers
Although NPWT has been used extensively in non healing leg ulcers, there is limited rigorous RCT evidence available concerning the clinical effectiveness of NPWT in the treatment of leg ulcers.
- Pressure ulcers
NPWT is increasingly being used to manage PUs, most likely due to its flexibility, which allows the caregivers to insert it into a more complex and articulated therapeutic strategy.
- Diabetic foot ulcer
The rationale for adopting NPWT in DFUs is related to its capacity of removing exudate, protecting the wound from exposure to the environment, reducing odor and helping debridement. Complications of NPWT
NPWT is generally safe and well tolerated. Some of the important complications include bleeding, infection, pain, organ damage, and possibly death. Such complications are most likely to occur when NPWT is applied to patients whose wounds have devitalized tissue or exposed vital structures (eg, organs, blood vessels, vascular grafts).
- Bleeding
Bleeding is the most serious complication of NPWT and can occur in hospitals, long-term care facilities, and at home. Minor bleeding during dressing changes due to granulation tissue at the base of the wound is common and is best managed with firm pressure to the wound surface.
Severe hemorrhage can occur during removal of foam that has become adherent to the granulation tissue below, especially in patients who are anticoagulated, or in patients with exposed vessels or vascular grafts. In patients with severe bleeding, direct pressure should be applied and emergency services contacted. Surgery may be needed to control bleeding.
- Infection
Infection related to the use of NPWT is often due to prior wound infection that was inadequately controlled prior to initiating NPWT. When infection is suspected the NPWT dressing in discontinued, the wound irrigated and debrided, wound cultures obtained, and empiric antibiotics initiated.
- Enterocutaneous fistula
Contraindications of NPWT
- Risk of bleeding
NPWT should not be applied in wounds with manifest bleeding or a risk of bleeding, as suction could result in a significant blood loss.
Additionally the bleeding can clot the foam and therefore stop any function of the NPWT device.
- Exposed vessels and vascular prostheses
FDA has stated this as one of the contraindications for the use of NPWT.
- Necrotic wound bed
Necrotic tissue acts as a barrier to new tissue growth. The use of NPWT must therefore be preceded by radical debridement.
- Untreated osteomyelitis
Due to the deep extension of a potential osteomyelitic focus, simple surface treatment is unlikely to be successful. In this case, treatment must include the radical removal of the focus of infection.
- Malignant wounds
NPWT is known to promote granulation tissue growth and is therefore used for the purpose of improving tissue perfusion and enhancing granulation tissue formation. As a consequence, it should not be used in the presence of malignant neoplastic tissue.
NPWT can be useful as a purely palliative measure in inoperable cases. When used as a purely palliative measure, it allows wounds to be covered in a hygienic and clean manner.
#Negative Pressure Wound Therapy#Negative Pressure Wound Therapy manufacturers#Negative Pressure Wound Therapy suppliers
0 notes
Text
Biomed Grid | A Review: Targeted Cancer Therapy as a Fight Against Brain Tumor
The Brain tumor can be treated by surgery, radiation, and chemotherapy. With the implications of all these strategies, the overall survival rate of a glioblastoma patient is only 14.6 months after diagnosis [1] . Moreover, the brains of children are very sensitive to tolerate these intense treatment strategies. So, there is a need to discover such agents that particularly target the malignant cells while causing no harm to normal cells [2] . Treatment of brain tumor is very difficult due to certain barriers that make it difficult for the therapeutic agent to reach the targeted infectious cell or tissue.
The major obstacle is the Brain Blood Barrier (BBB) that does not allow any drug or foreign substance to reach to the brain [3]. Another barrier is the enzymes present in BBB e.g. phosphatase enzyme that attack these molecules due to these barriers. So, most drugs have no access to the CNS [4]. Now the particles having to target specificity, i.e. antibody/Nanoparticles can cross this barrier and can reach these affected cells [5]. But the passage of targeted drugs. i.e. protein, oligonucleotides, peptides is interrupted very often [6].
With the identification of tumor-specific antigens, it became possible to develop such therapeutic methods that selectively target diseased cells. Targeted therapies are being used for the treatment of the brain tumor that specifically targets the pathways that are responsible for the disease [7].
Case Report
Brain tumor includes diminishing distinction and deviant multiplication of precursor cells. Recent research has described that cancer-causing events make the change from asymmetrical to symmetrical cell division. The most common malignant tumors in adults and children are Glioma and Medulloblastoma (MB). Glioma is further characterized to Grade I, II, III and grade IV [8]. Grade I is called Pilocytic astrocytoma and mostly occur in children. On the other hand, Grade II and III termed as oligodendroglia astrocytoma, oligoasrocytoma ependymoma is reported to occur in both adults and children’s equally [5] same as vast depict of MBs has accepted numerous subgroups of tumors [9]. Inspire of Glioma and MB cells exhibit definite glial and neuronal makeup, both types of brain tumors have been delineated to emerge from neural indication, competent to show neurogenesis. The ventricle present between the forebrain and spinal canal form glial cells [10]. Glial Fibrillary Acid Protein (GFAP)showing neural stem cells (NSCs) that are also termed as Type B1 cells are considered to sustain asymmetrical cell division to produce transit magnifying ancestors (TAPS, Type C cells), that stimulate differentiation into unripe neuroblast [11].
Obstacles in brain tumor treatment
There are many obstacles to the treatment of brain tumor due to the complex nature of the brain, different morphological characteristics of each type of brain tumor, inaccessibility of therapeutic agents to the tumor cells, difficulty in specifically targeting of tumor cells while giving no harm to surrounding normal cells and sometimes due to the development of resistance against chemotherapeutics agents [12]. One of the major obstacles is the brain blood barrier.
Brain blood barrier
Brain blood barrier (BBB) which protects the brain can be considered as the major obstacle to the therapeutic agent [3]. It includes endothelial cells, pericytes, astrocytes, and neurons. Brain capillary endothelial cells (BCEC) are the major boundary between the brain and cellular tissues. The function of pericytes is to maintain and support the BCEC [13]. Astrocytes are the nonneuronal cells that release signal to regulate the permeability of endothelium [1] . Neurons supply capillary to the BBB with nerves. So, the major barrier component is BCEC [14]. Another barrier is the enzymes present in the brain blood e.g. phosphatase enzyme that attack foreign molecules and limit their access to the CNS [6]. With the formation of brain tumor, infectious cells begin to attack healthy brain tissue and after growing to a certain volume they also give harm to the BBB and formation of blood-brain tumor barrier occurs [15]. Another barrier that limits the entry of therapeutic agents is the blood-cerebrospinal fluid barriers (CSF) [16]. It is also composed of epithelial cells which form a barrier and do not allow any therapeutic agent to enter the brain parenchyma. So, most of the molecules failed to reach the targeted site [17].
Targeted strategies for the Treatment of Brain Tumor
The mediate general survival of patients with glioblastoma (GBM) is only 14.6 months after recent multimodal treatment aggressive surgical abscission followed by coincident or consecutive radiation temozolomide chemotherapy [18].
BBB targeting strategies
The Blood Brain Barrier is not only responsible for the protection of the brain from harmful substances but is also involved in providing the necessary nutrients to the brain. It is the main challenge in drug delivery to the brain [15].
Absorptive-Mediated transcytosis
It furnishes a way for the transport of drugs beyond BBB by cationic protein or cell-Penetrating peptide. Classic cationic bovine serum albumin accumulated with pegylated nanoparticle (CBSANP) was designed for brain targeting drug delivery [19]. It was stated that plasmid PORF-hTRAIL (pDNA) subsume (CBSA-NP) abreast with glycoprotein in brain and tumor micro variability and assembled in tumor cells [19,20] arrogate other cationic protein called wheat germ agglutinin (WGA) associated to the surface of liposomes and also reveal elevated BBB transporter.CPP has been used to lessen the lipophilic barrier of the cell membrane and transport large diversity of baggage which involves peptides, DNA, antibodies, toxins and Nano drug carrier such as liposomes and micelles [21].
Transporter-mediated transcytosis
It is substrate selective so the drugs that intimate the endogenous substances would be delivered to the brain. Glucose Transporters (GLUT) which ease the delivery of glucose from the blood to the brain, have inclusive approach use in brain quarry. Another chief system is choline transporter which ties up positively charged ammonium group or simple cations [22]. The glazed nanoparticle was skill full to hybrid an in vitro replica of BBB (Bovine BCEC).
Receptor-mediated transcytosis
This is the technique that involves the only drug delivery that bosom imitates the endogenous carrier and then delivered to the brain. One of the most accepted receptor-mediated transcytoses for brain targeting is the Transferrin Receptor (TfR) [23]. These are stacked with polyphosphoester Hybrid Micelles (TPM). It expresses a strong anti- Glioma activity [11]. The low-density lipoprotein (LDC) and Receptor-related protein (LRP) have been noticed to mediate delivery of different ligand associated with Nano carrier beyond the BBB [24].
BBTB targeting strategies and drug delivery
It is present between brain tumor tissues and micro vessels devised by extremely peculiar endothelium cells (ECs) restricting the transforming of the hydrophilic molecule to tumors [25]. The techniques suggested for BBTB are mostly constructed on receptor highly intimate on the tumor as epidermal growth factor receptor and integrity. Arginine glycine aspartic acid (RGD) is going to be used for targeted drug transformation to the brain.
Antibody-Targeted Therapeutics
Antibodies can be used for this purpose, which can easily identify the foreign toxic substance. It can easily cross multiple barriers including BBB and selectively accumulate in tumor cells [26]. As in the brain tumor, new blood vessels are also formed termed as angiogenesis assisting in growth and proliferation of brain tumor. These molecular targets inhibit angiogenesis as a treatment of the brain tumor. An antibody named as Bevacizumab VEGF (Vascular endothelial growth factor) is very efficient because it targets the mediator of angiogenesis [1] . Cetuximab IgG1 antibody can block this receptor [1] . EPGR (Epidermal growth factor receptor) show interaction with the protein kinase C which signals to activate transcription. Panitimumab and Erlotinib can also be used as an inhibitor of EPGR. Erlotinib can be used in combination with radiotherapy to treat glioma and be tolerated by the patients. It can be taken orally. Nimotuzumba can also especially target tumor cells due to the overexpression of EPGR and give no harm to cancer cells [2] .
Nanoparticle targeted therapy
Nano Technology put great work on brain Tumor and Open the doors for future treatments and researches for the brain tumor. Nano Particles especially play a distinct role in Brain tumor’s treatment, surgery and pharmacology researches. Today researches in Nanoparticles have proved a major contributor role for more future work on the brain tumor.
Importance of Nano Particles
Nanoparticles are important in the treatment of the brain tumor because they can be used by two mechanisms:
a. Specific mechanisms
b. Nonspecific mechanisms
Specific mechanism based on the interaction of antigens on the surface of nanoparticles with tumor cell receptors. Non-specific mechanism based on preference extravasation of nanoparticles that access the blood-brain barrier (BBB or tumor). Nanoparticles only access the tumor because they have more retention effects than other particles that do not access the tumor.
Peptide vaccine
In nanoparticle, peptide vaccine helps to target the single or multiple Nano antigens. Some peptide vaccines are EGFRviii, IL- 13RA2, AIM-2, and MAGE-19 [27,28]. These vaccines are more responsive in patients and tumor patients survived 26 months on average. Peptide vaccine can cause profusion of the cell which does not indicate the targeted antigens. Targeting multiple antigens was the most effective treatment of the tumor [29].
Administration Techniques for Molecular Therapeutics
Molecular targeting agents can be administrated toward the targeted site by systematic, intracranial and cell-mediated delivery.
Systematic administration
Systematic Administration of targeted therapeutics is a very easy technique for cargo transportation involving dreary quantity [13]. One evident technique for transportation of these molecules is through Intravenous (IV) injections. The nanoparticle established remedial are frequently administrated in many doses to control the growth of the tumors [30]. Low-frequency enrapt ultrasound supplies confined interference of BBB [31-33]. The preclinical studies have shown that this technique can purely increase the central delivery of the remedial agents into tumor cells [31]. Another method to ease the non -invasion technique is Magnetic targeting at a specialized site [34,35] has been practiced in clinical trials (Clinical Trails Gov. Identifier NCT0005495, NCT00034333). It is reported that magnetic targeting produces a 5times enhancement in the total subjection of glioma cells to the nanoparticle over not specific and non- targeting tumors and increase in target selection for collection in the tumor versus normal brain cells [13]. The main drawbacks of systemic delivery are the danger for the accumulation of nanoparticle in Nano-targeted organs like the liver, kidney, and lungs. The nanoparticle is not hazardous but the longtime interaction of nanoparticle deposition in the brain has not fully addressed [36].
Intracranial administration
Delivery of therapeutic agent to the targeted site in the brain tumor is a hard job without affecting normal cells. We can enter a therapeutic agent directly into the tumor site that can easily pass the BBB barrier [37]. We can use polymers that are capable to target tumor cells while sparing normal cells. These polymers can be degradable or non-degradable [38]. This method is mostly used when localization of drug to the target is low. It increases the concentration of such an agent within affected cells only. It depends on transporting a large amount of therapeutic agent in a continuous flow [39].
Cell-mediated administration
Cell-mediated delivery of synthetic nanoparticle-based drug carrier is a very promising strategy to control the distribution of the drug and improve targeting. In this method, cells are found the ability to cross the tumor. Mesenchymal stem cell (MSC) and neural stem cells show the ability to target the brain tumor cells by nanoparticle.
MSCs with bound silica Nano rattle-doxorubicin can be targeted in vivo and in vitro ways on the tumor [40]. It was noticed that Nano capsule containing ferrociphenol can be entered into the tumor and can slow the growth of the tumor [41].
The ability of ph-mediated drug release capabilities. This can deliver doxorubicin conjugated to BBB. This method of combining stem cells and nanoparticle for treatment based upon local administration method.[36]
Another way of treatment is that to combine the carriers and nanoparticles and carriers are tracked via trucker. For this purpose iron oxide was used [40].
Issues and Limitations of Brain-Targeted Therapies
Development of targeted vehicle is only possible after complete understanding of the target. As the brain is complex in nature so it is very difficult to recognize the specific differences between the target and other cells [6]. Non-covalent drug delivery system often results in the release of drug at a non-specific point during its transport. Some targeted molecules are also degraded by the enzymes. Another problem is safety. Some of the targeted molecules cause toxicity problems. e.g. Nanoparticles show slow toxicity after their entry in the systemic circulation and their mechanism of action are not fully known. So, there is no idea of the appearance of any side effect due to their long-term accumulation on the targeted site. Most of the targeted approaches are used with conventional methods of treatment i.e. chemotherapy and radiotherapy for disease management [13]. It limits the use of targeted molecules that target a number of pathways involved in the formation of tumor i.e. broad ranging therapeutic agent must be used [2] .
Conclusion
A number of brain tumor patients are being treated by the targeted therapies which have many advantages over conventional treatment techniques e.g. surgery, radiation, and chemotherapy. The therapeutic agents have many advantages and one of the potential advantages is that these can easily cross the barriers that protect the brain and target only the malignant site and is not harmful to the normal healthy cells of the brain. But it also has some dark aspects i.e. sometimes, it causes non-specific transport, and also sometimes before reaching the site of action these are degraded by the enzymes, and, toxicity profiles. Although these therapeutic agents have some issues and limitations they are mostly used in combination with conventional techniques to improve their efficacy.

Read More About this Article: https://biomedgrid.com/fulltext/volume3/a-review-targeted-cancer-therapy-as-a-fight-against-brain-tumor.000672.php
For more about: Journals on Biomedical Science :Biomed Grid
#American Journal of Biomedical Science & Research#Journals on Medical Casereports#Open Access journals on surgery#Open access clinical and medical journal#Journals on medical research#Journals on Medical Microbiology
0 notes
Text
Surgical Wound Care-Juniper Publishers

Introduction
In general, the surgical wound care is very important as the management of surgical wound is concerned. The management of post-operative wounds is important to prevent potential complications such as surgical site infections and wound dehiscence from developing. So, general practitioners and the nurses for wound care, who are important part in the sub-acute management of post-operative wounds, should appreciate the physiology of wound healing and the principles of post-operative wound care [1].
Palliative wound care is complex, dynamic, and constantly evolving to balance the individual care needs of the palliative patient and his/her circle of care [2]. The patient's circle of care includes the members of the patient unit including family, significant others, caregivers, and other healthcare professionals who may be external to the current inter professional team [3]. When following a palliative wound care pathway, the focus shifts from traditional wound care, where healing and wound closure are the goals, to promoting comfort and dignity, relieving suffering, and improving quality of life [4]. Palliative care principles are adopted to meet the whole person care needs of terminally ill patients, as well as older and frailer people who often present with chronic debilitating diseases, advanced diseases associated with major organ failure (renal, hepatic, pulmonary, or cardiac), profound dementia, [5] complex psychosocial issues, diminished self-care abilities, and challenging wound-related symptoms, whether the wound has the potential to heal or not. The authors propose a paradigm that could be integrated along the continuum of wound care, and its relevance may vary with the individual's goals, disease processes, and wound condition.
Aim
The aim of this article is to update general practitioners and wound care nurses on the important aspects of surgical wound care since they have to have basic knowledge about the surgical wound care.This includes a review of the physiology behind wound healing, an update on wound cleansing and dressing methods, as well as a guide on how common surgical wound complications also should be managed.
Discussion
The main elements of surgical wound care include timely review of the wound, appropriate cleansing and dressing, as well as early recognition and active treatment of wound complications. Appropriate post-operative surgical wound care is essential in preventing potential complications, such as surgical-site infections (SSIs), wound dehiscence and haematomas. General practitioners play a major part in managing patient's postoperative wounds and it is important to appreciate the principles of post-operative wound management to minimise the incidence of wound complications.
Phases of Wound Healing
Wound healing is a dynamic process consisting of four continuous, overlapping, and precisely programmed phases. The events of each phase must happen in a precise and regulated manner. Interruptions, aberrancies, or prolongation in the process can lead to delayed wound healing or a non-healing chronic wound.
In adult humans, optimal wound healing involves the following the events:
Rapid hemostasis
Appropriate inflammation
Mesenchymal cell differentiation, proliferation, and migration to the wound site
Suitable angiogenesis
Prompt re-epithelialization (re-growth of epithelial tissue over the wound surface) and
Proper synthesis, cross-linking, and alignment of collagen to provide strength to the healing tissue.
The first phase of hemostasis begins immediately after wounding, with vascular constriction and fibrin clot formation. The clot and surrounding wound tissue release pro- inflammatory cytokines and growth factors such as transforming growth factor (TGF)-p, platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and epidermal growth factor (EGF). Once bleeding is controlled, inflammatory cells migrate into the wound (chemotaxis) and promote the inflammatory phase, which is characterized by the sequential infiltration of neutrophils, macrophages, and lymphocytes. A critical function of neutrophils is the clearance of invading microbes and cellular debris in the wound area, although these cells also produce substances such as proteases and reactive oxygen species (ROS), which cause some additional bystander damage.
Macrophages play multiple roles in wound healing. In the early wound, macrophages release cytokines that promote the inflammatory response by recruiting and activating additional leukocytes. Macrophages are also responsible for inducing and clearing apoptotic cells (including neutrophils), thus paving the way for the resolution of inflammation. As macrophages clear these apoptotic cells, they undergo a phenotypic transition to a reparative state that stimulates keratinocytes, fibroblasts, and angiogenesis to promote tissue regeneration. Inthis way, macrophages promote the transition to the proliferative phase of healing.
T-lymphocytes migrate into wounds following the inflammatory cells and macrophages, and peak during the late- proliferative/early-remodeling phase. The role of T-lymphocytes is not completely understood and is a current area of intensive investigation. Several studies suggest that delayed T-cell infiltration along with decreased T-cell concentration in the wound site is associated with impaired wound healing, while others have reported that CD 4+ cells (T-helper cells) have a positive role in wound healing and CD8+ cells (T-suppressor- cytotoxic cells) play an inhibitory role in wound healing. Interestingly, recent studies in mice deficient in both T- and B-cells have shown that scar formation is diminished in the absence of lymphocytes. In addition, skin gamma-delta T-cells regulate many aspects of wound healing, including maintaining tissue integrity, defending against pathogens, and regulating inflammation. These cells are also called dendritic epidermal T-cells (DETC), due to their unique dendritic morphology. DETC are activated by stressed, damaged, or transformed keratinocytes and produce fibroblast growth factor 7 (FGF-7), keratinocyte growth factors, and insulin-like growth factor-1, to support keratinocyte proliferation and cell survival. DETC also generate chemokines and cytokines that contribute to the initiation and regulation of the inflammatory response during wound healing. While cross-talk between skin gamma-delta T-cells and keratinocytes contributes to the maintenance of normal skin and wound healing, mice lacking or defective in skin gamma-delta T-cells show a delay in wound closure and a decrease in the proliferation of keratinocytes at the wound site.
The proliferative phase generally follows and overlaps with the inflammatory phase, and is characterized by epithelial proliferation and migration over the provisional matrix within the wound (re-epithelialization). In the reparative dermis, fibroblasts and endothelial cells are the most prominent cell types present and support capillary growth, collagen formation, and the formation of granulation tissue at the site of injury. Within the wound bed, fibroblasts produce collagen as well as glycosaminoglycans and proteoglycans, which are major components of the extracellular matrix (ECM). Following robust proliferation and ECM synthesis, wound healing enters the final remodeling phase, which can last for years. In this phase, regression of many of the newly formed capillaries occurs, so that vascular density of the wound returns to normal. One critical feature of the remodeling phase is ECM remodeling to an architecture that approaches that of the normal tissue. The wound also undergoes physical contraction throughout the entire wound-healing process, which is believed to be mediated by contractile fibroblasts (myofibroblasts) that appear in the wound.
The role of stem cells (SC) in cutaneous wound healing and tissue regeneration is a topic of increasing research attention, with a focus on the role of adult stem cells such as epidermal stem cells and bone-marrow (BM)-derived cells (BMDCs). Epidermal stem cells reside in the bulge area of hair follicles and in the basal layer of the epidermis and give rise to the keratinocytes that migrate andre-epithelialize wounds. Normal skin is also a target organ for BMDCs. Two main stem cell populations are present in the bone marrow: hematopoietic SC (HSC) and mesenchymal SC (MSC). BM-MSCs are able to differentiate into a variety of cell types, including adipocytes, osteoblasts, chondrocytes, fibroblasts, and keratinocytes. Endothelial progenitor cells (EPCs) derived from the HSC lineage are key cells that contribute to neovascularization. Both BM-MSCs and EPCs are involved in the cutaneous wound-healing process. Wound-induced hypoxia triggers the mobilization of bone marrow EPCs to the circulation, playing asignificant role in the process of neovascularization.
Several different cell types are involved in the wound- healing process, and, as described above, the cellular activities of any particular cell type may also vary during different stages of repair. The complexity and coordination of the healing process are major hurdles to therapeutic approaches, since any therapeutic must effectively be sequenced to the appropriate stage [6].
Types of Wound Healing
There are two main types of wound healing: primary healing and secondary healing. Most surgical wounds undergo primary closure in which there is minimal tissue loss and the wound edges can be satisfactorily approximated. This allows for primary healing in which there is rapid epithelialisation of the wound and minimal scarring [7].
Secondary healing refers to the process where a fullthickness wound is intentionally left open. This may be due to the presence of infection or an inability to satisfactorily approximate the wound edges. In secondary healing the wound heals by the natural way of granulation, eventual contraction and slow epithelialisation.4-6 Wounds that undergo secondary healing often result in larger scars [8,9].
The following are the most common types of chronic wounds treated at our Wound Healing Program:
Venous Leg Ulcers
Diabetic Ulcers
Pressure Ulcers
Non-healing wounds of traumatic origin
Our program also features Hyperbaric Oxygen Therapy (HBOT), a non-invasive treatment that involves high levels of concentrated oxygen which accelerates cell growth to create healing while it enhances the body's natural healing capabilities and promotes more rapid chronic wound recovery.
The most common indications for Hyperbaric Oxygen Therapy, approved by most insurance companies for the treatment of non-healing wounds, include:
Diabetic wounds of the lower extremities
Chronic refractory osteomyelitis
Compromised skin grafts
Osteoradionecrosis
Late effects of radiation therapy
Crush injuries
Acute carbon monoxide intoxication
Gas Gangrene [10].
Surgical Wound Care
Principles
Regardless of the mechanism of wound healing, the aims of post-operative wound care remain the same: to allow the wound to heal rapidly without complications, and with the best functional and aesthetic results [11].
Wounds intended to be healed by primary healing should, in particular, have their wound edges well approximated. In the initial phases of healing, there is only minimal tensile strength inthe wound as remodelling of the collagen fibres has not occurred. As such, additional support in the form of sutures, staples or tapes is required until full remodelling and epithelialisation occur.
Dressing of the Surgical Wound
Definition of terms
Clean versus sterile technique: Various definitions and descriptions of dressing technique for wound care exist. Terms have been used interchangeably and all are subject to individual interpretation. The following definitions provide a point of reference for the terms used in this document [12].
Sterile technique: Sterile is generally defined as meaning free from microorganisms [13] Sterile technique involves strategies used in patient care to reduce exposure to microorganisms and maintain objects and areas as free from microorganisms as possible. Sterile technique involves meticulous hand washing, use of a sterile field, use of sterile gloves for application of a sterile dressing, and use of sterile instruments. "Sterile to sterile" rules involve the use of only sterile instruments and materials in dressing change procedures; and avoiding contact between sterile instruments or materials and any non-sterile surface or products. Sterile technique is considered most appropriate in acute care hospital settings, for patients at high risk for infection, and for certain procedures such as sharp instrumental wound debridement [14-17].
Clean technique: Clean means free of dirt, marks, or stains. Clean technique involves strategies used in patient care to reduce the overall number of microorganisms or to prevent or reduce the risk of transmission of microorganisms from one person to another or from one place to another. Clean technique involves meticulous handwashing, maintaining a clean environment by preparing a clean field, using clean gloves and sterile instruments, and preventing direct contamination of materials and supplies. No "sterile to sterile" rules apply. This technique may also be referred to as non-sterile. Clean technique is considered most appropriate for long-term care, home care, and some clinic settings; for patients who are not at high risk for infection; and for patients receiving routine dressings for chronic wounds such as venous ulcers, or wounds healing by secondary intention with granulation tissue.
Aseptic technique: Asepsis or aseptic means free from pathogenic microorganisms. Aseptic technique is the purposeful prevention of the transfer of organisms from one person to another by keeping the microbe count to an irreducible minimum. Some authors have made a distinction between surgical asepsis or "sterile technique" used in surgery and medical asepsis or "clean technique" that involves procedures to reduce the number and transmission of pathogens.
No touch technique: No touch is a method of changing surface dressings without directly touching the wound or any surface that might come in contact with the wound. Clean gloves are used along with sterile solution/supplies/dressings that are maintained as clean [18].
Definition of infection: Infection has been defined as a continuum from contamination, colonization, critical colonization, biofilm, and infection.
Contamination: Contamination is the presence of non-replicating microorganisms on the surface of the wound. All open wounds have some level of bacterial burden that is ordinarily cleared by the host [19].
Colonization: In colonization, microorganisms attach to the wound surface and replicate but do not impair healing or cause signs and/or symptoms of infection. The bacteria are not pathogenic and do not require treatment. All chronic wounds are colonized to varying degrees.
Critical colonization: With critical colonization, the organisms attach to the wound surface, replicate and multiply to a level that affects skin cell proliferation and tissue repair without provoking systemic signs of infection. There is no invasion of the healthy tissue at this point.
Biofilm: Approximately 70% of chronic wounds have biofilm. When organisms adhere to the wound surface, they begin to develop biofilm, which is a complex system of microorganisms embedded in an extracellular, polysaccharide matrix that protects from the invasion of other organisms, phagocytosis, and many commonly used antibiotics and antiseptics. Biofilms are difficult to treat and eradicate. Recently it has been proposed that biofilm might be present in all chronic wounds.
Infection: Infection occurs when organisms on the wound surface invade the healthy tissue, reproduce, overwhelm the host resistance, and create cellular injury leading to local or systemic symptoms [20-24] Infection is often described quantitatively as a bacterial count of greater than 1015 colony-forming units (CFU) per gram of tissue. However, some organisms such as beta-hemolytic streptococci impair wound healing at less than 1015 CFU per gram of tissue [25]. According to Kravitz [26] infection should be defined as the presence of bacteria in any quantity that impairs wound healing.
Clinical signs of infection include lack of healing after 2 weeks of proper topical therapy, erythema, increase in amount or change in character of exudate, odor, increased local warmth, friable granulation tissue, edema or induration, pain or tenderness, fever, chills, elevated white blood cell count, and elevated glucose in patients with diabetes. In patients who are immunosuppressed or have ischemic wounds, signs of infection can be subtle. Signs of inflammation such as a faint halo of erythema and moderate amounts of drainage might be the only signs of an infected arterial wound [12].Studies have shown that in chronic wounds, increasing pain, friable granulation tissue, wound breakdown, and foul odor have high validity for infection [27,28].
Complications of Surgical Wound
Two common complications of surgical wounds are infections and wound dehiscence [29].
Disordered wound healing
Most wounds heal without complications and healing is not impaired in the elderly unless there are specific adverse factors or complications. Factors which may affect healing rate are [30]:
Poor blood supply.
Excess suture tension.
Long-term steroids.
Immunosuppressive therapy.
Radiotherapy.
Severe rheumatoid disease.
Malnutrition and vitamin deficiency.
Wound dehiscence
This affects about 2% of midline laparotomy wounds.
It is a serious complication with a mortality of up to 30%.
It is due to failure of wound closure technique.
It usually occurs between 7 and 10 days postoperatively.
Often, it is heralded by serosanguinous discharge from the wound.
It should be assumed that the defect involves the whole of the wound.
Initial management includes opiate analgesia, sterile dressing to the wound, fluid resuscitation and early return to theatre for re-suture under general anaesthesia.
Incisional Hernia [31]
This occurs in 10-15% of abdominal wounds, usually appearing within the first year but can be delayed by up to 15 years after surgery.
Risk factors include obesity, distension and poor muscle tone, wound infection and multiple use of the same incision site.
It presents as a bulge in the abdominal wall close to a previous wound. It is usually asymptomatic but there may be pain, especially if strangulation occurs. It tends to enlarge over time and become a nuisance.
Management: surgical repair where there is pain, strangulation or nuisance. The use of laparoscopic techniques and biosynthetic mesh is being evaluated [32,33].
Conclusion
The management of surgical wounds is very important part of post-operative recovery and the medicals and the wound care nurses should monitor the process of acute wound healing, prevent wound complications and treat appropriately if complications arise. They should care about the wound properly and prevent the wound from its complication which would lead into further complications.
For more Journals in Juniper Publishers please click on https://juniperpublishers.com/journals.php
For more articles in Journal of Nursing & Health Care please click on https://juniperpublishers.com/jojnhc/index.php
#Juniper Publishers#open access journals#Journal of Nursing & Health Care#Wound Care#Health Care#Primary Care#nurse practitioner
0 notes
Text
Stem Cells to Improve the Wound Healing-Juniper publishers
Abstract
The cellular therapy using Mesenchymal Stem Cells, specially its two subtypes - Bone-marrow Mesenchymal Stem Cells and Adipose Derived Stem Cells - can benefit by virtue of the possibility of differentiating in specialized cells that secrete and suppress growing factors and cytokines necessary in the lesion niche. When attracted by the pro-inflammatory sinalization of the lesion, they act using the paracrine signaling, decreasing the inflammation, increasing the angiogenesis and the cell migration and proliferation. The development in the researches regarding the association of the application of MSCs, with reconstructive surgery practices, leads to effective future results that can bring more benefits to the clinic practice of this field. This paper has the objective of briefly reviewing the literature about the usage of MSCs and its subtypes, the ADSCs, to improve the skin cicatrization.
Keywords: Mesenchymal stem cells; Adipose derived stem cells; Bone-Marrow Mesenchymal Stem cells; Skin flaps; Skin grafts; Wound contraction; Paracrine signaling; Pro-inflammatory Interleukins; Anti-inflammatory Interleukins.
Abbreviations: MSC: Mesenchymal Stem Cells; HLA-DR: Human Leukocyte Antigen; ADSC - Adipose derived stem cells; BM-MSC: Bone-Marrow Mesenchymal Stem Cells; SDF1: Stromal Cell-Derived Factor; PDGF: Platelet-Derived Growth Factor; IL: interleukin; TNFα: Tumor Necrosis Factor-α; IFNγ: Interferon-γ; VEGF: Vascular Endothelial Growth Factor; BFGF: Basic Fibroblast Growth Factor; EGF: Epidermal Growth Factor; KGF: Keratinocyte Growth Factor; HGF: Hepatocyte Growth Factor; PDGF: Platelet-Derived Growth Factor; TGF-β: Transforming Growth Factor-β; FGF: Fibroblast Growth Factor; α-SMA: Alpha-smooth muscle actin's; IRI: Ischemia-Reperfusion Injury
Introduction
Mesenchymal stem cells (MSCs) were first described by Friedenstein et al. [1,2]. MSCs are heterogeneous cell populations, called multipotent adult cells or somatic stem cells [3,4], capable of differentiation in most cell types in order to maintain and repair the organism. They are resident in different organs and tissues such as adipose tissue [4,5], bone marrow, umbilical cord, amniotic membrane [2,4], kidneys, liver, spleen, lungs, pancreas, tendons, synovial membranes, placenta, amniotic fluid and dental pulp [4]. The International Society for Cellular Therapy proposed three minimal criteria for defining MSCs:
a) Plastic adherence;
b) Positive expression (greater than 95% of the cell population) for CD105, CD73 and CD90 markers and negative, (maximum 2% of the population) for CD45, CD34, CD14, CD11b, CD79a, CD19 and Human leukocyte antigen (HLA-DR) surface molecules, and
c) Ability to differentiate into osteoblasts, adipocytes and chondroblasts under standard in vitro differentiating conditions [2,6].
Later, others researchers complemented this characterization with new markers: CD13, CD44, CD54, 106 and Stro-1 [4] and the presentation of fibroblast cellular morphology [3,4].
MSCs have a great potential to the tissue repairment and regeneration in reconstructive plastic surgery, preventing ischemic lesions in skin flaps [7-14] and in skin grafts [15,16]. MSCs are capable of reducing severe atrophy, retraction, fibrosis and ulceration skin signals, caused by radiotherapy [17,18]. Combined with the lipoinjection in face and body defects and in rejuvenating esthetic treatments, MSCs raise in 35% the survival rate and the microvasculature from the injected fat [18]. Also, diabetic patients with ischemic wounds show greater survival rates and lower number of amputations in affected limbs, when treated with Adipose derived stem cells (ADSCs) [19]. The MSCs improved the skin healing at induced burns in rats [20,21]. In dermatology, the MSCs are being clinically used for inflammatory diseases [22-28], Graft-Versus-Host Disease [23,25], Chrown Disease [29], Systemic Sclerosis, Lupus or dermatomyositis [24,28,30].
When compared with the usage of Growing Factors, that like any drug has a limited half-life, the cellular therapy using MSCs, specially in its two subtypes - the Bone-Marrow Mesenchymal Stem Cells (BM-MSCs) and ADSCs - can bring greater benefits because of: their expansion capacity in cellular culture; stable phenotypic expression; and possibility of differentiating in specialized cells, that secrete and suppress cytokines and growing factors in the injury [13,31-33]. However, long expansion periods of the cellular culture, to achieve effective therapeutic doses, raise the possibility of infection from the host. Because of that, the ADSCs still have to be heavily studied to the application in the clinic routine [2]. This way, this study has as objective briefly review the literature about the usage of MSCs and its subtypes, the ADSCs, to improve the skin healing.
Discussion
The MSCs are capable to answer and adjust their functions, when exposed to cells or typical factors of the lesion environment [33,34]. They have an attraction capacity to the inflammation areas [33-35] or tumors, because of the molecules CCL21 or HMGB1, known as homing. In case of lacking of this signalization, they are attracted in order of preference to the lungs, liver and spleen [35]. The MSCs show chemotaxis in vitro by the Stromal Cell-Derived Factor (SDF1), Platelet-Derived Growth Factor (PDGF), Insulin-Like Growth Factor-1, Interleukin-8 (1L-8) and Tumor Necrosis Factor-α (TNFα). In murine models, the MSCs used in the systemic administration are capable of getting into the damaged tissues [14,34,36].
The MSCs act, in many levels, in the three healing phases: inflammatory, proliferative and remodeling phase. Current studies indicate that the differentiation of the MSCs, that contributed to the tissue regeneration, is limited by the small survival rate of these cells in the damaged area. However, the paracrine signaling to the cells from the injury is the main mechanism of the MSCs, reducing the inflammation, stimulating the angiogenesis and inducing the cell migration and proliferation [33,37]. Nevertheless, other authors suggest that the differentiation of the MSCs in keratinocytes and endothelial cells have the same function as the paracrine signaling to accelerate the neovascularization and the reepithelialization of wounds [32] (Figure 1 & 2).
Some studies suggest that immunosuppressive potential is the same of both types of MSCs, ADSCs and BM-MSCs, in an inflammatory environment. Their local administration inhibited the T cells recruitment and proliferation [37-41] and decrease Interferon-γ (lFNγ),Tumor Necrosis Factor-α (TNFα), 1L-6 and IL-1β expression in the skin grafts [39-41]. In the inflammatory phase (one to three days), the expression of anti-inflammatory cytokines 1L-4 and 1L-10 is increased while 1L-2 pro- inflammatory cytokines, TNF-α and 1FN-γ are decreased, causing less local inflammatory reaction [37,42]. MSCs can also regulate macrophage activation and convert phenotype expression of M1 macrophages to M2 anti-inflammatory macrophages [39-41], which accelerates the wound healing. Additionally, MSCs act over the innate immunity to prevent the rejection of transplanted allografts, inhibiting the complement system activation [39]. The decrease of TFN-α also helps the reepithelialization after the proliferation phase [43]. The antimicrobial action from the MSCs are also important to limit the infection occurrence [37].
With the utilization of cell therapy in the proliferative phase (14 days) the production of important growth factors is increased: Vascular Endothelial Growth Factor (VEGF), Basic Fibroblast Growth Factor (bFGF) [32,37,42,43], Epidermal Growth Factor (EGF), Keratinocyte Growth Factor (KGF), Hepatocyte Growth Factor (HGF), Platelet-Derived Growth Factor (PDGF) and Transforming Growth Factor-beta (TGF-β). Through their paracrine action, the MSCs raise the migration and proliferation of keratinocytes, endothelial and epithelial cells. The fibroblasts proliferation is also enhanced, as well as the blood vessels' formation. This way, there is a better growing of a more vigorous granulation tissue [33,37,42,43]. The angiogenesis is a very important factor to the wound's healing, and the VEGF and the Fibroblast Growth Factor (FGF) are very important angiogenic factors [32].
Through the paracrine signaling, from the MSCs in the remodeling phase (21 days to one year), the fibroblasts produce collagen fibers in greater quantity and density, generating an increase in wound tensile strength [37,42,43]. At the same time, there is a smaller wound contraction and increased matrix-metalloproteinases production, that control the collagen deposition exacerbation, improving the aesthetic result and keeping the skin's function [33,42,43]. MSCs also contribute to the scar's appropriate remodeling with the increased secretion of VEGF and HGF, the adequate balance between TGF-β1 and TGF-β3 [37] and the increase of bFGF and TGF-β which inhibit the Alpha-Smooth Muscle Actin (α-SMA) expression, responsible for myofibroblast phenotype [32].
Studies indicate that there is also the possibility of using BM-MSCs and ADSCs to protect the damaged tissues caused by the 1schemia-Reperfusion 1njury (1R1) and to decrease the dysfunction of organs undergoing ischemia. The ADSCs are able to protect axial flaps from the 1R1 with results as satisfactory as the ones obtained in procedures without ischemia, because of the improvement in the angiogenic response and the blood perfusion elevation [10]. Many studies use the ADSCs to prevent ischemic injuries in skin flaps [7-14], as others [15,16] used them to improve the quality of skin grafts.
Conclusion
The MSCs - both BM-MSCs and ADSCs - are capable to answer and module their functions, when exposed to cells or typical factors of the lesion environment, attracted by the cell signaling to the inflammation areas. The researches developed till the moament point the paracrine signaling as the main mechanism from the MSCs, decreasing the inflammation, benefiting the angiogenesis and inducing the cell migration and proliferation in the damaged area. The progress on the researches about the association of MSCs with reconstructive surgery practices indicates good and productive results, which can benefit the clinical practice in this field.
To know more about Open Access Journal of Head Neck & Spine Surgery please click on:
https://juniperpublishers.com/jhnss/index.php
To know more about Open access Journals Publishers please click on : Juniper Publishers
To know more about juniper publishers: https://juniperpublishers.business.site/
#Head and Neck Surgery Journal#Journal of Head Neck & Spine Surgery#Journal of Spine Surgery#Juniper Publishers#Open Access Journals
0 notes
Text
Epithelial and Endothelial Cells- How Do They Differ?

While discussing epithelial and endothelial cells, it is not easy to distinguish them but researchers have come up with some distinct features. Endothelial cells cover the blood vessel's inner surface, while epithelial cells cover the outer surface of the internal organs and the body. The endothelial cells and epithelial cells are derived from the epithelium, but they have differences in position, structure, and function.
Link:- https://kosheeka.com/epithelial-and-endothelial-cells-how-do-they-differ-2/
#Epithelialcells #endothelialcells #Cellculture #customizedprimarycells #biotechcompany #stemcells #exosomes #stemcellresearchcenter #primarycells #regenerativemedicine #bioengineering
#research#cellculture#kosheeka#biotech#cell culture#biotechnology#primarycells#cell biology#primary cells#cellbiology
0 notes
Text
Secrets of Mammary Stem Cells Secretome- Juniper Publishers

Abstract
The role of mammary stem/progenitor cells and its secreted proteins in therapeutic application has not been evaluated yet. Here, author reviewed information pertaining to secreted proteins of mammary epithelial cells and mammary stem/progenitor cells and supported their ideas that secretome could potentially be explored for natural antimicrobials against supportive therapy of bovine mastitis.
Keywords: Mammary stem cell; Secretome; Mastitis; Bovine; Spectrophometer; Proteins; Laboratory; Lactoferrin; Receptors; Biomarkers; Pacific; Population; Antibiotics; Progenitor cells
Abbrevations: HMEC: Human Mammary Epithelial Cells; CM: Conditioned Medium; EGFR: Epidermal Growth Factor Receptor; AFDC: Adherent Fraction Derived Cells; MDC: Mammosphere Derived Cells; TGFβ: Transforming Growth Factor Beta; MDBK: Madin Darby Bovine Kidney Epithelial; LPS: Lipopolysaccharides
Opinion
Secretome are the cell secreted proteins released in the cell extracellular space. Groups of proteins present in the secretome are involved in signalling and cell communications. Study of such proteins, thus would be helpful in understanding the niche of the cell. Researchers of Pacific Northwest National Laboratory, Washington probably was the first group which demonstrated secretome of human mammary epithelial cells (HMEC) in 2008 [1]. They identified secretome of human mammary epithelial cells (HMEC)-conditioned medium (CM) cell lysates and showed regulation of matrix metalloproteases through epidermal growth factor receptor (EGFR). In 2009, Simpson and co-investigators have profiled three different secretome of basal MaSC, luminal progenitors and mature luminal cell lines. In this study, in addition to enriched expression of ephrin receptors and integrins, the activity of Wnt signalling pathway was uniquely detected in the MaSC [2]. In a recent publication of Scientific Reports journal, researchers from Cornell University, Ithaca, NY has defined secretome of mammosphere derived MaSC and opened up new doors for the possible treatment of bovine mammary gland infections [3].
For their studies, the team isolated cultured two different populations of bovine mammary cells- adherent fraction- derived cells (AFDC) and suspension mammosphere-derived cells (MDC). These two cell population were immunopheno typically different as analyzed by various cell surface markers. However, the expression of CD44 and CD49f was higher in MDC CM than the AFDC CM. Level of CD29 was high in CM of both these cells. Proteome analyses of AFDC and MDC CM, using mass spectrophometer, revealed 347 and 537 matched proteins and peptides functionally related to defense and immunity and tissue regeneration like angiogenesis and cell migration. Furthermore, two antimicrobial proteins namely lactoferrin and cathelicidine have been validated and quantified using Western blot (Figure 1) and observed differential expression of the proteins in CM of MDC versus AFDC. Two angiogenesis factors namely, angiopoetin 1 and vascular endothelial growth factor alpha, angiopoetin 1 showed high expression level in MDC CM. Likewise, levels of proteins involved in cell migration (transforming growth factor beta (TGFβ), Insulin-like growth factor-1 and hepatocyte growth factor) were estimated in the CM and found that the concentration of TGFβ was high in CM of MDC. TGFβ induces epithelial cell to mesenchymal type and promotes cell migration. Taken together, this study showed CM of AFDC and MDC contains various proteins which have roles in tissue regeneration and immune defence of the host.
Interestingly, proteins of AFMC and MDC also contained factors which protects bacterial toxin-induced MEC death. Loss of MEC damage is an important consequence of mastitis and it is induced by the toxins of Gram-positive bacteria and lipopolysaccharides (LPS) of Gram-negative bacteria. A significant reduction in growth of bacteria (measured by optical density) was observed when Madin-Darby Bovine Kidney Epithelial (MDBK) cells were grown in presence of Klebsiella (a Gram-negative bacteria) and Staphylococcus aureus (a Grampositive bacteria) in presence of MDC CM. The researchers also evaluated shelf-life of MDC CM for one week and found that frozen CM is biologically effective on MDBK cell migration. The storing of CM for long time would be advantageous for future clinical use in mastitis management even at remote places.
In summary, these studies providing rationale for the potential use of MDC secretome as supportive or adjunct therapy in bovine mastitis. Secretome could be an ideal biological source for naturally occurring anti-microbial and potentially beallogenically safe like antibiotics. In future, an important follow-on studies should include evaluation of novel proteins as biomarkers and identification of newly identified proteins with no to little annotations. In vivo application of MDC secretome in management of naturally occurring bovine mastitis, also warranted future investigations.
To know more about journal of veterinary science impact factor: https://juniperpublishers.com/jdvs/index.php
To know more about Open Access journals Publishers: Juniper Publishers
#Juniper Publishers#Juniper Publishers e-pub#Juniper Publishers Group#Veterinary Sciences#Online Journals Publishers
0 notes
Text
The Positive aspects and Issues of Functioning with Primary Cells
Cell culture studies supply a valuable complement to in vivo experiments, enabling for a far more controlled manipulation of cellular functions and processes. For decades, cell lines have played a vital part in scientific advancements, but researchers have turn out to be increasingly cautious when interpreting information generated from cell lines only. Aspects like misidentified and contaminated cell lines have spurred renewed interest in Primary Cell Tips. Additionally, cell lines frequently differ genetically and phenotypically from their tissue origin, whereas primary cells maintain a lot of with the essential markers and functions. Endothelial cell lines, by way of example, lack various functional markers, when main endothelial cells retain these crucial attributes. In spite of these benefits, getting a pure population of primary cells can be a complicated and arduous procedure. At ScienCell Investigation Laboratories, where we specialize in major cell culture, we comprehend the quite a few challenges that scientists face when functioning with primary cells. Primary cells, in contrast to cell lines, are particularly sensitive cells requiring extra nutrients not included in classical media. To optimize survival and development, primary cells execute greatest in specialty media customized for every single cell kind. An endothelial cell, as an example, has very various nutritional needs than an epithelial cell or a neuron, and as a result demands a distinctive medium. Regular cell culture media has relied on serum to provide the development components, hormones, lipids along with other undefined elements to help cellular growth. For primary cells, however, higher serum levels can result in differentiation or promote development of contaminating cells like fibroblasts. In addition, serum is plagued by rising fees and lot to lot variability. Formulating specialty media with small or no serum circumvents these problems though enabling greater customization to promote development of individual main cell sorts. Other practices, such as seeding primary cells on additional physiologically relevant substrates as an alternative to making use of synthetic polymers can drastically improve cell attachment, growth, and purity. Gene expression evaluation is crucial for understanding the transcriptome profiles of primary cells and how this directly influences their functionality. Standard reporter-gene assays and cDNA microarrays often require either transfection of exogenous material or significant quantities of high high-quality RNA. Primary cells, on the other hand, are notoriously difficult to transfect and the efficiency varies tremendously amongst diverse cell sorts. Furthermore, primary cells have a finite lifespan and restricted expansion capacity, generating it tough to obtain a high yield of RNA. To assist address these challenges, ScienCell has developed GeneQuery™ qPCR Array kits and custom primer design service for gene expression profiling. GeneQuery™ will allow researchers to directly measure protein expression patterns of primary cells, stem cells, tissues, and cell lines. Importantly, the qPCR arrays have been validated and optimized working with main cell cDNA. Because of the high sensitivity and specificity from the assay, even genes with very low abundance is usually analyzed. Gene expression evaluation of Tebu Bio: Primary Cell will enable researchers to better have an understanding of biological pathways and disease processes, like cell cycle regulation, stem cell biology, cancer development, and neurological disorders. Primary cells are critical tools in the pursuit of future scientific breakthroughs and therapies. For over fifteen years, ScienCell has worked towards developing the tools important for scientists to attain such advancements.
0 notes
Text
cord blood banking
Cord Blood Banking 7 Reasons to store you baby umbilical cord blood
1. Cord blood collection is simple and safe The cord blood is a rich source of hematopoietic stem cells and can be collected easily, without any risk for the mother or the newborn baby.
The cord blood stem cell collection is a simple procedure that is performed by specially trained medical staff and can also be performed during Caesarian section.
The samples, once cryopreserved, are capable of being stored indefinitely and therefore used at any point during the individual's lifetime. In fact, umbilical cord blood can be stored cryopreserved for >20 years with an efficient recovery of hematopoietic stem cells.
It is important to underline that the cord blood samples, called "cord blood units (CBUs)" will be collected and stored following standard procedures. Moreover, since all testing has been completed, cryopreserved CBU is an 'off-the-shelf' product.
The methodology for cryopreservation has been developed over time. Basically, UCB is processed and stored in nitrogen to maintain the viability and potential of the cell product. Upon demand, stem cells contained in the cord blood unit can be sent to the transplant centres, so that the patients can be treated without any delays.
2. Cord blood stem cells can be used to treat more than 80 diseases
Cord blood stem cells have been used to treat more than 80 different diseases including some cancers, blood disorders and immune deficiencies. Among these are leukaemia, aplastic anaemia, Hodgkin's disease and non-Hodgkin's lymphoma. Cord blood transplants are also accepted as a treatment for sickle cell anaemia, thalassemia and inherited blood disorders that are predominant in certain ethnic groups. Moreover, they are used to treat rare metabolic disorders that would otherwise be fatal for infants such as Krabbe disease and Sanfilippo syndrome.
Umbilical cord blood has been also used for the treatment of non-hematopoietic disorders. This increasing attention in umbilical cord blood implementation has been reflected in an increasing amount of ongoing clinical trials.
In fact, there are ongoing clinical investigations of cord blood for other diseases that are not traditionally treated with HSCs transplantation, such as those related to tissue regeneration (nervous system, pancreas, etc.) (Table 2). Therefore, some clinical studies are in progress to test the efficacy of these cell in the treatment of cerebral palsy, autism, hydrocephalus, type 1 and type 2 diabetes, congenital heart defects, spinal cord injuries, heart failure, stroke and neurological disorders such as multiple sclerosis. According to ongoing clinical trials, over the following few years, the list of diseases that can be treated with cord blood stem cells will grow several times.
Store umbilical cord blood stem cells are better than bone marrow stem cells
The following are the advantages of using umbilical cord blood stem cells over bone marrow stem cells for transplants:
a) Less time needed for processing (more quickly available for use), they can be simply isolated and stored for later usage; cord blood stem cells are relatively easy to process and collect (ample availability and the easy and safe modality of the collection of the cells for transplant);
b) Cord blood requires less stringent tissue-type matching between the potential recipient and donor than is required for bone marrow donors;
c) Reduced risk of transmission of viral infection. Cytomegalovirus infections can have devastating effects in profoundly immunosuppressed bone marrow transplant recipients. The incidence of CMV antibody positivity in the BM donor population is approximately the same as the general population. However, in the neonatal population CMV infection has a very low incidence and thus the risk of CMV transmission from a CB donor to a recipient is correspondingly low.
d) Graft versus host disease is a complication of stem cell transplants in which the donor's immune cells destroy the patient's healthy tissues. Cord blood has a lower incidence and severity of acute and chronic GVHD than bone marrow. In fact, GVHD has been shown to be less severe in CB recipients than BM donor recipients; this means that mismatches can be better tolerated from a CB donor. This is due to the fact that the relative immaturity of the immune cells present in umbilical cord blood reduces the matching requirement. Therefore, the patient's chance of finding a suitable donor is greatly increased;
e) Cord blood stem cells have a higher proliferative potential than bone marrow stem cells. Therefore, a fewer number of cells are required to recapitulate hematopoiesis, a process by which cellular elements of blood, such as erythrocytes, leukocytes, lymphocytes, natural killer cells and platelets are generated;
f) Since CBSCs are isolated in advance and banked, samples were immediately tested for HIV, hepatitis and other blood-borne infections, eliminating the long delay that is inherent to the use of bone marrow;
g) Research studies of cord blood transplant outcomes, including transplants with two or more cord blood units, show promising results in regenerative medicine.
3. Cord Blood is an alternative source of stem cells for hematopoietic and immunologic reconstitution of patients that do not have matched related donors
CB contains immune cells that are immature; as a result, transplants can be performed without 'perfect' human leukocyte antigen (HLA) matching between donor and recipient with an acceptable incidence of graft-versus-host disease (GVHD). The ability to use partially matched CB grafts significantly increases the probability of finding suitable units for patients. Thus, if the older sibling is ill and the mother is pregnant at the time, your doctor may recommend collecting cord blood for the needs of siblings. The collecting and storing cord blood for a family member (family member banking) may be recommended for a newborn who has a sibling with a disease that can be successfully treated with haematopoietic stem cell transplantation (HSCT).
4. Cord Blood Stem Cells can be used not only to treat hematologic diseases (blood disorders) but also to treat a wide variety of diseases including cardiovascular, ophthalmic, orthopaedic, endocrine and neurologic diseases
Cord blood, like bone marrow and peripheral blood, contains blood-forming cells, also known as hematopoietic stem cells (HSCs), that can produce all cell types found in blood such as red cells, white cells and platelets. For this reason, they have been used for several years in bone marrow transplants to treat people who have certain types of cancer and conditions including lymphoma, leukaemia, multiple myeloma, haemoglobinopathies, anaemias, myelodysplasia aplastic anaemia and myeloproliferative diseases. Therefore, cord blood stem cells have long been considered as a rich source of hematopoietic (blood-forming) stem cells for transplantation. However, recently, cord blood has moved from its usual hematologic applications to the field of regenerative medicine, a branch of translational research that seeks to replace diseased or damaged human tissues using cell-based technologies.
In fact, the past clinical experience has suggested that cord blood stem cells can differentiate into cell types other than blood and immune cells. Several researches and clinical trials have shown that cord blood stem cells can regenerate numerous tissue types when transplanted into humans. Therefore, cord blood stem cells can also be successfully used for the repair or regeneration of non-hematopoietic tissues such as the repair of heart, nerves, pancreas, etc.
Cord blood stem cells can regenerate several tissue types because it contains several cellular populations with varying degrees of ability. The stem cells within cord blood include hematopoietic (HSCs), endothelial, epithelial and more importantly mesenchymal stem cells (MSCs). MSCs are very important for the formation of a person's nervous system, circulatory tissues, sensory organs, skin, cartilage, bone and several other tissues. Therefore, in future, cord blood stem cells could be also used to treat a wide variety of diseases including cardiovascular, ophthalmic, orthopaedic, neurologic, endocrine diseases and many others.
5. Cord blood can be used for autologous or allogeneic hematopoietic stem cell transplantation (HSCT)
Allogeneic and autologous stem cell transplants are different procedures with different clinical indications. Cord blood may serve as a stem cell source of autologous (patient's own haematopoietic stem cells) or allogeneic HSC transplantation (collected from a related donor/sibling/ family member). They are used to treat a particular disease where the underlying disease process does not involve the bone marrow such as neuroblastomas, non-Hodgkin lymphoma, multiple myeloma, aplastic anaemia and certain types of cancer including Ewing sarcoma, Wilms tumour, medulloblastoma, neuroblastoma, breast and testicular cancer. Today, own cord blood stem cells are also being used in clinical trials (studies that explore whether a treatment is safe and effective for humans) for the treatment of cerebral palsy, autism, traumatic brain injury, spinal cord injury, acute burns and more.
In an allogeneic transplant, stem cells are collected from a donor and transplanted into the patient. Allogeneic transplants use healthy donor hematopoietic stem cells from an unaffected tissue-matched relative, most often a sibling. Allogeneic transplants are indicated in the treatment of high-risk acute and chronic leukaemia, bone marrow failure syndromes such as severe aplastic anaemia, haemoglobinopathies, relapsed leukaemia and lymphoma.
6. The entire family could be treated with a stem cell transplant
The chance of any sibling being a full human leukocyte antigen match to another sibling is 25%, (because of the inheritance of HLA) and this chance increases with the number of siblings. The use of HLA-matched related cord blood units (CBUs) may reduce the risk of GVHD and improve transplant outcomes over the use of unrelated cord blood transplantation.
Therefore, cord blood can be used for the treatment of a sibling of the donor. Cord blood you banked will be stored for you, thus if your child or a family member require a transplant later, you can easily use them. Moreover, if a sibling will develop a treatable condition, it could be possible to treat the condition with the newborn's stem cells. More importantly, in the near future, cord blood you stored at the time of birth may be used to treat a family member with the newest types of treatment. Indeed, studies in patients with leukaemia suggest that rates of GVHD following related cord blood transplants may be less than rates following unrelated cord blood transplantation.
7. Umbilical cord blood stem cells save lives
People decide to store their baby's cord blood for possible medical use in the future. At the moment, the medical technology only accepts stem cells from cord blood to treat hematologic diseases. However, the prospect is that medical technology will continue to advance and that the stored cord blood will be utilised for other purposes in the near future such as to repair tissues and organs.
Storing cord blood stem cells at the present allows a family to be part of every future therapy that may be found. bone marrow transplant recipients stem cell cord bloodcord blood banking cord blood bankcord blood definitionCord blood banking baby cord blood banking cord blood banking banking cord blood best cord blood banking cost blood banking cost cord blood banking cord blood banking cord blood banking
cord blood banking
0 notes
Text
Myopia is now more widespread - some may even say it may be an epidemic. Myopia (nearsightedness) has increased in the US population by 66% during the last 3 decades. The World Health Organization has categorized myopia with cataract, macular degeneration, infectious disease and vitamin A deficiency as on the list of premiere factors behind blindness and vision impairment on the globe. Myopia is divided into two groups - low and high. The low group is up to -6.00 diopters in correction and also the high (or pathological) group is greater than -6.00. The pathological group includes a much higher incidence of potentially blinding conditions including macular degeneration, retinal detachment, glaucoma, loss of visual acuity and color sensitivity. The prevalence of myopia varies by ethnic group reaching up to 70-90% in Singapore. In Japan approximately over the million people are afflicted by a vision impairment associated with high myopia that cannot be adequately corrected with glasses or lenses. The prevalence of pathological myopia in a few population based studies is up to 3% with the population. In addition to the visually disabling effects would be the economic costs - not simply for that treatment costs but also for your decrease of income due to visual disability. Since no universally accepted treatments are actually in a position to turnaround for the structural changes of pathological myopia (the lengthening of the eyeball and thinning of the retina) they have for ages been the objective of research scientists in vision and ophthalmologists and optometrists to know the factors that lead to these devastating modifications in the structure of the eyeball and to devise therapeutic strategies. The common solution of wearing glasses or lenses is temporary because so many children will get worse annually, causing more blur and thicker and heavier lenses. Although most researchers agree that you have a genetic aspect of the creation of myopia there's a growing volume of research implicating visual experiences early in life that affect eyeball growth and also the subsequent progression of myopia. Studies with animals (chickens, tree shrews while others) show that early visual experience affects the expansion from the eye and the eventual myopia that may result. Specifically, the mismatch between the focus of central and peripheral vision can induce myopia. A typical eyeglass or contact that gives sharp central focus will overcorrect the peripheral focus. This causes myopia to increase. Some medications have a very biochemical effect that will reduce the progression of myopia, nevertheless they normally have undesirable unwanted side effects. The newest research shows that specially designed bifocal lenses can slow down or even stop the growth of myopia. The most exciting studies reveal that orthokeratology can stop and in many cases reverse (during the treatment period) the myopia that has already occurred, but only up to specific amount, typically -6.00 diopters. What Can We Do about Myopia? Orthokeratology is vision correction without surgery. Orthokeratology, is additionally generally known as corneal refractive therapy (CRT), vision shaping treatment (VST), corneal molding or Ortho-K. It could be the gentle reshaping with the cornea to correct myopia (nearsightedness). The cornea will be the eye's equal of a wristwatch crystal. It is a clear, dome shaped structure that overlies the colored iris. Its tissue is incredibly thin (about 1/50th of an inch) and intensely pliable. Because the cornea separates the attention from air and because it carries a curvature that bends light for the back from the eye, it's accountable for 2/3rd of the eye's corrective power and plays a part in various conditions like nearsightedness (myopia), farsightedness (hyperopia), and astigmatism. We can compensate to the eye's focus defects by reshaping the cornea. It continues to be practiced more than 40 years. In the early years a few lenses were fit on the attention, each which has a progressively flatter fit, with the goal of reshaping the curvature of the eye. The techniques and success have greatly improved throughout the last 10 years. Now orthokeratology is accomplished by using engineered disposable lenses called reverse geometry lenses that gently flatten the cornea by pushing the central epithelial layers directly over the pupil for the periphery in the cornea. This movement of corneal cells causes the center with the cornea to become thinner and flatter thus focusing the sunshine more detailed the retina. Orthokeratology refocuses the images on the retina in the same way as LASIK, but reversibly. Orthokeratology is FDA approved and FDA certified training is necessary of eye doctors to suit overnight ortho-K lenses. Only a number of orthokeratology lenses are already approved for overnight orthokeratology from the FDA. A commonly used approved lens may be the Paragon CRT (Corneal Refractive Therapy) Lens. Corneal Molding has evolved into a method where many patients be successful while using first lens. Good results typically take just one week. The process is accomplished when you sleep using a computer designed reverse geometry contacts. The lenses are inserted at night and removed each day. The lenses, also generally known as vision retainers, safely and gently reshape the cornea changing a person's eye's focus. Most patients may have good vision during the day. Some patients might have to wear their lenses on 2-3 nights every week to maintain good vision. Ortho-K can produce results in a surprisingly short time. The length of treatment to realize your goals may differ from patient to patient. Factors which can get a new speed of treatment incorporate your initial level of myopia, the rigidity of one's cornea, the actual topography (shape) of your cornea, your tear quality as well as your expectations. Children are especially good candidates as their corneas are more pliable and because any intervention from a young age may benefit them for that rest of their lives. Patients thinking about orthokeratology commence with an eye exam and a free orthokeratology screening. After a comprehensive eye exam, including an orthokeratology consultation, corneal topography is conducted. These are topographical maps with the cornea. Everyone's topographical map is unique, much like our fingerprints. Corneal topography shows irregularities inside the cornea which is necessary to designing contacts that may mold your cornea. Corneal topography also permits us to diagnose corneal diseases for example keratoconus. Specular microscopy can be performed which permits us to see how the endothelial corneal cells are healthy and fulfilling their function of keeping the cornea from becoming waterlogged and from losing its transparency. Research also shows the quantity of time that kids spend outdoors inside daylight also slows down the advancement of myopia. There is best contact solution for dry eyes best rewetting drops for contacts still much for us to learn in combating nearsightedness, but we've got the various tools to at least create a significant impact in controlling myopia. Dr. David Littlefield
0 notes
Text
cord blood banking
Cord Blood Banking 7 Reasons to store you baby umbilical cord blood
1. Cord blood collection is simple and safe The cord blood is a rich source of hematopoietic stem cells and can be collected easily, without any risk for the mother or the newborn baby.
The cord blood stem cell collection is a simple procedure that is performed by specially trained medical staff and can also be performed during Caesarian section.
The samples, once cryopreserved, are capable of being stored indefinitely and therefore used at any point during the individual's lifetime. In fact, umbilical cord blood can be stored cryopreserved for >20 years with an efficient recovery of hematopoietic stem cells.
It is important to underline that the cord blood samples, called "cord blood units (CBUs)" will be collected and stored following standard procedures. Moreover, since all testing has been completed, cryopreserved CBU is an 'off-the-shelf' product.
The methodology for cryopreservation has been developed over time. Basically, UCB is processed and stored in nitrogen to maintain the viability and potential of the cell product. Upon demand, stem cells contained in the cord blood unit can be sent to the transplant centres, so that the patients can be treated without any delays.
2. Cord blood stem cells can be used to treat more than 80 diseases
Cord blood stem cells have been used to treat more than 80 different diseases including some cancers, blood disorders and immune deficiencies. Among these are leukaemia, aplastic anaemia, Hodgkin's disease and non-Hodgkin's lymphoma. Cord blood transplants are also accepted as a treatment for sickle cell anaemia, thalassemia and inherited blood disorders that are predominant in certain ethnic groups. Moreover, they are used to treat rare metabolic disorders that would otherwise be fatal for infants such as Krabbe disease and Sanfilippo syndrome.
Umbilical cord blood has been also used for the treatment of non-hematopoietic disorders. This increasing attention in umbilical cord blood implementation has been reflected in an increasing amount of ongoing clinical trials.
In fact, there are ongoing clinical investigations of cord blood for other diseases that are not traditionally treated with HSCs transplantation, such as those related to tissue regeneration (nervous system, pancreas, etc.) (Table 2). Therefore, some clinical studies are in progress to test the efficacy of these cell in the treatment of cerebral palsy, autism, hydrocephalus, type 1 and type 2 diabetes, congenital heart defects, spinal cord injuries, heart failure, stroke and neurological disorders such as multiple sclerosis. According to ongoing clinical trials, over the following few years, the list of diseases that can be treated with cord blood stem cells will grow several times.
Store umbilical cord blood stem cells are better than bone marrow stem cells
The following are the advantages of using umbilical cord blood stem cells over bone marrow stem cells for transplants:
a) Less time needed for processing (more quickly available for use), they can be simply isolated and stored for later usage; cord blood stem cells are relatively easy to process and collect (ample availability and the easy and safe modality of the collection of the cells for transplant);
b) Cord blood requires less stringent tissue-type matching between the potential recipient and donor than is required for bone marrow donors;
c) Reduced risk of transmission of viral infection. Cytomegalovirus infections can have devastating effects in profoundly immunosuppressed bone marrow transplant recipients. The incidence of CMV antibody positivity in the BM donor population is approximately the same as the general population. However, in the neonatal population CMV infection has a very low incidence and thus the risk of CMV transmission from a CB donor to a recipient is correspondingly low.
d) Graft versus host disease is a complication of stem cell transplants in which the donor's immune cells destroy the patient's healthy tissues. Cord blood has a lower incidence and severity of acute and chronic GVHD than bone marrow. In fact, GVHD has been shown to be less severe in CB recipients than BM donor recipients; this means that mismatches can be better tolerated from a CB donor. This is due to the fact that the relative immaturity of the immune cells present in umbilical cord blood reduces the matching requirement. Therefore, the patient's chance of finding a suitable donor is greatly increased;
e) Cord blood stem cells have a higher proliferative potential than bone marrow stem cells. Therefore, a fewer number of cells are required to recapitulate hematopoiesis, a process by which cellular elements of blood, such as erythrocytes, leukocytes, lymphocytes, natural killer cells and platelets are generated;
f) Since CBSCs are isolated in advance and banked, samples were immediately tested for HIV, hepatitis and other blood-borne infections, eliminating the long delay that is inherent to the use of bone marrow;
g) Research studies of cord blood transplant outcomes, including transplants with two or more cord blood units, show promising results in regenerative medicine.
3. Cord Blood is an alternative source of stem cells for hematopoietic and immunologic reconstitution of patients that do not have matched related donors
CB contains immune cells that are immature; as a result, transplants can be performed without 'perfect' human leukocyte antigen (HLA) matching between donor and recipient with an acceptable incidence of graft-versus-host disease (GVHD). The ability to use partially matched CB grafts significantly increases the probability of finding suitable units for patients. Thus, if the older sibling is ill and the mother is pregnant at the time, your doctor may recommend collecting cord blood for the needs of siblings. The collecting and storing cord blood for a family member (family member banking) may be recommended for a newborn who has a sibling with a disease that can be successfully treated with haematopoietic stem cell transplantation (HSCT).
4. Cord Blood Stem Cells can be used not only to treat hematologic diseases (blood disorders) but also to treat a wide variety of diseases including cardiovascular, ophthalmic, orthopaedic, endocrine and neurologic diseases
Cord blood, like bone marrow and peripheral blood, contains blood-forming cells, also known as hematopoietic stem cells (HSCs), that can produce all cell types found in blood such as red cells, white cells and platelets. For this reason, they have been used for several years in bone marrow transplants to treat people who have certain types of cancer and conditions including lymphoma, leukaemia, multiple myeloma, haemoglobinopathies, anaemias, myelodysplasia aplastic anaemia and myeloproliferative diseases. Therefore, cord blood stem cells have long been considered as a rich source of hematopoietic (blood-forming) stem cells for transplantation. However, recently, cord blood has moved from its usual hematologic applications to the field of regenerative medicine, a branch of translational research that seeks to replace diseased or damaged human tissues using cell-based technologies.
In fact, the past clinical experience has suggested that cord blood stem cells can differentiate into cell types other than blood and immune cells. Several researches and clinical trials have shown that cord blood stem cells can regenerate numerous tissue types when transplanted into humans. Therefore, cord blood stem cells can also be successfully used for the repair or regeneration of non-hematopoietic tissues such as the repair of heart, nerves, pancreas, etc.
Cord blood stem cells can regenerate several tissue types because it contains several cellular populations with varying degrees of ability. The stem cells within cord blood include hematopoietic (HSCs), endothelial, epithelial and more importantly mesenchymal stem cells (MSCs). MSCs are very important for the formation of a person's nervous system, circulatory tissues, sensory organs, skin, cartilage, bone and several other tissues. Therefore, in future, cord blood stem cells could be also used to treat a wide variety of diseases including cardiovascular, ophthalmic, orthopaedic, neurologic, endocrine diseases and many others.
5. Cord blood can be used for autologous or allogeneic hematopoietic stem cell transplantation (HSCT)
Allogeneic and autologous stem cell transplants are different procedures with different clinical indications. Cord blood may serve as a stem cell source of autologous (patient's own haematopoietic stem cells) or allogeneic HSC transplantation (collected from a related donor/sibling/ family member). They are used to treat a particular disease where the underlying disease process does not involve the bone marrow such as neuroblastomas, non-Hodgkin lymphoma, multiple myeloma, aplastic anaemia and certain types of cancer including Ewing sarcoma, Wilms tumour, medulloblastoma, neuroblastoma, breast and testicular cancer. Today, own cord blood stem cells are also being used in clinical trials (studies that explore whether a treatment is safe and effective for humans) for the treatment of cerebral palsy, autism, traumatic brain injury, spinal cord injury, acute burns and more.
In an allogeneic transplant, stem cells are collected from a donor and transplanted into the patient. Allogeneic transplants use healthy donor hematopoietic stem cells from an unaffected tissue-matched relative, most often a sibling. Allogeneic transplants are indicated in the treatment of high-risk acute and chronic leukaemia, bone marrow failure syndromes such as severe aplastic anaemia, haemoglobinopathies, relapsed leukaemia and lymphoma.
6. The entire family could be treated with a stem cell transplant
The chance of any sibling being a full human leukocyte antigen match to another sibling is 25%, (because of the inheritance of HLA) and this chance increases with the number of siblings. The use of HLA-matched related cord blood units (CBUs) may reduce the risk of GVHD and improve transplant outcomes over the use of unrelated cord blood transplantation.
Therefore, cord blood can be used for the treatment of a sibling of the donor. Cord blood you banked will be stored for you, thus if your child or a family member require a transplant later, you can easily use them. Moreover, if a sibling will develop a treatable condition, it could be possible to treat the condition with the newborn's stem cells. More importantly, in the near future, cord blood you stored at the time of birth may be used to treat a family member with the newest types of treatment. Indeed, studies in patients with leukaemia suggest that rates of GVHD following related cord blood transplants may be less than rates following unrelated cord blood transplantation.
7. Umbilical cord blood stem cells save lives
People decide to store their baby's cord blood for possible medical use in the future. At the moment, the medical technology only accepts stem cells from cord blood to treat hematologic diseases. However, the prospect is that medical technology will continue to advance and that the stored cord blood will be utilised for other purposes in the near future such as to repair tissues and organs.
Storing cord blood stem cells at the present allows a family to be part of every future therapy that may be found. bone marrow transplant recipients stem cell cord bloodcord blood banking cord blood bankcord blood definitionCord blood banking baby cord blood banking cord blood banking banking cord blood best cord blood banking cost blood banking cost cord blood banking cord blood banking cord blood banking
cord blood banking
Our Social Pages:
facebook.com linkedin.com
plus.google
youtube.com
0 notes
Text
modafinil
Name: modalert
Composition 1 bottle with the drug Abisil as an active component contains:
fir Siberian terpenes - 20%.
pharmachologic effect Abisil is a medicinal product for external use with the presence of pronounced reparative, anti-inflammatory, antimicrobial, analgesic, antiexudative properties. The breadth of pharmacological activity of the drug is due to the presence in its composition of organic compounds of natural origin, isolated from pine needles of Siberian fir.
The drug exhibits antibacterial properties against Gram-positive and Gram-negative microorganisms, even against strains with resistance to antibiotics.
The use of Abisil means promotes the rapid healing of wounds and other damage to the integrity of soft tissues, intensifies epithelization, and increases microcirculation at sites of injury.
The application of the drug to the affected areas of the skin is not characterized by its systemic absorption and local irritating effect.
It has been experimentally established that there is no toxicity, teratogenicity, mutagenicity, and carcinogenicity of the Abisil drug; therefore, it is allowed to be used in patients of different age groups, as well as during pregnancy and lactation.
Indications for use Means for external use Abisil is used as an antiseptic, anti-inflammatory and reparative drug:
in general surgical practice - with purulent-inflammatory lesions of the epidermis and soft tissues, such as: trophic ulcers, purulent wounds, characterized by the duration of the regeneration process (arising from the surgical treatment of various pathologies or resulting from trauma), fistula, pressure sores, Wounds resulting from burns or frostbite, abscesses and phlegmonous changes (including with maxillofacial localization); in dental practice: stomatitis, systemic inflammation of periodontium (periodontal disease), inflammatory gum disease (gingivitis) of varying severity, inflammatory lesion arising after tooth extraction (alveolitis), purulent-infectious inflammation resulting from dental prosthetics; in ENT-practice: otitis and eustachitis, sinusitis, rhinitis, pharyngitis, tonsillitis, laryngitis, postoperative complications in the removal of tonsils, adenoids; in dermatology: face, purulent lesion of the epidermis (pyoderma).
Mode of application Abisil is a remedy for external use only.
Apply a remedy to a damaged area of the skin or soft tissues 1-3 times a day a thin layer after pre-treatment with local antiseptics.
The duration of use of Abisil depends on the degree of epithelialization of the wound surface and is usually 5-10 days, unless the doctor has prescribed otherwise.
With purulent-inflammatory changes in the maxillofacial localization, after surgical manipulations (removal of the contents of abscesses and phlegmon) into the formed cavity, it is recommended to insert a tampon 1 time per day, impregnated with Abisil solution. The duration of this manipulation varies from 5 to 7 days, depending on the clinical picture.
When purulent-inflammatory changes in the oral cavity is recommended 2-3 times a day to apply a remedy to the injury site, it is also possible to use soaked tampons with a solution.
Inflammatory processes of the hearing aid are amenable to treatment by inserting into the external ear canal a tampon impregnated with a drug.
When purulent-inflammatory lesions of the endothelial mucosa of the nasal passages and sinuses of the nose, it is recommended to instill a drug 1-2 drops 3-4 times a day in each nasal passage. The treatment of sinusitis with Abisil means an injection into the maxillary sinus of a tampon moistened with a drug, but only after manipulation to remove its contents and antiseptic treatment by a qualified specialist by an otolaryngologist.
In order to treat inflammation of the throat, it is recommended to apply a thin layer of the drug to the affected areas of the mucosa 2-3 times a day.
The duration of use of the order modalert solution can be varied by the attending physician, depending on the severity of the inflammatory process and the response to ongoing therapy.
Side effects After applying the product to the affected area, a burning sensation may appear. If the patient has a predisposition to allergic reactions and hypersensitivity to the components of the drug - there may be an allergy.
Contraindications Abisil should not be used in the therapy of patients with hypersensitivity to Siberian fir.
Pregnancy It is allowed to use Abisil in the therapy of pregnant and lactating women, however, careful monitoring of the doctor for the condition of the patient and infant is recommended (with the therapy of a woman in the lactation period).
Drug Interactions The use of Abisil together with other external drugs should be avoided.
Overdose Information on overdose Abisilom absent.
0 notes
Text
Pseudoangiosarcomatous squamous cell carcinoma of the skin: A need for a more rigorous nomenclature for histopathological variants of squamous cell carcinoma
Abstract
Over the years, squamous cell carcinomas (SCC) that mimicked vascular lesions have been encompassed within different classifications and the underlying etiopathogenic mechanisms have been interpreted in different ways by different authors. Here, we present a case of SCC with pseudovascular areas in the right leg of a 96-year-old woman with chronic venous insufficiency. Histopathological examination closely resembled an angiosarcoma, but the immunohistochemical negativity for endothelial markers and the strong positivity for the pancytokeratin marker AE1/AE3 revealed the epithelial nature of the neoplasm. After a comprehensive review of all similar previously published cases, we believe that it is necessary to separate SCC with pseudoluminal structures composed of glandular-like areas (pseudoglandular or adenoid SCC) from those mimicking vascular lumina (pseudovascular and pseudoangiosarcomatous SCC). We would like to emphasize that acantholytic SCC, a definitive variant of SCC, can be further classified into the common or ordinary subtype of acantholytic SCC, that shows solid nests containing numerous acantholytic atypical keratinocytes without any mimickers for specific structures, and pseudoglandular, pseudovascular and pseudoangiosarcomatous subtypes when glandular or vascular structures are mimicked, respectively.
from # All Medicine by Alexandros G. Sfakianakis via alkiviadis.1961 on Inoreader http://ift.tt/2fJZ1FR from OtoRhinoLaryngology - Alexandros G. Sfakianakis via Alexandros G.Sfakianakis on Inoreader http://ift.tt/2wdPCg0
0 notes