#Clinical Trial Center
Explore tagged Tumblr posts
Text
Analisi Decentrate e Point of Care Testing: Il Convegno ad Alessandria per l'Innovazione Diagnostica
Appuntamento il 16 novembre per un dibattito sui PoCT e la loro importanza nella sanità moderna
Appuntamento il 16 novembre per un dibattito sui PoCT e la loro importanza nella sanità moderna. Analisi Decentrate e Point of Care Testing: Alessandria ospita il Convegno per l’Innovazione Diagnostica Sabato 16 novembre, l’Azienda Ospedaliero-Universitaria di Alessandria ospiterà il convegno “Le analisi decentrate e i Point of Care Testing: una strada in salita?” presso il Salone di…
#Alessandria eventi#Alessandria sanità#Alessandria today#analisi decentrate#analisi PoCT#Andrea Rocchetti#Annalisa Roveta#AOU AL#AOU Alessandria#Clinical Trial Center#convegno sanitario#cure sanitarie#DAIRI.#dati sanitari#diagnostica moderna#diritto sanitario#esperti sanitari#gestione clinica#gestione ospedaliera#gestione qualità#Google News#innovazione sanitaria#italianewsmedia.com#laboratori medici#Marta Betti#Microbiologia#Microbiologia clinica#nuove tecnologie#Ospedale#Pandemia
0 notes
Text
Clinical Trials for Eye Diseases: Why They Matter and How to Get Involved

Clinical trials are a crucial part of advancing medical science, especially for eye diseases that impact millions. These trials help doctors and researchers test new treatments and develop better ways to manage or even cure eye conditions. But the benefits go beyond just research—patients involved in clinical trials have access to cutting-edge therapies that aren’t yet available to the public. Here’s a look at why clinical trials for eye diseases are so important, what types are available, and how to get involved if you’re interested.
Why Clinical Trials Matter for Eye Diseases
Eye diseases can range from mildly annoying conditions, like dry eyes, to serious issues that can lead to vision loss, such as age-related macular degeneration (AMD) and diabetic retinopathy. Each type of eye disease has its challenges, and developing treatments that are both effective and safe requires thorough testing. Clinical trials provide a structured way to evaluate new therapies under controlled conditions, ensuring that they’re safe and work as intended.
For many eye diseases, clinical trials bring a few main benefits:
Advancing Treatment Options: Eye diseases are complex, and in many cases, treatments only address symptoms rather than providing a cure. Clinical trials allow researchers to develop new therapies that might go beyond current treatment limits.
Expanding Accessibility: Patients in clinical trials often receive treatments that are otherwise unavailable. This can be life-changing for those with conditions that have limited or no effective treatment.
Improving Quality of Life: For some eye conditions, like dry eye disease, symptoms can be very disruptive. Clinical trials test therapies that might significantly improve patients’ comfort and daily experiences.
Understanding Different Types of Eye Disease Clinical Trials
There are various types of clinical trials, and each has a specific focus. Here’s a breakdown of some common types relevant to eye diseases:
Treatment Trials: These focus on testing new drugs, therapies, or devices. For eye diseases, treatment trials often explore medications to slow down or halt vision loss, therapies for pain relief, and approaches to manage symptoms of conditions like dry eye disease.
Prevention Trials: These trials aim to prevent disease before it starts. For example, researchers might test a new supplement or eye drop that could reduce the risk of developing AMD.
Diagnostic Trials: These trials test new methods to detect eye diseases early or improve accuracy. For example, researchers may look into innovative imaging techniques to catch diabetic retinopathy or glaucoma in their earliest stages.
Quality of Life Trials: These trials focus on improving the daily lives of people with chronic eye conditions, addressing factors like comfort, independence, and mobility.
Natural History Studies: Sometimes, understanding how an eye disease progresses without intervention is valuable. These studies observe patients over time, allowing researchers to identify the stages and symptoms of a disease more accurately.
Clinical Trials for Dry Eye Disease: A Common Example
Dry eye disease is one of the most common reasons people seek eye care, and while there are treatments, there’s a need for more effective and lasting solutions. Many clinical trials focus on this area because dry eye can significantly impact quality of life. These trials test new eye drops, devices, and even lifestyle changes that might help manage dry eye more effectively.
Some dry eye clinical trials might include:
Testing New Eye Drops: There are already various over-the-counter and prescription drops, but clinical trials test formulas that could offer longer relief or address underlying causes rather than just symptoms.
Lifestyle and Environment Studies: Some trials look at how factors like screen time, diet, and environmental exposure impact dry eye. These studies can help create better guidance for people with this condition.
Device Trials: Certain devices, like warm compresses or light therapy tools, have shown potential for helping with dry eye. Clinical trials can establish how well these devices work and whether they are safe for long-term use.
How Clinical Trials Work: What to Expect
If you’re considering participating in a clinical trial, it’s helpful to know what’s involved. Clinical trials are conducted in “phases,” each of which serves a different purpose:
Phase 1: This is the first clinical trial phase, where a new treatment is tested on a small group to assess safety, dosage, and side effects. This phase is usually focused more on safety than effectiveness.
Phase 2: Here, the treatment is given to a larger group of people to see how effective it is and to continue assessing safety.
Phase 3: If a treatment has shown promise, it moves to phase 3, where it’s given to even more participants. This phase gathers data on how well the treatment works compared to existing therapies.
Phase 4: Once a treatment is approved, it may still be monitored in phase 4 to collect long-term data on effectiveness and safety.
Each trial has a clinical trials specialist, usually a doctor or researcher, who oversees the process. Participants are carefully monitored, and some trials even cover certain costs, like travel or specific tests.
How to Get Involved in a Clinical Trial
If you’re interested in participating in a clinical trial, here are some steps to help you get started:
Talk to Your Eye Doctor: Your doctor is often the best resource for finding clinical trials that match your needs. They can refer you to trials they believe would be a good fit.
Search Online Registries: Websites like ClinicalTrials.gov and the World Health Organization’s International Clinical Trials Registry Platform list ongoing trials, and you can search by condition, location, and more.
Visit Local Eye Care Centers: Many clinical trial centers are associated with large eye care institutions or universities, such as the clinical trials center at Albemarle Eye Center in Washington, NC, which may offer information on trials specific to eye diseases, including dry eye and other common conditions.
Contact Research Foundations: Some foundations dedicated to eye health or specific diseases, like the American Academy of Ophthalmology, have information on trials and may offer support for those interested in participating.
Benefits and Risks of Participating in Clinical Trials
Participating in a clinical trial can have several benefits:
Access to New Treatments: As a participant, you get early access to therapies that might be more effective than current options.
Close Monitoring: Clinical trials often provide thorough medical attention and regular check-ups, which can benefit those managing chronic conditions.
Contributing to Research: By participating, you help advance medical knowledge and potentially improve care for others with similar eye conditions.
However, there are risks to consider as well:
Unknown Side Effects: New treatments may have side effects that are not fully understood. Participants must weigh the potential for improvement with the risk of adverse reactions.
Time Commitment: Some trials require frequent visits or tests, which can be demanding. It’s important to know what level of commitment is expected before enrolling.
Key Considerations Before Joining a Clinical Trial
Here are a few factors to keep in mind:
Eligibility Requirements: Most trials have specific criteria, such as age, health status, and disease stage.
Informed Consent: You’ll be provided with detailed information about the trial, including potential risks. Make sure to read and understand this before signing up.
Privacy: All information in clinical trials is confidential, but it’s always wise to ask about data privacy.
Clinical Trials and the Future of Eye Health
Clinical trials are critical in developing better treatments for eye diseases. For conditions like dry eye, diabetic retinopathy, glaucoma, and more, these trials could mean the difference between managing symptoms and finding long-term relief or even a cure. As technology and medicine continue to advance, participating in a clinical trial can be a way for patients to actively contribute to these advancements and benefit from them directly.
Conclusion
Clinical trials for eye diseases offer hope, access, and opportunities for both patients and researchers. If you’re living with an eye condition and are interested in exploring new treatment options, consider getting involved in a clinical trial. It’s a unique way to contribute to science while receiving potential benefits that can improve your quality of life.
To learn more or find a clinical trial near you, Schedule An Appointment with Albemarle Eye Center in Washington, NC, or consult with a clinical trials specialist about your options.
0 notes
Text
हिमाचल में कैंसर के 32 हजार 909 मरीज, 3 हजार 138 अस्थमा से पीड़ित; धनीराम शांडिल
Himachal Pradesh Cancer Patients: हिमाचल प्रदेश में कैंसर के 32 हजार 909 और अस्थमा के 3 हजार 138 मरीज हैं. मौजूदा वक्त में चिकित्सा शिक्षा एवं अनुसंधान हिमाचल प्रदेश विभाग के तहत जिला शिमला, कांगड़ा, सिरमौर, मंडी और हमीरपुर गवर्मेंट मेडिकल कॉलेज और अस्पताल चलाए जा रहे हैं. इन महाविद्यालय एवं चिकित्सालय में यह मरीज अपना इला�� करवा रहे हैं. यह जानकारी हिमाचल प्रदेश विधानसभा के मानसून सत्र के दौरान…
#asthma#best cancer hospital in india#bladder cancer#blood cancer#breast cancer#cancer#cancer 101#cancer care#cancer causes#cancer cells#cancer center#cancer clinical trial#cancer cure#cancer help#cancer information#cancer news#cancer patients#cancer research#cancer research uk#cancer support#cancer symptoms#cancer test#cancer treatment#cancer trial#cancer types#cancers#civilstap himachal#colon cancer#Dhaniram Shandil#Himachal
0 notes
Text
My life changed when I found Abid Hearing Instruments! Their powerful, discreet hearing aids helped me reconnect with loved ones. #HearingLoss #HearingAids #Bangladesh
#hearing aids#hearing loss#hearing health#hearing aid technology#digital hearing aids#invisible hearing aids#rechargeable hearing aids#hearing aid benefits#hearing aid reviews#hearing aid comparison#hearing aid tips#hearing aid care#hearing aid accessories#hearing aid cleaning#hearing aid batteries#hearing aid insurance#hearing aid financing#hearing aid warranty#hearing aid specialist#hearing aid audiologist#hearing aid center#hearing aid clinic#hearing aid fitting#hearing aid adjustment#hearing aid repair#hearing aid maintenance#hearing aid consultation#hearing aid trial#hearing aid brands#hearing aid manufacturers
0 notes
Text
Random thoughts/hc I have about clinical trial (I did the double accept ending so this is for that).
(SPOILERS)
Angel works at home doing art and commissions while Lee still works his normal job. Lee helped them build a loose schedule and tried to help set up stuff to get the commission money. He doesn't know much about marketing (who wouldn't like their art) or making art so everything else is on angle and he encourages them however he can. (I don't know how artists make money or if patron existed in this timeline). Angle doesn't need to work but they mentioned kinda feeling like a burden so Lee wants to help them feel more independent.
The furry stuff Angel draws reminds Lee of the anthropomorphic animals in his childhood books. The religious ones his mother used to read him before she decided he was too old for it. He doesn't remember her reading them he just remembered that it was a thing and saw it happening with his younger siblings. When his mom would pass out before the younger kids he'd try to read to them but sometimes she'd wake up and yell at him to go back to his room. Angles animals are much more colorful and unique, full or life.
Angel starts living sticky notes with cute little doodles for Lee around the house. Their only sticky for a few day so Lee started to keep them in a folder but he feels super guilty over it because he feels like he's slowly repeating the shrine incident. He even keeps some napkins Angle doodled on. He eventually shows it to angle while apologizing for having it.
I feel like lee got super attached to objects as a child, especially those given as gifts. He kept the old socks he was given as a child even when they were riddled with holes, he kept broken glasses/cups, old clothes he outgrew, bus tickets from a day out, packaging/wrapping paper all hidden in a little space in his room. His parents found it and freaked out on him for hoarding it. He just didn't want anything to go to waste, he should be thankful for all he's given. Other than that he was a very organized kid. He wants to keep his place neat so now he only gets/keeps things he really wants
The blue Turkish floor lamp. I have thoughts on it. Lee likes the reflection from it and his stained glass window. As a child he was enamored by the stained glass at his church. Anyways I can't tell if I want angels hair to remind him of the lamp at home or if I want him to have the lamp because it reminds him of Angel. I'm leaning towards him already having it tho.
Angel starts attending a nearby community College part time for art classes. It's mostly to meet other people.
Sometimes they stay a bit late to hang out with someone or continue working on a piece so Lee would just wait in the car. Angel encourages him to wander around (angle was wondering behind the plexiglass in my run so I hc that they like wandering around places.) Lee ends up finding an entomology class so he just sits in the back happily listening the entire time. He trys to draw some of the bugs too (for scientific purposes). He might also find some biology classes too. He's not enrolled in the classes he's just there in the back. Maybe he listens to the professors like podcasts while studying. The students think he's a security guard the professors don't care. (Idk what community College is like but I once chilled in a horticultural class by accident and the professor just accepted it until I told him I didn't need the handout because I don't study this)
I also like angle going to a community center. They get to do art classes, maybe they volunteer to teach kids how to draw. The shy kids flock to them. Maybe Lee takes some beginner classes too.
Lee is very nervous to meet angels new friends. He's worried they'll think he's a creep even with the abridged version of how they got together. (I wanna maybe write a fic about this because Lee is definitely in the wrong but also their where both incredibly lonely so I wanna explore that)
Lee doesn't feel Like a creative person, he feels like he lacks imagination. He can't really make an image in his head and put it to paper like angel can. Angel tells him he is creative, he made the doll by hand (they emphasize not making another doll). As a kid he learned some sewing skills from his sister and mom but then got told it was “women's work” so now he usually only uses it to mend his clothes. He starts sewing again and makes some plushies (shrimps, rabbits, their sona, ect) then gets into clothes. He feels a little embarrassed about making clothing at first but then he realizes he can make angel outfits. (I might make a short fic of Lee making Angels bunny sona plush and than freaking out because it's a sona so he doesn't know if it's crossing the line again. Angle actually thinks this one is cute though.)
He was told to avoid “women's work” so now he sucks at cooking. He doesn't believe that, he was just never given the opportunity to learn and was never interested in learning once we left the religion.
Angel bakes cute animal themed feed. Lee thinks it's too pretty to eat but he can't keep it forever so the next best thing he can do is eat it. He likes it and feels that it brings him closer to angle. He's put on a few pounds from it.
(I'm projecting and also I thought this before I saw the reject ending) I feel like lee would really like chilling in corners. Like if he knows hes going to repeatedly go to a a place he's picking a corner to chill in and that's his corner now. His day is off if someone is in his corner. Why have you taken His mentally assigned spot?
____
I'm 100% projecting but angel tries to get into a physical hobby (Lee is very stoked about this!) So they do rock climbing and Bouldering. They don't wanna Crack their head open trying it outside so Lee buys them a subscription to a climbing gym. Angel kind of sucks at it at first but they get a climbing kick and keep trying. Their not very tall so they get good at launching themselves up to grab the next rock instead of stretching to reach it. It makes them feel like an animal (in a good way) and they get to let everything go and focus on completing a task. Lee feels like he's watching his Angel fly.
103 notes
·
View notes
Text
also preserved on our archive
by Rowan Walrath
Public and private funding is lacking, scrambling opportunities to develop treatments
In brief Long COVID is a difficult therapeutic area to work in. It’s a scientifically challenging condition, but perhaps more critically, few want to fund new treatments. Private investors, Big Pharma, and government agencies alike see long COVID as too risky as long as its underlying mechanisms are so poorly understood. This dynamic has hampered the few biotechnology and pharmaceutical companies trying to develop new medicines. The lack of funding has frustrated people with long COVID, who have few options available to them. And crucially, it has snarled research and development, cutting drug development short.
When COVID-19 hit, the biotechnology company Aim ImmunoTech was developing a drug for myalgic encephalomyelitis/chronic fatigue syndrome, better known as ME/CFS. As more people came down with COVID-19, some began to describe lingering problems that sounded a lot like ME/CFS. In many cases, people who got sick simply never seemed to get better. In others, they recovered completely—or thought they had—only to be waylaid by new problems: fatigue that wouldn’t go away with any amount of rest, brain fog that got in the way of normal conversations, a sudden tendency toward dizziness and fainting, or all the above.
There was a clear overlap between the condition, which patients began calling long COVID, and ME/CFS. People with ME/CFS have a deep, debilitating fatigue. They cannot tolerate much, if any, exercise; walking up a slight incline can mean days of recovery. Those with the most severe cases are bedbound.
Aim’s leaders set out to test whether the company’s drug, Ampligen, which is approved for ME/CFS in Argentina but not yet in the US, might be a good fit for treating long COVID. They started with a tiny study, just 4 people. When most of those participants responded well, they scaled up to 80. While initial data were mixed, people taking Ampligen were generally able to walk farther in a 6 min walk test than those who took a placebo, indicating improvement in baseline fatigue. The company is now making plans for a follow-on study in long COVID.
Aim’s motivation for testing Ampligen in long COVID was twofold. Executives believed they could help people with the condition, given the significant overlap in symptoms with ME/CFS. But they also, plainly, thought there’d be money. They were wrong.
“When we first went out to do this study in long COVID, there was money from . . . RECOVER,” Aim scientific officer Chris McAleer says, referring to Researching COVID to Enhance Recovery (RECOVER), the National Institutes of Health’s $1.7 billion initiative to fund projects investigating causes of, and potential treatments for, long COVID. McAleer says Aim attempted to get RECOVER funds, “believing that we had a therapeutic for these individuals, and we get nothing.”
Instead of funding novel medicines like Ampligen, the NIH has directed most of its RECOVER resources to observational studies designed to learn more about the condition, not treat it. Only last year did the agency begin to fund clinical trials for long COVID treatments, and those investigate the repurposing of approved drugs. What RECOVER is not doing is funding new compounds.
RECOVER is the only federal funding mechanism aimed at long COVID research. Other initiatives, like the $5 billion Project NextGen and the $577 million Antiviral Drug Discovery (AViDD) Centers for Pathogens of Pandemic Concern, put grant money toward next-generation vaccines, monoclonal antibodies, and antivirals for COVID-19. They stop short of testing those compounds as long COVID treatments.
Private funding is even harder to come by. Large pharmaceutical companies have mostly stayed away from the condition. (Some RECOVER trials are testing Pfizer’s COVID-19 antiviral Paxlovid, but a Pfizer spokesperson confirms that Pfizer is not sponsoring those studies.) Most investors have also avoided long COVID: a senior analyst on PitchBook’s biotech team, which tracks industry financing closely, says he isn’t aware of any investment in the space.
“What you need is innovation on this front that’s not driven by profit motive, but impact on global human health,” says Sumit Chanda, an immunologist and microbiologist at Scripps Research who coleads one of the AViDD centers. “We could have been filling in the gaps for things like long COVID, where pharma doesn’t see that there’s a billion-dollar market.”
The few biotech companies that are developing potential treatments for long COVID, including Aim, are usually funding those efforts out of their own balance sheets. Experts warn that such a pattern is not sustainable. At least four companies that were developing long COVID treatments have shut down because of an apparent lack of finances. Others are evaluating a shift away from long COVID.
“It is seen by the industry and by investors as a shot in the dark,” says Radu Pislariu, cofounder and CEO of Laurent Pharmaceuticals, a start-up that’s developing an antiviral and anti-inflammatory for long COVID. “What I know is that nobody wants to hear about COVID. When you say the name COVID, it’s bad . . ., but long COVID is not going anywhere, because COVID-19 is endemic. It will stay. At some point, everyone will realize that we have to do more for it.”
‘Time and patience and money’ Much of the hesitancy to make new medicines stems from the evasive nature of long COVID itself. The condition is multisystemic, affecting the brain, heart, endocrine network, immune system, reproductive organs, and gastrointestinal tract. While researchers are finding increasing evidence for some of the disease’s mechanisms, like viral persistence, immune dysregulation, and mitochondrial dysfunction, they might not uncover a one-size-fits-all treatment.
“Until we have a better understanding of the underlying mechanisms of long COVID, I think physicians are doing the best they can with the information they have and the guidance that is available to them,” says Ian Simon, director of the US Department of Health and Human Services’ Office of Long COVID Research and Practice. The research taking place now will eventually guide new therapeutic development, he says.
Meanwhile, time marches on.
By the end of 2023, more than 409 million people worldwide had long COVID, according to a recent review coauthored by two cofounders of the Patient-Led Research Collaborative (PLRC) and several prominent long COVID researchers (Nat. Med. 2024; DOI: 10.1038/s41591-024-03173-6). Most of those 409 million contracted COVID-19 and then long COVID after vaccines and antivirals became available. That fact undercuts the notion that the condition results only from severe cases of COVID-19 contracted before those interventions existed. (Vaccination and treatment with antivirals do correlate with a lower incidence of long COVID but don’t prevent it outright.)
“There is that narrative that long COVID is over,” says Hannah Davis, cofounder of the PLRC and a coauthor of the review, who has had long COVID since 2020. “I think that’s fairly obviously not true.”
The few biotech companies that have taken matters into their own hands, like Aim, are often reduced to small study sizes with limited time frames because they can’t get outside funding.
InflammX Therapeutics, a Florida-based ophthalmology firm headed by former Bausch & Lomb executive Brian Levy, started testing an anti-inflammatory drug candidate called Xiflam after Levy’s daughter came down with long COVID. Xiflam is designed to close connexin 43 (Cx43) hemichannels when they become pathological. The hemichannels, which form in cell membranes, would otherwise allow intracellular adenosine triphosphate (ATP) to escape and signal the NLRP3 inflammasome to crank up its activity, causing pain and inflammation.
InflammX originally conceived of Xiflam as a treatment for inflammation in various eye disorders, but after Levy familiarized himself with the literature on long COVID, he figured the compound might be useful for people like his daughter.
InflammX set up a small Phase 2a study at a site just outside Boston. The trial will enroll just 20 participants, including Levy’s daughter and InflammX’s chief operating and financial officer, David Pool, who also has long COVID. The study is set up such that participants don’t know if they’re taking Xiflam or a placebo.
Levy says the company tried to communicate with NIH RECOVER staff multiple times but never heard back. “We couldn’t wait,” he says.
Larger firms are similarly disconnected from US federal efforts. COVID-19 vaccine maker Moderna appointed a vice president of long COVID last year. Bishoy Rizkalla now oversees a small team studying how the company’s messenger RNA shots could mitigate problems caused by new and latent viruses, including SARS-CoV-2. But Rizkalla says Moderna has no federally funded projects in long COVID.
Federal bureaucracy has slowed down research in other ways. When long COVID appeared, Tonix Pharmaceuticals was developing a possible drug called TNX-102 SL to treat fibromyalgia. The two conditions look similar: they’re painful, fatiguing, and multisystemic, and fibromyalgia can crop up after a viral infection.
But it wasn’t easy to design a study to test the compound in long COVID. Among other issues, the US Food and Drug Administration initially insisted that participants have a positive COVID-19 test confirmed by a laboratory, like a polymerase chain reaction test, to be included in the study. At-home diagnostics wouldn’t count.
“We spent a huge amount of money, and we couldn’t enroll people who had lab-confirmed COVID because no one was going to labs to confirm their COVID,” cofounder and CEO Seth Lederman says. “We just ran out of time and patience and money, frankly.”
Tonix had planned to enroll 450 participants. The company ultimately enrolled only 63. The study failed to meet its primary end point of reducing pain intensity, a result Lederman attributes to the smaller-than-expected sample size.
TNX-102 SL trended toward improvements in fatigue and other areas, like sleep quality and cognitive function, but Tonix is moving away from developing the compound as a long COVID treatment and focusing on developing it for fibromyalgia. If it’s approved, Lederman hopes that physicians will prescribe it to people who meet the clinical criteria for fibromyalgia regardless of whether their condition stems from COVID-19.
“I’m not saying we’re not going to do another study in long COVID, but for the short term, it’s deemphasized,” Lederman says.
Abandoned attempts Without more public or private investment, it’s unclear how research can proceed. The small corner of the private sector that has endeavored to take on long COVID is slowly becoming a graveyard.
Axcella Therapeutics made a big gamble in late 2022. The company pivoted from trying to treat nonalcoholic steatohepatitis, a liver disease, to addressing chronic fatigue in people with long COVID. In doing so, Axcella reoriented itself exclusively around long COVID, laying off most of its staff and abandoning other research activities. People in a 41-person Phase 2a trial of the drug candidate, AXA1125, showed improvement in fatigue scores based on a clinical questionnaire (eClinicalMedicine 2023, DOI: 10.1016/j.eclinm.2023.101946), but Axcella shut down before it could get its planned 300-person follow-on study up and running.
The fate of AXA1125 may be to gather dust. Axcella’s former executives have moved on to other pursuits. Erstwhile chief medical officer Margaret Koziel, once a champion of AXA1125, says by email that she is “not up to date on current research on long COVID.” Staff at the University of Oxford, which ran the Phase 2a study, were not able to procure information about the planned Phase 2b/3 trial. A spokesperson for Flagship Pioneering, the venture firm that founded Axcella in 2011, declined to comment to C&EN.
Other firms have met similar ends. Ampio Pharmaceuticals dissolved in August after completing only a Phase 1 study to evaluate an inhaled medication called Ampion in people with long COVID who have breathing issues. Biotech firm SolAeroMed shut down before even starting a trial of its bronchodilating medicine for people with long COVID. “Unfortunately we were unable to attract funding to support our clinical work for COVID,” CEO John Dennis says by email.
Another biotech company, Aerium Therapeutics, did manage to get just enough of its monoclonal antibody AER002 manufactured and in the hands of researchers at the University of California, San Francisco, before it ended operations. The researchers are now testing AER002 in a Phase 2 trial with people with long COVID. Michael Peluso, an infectious disease clinician and researcher at UCSF and principal investigator of the trial, says that while AER002 may not advance without a company behind it, the study could be valuable for validating long COVID’s mechanisms of disease and providing a proof of concept for monoclonal antibody treatment more generally.
“[Aerium] put a lot of effort into making sure that the study would not be impacted,” Peluso says. “Regardless of the results of this study, doing a follow-up study now that we’ve kind of learned the mechanics of it with modern monoclonals would be really, really interesting.”
‘A squandered opportunity’ In 2022, the NIH’s National Institute of Allergy and Infectious Diseases (NIAID) put about $577 million toward nine research centers that would discover and develop antivirals for various pathogens. Called the Antiviral Drug Discovery (AViDD) Centers for Pathogens of Pandemic Concern, the centers were initially imagined as 5-year projects, enough time to ready multiple candidates for preclinical development. The NIH allocated money for the first 3 years and promised more funds to come later.
The prospect excited John Chodera, a computational chemist at the Memorial Sloan Kettering Cancer Center and a principal investigator at an AViDD center called the AI-Driven Structure-Enabled Antiviral Platform. Chodera figured that if his team were able to develop a potent antiviral for SARS-CoV-2, it could potentially be used to treat long COVID as well. A predominant theory is that reservoirs of hidden virus in the body cause ongoing symptoms.
But Chodera says NIAID told him and other AViDD investigators that establishing long COVID models was out of scope. And last year, Congress clawed back unspent COVID-19 pandemic relief funds, including the pool of money intended for the AViDD centers’ last 2 years. Lawmakers were supposed to come through with additional funding, Chodera says, but it never materialized. All nine AViDD centers will run out of money come May 2025.
“When we do start to understand what the molecular targets for long COVID are going to be, it’d be very easy to pivot and train our fire on those targets,” says Chanda from Scripps’s AViDD center. “The problem is that it took us probably 2 years to get everything up and going. If you cut the funding after 3 years, we basically have to dismantle it. We don’t have an opportunity to say, ‘Hey, look, this is what we’ve done. We can now take this and train our fire on X, Y, and Z.’ ”
Researchers at multiple AViDD centers confirm that the NIH has offered a 1-year, no-cost extension, but it doesn’t come with additional funds. They now find themselves in the same position as many academic labs: seeking grant money to keep their projects going.
Worse, they say, is that applying for other grants will likely mean splitting up research teams, thus undoing the network effect that these centers were supposed to provide.
“Now what we’ve got is a bunch of half bridges with nowhere to fund the continuation of that work,” says Nathaniel Moorman, cofounder and scientific adviser of the Rapidly Emerging Antiviral Drug Development Initiative, which houses an AViDD center at the University of North Carolina at Chapel Hill.
“This was a squandered opportunity, not just for pandemic preparedness but to tackle these unmet needs that are being neglected by biotech and pharma,” Chanda says.
Viral persistence Ann Kwong has been here before. The virologist was among the first industry scientists trying to develop antivirals for hepatitis C virus (HCV) back in the 1990s. Kwong led an antiviral discovery team at the Schering-Plough Research Institute for 6 years. In 1997, Vertex Pharmaceuticals recruited her to lead its new virology group.
Kwong and her team at Vertex developed a number of antivirals for HCV, HIV, and influenza viruses; one was the HCV protease inhibitor telaprevir. She recalls that a major challenge for the HCV antivirals was that scientists didn’t know where in the body the virus was hiding. Kwong says she had to fight to develop an antiviral that targeted the liver since it hadn’t yet been confirmed that HCV primarily resides there. People with chronic hepatitis C would in many cases eventually develop liver failure or cancer, but they presented with other issues too, like brain fog, fatigue, and inflammation.
She sees the same dynamic playing out in long COVID.
“This reminds me of HIV days and HCV days,” Kwong says. “This idea that pharma doesn’t want to work on this because we don’t know things about SARS-CoV-2 and long COVID is bullshit.”
Since January, Kwong has been cooking up something new. She’s approaching long COVID the way she did chronic hepatitis C: treating it as a chronic infection, through a start-up called Persistence Bio. Persistence is still in stealth; its name reflects its mission to create antivirals that can reach hidden reservoirs of persistent SARS-CoV-2, which many researchers believe to be a cause of long COVID.
“Long COVID is really interesting because there’s so many different symptoms,” Kwong says. “As a virologist, I am not surprised, because it’s an amazing virus. It infects every tissue in your body. . . . All the autopsy studies show that it’s in your brain. It’s in your gut. It’s in your lungs. It’s in your heart. To me, all the different symptoms are indicative of where the virus has gone when it infected you.”
Kwong has experienced some of these symptoms firsthand. She contracted COVID-19 while flying home to Massachusetts from Germany in 2020. For about a year afterward, she’d get caught off guard by sudden bouts of fatigue, bending over to catch her breath as she walked around the horse farm where she lives, her legs aching. Those symptoms went away with time and luck, but another round of symptoms roared to life this spring, including what Kwong describes as “partial blackouts.”
Kwong hasn’t been formally diagnosed with long COVID, but she says she “strongly suspects” she has it. Others among Persistence’s team of about 25 also have the condition.
“Long COVID patients have been involved with the founding of our company, and we work closely with them and know how awful the condition can be,” Kwong says. “It is a big motivator for our team.”
Persistence is in the process of fundraising. Kwong says she’s in conversations with private investors, but she and her cofounders are hoping to get public funding too.
On Sept. 23, the NIH is convening a 3-day workshop to review what RECOVER has accomplished and plan the next phase of the initiative. Crucially, that phase will include additional clinical trials. RECOVER’s $1.7 billion in funding includes a recent award of $515 million over the next 4 years. It’s not out of the question that this time, industry players might be invited to the table. Tonix Pharmaceuticals’ Lederman and Aim ImmunoTech’s McAleer will both speak during the workshop.
The US Senate Committee on Appropriations explicitly directed the NIH during an Aug. 1 meeting to prioritize research to understand, diagnose, and treat long COVID. It also recommended that Congress put $1.5 billion toward the Advanced Research Projects Agency for Health (ARPA-H), which often partners with industry players. The committee instructed ARPA-H to invest in “high-risk, high-reward research . . . focused on drug trials, development of biomarkers, and research that includes long COVID associated conditions.” Also last month, Sen. Bernie Sanders (I-VT) introduced the Long COVID Research Moonshot Act, which would give the NIH $1 billion a year for a decade to treat and monitor patients.
It’s these kinds of mechanisms that might make a difference for long COVID drug development.
“What I’ve seen a lot is pharma being hesitant to get involved,” says Lisa McCorkell, a cofounder of the PLRC and a coauthor of the recent long COVID review. “Maybe they’ll invest if NIH also matches their investment or something like that. Having those public-private partnerships is really, at this stage, what will propel us forward.”
Chemical & Engineering News ISSN 0009-2347 Copyright © 2024 American Chemical Society
#mask up#covid#pandemic#wear a mask#covid 19#public health#coronavirus#sars cov 2#still coviding#wear a respirator#long covid#covid conscious#covid is not over#wear a fucking mask
149 notes
·
View notes
Text
Oh, Camellia, won't you take me away? - A Hanahaki!Eddie Munson story (sneak peek!)


eddie munson x fem!reader
summary: eddie munson had been a constant during your short time in hawkins, indiana, which made it that much harder when you had to leave. four years and a clinical trial later, you'd thought you'd conquered an otherwise fatal disease. what you weren't expecting, though, was the man that nearly killed you to walk back into your life, threatening to undo all of the progress you'd made towards healing - both physically and emotionally.
cw: hanahaki!au, angst, descriptions of light gore, childhood trauma, sexual themes and content
a/n: here is a snippet from the hanahaki eddie fic that has been bouncing around in my brain over the past week. feedback welcomed!

Water flowed out across the floor in a surge that mimicked crashing ocean waves. You cursed as you scrambled to right the plastic Procona and liquid sloshed awkwardly, lapping at your fingertips. It was a surprising amount from a relatively small bucket.
“Everything alright out there?” called a gruff voice from the back office.
You sighed. “Just fine, Bill! Minor spill. Nothing major.”
A muffled grumble could be heard from the owner’s space behind you, but you paid it no mind. It only took a few steps for you to grab the mop and start cleaning up the water all over the workspace floor, and to your relief, it really wasn’t as much as it seemed.
The nearly four years you’d spent at Indiana Floral Company had seemed to fly by in a blink of an eye. You weren’t expecting an on the spot interview when you’d first stopped into the shop, but the owner Bill had been impressed at your willingness to learn and your natural eye for design and hired you immediately. Probationary, of course.
So under Bill’s tutelage, your floral design skills blossomed. The basic knowledge of plants you’d brought from years of spending time gardening with your Grandma grew. You went from simply identifying lilies to knowing the difference between Asiatic and Oriental and their best growing seasons. You could identify roses based on subtle color differences and had learned how to take the most tightly closed bud and blow it open with a little humidity, a plastic bag, and very careful preening. And though you didn’t like to brag, you could match corsage ribbon to prom dresses better than anyone in town.
As time wore on, Bill had shared that years of design had wrecked his body and that it was time to begin passing the torch. Since Indiana Floral Company was one of the top floral design studios in town, the responsibility embedded in passing said torch was sobering. But after a year and a half of earning your stripes, you landed a head designer role and began training to take over the small family business.
Humming a nondescript tune, you refilled the earlier bucket with water and flower food before chopping the ends off of a bunch of de-thorned roses with the guillotine-like stem cutter. A clunk thrummed out when you dropped the two dozen stems into the water. Each blossom peered at you with a center like a curled eye — delicate sandy cream — perfect for the event you were designing later this weekend.
A ring of the bells on the front door broke your focus. You wiped your hands on the rag shoved haphazardly into your apron and turned at the sudden sound of Bill’s voice.
“The 1:30 initial wedding consult must be early. You mind taking this one, kid?” His head peeked around the office door. “I started the file – it’s on the cash wrap.” He looked tired; the man should have retired two years ago.
With a slight smile, you nodded. “Got it.”
It was impossible to see who had entered due to the amount of plants, gift items, and displays you’d designed around the small space (“customers shop with their eyes first, kid; you gotta draw them in before you let them see the price tag” Bill had said). But as soon as you rounded the front display, your stomach dropped clear out of your body and onto the floor.
Maybe it was the habitual need to weave around the labyrinth of flora and gifts that had lowered your defenses. Or perhaps it was the fact that this was a typical boring Wednesday afternoon in April. Hell, it could have been the questionable sandwich you had for lunch that you found at the back of the minifridge.
But one thing was clear: you hadn’t expected to see Eddie Munson and Chrissy Cunningham hand in hand looking around at the array of merchandise you’d set out in preparation for Mother’s Day.
“Hi!” A saccharine voice matched the sickeningly sweet smile on the strawberry blonde in front of you. “We’re here for a wedding consultation at 1:30. Sorry we’re a bit early — we didn’t want to be late!”
Time stood still. Or maybe that was just you — frozen as you stared the couple down with a look of surprise plastered across your features. You didn’t think you could move (or even speak, for that matter).
However, for the first time in almost four years, you felt your chest tighten and a sharp prickling sensation snake up your windpipe. You licked your dry lips (hadn’t you just put on chapstick?) and attempted to swallow with no success. Instead, your throat constricted, and there it was: an involuntary, yet ever so familiar metallic cough.

image credit: pinterest dividers: @saradika-graphics
tagging some moots that might be interested: @chickpeadumpsterfire @voyeurmunson @joshlmbrt @mediocredreams @littlexdeaths @anamelessfool
#eddie munson x reader#eddie munson fanfic#eddie munson#eddie munson x you#eddie munson x female reader#eddie munson angst#eddie munson hanahaki au!#hanahaki#hanahaki au#hanahaki!eddie munson#eddie munson hanahaki#eddie stranger things#stranger things#stranger things fic#sneak peek#preview#my writing#hannie’s writing
170 notes
·
View notes
Text
Stephanie Armour at KFF Health News, via AlterNet:
Researchers racing to develop bird flu vaccines for humans have turned to a cutting-edge technology that enabled the rapid development of lifesaving covid shots. There’s a catch: The mRNA technology faces growing doubts among Republicans, including people around President Donald Trump. Legislation aimed to ban or limit mRNA vaccines was introduced this year by GOP lawmakers in at least seven states. In some cases, the measures would hit doctors who give the injections with criminal penalties, fines, and possible revocation of their licenses. Some congressional Republicans are also pressing regulators to revoke federal approval for mRNA-based covid shots, which President Donald Trump touted as one of the signature achievements of his first term. The opposition comes at a critical juncture because vaccines using mRNA have applications well beyond avian flu and covid. They hold the promise of lifesaving breakthroughs to treat many diseases, from melanoma to HIV to Zika, according to clinical trials. The proposed bans could block access to these advances.
MRNA is found naturally in human cells. It is a molecule that carries genetic material and, in a vaccine, trains the body’s immune system to fight viruses, cancer cells, and other conditions. An advantage of mRNA technology is that it can be developed more quickly to target specific variants and is safer than developing a vaccine made from inactivated virus. “Right now, if we had a bird flu pandemic, we would have a shortage of the vaccine we need,” said Michael Osterholm, director of the University of Minnesota’s Center for Infectious Disease Research and Policy. “The one thing that could save us is mRNA vaccine. The challenge would be if mRNA is banned. This is truly dangerous policy.”
The pushback conflicts with innovations championed by Trump. He assembled tech tycoons at the White House just after his inauguration to announce Stargate, a $500 billion artificial intelligence initiative that could help transform cancer treatment by creating tumor-targeting mRNA vaccines. The fledging partnership between Oracle, SoftBank Corp., and OpenAI, co-founded by Elon Musk, envisions leveraging AI in part to improve health outcomes. Patients would undergo blood tests and AI would be used to find cancer.
[...] But some politically conservative doctors, lawmakers, and researchers question the safety of mRNA vaccines, especially covid shots made with the technology. Robert F. Kennedy Jr. unsuccessfully petitioned the FDA in 2021 to rescind approval for covid shots and called them “the deadliest vaccine ever made” — a controversial statement that has been refuted. Now that he’s newly confirmed as Health and Human Services secretary, Kennedy is poised to oversee federal approvals of vaccines, with the power to shape policy such as immunization schedules and appoint vaccine opponents to committees that advise on the approval of shots.
[...] Support for an mRNA ban is coming from other sources too. Florida Gov. Ron DeSantis on March 5 urged the Centers for Disease Control and Prevention to stop recommending the covid-19 vaccine for children and called for a state ban on mRNA vaccine mandates. In February, Rep. Thomas Massie (R-Ky.) said on X that the “FDA should immediately revoke approval of these shots,” and Sen. Ron Johnson (R-Wis.) is leading an investigation into the safety of the vaccines. Trump in February signed an order to strip federal funds from schools that require covid shots for attendance. Vaccine skepticism has become pronounced among Republicans since the pandemic. Four in 10 Republicans who responded to a KFF poll published in January said it was “probably” or “definitely true” that “more people have died from covid-19 vaccines than from the virus itself.” Just a quarter of Republicans reported holding that view in 2023. [...]
Networks of Opposition
Groups opposed to the mRNA technology have built a vast and well-funded legal, marketing, and social media network. Members hold conferences to discuss strategies, fund lawsuits against vaccine mandates, and produce reports on the covid vaccines. As for state legislative efforts, measures introduced this year have varied and their progress has been mixed. Montana’s measure, for instance, was blocked. Idaho lawmakers in February held a hearing on its bill, which calls for a 10-year moratorium on mRNA vaccines. Idaho’s proposal, likely to be amended, as well as Iowa’s and Montana’s have featured criminal penalties for providers who administer all or certain mRNA vaccines. In addition, some state bills, such as legislation in Pennsylvania and Tennessee, focused on the use of the vaccine in livestock and food production.
Various bills are pending in the Texas Legislature to restrict mRNA vaccines in both livestock and humans. South Carolina’s pending bill would require anyone administering certain covid mRNA vaccines to inform patients that the shot is contaminated with fragments of “bacterial plasmid DNA.” Covid mRNA shots may have minute amounts of residual DNA from production processes but they are heavily degraded and pose no risk, according to the Global Vaccine Data Network, which evaluates vaccine safety concerns. Speakers at some legislative proceedings have included representatives from Children’s Health Defense, an activist, anti-vaccine group founded by Kennedy. The Florida surgeon general in January 2024 called for a halt in the use of covid mRNA vaccines. And in Texas, Attorney General Ken Paxton in January moved to appeal a lawsuit he filed claiming Pfizer misrepresented the safety of its mRNA shot. Efforts to restrict the shots have raised the profile of groups such as the Independent Medical Alliance, which advocates for mRNA-based covid vaccines to be withdrawn from the market.
The normalization of anti-vaxxer sentiment in a sizable chunk of the GOP in recent years is leading to disastrous consequences, such as the recent attacks on mRNA technology used for vaccinations.
40 notes
·
View notes
Text
Curious about how clinical trials are shaping the way we treat eye conditions? 👁️ From groundbreaking surgeries to new therapies for chronic conditions like glaucoma and macular degeneration, Albemarle Eye Center is at the forefront of innovation in Kitty Hawk, NC! 🌟 Want to know more? 👇 Read the full blog now.
URL: https://albemarleeye.com/service/clinical-trials/
0 notes
Text
Medical research has shortchanged women for decades. This is particularly true of older women, leaving physicians without critically important information about how to best manage their health.
Late last year, the Biden administration promised to address this problem with a new effort called the White House Initiative on Women’s Health Research. That inspires a compelling question: What priorities should be on the initiative’s list when it comes to older women?
Stephanie Faubion, director of the Mayo Clinic’s Center for Women’s Health, launched into a critique when I asked about the current state of research on older women’s health. “It’s completely inadequate,” she told me.
One example: Many drugs widely prescribed to older adults, including statins for high cholesterol, were studied mostly in men, with results extrapolated to women.
“It’s assumed that women’s biology doesn’t matter and that women who are premenopausal and those who are postmenopausal respond similarly,” Faubion said.
“This has got to stop: The FDA has to require that clinical trial data be reported by sex and age for us to tell if drugs work the same, better, or not as well in women,” Faubion insisted.
Consider the Alzheimer’s drug Leqembi, approved by the FDA last year after the manufacturer reported a 27% slower rate of cognitive decline in people who took the medication. A supplementary appendix to a Leqembi study published in the New England Journal of Medicine revealed that sex differences were substantial — a 12% slowdown for women, compared with a 43% slowdown for men — raising questions about the drug’s effectiveness for women.
This is especially important because nearly two-thirds of older adults with Alzheimer’s disease are women. Older women are also more likely than older men to have multiple medical conditions, disabilities, difficulties with daily activities, autoimmune illness, depression and anxiety, uncontrolled high blood pressure, and osteoarthritis, among other issues, according to scores of research studies. (Read more at link)
While it certainly isn’t a panacea, for certain things I will only see a female doctor. No one is immune to bias and I’ve had bad experiences with some women, overall I feel more listened to and more at ease at appointments. Medical sexism is way too real, and we should use any tool we can to minimize it.
#disability#chronic pain#ableism#spoonies#chronic illness#sexism#feminism#medical bias#medical sexism#article
100 notes
·
View notes
Text
Reasons Why I Think Jerome Adams is Oedipus. [CHAPTER 13 SPOILERS]
So in the recent events of Main Story 13, we see that Luke finally confronts Oedipus about the drug or, should I say "cure", to Luke's illness. I had my suspicions before, but we have incriminating evidence of this being true in this chapter. Let's go through the facts together:
Oedipus
There are things we know about Oedipus:
He has contacted two individuals within the Story; Skye Harper, the nurse who murdered both Tyson Turner and Gerard Boone's mother by injecting the NXX drug into them; and Luke, receiving an unknown drug with a note from Oedipus that says "try to live on" and meeting him under the guise of a Teddy Bear mascot, sending children to bid Luke his little cryptic messages.
Knowing these facts we can concur two facts from this:
Oedipus is someone who has access to the NXX drug.
Oedipus has access to the hospital to be able to sneak the drug into Skye Harper's hand and a part-time job as a mascot (or several) to be able to relay the message to Luke.
Oedipus knows a lot, he's always at the right place and the right time: about the NXX investigations and the whereabouts of the NXX team if he can figure out who Luke is and where he is.
The only person who would fit this criterion is a little freaky Where's Waldo ass mf with a ridiculous amount of part-time work he could probably use as covers aka Jerome Adams. (Seriously, it's like he knows to be at the right place at the right time every time.)
Here is his character description from the wiki:
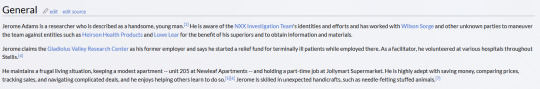
Thus we can see that Jerome meets all the criteria because: 1. Access to the NXX Drug - "Jerome claims the Gladiolus Valley Research Center as his former employer", We have also seen several scenes of him previously in the Gladiolus Valley Research Center conversing with Wilson Surge,
2. Access to the hospital - "As a facilitator, he volunteered at various hospitals throughout Stellis." 3. He's aware of the NXX Team's presence and can be at the right time and place. Holding multiple part-time jobs such as a convenience store clerk and a food delivery service (and this time a bear mascot) gives him cover for his activities.
But the most incriminating evidence we have is actually within the exchange between Luke and Oedipus himself.

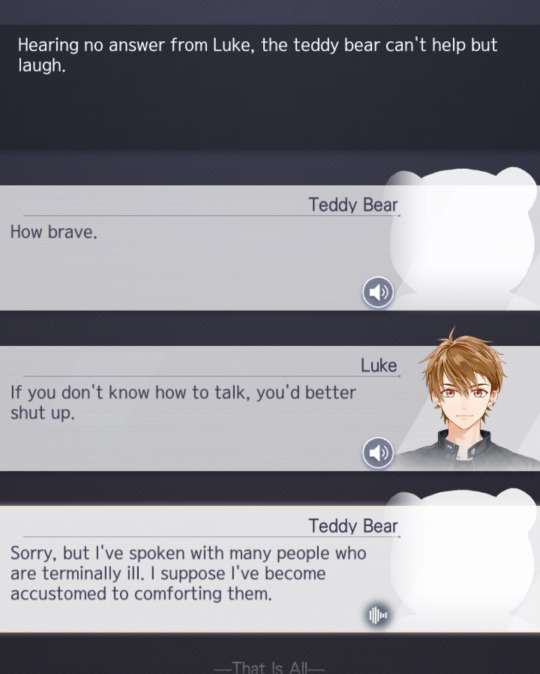
This is undoubtedly something Jerome Adams would do because: as established in the previous chapters, Jerome Adams works in a hospital and he has handled kids as well. This exchange coincides with all the facts previously mentioned. Ultimately, there's just too many threads that connect Oedipus and Jerome Adams together.
But why is he doing this? I can think of two reasons:
The reason why he is choosing to cooperate with Luke: In this chapter, we can see that both Oedipus and Luke have something to gain from this. Oedipus can run "clinical trials" for the NXX drug on Luke, and well... Luke doesn't have much of a choice here either, does he?
Despite Jerome freaky freaky ways, ultimately, I believe that Jerome has "good intentions" despite his very... twisted way about going about things. I can't wait to see what the new chapters have in store about is backstory and his ties with the NXX drug.
I think there's a reason why Jerome/Oedipus seems to always be hovering around the NXX team. Perhaps we're getting closer to the truth or perhaps he's trying to cooperate with us. But, I don't think Jerome/Oedipus is an enemy if anything, he's more like a third party that is working independently. I could imagine him working together with the NXX team (for a short while perhaps before they start getting at each other's throat again), or maybe even sacrificing himself in the end to ensure the NXX team gets to the root of the problem. That said, I have several other theories storming up in my brain regarding the NXX drug and how all the boys tie in all of this as well.... in another post.
53 notes
·
View notes
Text
I'm trying to come up with fun classes Angel and Lee can take at a local community center. Like, I'm picturing Angel taking a yoga class because people always say yoga is good for your mental health but it actually starts to go on them. They don't know if they are actually medicating but at the end of class when they lay down on their mat with their eyes closed, other people fade away, and they get great ideas.
Their back doesn't hurt, and they can stretch in new positions for drawing; it's nice. They start to feel connected to others through the repetitive group activity and even start to talk to them. For the first time, it's like their body and brain are on the same page.
Lee is happy Angel is happy. Angel shows Lee is position where you sit on a floating chair and pinch your ear load between your thumb and index finger to focus. Lee laughs it off because he doesn't actually think Angel is serious about this (it's unlike them to be like this). It fucks with them a bit because they know their mentally ill but the yoga stuff actually makes them happier but them it ends and they go back to feeling like shit again. What if their mom was right and they just needed to play outside more? Why do they make everything worse?
They take Lee to a class, and after checking him out for a bit, Angel starts to talk to their yoga buddies. They pull Angel aside to grill them about Lee. Angel knows he's not the best socially and prepares to defend him, but they start asking how old he is. It throws Angel off he's like 7-10 ish years older than them, but it's not like that. Then, they ask how they met, and the abridged version is that they ran into Lee again after completing the clinical trial with them. It's a much sweeter version of the story, but then they start telling Angel that's an odd coincidence and asking if they knew where they worked. They tell Angel that Lee is giving them "a bad vibe." and that they should "reflect on if he's really the right guy". They can't believe they've known him or less than a year and they're already moved in, they speak about him like they "idolize" him (I think it would be really funny to say that about Angel). They don't know angel, they don't know what they've been through together, they don't know what Lees done for them. Angel tells them that Lee's a nice guy, and he's done a lot for them. They just get told that those things don't give him a pass to do anything bad to Angel; if he does end up doing something bad to Angel.
Angel feels like a child again; their not going to come to the fun class if their just going to get lectured. They leave. In the car, Angel tells Lee that they're not coming back. He asks them why since Lee thought it would be a great opportunity to socialize with their friends but Angel just tells them those aren't their friends; they don't understand him. Part of Lee feels sick for feeling relieved. If angel has people that make them happier, what do they need him for?
(I did a yoga class yesterday, and I have these guys rotating in my head at the moment so they came together to make this. I do wanna make an actual fic of Angel hyping up Lee to a group of people then someone saying they idolize him, it would be very funny to me but a mindfuck for Angel.)
#im kinda tired and writing in a rush so they may be incoherent#clinical trial game#clinical trial#lee smith#angle martinez
30 notes
·
View notes
Text
Also preserved in our archive
A wearable electrical nerve stimulation device can provide relief to people experiencing the persistent pain and fatigue linked to long COVID, a study co-led by UCLA and Baylor College of Medicine researchers suggests.
Long-COVID, a complex and lingering condition following COVID-19 recovery, affects approximately 1 in 13 adults in the U.S. Symptoms such as widespread pain, fatigue, and muscle weakness often continue to disrupt daily activities, including walking and basic tasks.
The study, published in the peer-reviewed Scientific Reports, focused on a wearable Transcutaneous Electrical Nerve Stimulation (TENS) device, which uses low-voltage electrical currents to reduce pain, fatigue, and mobility issues associated with long-COVID.
The project was co-led by Dr. Bijan Najafi, research director of the Center for Advanced Surgical & Interventional Technology at UCLA Health and co-director of NSF IUCRC Center to Stream HealthCare in Place (C2SHIP), who said the device could have wider applications.
"While this study focused on managing pain and fatigue caused by long COVID, it may also have potential applications for addressing similar symptoms in individuals with other respiratory diseases, those who have experienced extended ICU stays and developed post-hospitalization weaknesses, and conditions involving chronic fatigue and pain, such as fibromyalgia or chemotherapy-related side effects," Najafi said. "But further studies are needed to confirm these potential uses."
In the study, 25 participants with chronic musculoskeletal pain, fatigue, and gait difficulties were assigned either a high-dose (active) TENS device or a low-dose (placebo) device. Both groups used the TENS device for three to five hours daily over a four-week period.
Researchers measured participants' pain levels, fatigue, and walking performance before and after the therapy period. Findings indicated that the high-dose TENS group experienced notable improvements in pain relief (26.1% more relief compared to placebo) and walking ability (8% during fast walking), suggesting that wearable TENS therapy may help reduce long-COVID's impact on daily life.
The high-dose TENS group also reported a slightly higher perceived benefit (71.2%) compared to the low-dose group (61.4%), underscoring the potential of wearable TENS technology to support long-COVID recovery.
One factor in the study's success was likely the high rate of daily device usage, Najafi said. The wearable nature of the TENS device allowed participants to use it seamlessly throughout the day, without disrupting their routines.
"This wearable TENS system offered immediate, on-demand relief from pain and fatigue, making it easy to integrate into daily activities," Najafi said.
He also cautioned that more research is needed. "This study provides some hope for finding an effective, non-invasive solution for managing lingering COVID-19 symptoms that continue to affect millions," he said. "But our sample size was limited, so further research is needed to confirm these findings."
Study co-authors are Alejandro Zulbaran-Rojas, Rasha Bara, Myeounggon Lee, Miguel Bargas-Ochoa, Tina Phan, Manuel Pacheco, Areli Flores Camargo, Syed Murtaza Kazmi, Mohammad Dehghan Rouzi, Dipaben Modi, and Fidaa Shaib of Baylor College of Medicine.
More information: Alejandro Zulbaran-Rojas et al, Transcutaneous electrical nerve stimulation for fibromyalgia-like syndrome in patients with Long-COVID: a pilot randomized clinical trial, Scientific Reports (2024). DOI: 10.1038/s41598-024-78651-5 www.nature.com/articles/s41598-024-78651-5
#mask up#public health#wear a mask#pandemic#covid#wear a respirator#covid 19#still coviding#coronavirus#sars cov 2
98 notes
·
View notes
Text
Clinical Trial - Partial Dog TF
Logan didn’t think much of it when the lab techs handed him a clipboard and a consent form.
“Simple injection. Harmless,” they said, smiling reassuringly. “Short-term study, minimal side effects. Just monitor yourself for the next 48 hours.”
Five thousand dollars for a single shot? Easy money.
The injection was over in seconds — a cold sting in his upper arm — and Logan left the sterile building feeling victorious, texting his friends and joking about blowing the money on something stupid.
He didn’t feel any different. Not at first.
It started a few hours later, at home.
A weird heat in his lower back, like he’d pulled a muscle. His feet throbbed too, like he’d been walking all day. Brushing it off, Logan downed some water and laid back to binge a show — only to jolt upright with a sharp jab from his spine, followed by a loud tear in the back of of his shorts, just below the waistband.
He felt something long tickling his butt cheeks, swaying back and forth. His hand shot behind him instinctively — and he gasped.
Something moved under his fingers. Something thick and twitching.
Logan stumbled to the bathroom mirror, yanking down his shorts. His heart slammed into his throat.
Sprouting just above his ass was a furry dog tail, shivering and spasming like it was testing itself for the first time. Golden-brown fur, thick and soft, covered the entire thing.
“What the f—” he croaked, but the words stuck in his throat, broken by a dry, heavy pant that rolled off his tongue.
Another burning wave hit, centered around his legs this time. He watched in horror as fur burst out from the pours of his skin sprouting along his thighs, racing downward like a time-lapse of a forest growing. His calves, ankles, then his feet—all covered in fur.
CRRRIP — Logan stumbled back as the nails on his toes thickened and curved, claws punching right through his ankle socks.
He tore the ruined socks off and nearly puked. The soles of his feet had changed, thickening into rough, dark pads — paw-like, rough and tender at the same time.
“This isn’t happening,” he panted, tongue lolling involuntarily from his mouth, too long, too heavy, hanging stupidly against his chin. He tried to suck it back in, but the damn thing wanted to hang free.
And then he noticed the bulge at his groin.
Shaking, Logan lowered his gaze and found that his human cock was gone, replaced by a sheath nestled tightly against his now furry pelvis. Thick, animalistic, breathing with an embarrassing, rhythmic twitch.
“No, no, no—” he gasped again, gripping the counter for support. His tail wagged once, betraying his panic.
Logan tried to tell himself it would stop. That he could hide it. That he could be normal.
The next morning, he fastened his tail to the back of his right thigh, using a couple of long velcro straps and squeezed himself into his work clothes — an oversized button-up, dress pants, and black dress socks to cover the horror show his legs had become.
The shoes were a nightmare. His padded soles and new clawed toes screamed against the tight leather confines. Every step was a blend of pressure and sharp stabbing pain. Halfway to the car, he could feel the socks ripping at the front where his claws scraped and pushed through, straining against the material.
At the office, it only got worse.
The tail, while fastened, twitched anxiously whenever someone spoke to him. He had to awkwardly clench his lower back muscles just to stop it from wagging visibly under the fabric.
Typing at his desk became a panting, sweaty ordeal. His tongue wouldn’t behave, constantly peeking between his lips. His coworkers started giving him weird looks — was he... panting?
Logan ducked into the bathroom, locking the stall door.
He tore off his shoes with a grunt, rubbing his aching padded feet. His socks were ruined — split open where the claws punctured through. He unfastened his tail letting it wag around a bit.
Shame washed over him.
He wasn’t Logan anymore — at least not fully. He was something else. Some freakish hybrid stuck between man and beast.
When he pissed, it was even worse. Squatting down on all fours, lifting a leg, struggling to aim from the unfamiliar sheath, he felt humiliated. The urine dribbled in an awkward arc, unpredictable, animalistic. His cheeks burned with humiliation.
By lunch, Logan could barely walk straight. The pressure against his lower half was overwhelming — fur itching under his pants, tail begging to move, paws screaming from confinement. His tongue hung out more and more without him even realizing it.
He caught a glimpse of himself in a window reflection.
Dress shirt, slacks... but underneath it all, the unmistakable shape of a tail wagging, a faint golden sheen of fur visible where the pants rode up too high. A handsome young man... degraded into something else, hidden behind a crumbling human costume.
He bit down hard on his tongue — literally — forcing it back into his mouth. Tears pricked at the corners of his eyes.
Hold it together. Just get through the day.
But some part of him — that deep, primal part awakening under the fur — knew it was already too late.
He wasn’t fooling anyone. Least of all himself.

24 notes
·
View notes
Text
I'm going to condense more than five pages of ramblings here. So my HCs on Republic City organized crime are presented in one excessively long post.
(If you have your own or don't like any of them, let me know, I'm dying to talk to someone with this)

🔥Agni Kai🔥
Its origins are traced back to the dissidents of the Fire Nation armies, groups that were left without government support when Zuko took power, refused to hand over their weapons and continued to survive with violence as their calling card. They did not have any kind of order or clear leader until their arrival in Republic City, a place they avoided for a long time until the Earth Kingdom forces began to dwindle their numbers. The first leader recognized in their history is Sozan, a prodigious firebender capable of using lightning when it was still an elite technique, she became a legend at a young age, many pointed to her as a lost daughter of Princess Azula herself, Princess Azula herself or a pupil of the Wandering Princess, her royal past was lost over the years and she herself fueled those myths to aggrandize her figure.
She formed the Agni Kai and quickly positioned them as one of the most prominent mafias in the city, standing out for their effective assassins and their loan systems. She had several children who stayed away from the criminal life; she prepared her own pupil, Zolt the Lightning, who would relieve her after a confrontation with Toph that left her unable to continue fighting.
Contrary to what Sozan expected, Zolt quickly abandoned his position as leader, taking the best members of the Agnis and taking them to his own gang, leaving the group weak. The gang was close to disappearing until the death of Avatar Aang, where Sayuri, Sozan's eldest granddaughter, appeared out of nowhere and revived her group at the young age of sixteen, much colder and more methodical than her grandmother, she has strengthened the Sozinist discourse in the group and they have established themselves in the borders where they control the main drug lines.
The uprising of the Equalists and the Harmonic Convergence severely affected the Agni Kai, it is rumored that they are about to disappear.

🌊Red Monsoons🌊
The smallest of the gangs, made up mostly of waterbenders, began with a group of the Northern tribes that migrated when the city was founded led by Igaluk, who attracted more people promising to quickly achieve a higher standard of living, most of them were women who left the North in search of freedom from their customs. Once Katara intervened to cut the corruption that fueled the medical areas centered on the theft of supplies and clandestine clinics, the Monsoons saw things black, almost disappearing, it was when Igaluk opened the doors to Yakone, who would arrive to change the course of the gang with his bloodbending, copiously avoiding confrontation with Katara and focusing on expanding the territory by training more and more benders who were attracted and intimidated by his power.
The medical approach was maintained and the Monzones rose above all other gangs becoming known only as “The Followers of Yakone” for almost two decades. It was after the trial that the other gangs came after the Monzones, taking advantage of the fact that they were a headless body and cornered them again. Their golden age had ended.
Najak was then the one who took command when the killing ended, migrating the group's focus to other more political panoramas, before this he helped consolidate the Triple Threat with Zolt and separated when there were differences of thought, he had some freedom with the Monsoons due to his good terms with the Triad, his smuggling of exotic materials such as endangered animals or really rare minerals positioned the Monsoons in the highest circles as a trustworthy organization, added to his dealings with politicians. Najack died during the Robbery of the Fangs, a raid on the Ba Sing Se palace that resulted in one of the largest thefts ever executed.
After her came Ookpik, who had been under Najak's tutelage and who has continued in his footsteps with less success, he does not have the social skills and connections that distinguished his predecessor, he has remained in office because he is a killing machine who they have not managed to bring down. After the harmonic convergence they officially entered a slow death.

🪨Terras🪨
They were never really considered a group, they lacked organization and ideology, they were formed from the many gangs of the Earth Kingdom that arrived in Republic City, they grouped together to dominate the underworld of the city when it was just being built. Their first big blow was during the inauguration of the Council while the Earth King Kuei was giving his speech, they destroyed the entire building, rumors say that Chief Beifong began her military career due to this event.
Once other groups such as the Agni Kai or the Red Monsoons began to be born, the Terras began to lose territory rapidly due to their disorganization. This did not change until the arrival of the first leaders: The Tuo brothers.
Quiang and Jing Lei known for being two bastards of one of the aristocratic families of Ba Sing Se used their education and knowledge to dominate the underworld, literally. They created thousands of tunnels under the city, using the drainage systems to move their goods, introduced many new drugs and founded the first Red Light District, quickly abandoning their other lines to focus on prostitution.
They maintained their reign for a very long time, they saw great figures like Sozan and Yakone fall, they would have kept things that way if it weren't for the fact that Quiang was killed during a trip to the Ember Islands betrayed by his right hand, that deeply frightened Jing Lei, who took all the money he could and left the city with his nephews. The leader position was assumed by Minwei, one of the most famous actors of the red light district, noted for his performance, despite being a non-master he had such magnetism that he was quickly accepted, however under his command the conditions in the Red Light District became harsher fattening the networks of human trafficking, his reign of terror was fleeting, his capture occurred only six years later at the hands of Major General Lin Beifong, merit that would give him the leadership months later. The last known leader was Feiju, Minwei's right-hand man, who collaborated with the police to turn him in and continued to push the bigwigs in his group into the jaws of the police until he was able to get his charges greatly reduced and was able to retire without ever stepping foot in prison.
The Terra ceased to exist as a gang a couple of years before Avatar Korra's arrival in the city after losing the turf war against the Creeping Crystals.

⚜️Creeping Crystals Triad⚜️
They started in Ba Sing Se under a branch of the Dai Li that researched and used the properties of their crystals for combat. Once the restructuring took place after the war, much of their research was banned, forcing those less happy to continue in the shadows, spreading terror in the most disadvantaged neighborhoods until the Dai Li high command intervened in an effort to clear their name and disassociate themselves from that part of their past. There were many captures in the process, however there was a part that survived and escaped to Republic City with their knowledge under their shoulders.
There they spread terror left and right with genamite, weakened the Agni Kai by stealing much of their territory and suffocated the Red Monsoons who had just lost Yakone, with the Terras they were more negotiative, actively participating in commercial and territorial deals with the Tuo brothers, the leader of that time was the former Dai Li Haoyu, who would be captured by Chief Toph Beifong after he attacked the Police Academy with Genamite during the graduation of the so-called "Golden Generation", where Academy personnel and civilians died.
After this, the gang was considered dead for a good couple of years, its territory was claimed by other gangs and the use of genomite was heavily regulated by the Metal Police supported by Kyoshi warriors who infiltrated the gang and identified their routes; the fall of the gang began to be used as a warning not to mess directly with the chief Toph Beifong. Toph's predecessor, Jing Shezen, kept his foot on the gang's neck until passing the baton to Lin, under his mandate the laws were toughened initiating the deportation of these criminals to Ba Sing Se where they faced inhuman treatment in the reign of Hou-Ting.
It was considered the death of the gang until luck smiled on them.
The council subjected Lin Beifong to a trial for her attack against the Temple of the Island and removed her from her functions for almost two years as a sanction, a fact that coincided with the appearance of Jargala Omo.
Born in the villages surrounding Ba Sing, she brought with her new injections of capital and gathered former gang members, spreading terror in the city, causing escapes in the most renowned prison to return the gang to the maps.
Her biggest coup was against the Police Council using Genamite. This led the council to lift Lin's restrictions allowing a war without quarter between both women that culminated in a deal under the table that calmed the waters after the Creeping Crystals could not sustain the battle against the constant injection of capital and personnel at the hands of the chief in the police forces.
Their numbers dropped considerably during the Equalist uprising and the Harmonic Convergence rekindled the territorial fight, Jargala has managed to maintain his group but finds himself at a critical moment.

🔸Triple Threat Triad🔸
One of the newest gangs, founded by Zolt after taking power from the Agni Kai, had been in contact with members of other gangs for a couple of years, building ties for this project.
They dominated the commercial area with loans, extortion, illegal betting and systematic robberies. The diversity of the members of their gang attracted many people, who saw there an opportunity to exploit their skills without fear of discrimination and extremist discourses that were handled by other gangs.
They quickly expanded their movements, taking advantage of the falls of other groups to take over the territory. They came to have control of almost the entire city. Such was their territorial dominance and the impunity that their members wielded that rumors that they were working with the police were not long in coming.
They subjected the Metal Police to scrutiny never seen before, uncovering moles and corrupt dealings that forced the restructuring of the judicial forces. The growth of the triad was halted during this event, called "The Bay Trials", led by the then Chief Inspector of the Organized Crime Department Lin Beifong, who was spared scrutiny for her active role in the trials.
More than thirty-five people from the Triple Alliance were captured and their activity dropped considerably. However, in less than a year they had filled the positions and reorganized their methods, their ability to adapt kept them from disappearing.
Their most formidable rival appeared with the arrival of Jargala to the Creeping Crystals. This enmity did not last long, as in order to survive the hunt of the then Chief Lin Beifong, Jargala sought her support. Unfortunately, Zolt had his own dealings with the police and helped sabotage his new ally to stay ahead, this betrayal was not discovered. The "deal" lasted until the uprising of the Equalists, who removed Zolt from his power by taking away his firebending, leaving the leadership in the hands of Viper, his second in command, who saw the reduction of his territory, the loss of the dealings with the police and the loss of his best men during that stage. After the Harmonic Convergence, talk of their downfall began, pushing for less favorable alliances with other gangs in the face of the hunt that the dissidents of the Agni Kais began to direct against them. However, luck smiled on them again and after Kuvira's attack, the Triple Alliance recovered much of its territory by temporarily allying itself with the police under the table to stifle the new gangs that wanted to take advantage of the chaos.
They are negotiating a merger with the Jargala gang in order to be able to disengage from the authorities and stabilize.

🐺White Fang🐺
It was a small group, made up mainly of teenagers and children, founded by Amaruk, son of a non-bending Southerner and a northern waterbender, raised in one of the concentrated neighborhoods of natives of the Poles, he was always steeped in that culture.
During one of the gang fights between the Red Monsoons and the Agni Kai, the neighborhood was plunged into disaster because the gas pipes were affected and fires did not take long to appear, there were many losses and families moved towards the borders of the City. Amaruk soon began dealing with the merchants who came and went helping with small orders, he was so elusive and a good businessman that they began to nickname him "Viper", he learned water control from his father and taught other children in the area in exchange for charging them favors, little by little he prepared his neighborhood companions to help him carry messages, drugs and other packages that adults were willing to pay, that evolved into hired thugs, going to break furniture in stores that did not pay their fees or directly hurting debtors of other larger gangs. They would be absorbed by Zolt during the founding of the Triple Alliance when he offered Viper to be his second.

🎭Equalists🎭
Their origins can be traced back to the Fire Nation colonies in the Earth Kingdom, where there were scattered groups of non-benders who spoke of elemental supremacy and invited questioning and confrontation. The one who would unify them and give them purpose would be a foreign figure who led under the name of "Amon." His excellent oratory and his strange ability to withdraw the power of the benders encouraged many to follow him religiously.
The one who introduced the chip lock to the group was a former Kyoshi warrior named Kushi, who imparted her knowledge after resigning from her position as director of one of the Kyoshi Academies in the Fire Nation when one of her students burned down the facilities and was not punished accordingly due to the intervention of her aristocratic parents.
The right-hand position was in her hands until the arrival of the Lieutenant, who quickly surpassed her in skill and took the movement to Republic City, Kushi would leave the group after the introduction of Hiroshi Sato due to his rejection by the higher echelons.
They managed to terrorize the world's capital and take power over them by appeasing both gangs and national forces such as the police or the fleets of the United Forces, their success seemed imminent until the revelation of Amon as a waterbender. The moral blow that this produced greatly destabilized the members of the terrorist group, who were quickly suffocated, there were arrests, extrajudicial executions, deportations, severe sentences and a huge rejection by the population after the identities of the members were revealed by a leak of information from the police files; many dispersed and the movement was considered dead for a couple of years until Kuvira's attack on Republic City.
Kushi came out of retirement to raise the Equalists again, now outside of Republic City and focused on being a protective force against the benders, unfortunately they have begun to fall into protection fees and, again, acts of terrorism.

That's not all, there's another file talking about events like "The Yakone Trial", detailing the attack on the Academy and so on because I'm unemployed (Metaphorically)
And that, I hope at least they entertain themselves lol I have to go collect street names from Republic City on AO3
#avatar the last airbender#avatar the legend of korra#tlok#headcanon#toph beifong#lightning zolt#jargala omo#viper#lin beifong#ocs#metalbending police#cristal creeping#triple threat triad#red monsoons#Corrupt police#I have drawings of half of the OCs I mention
20 notes
·
View notes
Text
Do you know which book this is from?

Please reblog the polls, but KEEP IT SPOILER-FREE to make people read the excerpt with an open mind 💖📚 Title and author will be revealed after the poll's conclusion.
Note: this excerpt is too long for Tumblr’s alt text character limit, so for this poll, the alt text is below the read more.
Edit: The results are up here!
In 1999 the RAND Corporation published a report (the first and, so far, last of its kind) with a "conservative estimate" that more than 307 million tissue samples from more than 178 million people were stored in the United States alone. This number, the report said, was increasing by more than 20 million samples each year. The samples come from routine medical procedures, tests, operations, clinical trials, and research donations. They sit in lab freezers, on shelves, or in industrial vats of liquid nitrogen. They're stored at military facilities, the FBI, and the National Institutes of Health. They're in biotech company labs and most hospitals. Biobanks store appendixes, ovaries, skin, sphincters, testicles, fat, even foreskins from most circumcisions. They also house blood samples taken from most infants born in the United States since the late sixties, when states started mandating the screening of all newborns for genetic diseases.
And the scale of tissue research is only getting bigger. "It used to be, some
researcher in Florida had sixty samples in his freezer, then another guy in Utah had some in his," says Kathy Hudson, a molecular biologist who founded the Genetics and Public Policy Center at Johns Hopkins University and is now chief of staff at NIH. "Now we're talking about a massive, massive scale." In 2009 the NIH invested $13.5 million to develop a bank for the samples taken from new borns nationwide. A few years ago the National Cancer Institute started gathering what it expects will be millions of tissue samples for mapping cancer genes; the Genographic Project began doing the same to map human migration patterns, as did the NIH to track disease genes. And for several years the public has been sending samples by the millions to personalized DNA testing companies like 23andMe, which only provide customers with their personal medical or genealogical information if they first sign a form granting permission for their samples to be stored for future research.
Scientists use these samples to develop everything from flu vaccines to penis-enlargement products. They put cells in culture dishes and expose them to radiation, drugs,
cosmetics, viruses, household chemicals, and biological weapons, and then study their responses. Without those tissues, we would have no tests for diseases like hepatitis and HIV; no vaccines for rabies, smallpox, measles; none of the promising new drugs for leukemia, breast cancer, colon cancer. And developers of the products that rely on human biological materials would be out billions of dollars.
How you should feel about all this isn't obvious. It's not as if scientists are stealing your arm or some vital organ. They're using tissue scraps you parted with voluntarily. Still, that often involves someone taking part of you. And people often have a strong sense of ownership when it comes to their bodies. Even tiny scraps of them. Especially when they hear that someone else might be making money off those scraps, or using them to uncover potentially damaging information about their genes and medical histories. But a feeling of ownership doesn't hold up in court. And at this point no case law has fully clarified whether you own or have the right to control your tissues. When they're part of your body, they're clearly yours. Once they're excised, your rights get murky.
Kathy Hudson, who has conducted focus groups about the public's feelings on the tissue issue, says she believes that tissue rights have the potential to become a bona fide movement.
"I could see people starting to say, 'No, you can't take my tissues,' " she told me. "All I can say is, we better deal with the problems now instead of waiting until that happens."
There are, essentially, two issues to deal with: consent and money. For most people, knowing if and how their tissues are being used in research is a far bigger issue than profiting from them. Yet when this book went to press, storing blood and tissues for research did not legally require informed consent, because the law governing such things doesn't generally apply to tissue research.
The Federal Policy for the Protection of Human Subjects, also known as the Common Rule, requires informed consent for all human-subject research. But in practice, most tissue research isn't covered because: (1) it's not federally funded, or (2) the researcher never learns the identity of the "donors" or has firsthand contact with them, in which case it's not considered research on humans. So in the end, the Common Rule doesn't actually govern most tissue research.
Today, if doctors want to gather tissues from patients strictly for research purposes—as in Henrietta's case—they are required to get informed consent. But storing tissues from diagnostic procedures like, say, mole biopsies, and using them in future research doesn't require such consent. Most institutions still choose to get permission, but there's no uniformity in the way that's done. A few hand out enough information to fill a small book, explaining exactly what will be done with all patient tissues. But most just include a short line in an admission form saying that any tissues removed may be used for education or research.
#poll#lit#literature#tumblr poll#tumblr polls#polls#book excerpt#poll time#nonfiction#booklr#bookblr
16 notes
·
View notes