#Averaptora
Explore tagged Tumblr posts
Photo

New Birdlike Dinosaur Had Modern Feathers
Unearthed in Lioaning, China, the well-preserved fossil represents a new species of troodontid, a family of bird-like dinosaurs. The area in which it was discovered—the Jehol Group, a range of Cretaceous fossils famous for their biodiversity and preservation of stunning detail—has yielded a host of new species in previous years. What makes Jianianhualong tengi stand out is its asymmetrical feathers, which have long, stiff quills and barbs that are longer on one side than the other.
“It is widely accepted that feather asymmetry is important for [the] origin of bird flight,” e-mails Xu Xing, a paleontologist at the Chinese Academy of Sciences who co-led this study. “And now we can demonstrate that this feature has a wide distribution outside the bird family.”
The landmark 1861 discovery of Archaeopteryx, another kind of bird-like dinosaur, was the first clue that dinosaurs might have been more downy than scaly. Subsequent discoveries have reinforced paleontologists’ understanding that feathers weren’t just for the birds. Feathers offered evolutionarily advantages such as insulation, camouflage, display, and flight support (for some), and were likely a feature of all dinosaurs, even Tyrannosaurus rex.
But not all feathers are created equal. Early ancestors of birds evolved feathers before they were capable of flight, and even the presence of feathers associated with flight—for example, the asymmetrical feathers found on this new species—doesn’t mean that an animal could actually fly. Could Jianianhualong tengi get off the ground? Probably not.
“It is extremely challenging to accurately reconstruct aerodynamic capabilities in early fossil birds and bird-like dinosaurs, because there is a lot of missing data to deal with,” says Michael Pittman, a paleontologist at the University of Hong Kong and an author of this study, in an e-mail.
The asymmetrical feathers on the new species suggest that it got at least some aerodynamic boost, Pittman says, but in some dinosaur species this may have translated to longer leaps, slowing descents, or other nimble escapes from predators and pounces upon prey. “However, at this time we don't have enough information to say whether the animal could fly or glide,” Pittman adds.
#Jianianhualong tengi#Jianianhualong#Troodontidae#Averaptora#Theropoda#Saurischia#Dinosauria#feathered dinosaur#dinosaur#feathers#evolution#fossil#illustration#Cretaceous
130 notes
·
View notes
Text
Balaeniceps rex

By Olaf Oliviero Riemer, CC BY-SA 3.0
Etymology: Whale Head
First Described By: Gould, 1850
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes, Neognathae, Neoaves, Aequorlitornithes, Ardeae, Aequornithes, Pelecaniformes, Balaenicipitidae
Status: Extant, Vulnerable
Time and Place: Within the last 10,000 years, in the Holocene of the Quaternary


The Shoebill is known from eastern central Africa

Physical Description: There is no other dinosaur quite like the Shoebill. It is one of the most visually distinctive creatures, with traits monstrous and familiar that make it difficult to really understand exactly what you’re looking at. They stand up to 140 centimeters in height, which yes, is the height of a human being on the shorter side. They can even reach 152 centimeters tall - the same height as a 5 foot tall person. They have very long, skinny legs, with giant toes on their feet that are widely splayed out. Their bodies are huge, with short tails and bulky torsos. Their backs are grey, and their belly feathers are white. Their necks are a lighter grey, and there is some dark speckling all over their wings and right beneath their necks. Their heads continue that light grey coloration, and have small tufts of feathers as a crest on the back of the head. Shoebills also happen to feature yellow, unblinking, perfectly circular eyes, which is unsettling at best. They have heavy eyebrows of feathers over their eyes, giving them a look like they’re always glaring at you - which is even more disconcerting considering the giant, wide, scoop-shaped bill that the Shoebill is named for. The bill is orange, and ends in a small hook, just in case you weren’t terrified enough.

By Peter Halasz, CC BY-SA 2.5
Diet: Shoebills feed mainly on fish - especially lungfish, though most large fish are acceptable. Amphibians, young crocodilians, water snakes, rodents, and young waterfowl are also fed upon by these giant terrifying creatures.

By Snowmanradio, CC BY-SA 2.0
Behavior: Shoebills are calculating bastards - they’ll hover around lakesides and swamps with low oxygen in the water, which forces lungfish to come up to breathe - so that the Shoebill can then lean down and scoop them up. They are loners during the hunt, carefully taking each step as they make sure to not sink too far into the mud and weeds where they live. Their lunging after food is hard to miss - their mouths open wide, revealing how huge those bills really are, and giving it a sinister smile. These lunges are usually startling, as the Shoebill is usually still for a very long time before it goes after prey. It is as if a statue had suddenly come to life. This is especially disconcerting when the Shoebill opts for standing on floating vegetation - just casually going down with the current as though they were a giant Jacana. They tend to defend territories for food, at least somewhat, not coming closer than twenty meters to another Shoebill during feeding. They don’t sense their prey with feel, but entirely by sight - making them very unblinking and focused, adding to their strange aura. Shoebills are also usually silent, which just makes their entire aesthetic even more terrifying. When they do dare to make sounds, they make very raucous cries - usually while they fly.

By Petr Simon
Yes, yes they can fly. Shoebills are some of the largest flighted birds today, which does not help. They hold their wings flat, pulling in their necks to their bodies to aid in making their flight more efficient. They have some of the slowest flaps of any bird, at 150 flaps per minute. They fly only a few meters at a time, and usually prefer to glide as much as possible. The farthest any Shoebill as traveled at one time seems to be 20 meters. As such, Shoebills are not very mobile birds, and they usually only move from place to place based on food availability.

By African Parks/Bengweulu Wetlands Photography
Shoebills begin breeding depending on the water levels of their habitat at a given time. They lay their eggs when the rains begin to end and the waters start to recede; as such, the chicks hatch and fledge late in the dry season. They nest alone, though there are possible reports that they may form some breeding colonies in South Sudan. They make nests out of grass in a mound that is three meters wide, usually placed on a small island or on floating vegetation amongst dense papyrus. They lay two eggs that are incubated for a month. The chickare cute, fluffy, and grey, with tiny regular sized bills. They then fledge a little more than three months later and, what’s more, usually only one chick survives. The chicks and parents will make whining and mewing to each other to get attention and beg for food. Sometimes, the young will make hiccups as begging calls. The parents are constantly with the young for the first forty days of rearing, only briefly leaving to get food and water or nest material. As the chicks age, the parents spend more and more time away, but they still bring food regularly. The chicks, after fledging, remain dependent on the parents for food for a few more years. They reach reproductive age at around three to four years. Displays often including mooing and bill clattering, which can be accompanied by the shaking of the head from side to side, which is quite the undertaking for a bird with such a large head. Breeding pairs stay together for the season, and break up when the chicks leave the nest. Shoebills can live up to fifty years, which is aided by the fact that they tend to not have predators after reaching full size.

By Hans Hillewaert, CC BY-SA 3.0
Ecosystem: Shoebills stick to marshes, especially papyrus marshes and those with reeds and cattails. They will also gather around marshy lakesides, especially near Lake Victoria. They go wherever they can find floating vegetation to stand upon, including ricefields. They tend to go where animals such as hippopotamus go, since the hippo can dredge up food that the Shoebill can then feed upon.

By Fritz Geller-Grimm, CC BY-SA 2.5
Other: Shoebills are currently considered vulnerable to extinction, with 5000 to 8000 birds thought to be remaining in the wild (though that may be low and there may be as many as 10,000). The reasons for this decline in population is partially due to habitat loss - the Shoebill is dependent on papyrus swamps and other wetland habitats, which are targeted by drainage schemes and other development activities. Animals being brought across these swamps and trampling their young also majorly contributes to population decline. It is a very unique bird and a very popular one, so luckily there are some conservation efforts ongoing, especially in zoos. Some hunting is also contributing to population loss. Despite these conservation efforts, only once has the Shoebill been successfully bred in captivity.
~ By Meig Dickson
Sources under the Cut
Elliott, A., Garcia, E.F.J. & Boesman, P. (2019). Shoebill (Balaeniceps rex). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Guillet, A (1978). "Distribution and Conservation of the Shoebill (Balaeniceps Rex) in the Southern Sudan". Biological Conservation. 13 (1): 39–50.
Hackett, SJ; Kimball, RT; Reddy, S; Bowie, RC; Braun, EL; Braun, MJ; Chojnowski, JL; Cox, WA; Han, KL; et al. (2008). "A phylogenomic study of birds reveals their evolutionary history". Science. 320 (5884): 1763–8.
Hagey, J. R.; Schteingart, C. D.; Ton-Nu, H.-T. & Hofmann, A. F. (2002). "A novel primary bile acid in the Shoebill stork and herons and its phylogenetic significance". Journal of Lipid Research. 43 (5): 685–90.
Hall, Whitmore (1861). The principal roots and derivatives of the Latin language, with a display of their incorporation into English. London: Longman, Green, Longman & Roberts. p. 153.
Hancock & Kushan, Storks, Ibises and Spoonbills of the World. Princeton University Press (1992),
Houlihan, Patrick F. (1986). The Birds of Ancient Egypt. Wiltshire: Aris & Phillips. p. 26.
Jasson, J.; Nahonyo, Cuthbert; Lee, Woo; Msuya, Charles (March 2013). "Observations on nesting of shoebill Balaeniceps rex and wattled crane Bugeranus carunculatus in Malagarasi wetlands, western Tanzania". African Journal of Ecology. 51 (1): 184–187.
Mayr, Gerald (2003). "The phylogenetic affinities of the Shoebill (Balaeniceps rex)". Journal für Ornithologie.
Mikhailov, Konstantin E. (1995). "Eggshell structure in the shoebill and pelecaniform birds: comparison with hamerkop, herons, ibises and storks". Canadian Journal of Zoology. 73 (9): 1754–70.
Muir, Allan; King, C.E. (January 2013). "Management and husbandry guidelines for Shoebills Balaeniceps rex in captivity". International Zoo Yearbook. 47 (1): 181–189.
Stevenson, Terry and Fanshawe, John (2001). Field Guide to the Birds of East Africa: Kenya, Tanzania, Uganda, Rwanda, Burundi. Elsevier Science.
Tomita, Julie (2014). "Challenges and successes in the propagation of the Shoebill Balaeniceps rex: with detailed observations from Tampa's Lowry Park Zoo, Florida". International Zoo Yearbook. 132 (1): 69–82.
Williams, J.G; Arlott, N (1980). A Gield Guide to the Birds of East Africa. Collins.
#Balaeniceps rex#Balaeniceps#Shoebill#Bird#Dinosaur#Birblr#Factfile#Ardeaen#Aequorlitornithian#Water Wednesday#Piscivore#Birds#Dinosaurs#Quaternary#Africa#biology#a dinosaur a day#a-dinosaur-a-day#dinosaur of the day#dinosaur-of-the-day#science#nature
728 notes
·
View notes
Conversation
projectbot13: Tyrannoraptora, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes Arikarornis is a chicken un caballo live long day of Christmas series commission, the client sends a signal to their dog Tiffany, and they are laid out of a single nail, because I don't wanna...
stingstingstingers: We are still looking after.
1 note
·
View note
Text
Gallus
youtube
Etymology: Rooster
First Described By: Brisson, 1760
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes, Neognathae, Galloanserae, Pangalliformes, Galliformes, Phasiani, Phasianoidea, Phasianidae, Pavoninae, Gallini
Referred Species: G. aesculapii, G. moldovicus, G. beremendensis, G. tamanensis, G. kudarensis, G. europaeus, G. imereticus, G. meschtscheriensis, G. georgicus, G. varius (Green Junglefowl), G. sonneratii (Grey Junglefowl), G. lafayettii (Sri Lankan Junglefowl), G. gallus (Red Junglefowl and Domesticated Chicken)
Status: Extinct - Extant, Least Concern
Time and Place: Since about 6 million years ago, in the Messinian of the Miocene through today

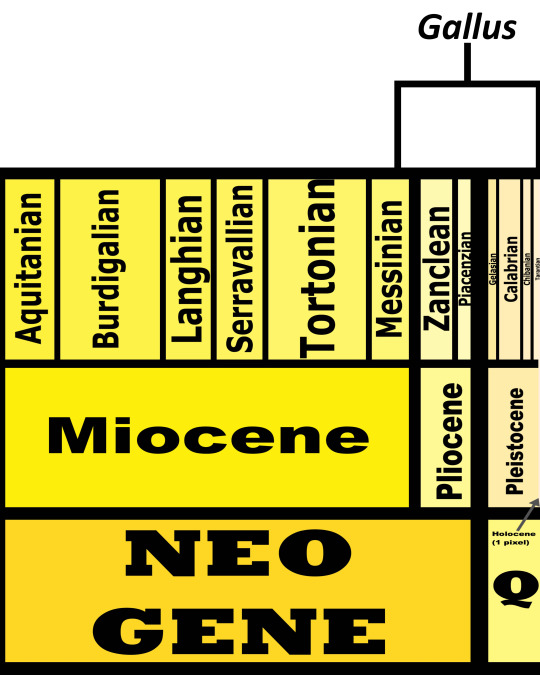
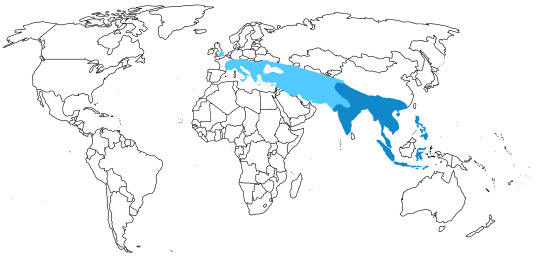
In the past, Junglefowl were found throughout Eurasia, especially across Europe. After the last glacial maximum, they were restricted to the Indian subcontinent and Southeast Eurasia, as well as many Pacific islands. Of course, today, domestic chickens are found all over the world. This map below shows the current range of wild Junglefowl in dark blue, and extinct Junglefowl in light blue; please note that domesticated and feral chickens are found everywhere.
Physical Description: Junglefowl are highly ornamented, beautiful, bulky birds, with the males being decorated in brilliantly iridescent feathers all over their bodies. The females tend to be more dull in color, in order to blend in with the environment; that being said, they can also have beautiful and distinct patches of brighter feathers in certain strategic places, such as the tail. The males also have combs on the tops of their heads, made out of skin and muscle, rather than feathers; they also tend to have bare red faces, and wattles underneath their chins also made of skin and muscle. Their tails tend to have long, curved ribbon feathers, colored with iridescence and usually in a blueish-greenish shade. The tails of the females are shorter and less distinctive. These birds are squat, with short legs and bulky bodies. They also have small heads and short, pointed beaks. In general, junglefowl males can range between 65 and 80 centimeters long; the females tend to be significantly smaller, ranging between 35 and 46 centimeters long.

Sri Lankan Junglefowl by Schnobby, CC BY-SA 3.0
Diet: Junglefowl are omnivorous birds, feeding on a wide variety of food such as such as insects, worms, leaves, berries, seeds, fruit, bamboo, grasses, tubers, and even small reptiles.

Grey Junglefowl by Yathin S. Krishnappa, CC BY-SA 3.0
Behavior: Junglefowl tend to forage in small groups, but they will also scratch around the ground for food alone, using their feet to release food that might be trapped under the most shallow layer of ground or leaf litter. They peck, very distinctly, at the ground - bobbing their bodies back and forth as they move around, pecking in short spurts to gather the food they look for. They are very opportunistic feeders, switching back and forth between different food sources based on what is more available in a given season. They can even associate, happily, with other birds and even mammals of all things, using the environmental disturbance they cause in order to find food.

Green Junglefowl by Francesco Veronesi, CC BY-SA 2.0
Junglefowl make some of the most distinctive calls of any bird, though of course, each language seems to have its own onomatopoeia to describe it. They make very distinctive clucks, cackling, and even cooing sounds depending on the situation. Males do make “cock-a-doodle-do” calls, though they can vary in tone and loudness, as well as the syllables involved, from species to species. These calls are actually advertising calls, made by the males, in order to attract females! The females tend to be quieter than the males, though domesticated female chickens are not quiet animals by a longshot. Junglefowl do not migrate, and tend to stay limited within their preferred habitats (though, of course, domesticated chickens have been bred to deal with a wider variety of climate better than their wild relatives.)

Red Junglefowl by Harvinder Chandigarh, CC By-SA 4.0
Junglefowl can breed throughout the year (it’s why they were domesticated), though some populations tend to favor the dry season over the wet season (primarily due to less danger with the daily weather - these guys do hail from the monsoon lands!) As a general rule, junglefowl are polygamous - males will mate with a variety of females throughout the year, with the females doing the bulk of the work in nest construction and child care (which makes sense, since they blend in so well with the environment). Some species - such as the Grey Junglefowl - do show monogamous behavior from time to time, with males sticking with one female for long periods of time. In a classic case of sexual selection, females tend to prefer males with more brilliant combs (rather than focusing on plumage color, though this could be different in non-domesticated species). The female will lay between 2 and 6 eggs (some species laying more than others) in a depression amongst dense vegetation; the female will incubate the eggs for three weeks before the chicks hatch. The chicks are extremely fluffy and cute when hatching, usually covered in soft brown feathers (though domesticated ones are more yellowish). The chicks are able to fly after one week, and males will become sexually mature sometime between 5 and 8 months. They are not the strongest fliers, usually preferring short bursts of activity rather than sustained flight.

Domesticated Chicken Chicks by Uberprutser, CC BY-SA 3.0
Extremely social birds, chickens have a very noticeable pecking order - with individual chickens dominating over others in order to have priority for food and nesting location. This pecking order is disrupted when individuals are removed from a flock; adding new chickens also causes fighting and injury until a new pecking order is established. This family structure was exploited by early humans, in order to become the “top chicken” and domesticate the species. Interestingly enough, chickens do gang up on inexperienced predators - foxes have even been killed in such encounters! Despite stereotypes to the contrary, chickens are extremely intelligent animals - studies have shown they have higher intellectual capabilities than human toddlers - they are self aware, are able to count, and do trick one another into actions (aka, they can lie and manipulate other chickens). What’s more, despite their pecking order fights, they are very affectionate and empathetic birds - prone to cuddling with other flock members, and checking in to make sure the flock is alright. They show very rapid learning ability, and are able to grasp basic number theory only after a few weeks from hatching. In addition to being logical with numbers, they can reason out many other things - including forming teams to play kickball! Bird-brain, indeed!

Red and Green Junglefowl by Francesco Veronesi, CC BY-SA 2.0
Ecosystem: Junglefowl primarily live in dense, humid rainforest and wet woodland. They can also be found in savanna, scrub habitat, coastal scrub, mountain forest, and also in human plantations and farmland (as wild species spreading into human-created habitat). They do prefer lower elevations to higher ones, as a general rule. They are fed upon by a wide variety of creatures - larger birds, predatory mammals, and large lizards and crocodilians. Of course, the biggest predator of junglefowl is probably People! Just, statistically speaking.

Sri Lankan Junglefowl by Steve Garvie, CC BY-SA 2.0
Other: Junglefowl are, thankfully, not threatened with extinction. In fact, they are extremely common birds throughout their range. Domesticated chickens even regularly go feral (ie, return to wild living despite being descended from fully domesticated populations), spreading into places far from their original range such as Latin America, Hawai’i, and Africa. There are many extinct species of Junglefowl; they used to have a much wider range into Europe, but went extinct during the last Glacial Maximum, when things got too cold for them everywhere but Southeastern Asia. They then thrived in those jungle habitats, before being domesticated by people during the Holocene.

Domesticated Chicken by Berit, CC BY 2.0
Chickens were domesticated from the Red Junglefowl sometime around 5,000 years ago in Southeastern Asia. It was probably domesticated multiple times - with hybridization occurring afterwards. It spread throughout the world, reaching Greece by the fifth century BCE, though they were in Egypt potentially one thousand years earlier (or even more!!!). They were domesticated due to their frequent laying schedule - made more so by selective breeding, of course - and easily exploitable family structure. They were domesticated to breed even more frequently, leading to an abundance of adult animals - and the females even lay unfertilized eggs, giving us another source of delicious food. They also have been bred to come in many sizes, shapes, and brilliant colors of plumage. Because of their high empathetic capacity, chickens are amazingly good pets - plus, they’re domesticated, which gives them a leg up over parrots. Docile breeds, such as silkies, are great pets for children, including children with disabilities. Chickens are so fundamental to human society, that aphorisms often feature them - and they serve as symbols on heraldry, their feathers are featured in clothing, and it’s hard to escape notice of chickens wherever we go in the world today.
youtube
Chickens are the most common bird in the entire world, being bred throughout the world and able to live in harsher climates than their original range (due to domestication and specially designed coops); there are probably over 50 billion members of the genus Gallus present on the planet today. They are so common that they are a model organism - in order to understand birds as a whole, scientists do extensive studies on chickens in order to understand avian evolution. The genes and development of chickens are probably better understood than any other living kind of dinosaur. This is of special interest to members of this blog, as chicken genes have been manipulated to give them teeth (though without enamel) and longer tails - much like their non-avian dinosaur ancestors. One study even raised chickens to walk around with plungers stuck to their butts like a bony tail - and showcased how the chickens changed their head-bobbing and walking to match the redistributed weight, which makes a decent hypothesis for how non-avian dinosaurs like Velociraptor and Tyrannosaurus were able to walk (see above)!

By Scott Reid
Species Differences: Among the living species, there are distinct differences in the coloration of the males. While the females all tend to be brown and black spotted, with some patches of red on the tails and wings in some species, the males have brilliantly different colors all over. Red Junglefowl - the wild kind - are a mid sized species, and are named accordingly for their coloration. The males tend to have reddish orange heads, with green wings and bellies; their backs and back of their wings are alls reddish, though they have brilliantly green tails. Sri Lankan Junglefowl are also reddish, but instead of having green undersides to their wings and green tails, they have blueish-grey feathers in those locations. The Sri Lankan Junglefowl is also one of the smallest living species. The Grey Junglefowl also has greyish-blue tail and wing feathers, except it has a firey orange underbelly and wing top. It has grey feathers all over its body, and orange and white and black speckles on its neck. It is the largest known species. Finally, the smallest species, the Green Junglefowl, is much more than green - it is almost a rainbow of colored feathers! Its tail is green, as is its neck; but the rump tends to be yellow, the top of the wing red, and the wattle and comb aren’t red - but purple, red, yellow, and even blue! Extinct species tend to blur the line between junglefowl and their close relatives such as Peafowl (see the oldest known species, G. aesculapii, above); but in many ways, they differ mainly by living in Europe and Western Asia, rather than Southeast Asia and India.
~ By Meig Dickson
Sources Under the Cut
Ali, A.; Cheng, K. M. (1985). "Early Egg Production in Genetically Blind (rc/rc) Chickens in Comparison with Sighted (Rc+/rc) Controls". Poultry Science. 64 (5): 789–794.
Ali, S.; Ripley, S. D. Handbook of the birds of India and Pakistan. 2 (2nd ed.). Oxford University Press. pp. 106–109.
Allen, J.A. (1910). "Collation of Brisson's genera of birds with those of Linnaeus". Bulletin of the American Museum of Natural History. 28: 317–335.
Arshad MI; M Zakaria; AS Sajap; A Ismail (2000), "Food and feeding habits of Red Junglefowl", Pakistan J. Bio. Sci., 3 (6): 1024–1026.
Berhardt, Clyde E. B. (1986). I Remember: Eighty Years of Black Entertainment, Big Bands. Philadelphia: University of Pennsylvania Press. p. 153.
Brinkley, Edward S., and Jane Beatson. "Fascinating Feathers ." Birds. Pleasantville, N.Y.: Reader's Digest Children's Books, 2000. 15.
Brisbin, I. L. Jr. (1969), "Behavioral differentiation of wildness in two strains of Red Junglefowl (abstract)", Am. Zool., 9: 1072
Brisson, Mathurin Jacques (1760). Ornithologie, ou, Méthode contenant la division des oiseaux en ordres, sections, genres, especes & leurs variétés (in French and Latin). Volume 1. Paris: Jean-Baptiste Bauche.
Carter, Howard (April 1923). "An Ostracon Depicting a Red Jungle-Fowl (The Earliest Known Drawing of the Domestic Cock)". The Journal of Egyptian Archaeology. 9 (1/2): 1–4.
Cheng, Kimberly M. and Burns, Jeffrey T. (1988). "Dominance relationship and mating behavior of domestic cocks--a model to study mate-guarding and sperm competition in birds" (PDF). The Condor. 90 (3): 697–704.
Chickens team up to 'peck fox to death'". The Independent. March 13, 2019. Archived from the original on March 15, 2019. Retrieved March 13, 2019.
Clements, J. F., T. S. Schulenberg, M. J. Iliff, D. Roberson, T. A. Fredericks, B. L. Sullivan, and C. L. Wood. 2017. The eBird/Clements checklist of birds of the world: v2017
Collias, N. E. (1987), "The vocal repertoire of the red junglefowl: A spectrographic classification and the code of communication", The Condor, 89 (3): 510–524.
Condon, T. P., Morphological and Behavioral Characteristics of Genetically Pure Indian Red Junglefowl, Gallus gallus murghi, archived from the original on 29 June 2007.
Dohner, Janet Vorwald (January 1, 2001). The Encyclopedia of Historic and Endangered Livestock and Poultry Breeds. Yale University Press. ISBN 978-0300138139.
Eriksson, J.; et al. (2008). "Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken". PLoS Genetics. 4 (2). E1000010.
Evans, Christopher S.; Evans, Linda; Marler, Peter (July 1, 1993). "On the meaning of alarm calls: functional reference in an avian vocal system". Animal Behaviour. 46 (1): 23–38.
Evans, C. S.; Macedonia, J. M.; Marler, P. (1993), "Effects of apparent size and speed on the response of chickens, Gallus gallus, to computer-generated simulations of aerial predators", Animal Behaviour, 46 (1): 1–11.
Finn, Frank (1911). The game birds of India and Asia. Thacker, Spink and Co., Calcutta. pp. 21–23.
Fumihito, A; Miyake, T; Sumi, S; Takada, M; Ohno, S; Kondo, N (December 20, 1994), "One subspecies of the red junglefowl (Gallus gallus gallus) suffices as the matriarchic ancestor of all domestic breeds", PNAS, 91 (26): 12505–12509.
Fumihito, Akishinonomiya; Tetsuo Miyake; Masaru Takada; Ryosuke Shingut; Toshinori Endo; Takashi Gojobori; Norio Kondo & Susumu Ohno (1996). "Monophyletic origin and unique dispersal patterns of domestic fowls". Proc. Natl. Acad. Sci. 93 (13): 6792–6795.
Gaudry, A. 1862. Note sur les débris d'oiseaux et de reptiles trouvés a Pikermi (Grece), suivie de quelques remarques de paléontologie générale. Bulletin de la Société Géologique de France 19:629-640
Gill, Frank; Donsker, David, eds. (2017). "Pheasants, partridges & francolins". World Bird List Version 7.3. International Ornithologists' Union. Retrieved 22 November 2017.
Green-Armytage, Stephen (October 2000). Extraordinary Chickens. Harry N. Abrams.
Grouw, Hein van, Dekkers, Wim & Rookmaaker, Kees (2017). On Temminck's tailless Ceylon Junglefowl, and how Darwin denied their existence. Bulletin of the British Ornithologists' Club (London), 137 (4), 261-271.
Jobling, J. A. 2010. The Helm Dictionary of Scientific Bird Names. Christopher Helm Publishing, A&C Black Publishers Ltd, London.
Lawler, A. (2014). Why Did the Chicken Cross the World?: The Epic Saga of the Bird that Powers Civilization. Atria Books.
Lehr Brisbin Jr., I., Concerns for the genetic integrity and conservation status of the red junglefowl, Savannah River Ecology Laboratory, Drawer E, Aiken, SC 29802 (with permission from SPPA Bulletin, 1997, 2(3):1-2): FeatherSite.
Linnaeus, Carl (1748). Systema Naturae sistens regna tria naturae, in classes et ordines, genera et species redacta tabulisque aeneis illustrata (in Latin) (6th ed.). Stockholmiae (Stockholm): Godofr, Kiesewetteri. pp. 16, 28.
Linnaeus, Carl (1758). Systema Naturæ per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis (in Latin). Volume 1 (10th ed.). Holmiae (Stockholm): Laurentii Salvii. p. 158.
Liu, Yi-Ping; Wu, Gui-Sheng; Yao, Yong-Gang; Miao, Yong-Wang; Luikart, Gordon; Baig, Mumtaz; Beja-Pereira, Albano; Ding, Zhao-Li; Palanichamy, Malliya Gounder; Zhang, Ya-Ping (2006), "Multiple maternal origins of chickens: Out of the Asian jungles", Molecular Phylogenetics and Evolution, 38 (1): 12–19.
Madge, S.; Philip J. K. McGowan; Guy M. Kirwan (2002). Pheasants, Partidges and Grouse: A Guide to the Pheasants, Partridges, Quails, Grouse, Guineafowl, Buttonquails and Sandgrouse of the World. A&C Black.
Mayr, G. 2017. Avian Evolution: The Fossil Record of Birds and its Paleobiological Significance. Topics in Paleobiology, Wiley Blackwell. West Sussex.
Marino, L. 2017. Thinking chickens: a review of cognition, emotion, and behavior in the domestic chicken. Animal Cognition, doi: 10.1007/s10071-016-1064-4.
McGowan, P.J.K., Kirwan, G.M. & Boesman, P. (2019). Green Junglefowl (Gallus varius). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
McGowan, P.J.K. & Kirwan, G.M. (2019). Grey Junglefowl (Gallus sonneratii). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
McGowan, P.J.K. & Kirwan, G.M. (2019). Red Junglefowl (Gallus gallus). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
McGowan, P.J.K., Kirwan, G.M. & Boesman, P. (2019). Sri Lanka Junglefowl (Gallus lafayettii). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Morejohn, G. Victor (1968). "Breakdown of Isolation Mechanisms in Two Species of Captive Junglefowl (Gallus gallus and Gallus sonneratii)". Evolution. 22 (3): 576–582.
Morejohn, G. V. (1968). "Study of the plumage of the four species of the genus Gallus". The Condor. 70 (1): 56–65.
Nishibori, M.; Shimogiri, T.; Hayashi, T.; Yasue, H. (2005). "Molecular evidence for hybridization of species in the genus Gallus except for Gallus varius". Animal Genetics. 36 (5): 367–375.
Perry-Gal, Lee; Erlich, Adi; Gilboa, Ayelet; Bar-Oz, Guy (2015). "Earliest economic exploitation of chicken outside East Asia: Evidence from the Hellenistic Southern Levant". Proceedings of the National Academy of Sciences. 112 (32): 9849–9854.
Peters, James Lee, ed. (1934). Check-list of Birds of the World. Volume 2. Cambridge, Massachusetts: Harvard University Press. p. 118.
Peterson, A.T. & Brisbin, I. L. Jr. (1999), "Genetic endangerment of wild red junglefowl (Gallus gallus)", Bird Conservation International, 9: 387–394.
Piper, Philip J. (2017). "The Origins and Arrival of the Earliest Domestic Animals in Mainland and Island Southeast Asia: A Developing Story of Complexity". In Piper, Philip J.; Matsumura, Hirofumi; Bulbeck, David (eds.). New Perspectives in Southeast Asian and Pacific Prehistory. terra australis. 45. ANU Press.
Pritchard, Earl H. "The Asiatic Campaigns of Thutmose III". Ancient Near East Texts related to the Old Testament. p. 240.
Roehrig, Catharine H.; Dreyfus, Renée; Keller, Cathleen A. (2005). Hatshepsut: From Queen to Pharaoh. New York: Metropolitan Museum of Art. p. 268.
Sacci, MA; K Howes; K Venugopal (2001). "Intact EAV-HP Endogenous Retrovirus in Sonnerat's Jungle Fowl". Journal of Virology. 75 (4): 2029–2032.
Sherwin, C.M.; Nicol, C.J. (1993). "Factors influencing floor-laying by hens in modified cages". Applied Animal Behaviour Science. 36 (2–3): 211–222.
Siddharth, Biswas (2014). "Gallus gallus domesticus Linnaeus, 1758: Keep safe your domestic fowl from your domestic foul". Ambient Science. 1 (1): 41–43.
Smith, Jamon. Tuscaloosanews.com "World’s oldest chicken starred in magic shows, was on 'Tonight Show’" Archived February 20, 2019, at the Wayback Machine, Tuscaloosa News (Alabama, USA). August 6, 2006.
Smith, Page; Charles Daniel (April 2000). The Chicken Book. University of Georgia Press.
Stonehead. "Introducing new hens to a flock " Musings from a Stonehead". Stonehead.wordpress.com. Archived from the original on August 13, 2010.
Storer, R. W. (1988). Type Specimens of Birds in the Collections of the University of Michigan Museum of Zoology. University of Michigan, Miscellaneous publications No. 174.
Storey, A.A.; et al. (2012). "Investigating the global dispersal of chickens in prehistory using ancient mitochondrial DNA signatures". PLoS ONE. 7 (7): e39171.
"Top cock: Roosters crow in pecking order". Archived from the original on January 15, 2018. Retrieved January 14, 2018.
Wong, GK; et al. (December 2004). "A genetic variation map for chicken with 2.8 million single-nucleotide polymorphisms". Nature. 432 (7018): 717–722.
#Gallus#Chicken#Dinosaur#Bird#Junglefowl#Birds#Dinosaurs#Birblr#Palaeoblr#Factfile#Terrestrial Tuesday#Pheasant#Galloanseran#Landfowl#Quaternary#Neogene#Eurasia#Australia & Oceania#India & Madagascar#Omnivore#Gallus gallus#Gallus varius#Gallus lafayettii#Gallus sonneratii#Green Junglefowl#Grey Junglefowl#Sri Lankan Junglefowl#Red Junglefowl#Gallus aesculapii#paleontology
762 notes
·
View notes
Text
Titanis walleri

By Scott Reid
Etymology: Titan
First Described By: Brodkorb, 1963
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes, Neognathae, Neoaves, Inopinaves, Telluraves, Australaves, Cariamiformes, Phorusrhacoidea, Phorusrhacidae, Phorusrhacinae
Status: Extinct
Time and Place: Between 5 and 1.8 million years ago, from the Zanclean to the Gelasian ages of the Pliocene through Pleistocene
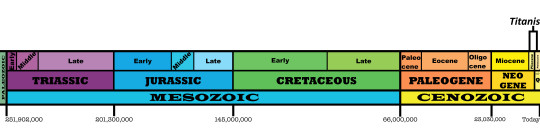
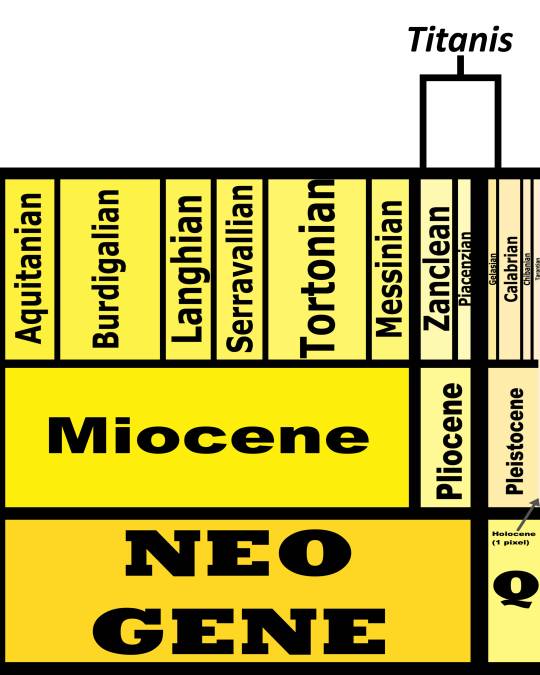
Titanis is known from the Santa Fe River and Nueces River Formations of Florida and Texas

Physical Description: Titanis was a Terror Bird, one of the largest known Terror Birds, a group of large flightless predatory birds that terrorized the Americas during the Cenozoic Era, right up until humans would have appeared on the scene. Titanis is one of the latest members of this group, and the one that made the journey up to North America - most Terror Birds are from South America. It would have been 2.5 meters tall - much taller than a person - and would have weighed 150 kilograms. There was a lot of variance in size and height, however, indicating that Titanis may have had at least some sexual dimorphism. It had a short tail and round body, with long and powerful legs. In fact, it also had very robust toes - and one of the strongest middle toes known for a Terror Bird. It had very small, useless wings, that were very much locked in against the body - they didn’t have a lot of folding power compared to other birds. This indicates the wings really were… useless. They didn’t use them for raptor prey restraint or anything else, making them distinctly different from the Dromaeosaurids of long past. Titanis had a very thick neck, which would have supported a large head with a very impressive and terrifying hooked bill - complete with extensive crunching power!

By Dmitry Bogdanov, CC BY-SA 3.0
Diet: As a Terror Bird, Titanis primarily ate large mammals - and some medium and small sized mammals, of course, but basically it was able to cronch anything around it.
Behavior: Terror Birds are most closely related to modern Seriemas, and so a lot of their behavior has been guessed based on Seriemas today. As such - and given that it didn’t have much in the way of wings - Titanis probably mainly relied on its feet in kicking its prey to death. It would chase its food down, kick it, and potentially pin it down. Then, final death blows would have been delivered with the powerful cronch of its beak, though of course the hook on the beak would have allowed increased tearing and shredding. While modern Seriemas are solitary, it is possible that Titanis and other Terror Birds may have used groups to take down larger prey, though they probably would have been more groups of convenience than formal packs. It is possible they had similar breeding habits to living Seriemas, but even that is a question - their larger size, different niche, and general different time periods would provide large differences. And we don’t even know the actual breeding habits of Seriemas very well! So, that being said, Titanis would have probably been fairly territorial over their nests, and both parents were probably involved in the care of the nest and then the young, even after fledging. The young would follow the parents around until reaching maturity. As such, it’s possible that the parents may have hunted for the young, and brought food back for them until they were old enough to hunt for themselves. Then, upon leaving the parents, they probably would have been fairly solitary until finding a mate of their own.

By José Carlos Cortés
Ecosystem: Titanis primarily lived in open grassland habitats in the southern parts of the United States, clearly extending from Texas through to Florida and probably found all over that range. It stuck to warmer, probably wetter habitats, though the exact environments it lived in aren’t very well studied in terms of general flora. Fauna, however, is well known. Titanis lived alongside a wide variety of other animals - in Citrus County, it was found with a variety of frogs, turtles, lizards, rabbits, horses, shrews, bears, dogs, mustelids, and cats (including Smilodon), armadillos, sloths, the Mastodon, cows, peccaries, camels, and deer. There were, of course, many dinosaurs as well - in addition to Titanis, there were waders (indicating a non-insignificant amount of water in this ecosystem, possible coastline, swamps, or lakes), vultures, pheasants, ducks, falcons, owls, pigeons (including the passenger pigeon), woodpeckers, blackbirds, corvids, sparrows, finches, flycatchers, cardinals, rails, grebes, herons, bitterns, and buzzards. Basically, a fairly typical array of North American birds! In Gilchrist County, Titanis also lived alongside similar creatures, including Smilodon, though without the Mastodon - though there was Rhynchotherium! Unfortunately, its Texan relatives aren’t well known, though it stands to reason that it would have been similar to other locations.
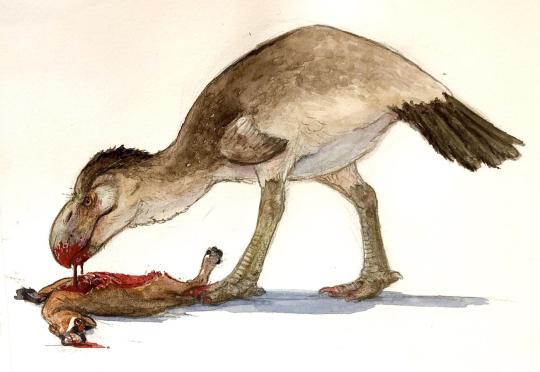
By Ripley Cook
Other: Titanis is one of the largest known Terror Birds, and one of the largest known ones discovered early in our understanding of Terror Birds. In fact, we knew about Titanis so early on that there are a lot of old depictions of it - including ones where it has… hands. Clawed hands. That is very much wrong and cringy, but hey, there are pictures of it! Titanis is also fascinating because of its place in Earth’s History - it is one of the (only?) known Terror Birds from North America. This occurred due to the Great American Interchange, a sort of mini-columbian exchange where North America and South America combined, leading to the mixing of animals from both continents together. The traditional narrative says that Terror Birds went extinct because sabre-toothed cats came in from North America, but this is flawed for three very big reasons: 1) there were already Sabre Toothed animals filling that niche in South America, they were just Marsupials; 2) Terror Birds stuck around for a long time after the Interchange, and 3) Terror Birds reached North America in return! So Titanis helps to showcase that Terror Birds were doing just fine during this ecological exchange. So why did it - and other Terror Birds - go extinct? Probably the Ice Age, though for now, we can’t be sure. Regardless, they went extinct… probably before people got there. There are fossils that might be Titanis from 15,000 years ago, which would indicate they were still there when people got there. Which is terrifying. And also might point to humans being the cause of their extinction. Still, that seems unlikely, and they were definitely on the decline before then - so the Ice Age seems like the most logical explanation.
~ By Meig Dickson
Sources Under the Cut
Alroy, John, Ph.D. Synonymies and reidentifications of North American fossil mammals, 2002. John P. Hunter, Ohio State University, Mammalian Paleontology.
Alvarenga, H. M. F.; Höfling, E. (2003). "Systematic revision of the Phorusrhacidae (Aves: Ralliformes)". Papéis Avulsos de Zoologia. 43 (4): 55–91.
Alvarenga, H.; Jones, W.; Rinderknecht, A. (May 2010). "The youngest record of phorusrhacid birds (Aves, Phorusrhacidae) from the late Pleistocene of Uruguay". Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 256 (2): 229–234.
Baskin, J. A. (1995). "The giant flightless bird Titanis walleri (Aves: Phorusrhacidae) from the Pleistocene coastal plain of South Texas". Journal of Vertebrate Paleontology. 15 (4): 842–844.
Bertelli, S., L. M. Chiappe, and C. Tambussi. 2007. A new phorusrhacid (Aves: Cariamae) from the Middle Miocene of Patagonia, Argentina. Journal of Vertebrate Paleontology 27(2):409-419.
Brodkorb, P. (1963). "A giant flightless bird from the Pleistocene of Florida". Auk. 80 (2): 111–115.
Carroll, R. L. 1988. Vertebrate Paleontology and Evolution 1-698
Chandler, R.M. (1994). "The wing of Titanis walleri (Aves: Phorusrhacidae) from the Late Blancan of Florida". Bulletin of the Florida Museum of Natural History, Biological Sciences. 36: 175–180.
Cracraft, Joel, A review of the Bathornithidae (Aves, Gruiformes), with remarks on the relationships of the suborder Cariamae. American Museum Novitates ; no. 2326.
De Iuliis, G., and C. Cartelle. 1999. A new giant megatheriine ground sloth (Mammalia: Xenarthra: Megatheriidae) from the late Blancan to early Irvingtonian of Florida. Zoological Journal of the Linnean Society 127:495-515
Degrange, Federico J.; Tambussi, Claudia P.; Moreno, Karen; Witmer, Lawrence M.; Wroe, Stephen; Turvey, Samuel T. (18 August 2010). "Mechanical Analysis of Feeding Behavior in the Extinct "Terror Bird" Andalgalornis steulleti (Gruiformes: Phorusrhacidae)". PLoS ONE. 5 (8): e11856.
Ehret, D. J., and J. R. Bourque. 2011. An extinct map turtle Graptemys (Testudines, Emydidae) from the Late Pleistocene of Florida. Journal of Vertebrate Paleontology 31(3):575-587
Emslie, S. D. 1998. Avian community, climate, and sea-level changes in the Plio-Pleistocene of the Florida Peninsula. Ornithological Monographs (50)1-113
Emslie, S. D., and N. J. Czaplewski. 1999. Two new fossil eagles from the late Pliocene (late Blancan) of Florida and Arizona and their biogeographic implications. Smithsonian Contributions to Paleobiology 89:185-198.
Franz, R., and I. R. Quitmyer. 2005. A fossil and zooarchaeological history of the gopher tortoise (Gopherus polyphemus) in the Southeastern United States. Bull. Fla. Mus. Nat. History 45(4):179-199.
Gibbons, J. W., and M. E. Dorcas. 2004. North American Watersnakes: A Natural History. 1-438.
Gould, G.C. & Quitmyer, I.R. (2005). "Titanis walleri: bones of contention". Bulletin of the Florida Museum of Natural History. 45: 201–229.
Hulbert, R. C. 1995. Equus from Leisey Shell Pit 1A and other Irvingtonian localities from Florida. Bulletin of the Florida Museum of Natural History 37(17):553-602.
Hulbert, R. C. 2010. A new early Pleistocene tapir (Mammalia: Perissodactyla) from Florida, with a review of Blancan tapirs from around the state. Bulletin of the Florida Museum of Natural History 49(3):67-126.
MacFadden, Bruce J.; Labs-Hochstein, Joann; Hulbert, Richard C.; Baskin, Jon A. (2007). "Revised age of the late Neogene terror bird (Titanis) in North America during the Great American Interchange". Geology. 35 (2): 123–126.
Marsh, O. C. (1875). "On the Odontornithes, or birds with teeth". American Journal of Science. 10 (12): 403–408.
Mayr, G. 2009. Paleogene Fossil Birds. Springer-Verlag Berlin Heidelberg.
Mayr, G. 2017. Avian Evolution: The Fossil Record of Birds and its Paleobiological Significance. Topics in Paleobiology, Wiley Blackwell. West Sussex.
McDonald, H. G. 1995. Gravigrade xenarthrans from the early Pleistocene Leisey Shell Pit 1A, Hillsborough County, Florida. Bulletin of the Florida Museum of Natural History 37(11).
Meachen, J. A. 2005. A new species of Hemiauchenia (Artiodactyla, Camelidae) from the late Blancan of Florida. Bulletin of the Florida Museum of Natural History 45(4):435-447.
Meylan, P. 2001. Late Pliocene anurans from Inglis 1A, Citrus County, Florida. Bulletin of the Florida Museum of Natural History 45(4):171-178.
McFadden, B.; Labs-Hochstein, J.; Hulbert, Jr., R. C.; Baskin, J. A. (2006). "Refined age of the late Neogene terror bird (Titanis) from Florida and Texas using rare earth elements". Journal of Vertebrate Paleontology. 26 (3): 92A (Supplement).
Morgan, G. S., and R. B. Ridgway. 1987. Late Pliocene (late Blancan) vertebrates from the St. Petersburg Times site, Pinellas County, Florida, with a brief review of Florida Blancan faunas. Papers in Florida Paleontology 1:1-22.
Morgan, G. S. 1991. Neotropical Chiroptera from the Pliocene and Pleistocene of Florida. In T. A. Griffiths, D. Klingener, (eds.), Bulletin of the American Museum of Natural History 206:176-213.
Morgan, G. S., and R. C. Hulbert. 1995. Overview of the geology and vertebrate biochronology of the Leisey Shell Pit Local Fauna, Hillsborough County, Florida. Bulletin of the Florida Museum of Natural History 37(1).
Morgan, G. S., and J. A. White. 1995. Small mammals (Insectivora, Lagomorpha, and Rodentia) from the early Pleistocene (early Irvingtonian) Leisey Shell Pit Local Fauna, Hillsborough County, Florida. Bulletin of the Florida Museum of Natural History 37(13).
Robertson, J. S. 1976. Latest Pliocene mammals from Haile XV A, Alachua County, Florida. Bulletin of the Florida State Museum 20(3):1-186.
Ruez, D. R. 2001. Early Irvingtonian (latest Pliocene) rodents from Inglis 1C, Citrus County, Florida. Journal of Vertebrate Paleontology 21(1):153-171.
Tambussi, Claudia P.; de Mendoza, Ricardo; Degrange, Federico J.; Picasso, Mariana B.; Evans, Alistair Robert (25 May 2012). "Flexibility along the Neck of the Neogene Terror Bird Andalgalornis steulleti (Aves Phorusrhacidae)". PLoS ONE. 7 (5): e37701.
Tedford, R. H., X. Wang, and B. E. Taylor. 2009. Phylogenetic Systematics of the North American Fossil Caninae (Carnivora: Canidae). Bulletin of the American Museum of Natural History 325:1-218.
Webb, S. D. 1974. Chronology of Florida Pleistocene mammals. In S. D. Webb (ed.), Pleistocene Mammals of Florida 5-31.
Webb, S. D., and K. T. Wilkins. 1984. Historical Biogeography of Florida Pleistocene Mammals. In H. H. Genoways and M. R. Dawson (eds.), Carnegie Museum of Natural History Special Publication 8:370-383.
White, J. A. 1991. A new Sylvilagus (Mammalia: Lagomorpha) from the Blancan (Pliocene) and Irvingtonian (Pleistocene) of Florida. Journal of Vertebrate Paleontology 11(2):243-246.
#Titanis walleri#Titanis#Bird#Terror Bird#Dinosaur#Dinosaurs#Raptor#Bird of Prey#Birds#Palaeoblr#Birblr#Factfile#Australavian#Cariamiform#Theropod Thursday#North America#Neogene#Quaternary#Carnivore#paleontology#prehistory#prehistoric life#biology#a dinosaur a day#a-dinosaur-a-day#dinosaur of the day#dinosaur-of-the-day#science#nature
471 notes
·
View notes
Text
Pelagornis
By Ripley Cook
Etymology: Sea Bird
First Described By: Lartet, 1857
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes, Neognathae, Pelagornithidae
Referred Species: P. chilensis, P. longirostris, P. mauretanicus, P. miocaenus, P. orri, P. sandersi, P. stirtoni, P. tenuirostris, P. wetmorei
Status: Extinct
Time and Place: Between 30 and 2.5 million years ago, from the Rupelian of the Oligocene through the beginning of the Pleistocene (in the Gelasian age)
Pelagornis, being an extremely common seabird, is known from nearly everywhere around the world, usually associated with the coast.
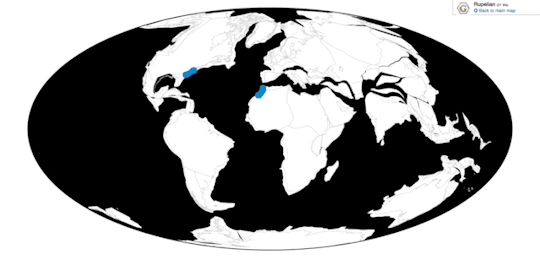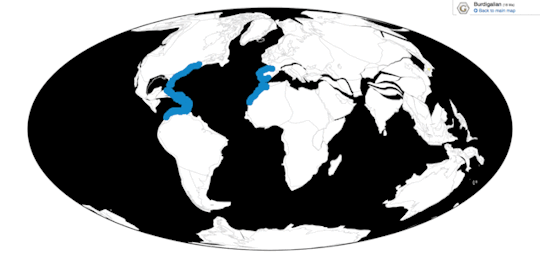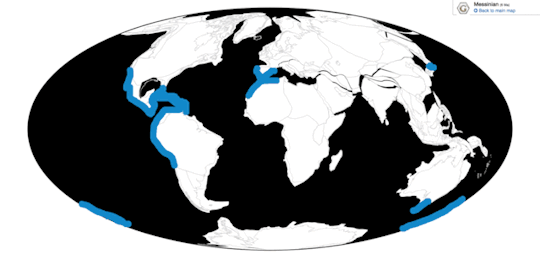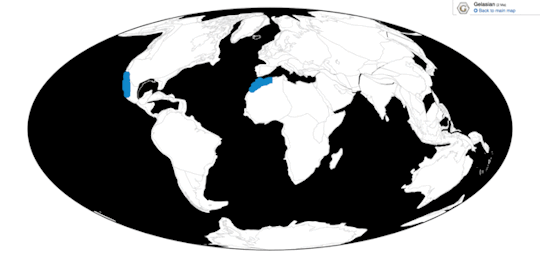
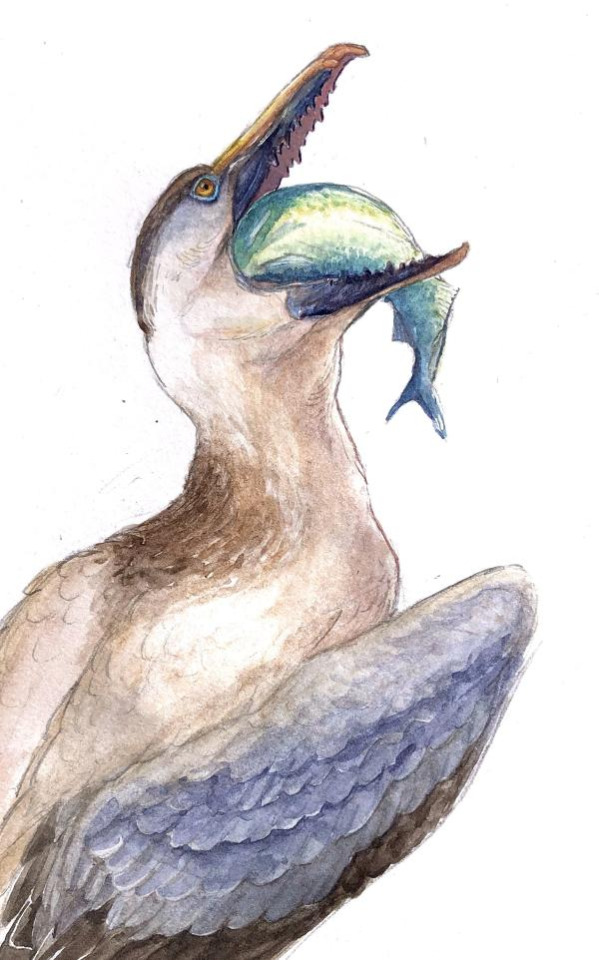
Physical Description: Despite the incredibly generic name, Pelagornis was quite an interesting bird. Like other pseudotooth birds, both its upper and lower beak bore toothlike spikes, in an alternating small/big/small/big pattern. Its beak was robust and fairly long compared to the back of the skull. These pseudoteeth appear to have grown in relatively late in Pelagornis’s growth, implying the keratin covering the beak may not have been fully hardened until close to adulthood. Interestingly enough, fossil evidence indicates that Pelagornis probably held its head upright at a vertical angle.

By José Carlos Cortés
Pelagornis was fucking huge, m’kay. P. sandersi has an estimated wingspan between 6.1 and 7.4 meters! This makes Pelagornis the bird with the largest wingspan (but not the heaviest flying bird - that record belongs to Argentavis). Its wings were even more proportionally long and narrow than those of the largest flying birds alive today, the albatrosses. In comparison, its body was fairly small. There were, of course, some species of Pelagornis that were smaller than this, reaching only 4 meters long in terms of wingspan. Still, this large wingspan size is really only characteristic of these birds in flight - compressed, they would have looked much smaller, especially given that they were very light weight. They had stout legs and shorter tails, which indicates that they weren’t very good walkers, and spent most of their time in the air or sitting on the land.

By Jack Wood
Diet: Probably fish. The pseudoteeth are likely an adaptation to grab and hold onto large fish. Similar toothlike serrations are seen, albeit much less exaggerated, in modern mergansers, which also eat fish. In addition, the vertical position of the head would have allowed Pelagornis to skim-feed, grabbing fish and other aquatic organisms from the top layer of the ocean and scooping them into their mouths. Thus, the fake-teeth would have allowed Pelagornis to grab onto fish better than non-toothed skim feeding birds. It may have also used these sharp fake teeth in order to grab onto the slipperiest fish and cephalopods - rather than harder shelly animals.

By Scott Reid
Behavior: As with modern seabirds, Pelagornis likely spent most of its time out at sea. Gliding on oceanic thermals would have helped to support its huge body in the air without wasting energy just to stay aloft - which was important, since it wasn’t very good at flapping its wings and would have had trouble staying aloft long enough to get food if it had to flap too frequently. Think an albatross, but a giant, evil albatross. Landing and taking off would have been more awkward, though. It probably needed to take advantage of headwinds, drops in elevation and/or air gusts to get into the air at all. Albatrosses also kinda have this problem, but nowhere near to the same extent. The late appearance of the pseudoteeth implies that Pelagornis may have fed its young back on land like many modern seabirds before they could feed themselves out at sea. As such, they would have sought out good nesting sites, which may correspond to where fossils of Pelagornis are found - indicating that their spread around the world was greater than that we know of. Since it was a sea bird, it probably would have been very social, living in large colonies - and it would have cared for its young in similar social groups. In fact, it seems more likely than not that it would have laid its nests on cliffs and in rocky areas and plateaus, where being able to take off would have been easier than flatter, sandier beaches. Whether or not these animals were as noisy as modern seabirds is really another question altogether.

By Jack Wood
Interestingly enough, Pelagornis had a salt gland in the eye that would have allowed it to excrete excess salt, which was an extremely helpful trait when Pelagornis ate almost entirely seafood. That seafood diet didn’t meant it wasn’t a danger, however - today, seabirds will venture away from the coasts in order to scavenge food on the beach, and they are certainly defensive of their nests, young, and territory. Also fascinatingly, it had a very very very long skull - with all of those pseudoteeth packed in - which had similar shapes and organization as to the extinct really toothed birds of the Mesozoic. This implies that there was a certain amount of evolutionary regression in Pelagornis, allowing it to better support its teeth and chomping ability than it would otherwise. There is also an interesting furrow in the skull, which allowed it to be better support the head and possibly to better grab prey in the ocean.

By Scott Reid
Ecosystem: Pelagornis lived around coastlines worldwide. Because of this, it is difficult to pinpoint with certainty the types of animals it lived with. In fact, it was so long-lived and widespread it is more likely than not that Pelgaornis interacted with any ocean-going creature or animal found along the coast. It doesn’t seem to have a preference in the fossil record between rocky coasts or beaches, though it did seem to stay in at least somewhat warmer ecosystems and where cliffs would have been present for easier take-offs (and it is reasonable to suppose that cliff areas would have been its preferred place for nesting). Some notable animals it would have interacted with include extinct penguins, cetaceans, the famed giant shark Megalodon and… humans. Yup, Pelagornis is known from locations where early members of genus Homo ventured to. So, if you can imagine being afraid of a giant bird with fake teeth a little too well, that would be the instincts of your ancestors talking.

By Scott Reid
Other: Pelagornis is a fun time, classification wise, for multiple reasons: one, a whole bunch of different types of Pseudotoothed birds are actually, apparently, species of Pelagornis; and two, we don’t really know what Pseudotoothed birds really are. So, let’s break this down into those two parts. What’s going on with the species? Well, in the 2010s, a lot of research has been made that shows a bunch of the Neogene Pseudotoothed birds that we’ve counted as different genera are actually… just… part of Pelagornis. Why Wikipedia has not chosen to update their information as to this effect is beyond me, but the fact remains is that a lot of Pseudotoothed birds are just different shades of Pelagornis, primarily due to the fact that they really… aren’t different. In fact, a lot of the differences were just based on time and place, and the fact that Pseudotoothed birds weren’t really well known at all. The loss of Osteodontornis is a bit of a bummer, but there aren’t any major differences between this genus and Pelagornis, so it’s gone. We’ve also lost Pseudodontornis, you know, the name that actually means “fake toothed bird”, unlike the crappy name for Pelagornis, which just means Sea Bird. Like, come on people. Why are we here. Just to suffer. We’ve also lost Palaeochenoides, Neodontornis, and Tympanonesiotes. Hence the extreme amount of art in this article - the last time I covered Pseudotoothed birds, these were separate. So we have an abundance of terrifying tooth art.

By José Carlos Cortés
Finally - what the heck are Pseudotoothed birds? We don’t know. We really don’t know where they go. Are they related to the sea birds we have today (the Aequorlitornithes)? Are they related to ducks? Are they something else entirely? We have no idea, because, frankly, they seem to just appear in the fossil record without any sort of origin whatsoever. Like magic. Suddenly, toothed birds were back like the asteroid never hit. Honestly if I were to hazard a guess, based on the fossil characteristics, they’re probably none of the above - but an early branching group of Neognathous (aka, all birds that aren’t ratites and their cousins) birds that evolved from a non-easily fossilized ancestor. Whether that ancestor had weak bones or just lived in places where fossils don’t happen is a different question entirely, but either way, so far we have nothing. They just appear, in the Paleocene, out of nowhere. And, eventually, Pelagornis also disappeared.

By Jack Wood
Why did Pelagornis, the latest surviving species disappear? The most likely answer is climate change. The onset of the ice age would have caused extreme changes to the water patterns, currents, and air flow. Since Pelagornis didn’t flap its wings much, and relied almost entirely on soaring and thermals, it probably would have been greatly affected by changes in these weather patterns. So, changes in the ocean and the air by the ice age would have decreased its ability to reach food, and then the dramatic changes in its home climate would have been a further death knell. Interestingly enough, they only began to become uncommon right before they became extinct - indicating that Pelagornis really was finished off by this change in climate. Which is sad, because that’s right around when humans were becoming more of a thing, and it would have been nice to see one of these things in life. Except it wouldn’t have been. Because they’re terrifying. But I laugh in the face of danger. I think. I dunno I just think they’re neat.

By Scott Reid
Species Differences: The different species of Pelagornis differ primarily due to location and time, though there are some differences in shape and size - those fossils that were once assigned to Tympanonesiotes, for example, were on average smaller than other members of this genus. The largest known species was decidedly Pelagornis sandersi, though the best known species is Pelagornis chilensis. For now, however, Pelagornis is kind of a mess, since so much research is needed on this species complex to make sure things are where they belong and one genus is enough, so species differences are difficult to parse out until more research has been published on the subject. Just know that there were a lot of Pelagornis - and they came in all kinds of different shapes and sizes all over the place.
~ By Meig Dickson and Henry Thomas
Sources Under the Cut
Becker, J.J. (1987): Neogene avian localities of North America. Smithsonian Research Monographs 1. Prentice Hall & IBD.
Bourdon, Estelle (2005): Osteological evidence for sister group relationship between pseudo-toothed birds (Aves: Odontopterygiformes) and waterfowls (Anseriformes). Naturwissenschaften 92(12): 586–591.
Brodkorb, Pierce (1963): Catalogue of fossil birds. Part 1 (Archaeopterygiformes through Ardeiformes). Bulletin of the Florida State Museum, Biological Sciences 7(4): 179–293.
Cenizo, M., C. Acosta Hospitaleche, and M. Reguero. 2016. Diversity of pseudo-toothed birds (Pelagornithidae) from the Eocene of Antarctica. Journal of Paleontology 89 (5): 870 - 881.
Hastings, A. K., and A. C. Dooley. 2017. Fossil-collecting from the middle Miocene Carmel Church Quarry marine ecosystem in Caroline County, Virginia. The Geological Society of America Field Guide 47:77-88
Hopson, James A. (1964): Pseudodontornis and other large marine birds from the Miocene of South Carolina. Postilla 83: 1–19.
Ksepka, D.T. 2014. Flight performance of the largest volant bird. PNAS 111: 10624-10629.
Louchart, A., Sire, J.-Y., Mourer-Chauvire, C., Geraads, d., viriot, L., de Buffrenil, V. 2013. Structure and Growth Pattern of Pseudoteeth in Pelagornis mauretanicus (Aves, Odontopterygiformes, Pelagornithidae). PLoS One 8(11): e80372.
Mayr, G. 2009. Paleogene Fossil Birds. Springer-Verlag Berlin Heidelberg.
Mayr, G., D. Rubilar-Rogers. 2010. Osteology of a new giant bony-toothed bird from the Miocene of Chile, with a revision of the taxonomy of Neogene Pelagornithidae. Journal of Vertebrate Paleontology 30 (5): 1313-1330.
Mayr, G., J. L. Goedert, S. A. McLeod. 2013. Partial Skeleton of a Bony-Toothed Bird from the Late Oligocene/Early Miocene of Oregon (USA) and the Systematics of Neogene Pelagornithidae. Journal of Paleontology 87 (5): 922 - 929.
Mayr, G. 2017. Avian Evolution: The Fossil Record of Birds and its Paleobiological Significance. Topics in Paleobiology, Wiley Blackwell. West Sussex.
McKee, Joseph W.A. (1985). "A pseudodontorn (Pelecaniformes: Pelagornithidae) from the middle Pliocene of Hawera, Taranaki, New Zealand". New Zealand Journal of Zoology. 12 (2): 181–184.
Mlíkovský, Jirí (2002): Cenozoic Birds of the World, Part 1: Europe. Ninox Press, Prague.
Olson, Storrs L. (1985): The Fossil Record of Birds. In: Farner, D.S.; King, J.R. & Parkes, Kenneth C. (eds.): Avian Biology 8: 79-252.
Ono, Keiichi (1989). "A Bony-Toothed Bird from the Middle Miocene, Chichibu Basin, Japan". Bulletin of the National Science Museum Series C: Geology & Paleontology. 15 (1): 33–38.
Rincón R., Ascanio D. & Stucchi, Marcelo (2003). "Primer registro de la familia Pelagornithidae (Aves: Pelecaniformes) para Venezuela [First record of Pelagornithidae family from Venezuela]" (PDF). Boletín de la Sociedad Venezolana de Espeleología (in Spanish and English). 37: 27–30.
Scarlett, R.J. (1972): Bone of a presumed odontopterygian bird from the Miocene of New Zealand. New Zealand Journal of Geology and Geophysics 15(2): 269-274.
Zouhri, S., P. Gingerich, S. Adnet, E. Bourdon, S. Jouve, B. Khalloufi, A. Amane, N. Elboudali, J.-C. Rage, F. Lapparent De Broin, A. Kaoukaya and S. Sebti. 2018. Middle Eocene vertebrates from the sabkha of Gueran, Atlantic coastal basin, Saharan Morocco, and their peri-African correlations. Comptes Rendus Geoscience 350(6):310-318
#Pelagornis#Pseudotoothed bird#Pelagornithid#Bird#Dinosaur#Birds#Dinosaurs#Factfile#Birblr#Palaeoblr#Neogene#Quaternary#Paleogene#North America#South America#Eurasia#Australia & Oceania#Africa#Piscivore#Water Wednesday#Neognath#Osteodontornis#Pseudodontornis#paleontology#prehistory#prehistoric life#biology#a dinosaur a day#a-dinosaur-a-day#dinosaur of the day
288 notes
·
View notes
Text
Nyctibius

Common Potoo by Gmmv1980, CC BY-SA 4.0
Etymology: Night Feeder
First Described By: Vieillot, 1816
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes, Neognathae, Neoaves, Strisores, Caprimulgiformes, Nyctibiidae
Referred Species: N. bracteatus (Rufous Potoo), N. grandis (Great Potoo), N. aethereus (Long-Tailed Potoo), N. leucopterus (White-Winged Potoo), N. maculosus (Andean Potoo), N. griseus (Common Potoo), N. jamaicensis (Northern Potoo)
Status: Extant, Least Concern
Time and Place: From 12,000 years ago through today, in the Holocene of the Quaternary
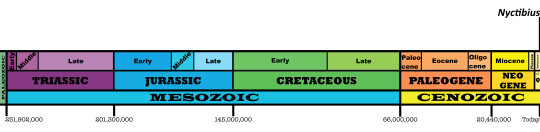
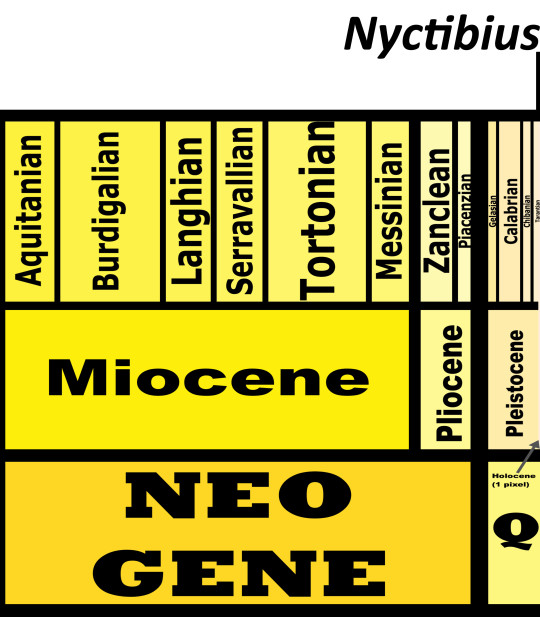
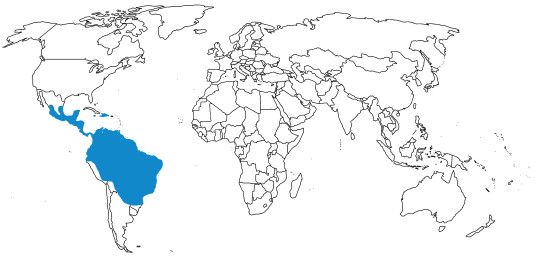
Potoos are known from Central and South America, around the Equator
Physical Description: Potoos are some of the weirdest birds alive today, looking about as ridiculous and muppet-like as any bird can really look. I’m honestly not sure if there is another living dinosaur that looks more like a muppet - and, of course, we don’t know if any extinct dinosaurs could have taken home the gold. The only probable and possible contender is the Frogmouth, which may just be the slightest amount more muppet-like, but it’s a close contest. They have distinctive faces, that are more feather than underlying tissue - their beaks stick out a bit, with a small hooked beak at the end. They have a large mouth, covered in fluff. Their eyes have a general sunken in appearance, which makes them look very large compared to the rest of the face. Their heads are very large compared to the rest of their bodies, and they have long bodies with short wings and long, fluffy tails. So, when they stand up, they look… well, they look like a stump, or a log standing up. They can then make themselves skinnier, which makes their eyes stand out compared to the rest of their bodies… which gives them the general appearance of a completely ridiculous animal. They can range in size from 21 to 58 centimeters, and range in color from reddish brown, to more orange brown, to more grey in color. This genus is not sexually dimorphic, though some species have variants in color.

By Charles J. Sharp, CC BY-SA 4.0
Diet: Potoos eat a lot of insects - from beetles to moths, to mantids, ants, termites, cicadas, leafhoppers, and grasshoppers.

Rufous Potoo by Eric Gropp, CC BY 2.0
Behavior: Potoos will hunt by standing extremely still on their perches - often, again, making themselves to look like a continuous log - and waiting for food to appear. Since they’re nocturnal, they are easily missed by the insects, as they blend into the background around them. Then, when they spot the prey, they launch forward, jutting forward to catch the insects and swallow them. Some species are more clumsy in this endeavor than others, though some are able to make leaps over several meters in order to grab the food they desire. They then return to the same post, returning to their previous log-like stillness as they wait for more food to appear. They’ll look around for the food by turning their heads rapidly from side to side - weirdly like owls, though they are not closely related to them at all. These perches can be only one meter off the ground, or up to 19 meters, depending on the forest around the potoo in question.

Common Potoo by the American Bird Conservancy
Potoos aren’t the most musical or birds, but they are loud - they make harsh, guttural “bwa-bwa-bwa” calls, similar to laughing or wailing. They can also make drawn out, descending rasps, that are… somewhat more musical at least. They tend to make their sounds mostly at dusk, right before dawn, and also on moonlit nights - so when it is Dark, but not too dark. These sounds, of course, do vary from species to species. There are some courtship calls, including descended calls made by females during the mating ritual, but they aren’t a major feature of these events. So, instead of picturing the great wolves as your moonlight singers, remember: the Potoos can and WILL be making these weird urts, laughs, and whistles, every time the moon is full and out in the sky. Potoos do not migrate, but they do appear to move sporadically in response to season changes and mating territory disturbances.

Long-Tailed Potoo by Lee R. Berger, CC BY-SA 3.0
As for nesting, Potoos are not… fantastic at the prospect, because of their tiny legs and weird, weird beaks. This makes them not great at both sitting on the nest and feeding the chicks. Still, they do it anyway, and clearly well enough since they aren’t endangered with extinction. They are monogamous, with both parents working together to incubate the egg and raise the chick, and they don’t build a nest - instead, the egg is laid in a depression on the branch, usually on top of a rotting stump. The male incubates the egg during the day, while the female will do so with the male at night. The chick is hidden almost entirely through camouflage. They hatch about a month later, and then stay in the nest for two more months, being protected by the parents and fed by them as well. They look… like clumps of fungus. Hiding underneath the log of their parents. The parents will defend themselves and the nest with mobbing behavior, crowding a predator and dive bombing it, and also calling at it loudly. In short, these birds are a Giant, Giant mess of Chaos.

White-Winged Potoo by Mark Sutton
Ecosystem: Potoos are known primarily from rainforests, and can be found at any level of the forest - some Potoos are known from the understorey, some from the middle, and some from the canopy - it really depends. They’re also found in very swampy forests, depending on the species and the habitats in question. They tend to stick to where there are easily accessible sources of water, regardless, especially rivers and lakes in the jungle. They stick to the deep interior of the forest, not venturing to forest edges much unless driven to by necessity. Some species are also found in mountain forest habitats. They have few natural predators after reaching adult size, though the young are hunted upon by monkeys and falcons.

Andean Potoo by Isirvio, CC BY-SA 2.0
Other: Potoos are a part of the Stirsorians, a group of WEIRD BIRDS that are adapted for a variety of extremely unique ecological niches, usually depending on their flight style. Close relatives of the Potoo include the Frogmouth, Nightjars, Oilbirds, Swifts, and Hummingbirds, among others. Potoos are highly adapted for their nocturnal lifestyle, adapted to blend in with their forested surroundings above all else. None are threatened with extinction at this time, though of course some species are rarer than others, and all are vulnerable to climate change and extensive habitat destruction in the American Rainforests. They are also quite uncommon birds, which of course affects their vulnerability as well.

Northern Potoo by Dominic Sherony, CC BY-SA 2.0
Species Differences: The different species of Potoo vary mainly on size, coloration, habitat, and location. The Rufous Potoo is one of the most notable, being very red in color and also the smallest species; it is known from northern Amazonia, in the middle and lower storeys of the forest. Great Potoos are the heaviest species, and greyish to yellowish brown; they are found in the canopy of Amazonia. The Long-Tailed Potoo is the longest species, and is a darker brown; it is found in lowland forest in Amazonia. The White-Winged Potoo has - you guessed it - white wings, and is small in size; it is found in the canopy of lowland Amazonia rainforest. The Andean Potoo is very dark and Extremely Muppety, and is found in mountain forests in the Andes Mountains. The Common Potoo is the most middle brown of them all and very middle in size, so the Averagest Potoo of them All; it is found in wet open woodland, usually at forest edges and the canopy, throughout Northern South America. The Northern Potoo is similar to the Common Potoo but usually larger, and it is also found in forest edges, but in Central America and the Carribean.
~ By Meig Dickson
Sources Under the Cut
Clements, J. F., T. S. Schulenberg, M. J. Iliff, D. Roberson, T. A. Fredericks, B. L. Sullivan, and C. L. Wood. 2017. The eBird/Clements checklist of birds of the world: v2017.
Cohn-Haft, M. (2019). Andean Potoo (Nyctibius maculosus). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Cohn-Haft, M. (2019). Common Potoo (Nyctibius griseus). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Cohn-Haft, M. & Kirwan, G.M. (2019). Great Potoo (Nyctibius grandis). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Cohn-Haft, M. & Kirwan, G.M. (2019). Long-tailed Potoo (Nyctibius aethereus). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Cohn-Haft, M. & Kirwan, G.M. (2019). Northern Potoo (Nyctibius jamaicensis). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Cohn-Haft, M. & Kirwan, G.M. (2019). Rufous Potoo (Nyctibius bracteatus). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Cohn-Haft, M. (2019). White-winged Potoo (Nyctibius leucopterus). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
#Nyctibius#Potoo#Strisorian#Dinosaur#Bird#Birds#Birblr#Dinosaurs#Factfile#North America#South America#Quaternary#Insectivore#Flying Friday#Nyctibius bracteatus#Nyctibius grandis#Nyctibius aethereus#Nyctibius leucopterus#Nyctibius maculosus#Nyctibius griseus#Nyctibius jamaicensis#Rufous Potoo#Great Potoo#Long-Tailed Potoo#White-Winged Potoo#Andean Potoo#Common Potoo#Northern Potoo#biology#a dinosaur a day
273 notes
·
View notes
Text
Larosterna inca

By Cristóbal Alvarado Minic, CC BY 2.0
Etymology: Gull-Tern
First Described By: Blyth, 1852
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes, Neognathae, Neoaves, Aequorlitornithes, Charadriiformes, Lari, Larida, Laridae, Sterninae
Status: Extant, Near Threatened
Time and Place: Since 126,000 years ago, from the Chibanian of the Pleistocene through the Holocene of the Quaternary
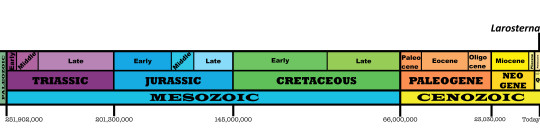
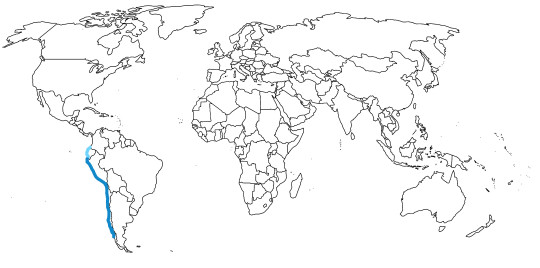
Inca Terns are known from the Pacific Coast of South America
Physical Description: Inca Terns are extremely visually distinctive birds, thanks to their bright red beaks and weird villainous-moustache feather plumes. These birds range in size between 39 and 42 centimeters in length, and are grey over most of their bodies. Their tails are distinctively black, and the wings are grey before ending in a distinctive white band and then continuing to black tips when folded. Their beaks are bright red, large, and slightly curved. They have a small yellow patch of feathers under their eyes, and a very long, curly white feather ribbon going from right under their eye down their neck. Their legs are short and dark red as well. The juveniles tend to be more brown all over before becoming darker with age.
Diet: Inca Terns feed primarily on small fish, plankton, and scraps.

By Cristóbal Alvarado Minic, CC BY 2.0
Behavior: These terns will stick to fishing boats in large flocks, hovering around them in order to opportunistically feed off of food brought up by fishing activity. They often will detect large sea mammals and fly away - rapidly - to avoid them, and also to grab the food that is welled up by them. They can often live in flocks of up to 5000 members. They forage by plunging in the water and diving for food, as well as dipping a little on the surface. They do not migrate, and are extremely loud at their colonies - making a variety of cackling and mewing sounds.

By Cristóbal Alvarado Minic, CC BY 2.0
Inca Terns breed throughout the year, with nests and eggs found in both the summer and the winter. Courting and mating birds are also found all over their range throughout the year. They build nests in fissures, burrows, and caves, as well as underneath rocks and boulders on the shore. They often build nests in mixed-species flocks with petrels and cormorants, though they will hide the nests more when vultures and other predators are present. They lay about two eggs which are incubated for four weeks, they hatch as small fluffy grey blobs that fledge in four more weeks. They are dependent on the parents for another month. The parents are monogamous, with both helping to take care of the young; interestingly enough, their fascinating plumage isn’t for sexual display, but rather to indicate the health of the individuals. In fact, the length of the villain moustache is the clearest indicator of individual health. These birds can live up to 25 years, though it is usually significantly less in the wild.

By Josue Hermoza, CC BY-SA 4.0
Ecosystem: Inca Terns primarily live in rocky coasts or where sandy beaches are surrounded by cliffs, since those are their primary nesting habitats. They are fed upon by cats, rats, and sea lions, as well as some raptors like falcons, and large seabirds do feed on the nests.

By Olaf Oliviero Riemer, CC BY-SA 3.0
Other: Inca Terns are considered near threatened, primarily due to fluctuations in food from El Niño - they have dramatic population drops in response, but then rebound quickly when it stops, indicating potential emigration rather than starvation. There is some hunting by humans, but not enough to cause these population drops. There are breeding programs present, especially in zoos, where Inca Terns do quite well. Inca Terns have actually been around since the last Ice Age, where their range was much more northward, indicating they have shifted their habitat with the warming of the planet.
Sources Under the Cut
Campbell, K. E. 1976. The late Pleistocene avifauna of La Carolina, southwestern Ecuador. Smithsonian Contributions to Paleobiology 27:155-168.
Clements, J. F., T. S. Schulenberg, M. J. Iliff, D. Roberson, T. A. Fredericks, B. L. Sullivan, and C. L. Wood. 2017. The eBird/Clements checklist of birds of the world: v2017.
Gochfeld, M. & Burger, J. (2019). Inca Tern (Larosterna inca). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Harrison, Peter (1988): Seabirds (2nd edition). Christopher Helm, London.
#Larosterna inca#Larosterna#Inca Tern#Bird#Dinosaur#Birds#Dinosaurs#Birblr#Palaeoblr#Factfile#Charadriiform#Tern#Prehistoric Life#Prehistory#Paleontology#Quaternary#South America#Piscivore#Aequorlitornithian#Water Wednesday#biology#a dinosaur a day#a-dinosaur-a-day#dinosaur of the day#dinosaur-of-the-day#science#nature
235 notes
·
View notes
Text
Irania gutturalis

By Amrou-A, CC BY-SA 4.0
Etymology: From Iran
First Described By: de Filippi, 1863
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes, Neognathae, Neoaves, Inopinaves, Telluraves, Australaves, Eufalconimorphae, Psittacopasserae, Passeriformes, Eupasseres, Passeri, Euoscines, Passerides, Core Passerides, Muscicapida, Muscicapoidea, Muscicapidae, Saxicolinae
Status: Extant, Least Concern
Time and Place: Since 10,000 years ago, in the Holocene of the Quaternary
White-Throated Robins are known from the Middle East in the Summer and Eastern Africa in the Winter
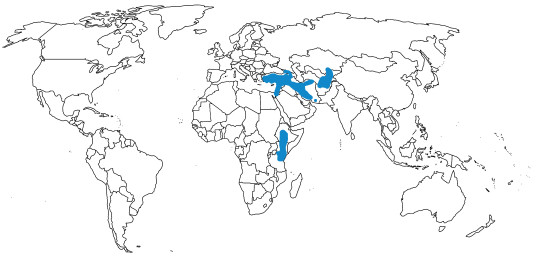
Physical Description: White-Throated Robins are beautiful passerines that actually kind of resemble American Robins in coloration, though they are not closely related at all and this is simply a case of convergent evolution. They range in size between 15 and 17 centimeters long, with grey backs and short black tails. Their wings tend to end in darker grey wingtips. They have a white stripe over their eyes, with a black side of their faces. As the name would suggest, their throats tend to be white in color. They have short, very pointy beaks and long grey legs. The reasons for their name is the coloration of their bellies - most males are a dark red-orange, and the females are as well, but with brown spotting and more white color on their belly. Some males are more of a yellow color than red-orange, which is fascinating. The juveniles tend to look like the females, but more dull in color.
Diet: White-Throated Robins primarily feed upon invertebrates and fruits, include a variety of beetles and ants, as well as berries.

By John A. Thompson
Behavior: These robins will forage among the low twigs on the ground, as well as in bushes and trees. They usually skulk around, spending most of their time in dense undergrowth and hiding in the thicket in order to avoid danger. They are very faithful to their preferred foraging sites, and will even defend them as their territory during the winter foraging season. They are highly migratory, wintering in eastern Africa - from Eritrea to Tanzania - and then leaving their winter sites by the end of March, passing through Kenya and Ethiopia through to Western Asia & Turkey, though some get as far west as Greece and some as far south as Israel. White-Throated Robins then stay in their breeding sites until the end of August, moving back to Africa by going across the Middle East.
Given this very noticeable migration, White-Throated Robins are extremely social and coordinated, making noticeable sounds to one another based on the situation at hand. Their songs are loud, vigorous warbling for multiple seconds with a variety of pauses and phrases, including flute-like whistling and scratchy chatters. These songs are often made in flight as well. Sometimes, the males of this species will mimic other birds. They also make warbles, hard “tec-tec-tec” calls, and more trilling calls as well. They can be, and usually are, extremely loud.
White-Throated Robins tend to breed in dry, rocky slopes with some bushes provided for cover. They usually lay one brood per season, in a nest made of twigs and plant stalks and lined with feathers, usually made in the shape of a flat cup. They’re placed low to the ground in a tree, bush, or stump, and they often place their nests in the same sites from year to year. They lay between four and six pale green-blue eggs, with brown spotting. The eggs are incubated for two weeks, and the young stay for a little bit longer than a week within the next. They then can flutter around at two weeks of age, and fly fully at three weeks. They stick with the parents for another two weeks, before being fully independent.

By Westan Mese, CC BY-SA 4.0
Ecosystem: White-Throated Robins live in scrubland, steppe, stony arid hillsides, semi-desert, and mountainous regions during the breeding season, usually newer juniper and weedy terrain. They will also go to locations with birch and crab apple. In the winter they tend to live in semi-arid scrub and thickets, including acacia woodland and gardens. They don’t tend to reach higher elevations. White-Throated Robins are often preyed upon by lizards, snakes, and Common Magpies.
Other: Funnily enough, despite being called a robin and being similar in appearance to the American Robin - which is actually a thrush - White-Throated Robins are actually chats. Because passerine phylogenetics is a mess. They are not threatened with extinction, and there are probably millions of White-Throated Robins alive today.
~ By Meig Dickson
Sources Under the Cut
Collar, N. (2019). White-throated Robin (Irania gutturalis). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Jobling, J. A. 2010. The Helm Dictionary of Scientific Bird Names. Christopher Helm Publishing, A&C Black Publishers Ltd, London.
#Irania gutturalis#Irania#White-Throated Robin#Bird#Dinosaur#Factfile#Birds#Dinosaurs#Birblr#Passeriform#Songbird#Perching Bird#Thrush#Omnivore#Africa#Eurasia#Quaternary#Songbird Saturday & Sunday#biology#a dinosaur a day#a-dinosaur-a-day#dinosaur of the day#dinosaur-of-the-day#science#nature
218 notes
·
View notes
Text
Eudyptula minor

By J. J. Harrison, CC BY-SA 3.0
Etymology: Good Diver
First Described By: Bonaparte, 1856
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes, Neognathae, Neoaves, Aequorlitornithes, Ardeae, Aequornithes, Austrodyptornithes, Sphenisciformes, Spheniscidae
Status: Extant, Least Concern
Time and Place: From 12,000 years ago until today, in the Holocene of the Quaternary
Little Penguins are known from the coast of Australia and New Zealand
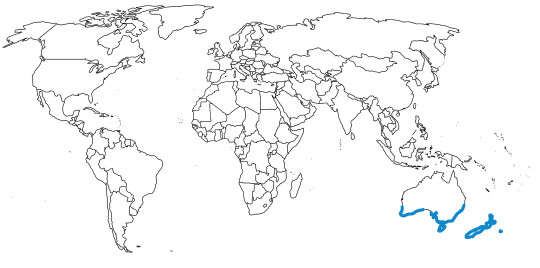
Physical Description: Little Penguins are some of the most adorable penguins alive today, and the reason is clear: they’re smol! Little Penguins range in size between 40 and 45 centimeters in length, and none weigh more than 2.1 kilograms. They are a blue-grey on their backs and white on their bellies and necks. They have very small flippers, which can be entirely blue or blue only in the center with white banding around it. They have short little tails and small feet, which are formed into lightly orange flippers. Their beaks are short and round, and either light in color or dark depending on the population. The juveniles tend to be a somewhat duller color than the adults, but usually very similar overall.

By Magnus Kjaergaard, CC BY 3.0
Diet: Little Penguins feed mainly on fish that form schools in the pelagic zone of the ocean (rather than closer to the coast). They’ll also feed on cephalopods and crustaceans.

By Francesco Veronesi, CC BY-SA 2.0
Behavior: These nocturnal penguins will capture their food via pursuit-diving, mainly swimming around schools of fish in tighter and tighter circles until finally - woosh! - they dive into the middle and grab as much food as they can. In shallower water, they do pursue the fish more directly. They can dive as deep as 50 meters underwater, sometimes more - including 69 meters deep. They go up to 62 kilometers away from the nesting and sleeping colony sites, though they can usually only go a fraction of that in a single day and longer distances are reserved for multiple day trips. Females tend to forage more than the males. They feed alone, though they do forage in groups. They are extremely noisy in their colonies, making a variety of trills, brays, growls, grunts, yelping, trumpeting, and wailing sounds to one another. They do not migrate, but do stay near the breeding colony extensively during the moulting and nesting season. Juveniles will leave the colony for a large amount of time, but do eventually return to breed where they were born.

By Phillip Island Tourism, CC BY-SA 4.0
Little Penguins begin breeding in July and continue through December, though it varies from colony to colony and from year to year. They tend to stick to one nest site for their whole lives and are also fairly monogamous, though they do divorce from their partners around 76% of the time. If they are unsuccessful in raising any chicks one year, the next year they are more likely to divorce than not. The nests are made in a little bit far apart from one another in the colonie, usually a burrow in the sand lined with plant material. Usually two eggs are laid in the nest and are incubated for a little more than one month by both parents. The chicks hatch extremely fluffy and greyish-brown; they then molt to look dark brown and grey another month later. At this time young birds will form creches together for two more months, hanging out and learning from each other. They then fledge and become juveniles two more months later. They become sexually mature at two to four years of age, and can live for up to 20 years (though shorter is more common). The chicks and nesting colonies tend to feed at dusk.

By Mark Nairn, CC BY 3.0
Ecosystem: Little Penguins live mainly in sandy and rocky coasts, usually near the bases of cliffs and in sand dunes. They will spend most of their time in temperate to sub-arctic marine waters, many miles offshore. They are preyed upon by cats, dogs, rats, foxes, lizards, snakes, ferrets, stoats, seals, and some other ocean predators. They are mostly affected by introduced mammalian predators at this time.

By fir0002, GFDL 1.2
Other: While Little Penguins are, overall, doing alright and are even common in terms of population management; however, a number of human-created threats are affecting certain colonies and bringing many of them to the verge of collapse. Uncontrolled hunting by domesticated dogs and cats are major causes of colony destruction, as are oil spills, fishing interactions (including being caught in nets), human interference and development, as well as cullings by humans attempting to manage populations of other birds. As such, Little Penguins are of major interest for ecological groups in order to preserve their populations in light of these threats. Fossils of this species are known from its current range from the recent ice age.
~ By Meig Dickson
Sources Under the Cut
Baird, R. F. 1992. Fossil avian assemblage of Pitfall Origin from Holocene sediments in Amphitheatre Cave (G-2), South-western Victoria, Australia. Records of the Australian Museum 44:21-44.
Banks, Jonathan C.; Mitchell, Anthony D.; Waas, Joseph R.; Paterson, Adrian M. (2002). "An unexpected pattern of molecular divergence within the blue penguin (Eudyptula minor) complex". Notornis. 49 (1): 29–38.
Bethge, P; Nicol, S; Culik, BM & RP Wilson (1997) "Diving behaviour and energetics in breeding little penguins (Eudyptula minor)". Journal of Zoology 242: 483-502.
Boessenkool, S., J. J. Austin, T. H. Worthy, P. Scofield, A. Cooper, P. J. Seddon, and J. M. Waters. 2009. Relict or colonizer? Extinction and range expansion of penguins in southern New Zealand. Proceedings of the Royal Society B 276:815-821.
Carroll, R. L. 1988. Vertebrate Paleontology and Evolution 1-698.
Chiaradia, A., Forero, M. G., Hobson, K. A., and Cullen, J. M. (2010) Changes in diet and trophic position of a top predator 10 years after a mass mortality of a key prey. – ICES Journal of Marine Science, 67: 1710–1720.
Clements, J. F., T. S. Schulenberg, M. J. Iliff, D. Roberson, T. A. Fredericks, B. L. Sullivan, and C. L. Wood. 2017. The eBird/Clements checklist of birds of the world: v2017.
Flemming, S.A., Lalas, C., and van Heezik, Y. (2013) "Little penguin (Eudyptula minor) diet at three breeding colonies in New Zealand". New Zealand Journal of Ecology 37: 199–205.
Fordyce, R. E. 1991. The Australasian marine vertebrate record and its climatic and geographic implications. In P. Vickers-Rich, J. M. Monaghan, R. F. Baird, T. H. Rich (eds.), Vertebrate Palaeontology of Australasia 1165-1190.
Grosser, Stefanie; Burridge, Christopher P.; Peucker, Amanda J.; Waters, Jonathan M. (2015-12-14). "Coalescent Modelling Suggests Recent Secondary-Contact of Cryptic Penguin Species". PLOS ONE. 10 (12): –0144966.
Grosser, Stefanie; Rawlence, Nicolas J.; Anderson, Christian N. K.; Smith, Ian W. G.; Scofield, R. Paul; Waters, Jonathan M. (2016-02-10). "Invader or resident? Ancient-DNA reveals rapid species turnover in New Zealand little penguins". Proceedings of the Royal Society B: Biological Sciences. 283 (1824): 20152879.
Littlely, Bryan (10 October 2007). "Fur seals threat to Granite Island penguins". The Advertiser. p. 23.
Martínez, I., Christie, D.A., Jutglar, F. & Garcia, E.F.J. (2019). Little Penguin (Eudyptula minor). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Numata, M; Davis, L & Renner, M (2000) "[Prolonged foraging trips and egg desertion in little penguins (Eudyptula minor)]". New Zealand Journal of Zoology 27: 291-298.
Rawlence, N. J., A. Kardamaki, L. J. Easton, A. J. D. Tennyson, R. P. Scofield and J. M. Waters. 2017. Ancient DNA and morphometric analysis reveal extinction and replacement of New Zealand’s unique black swans. Proceedings of the Royal Society B 284:20170876.
Rodríguez, A., Chiaradia, A., Wasiak, P., Renwick, L., and Dann, P.(2016) "Waddling on the Dark Side: Ambient Light Affects Attendance Behavior of Little Penguins." Journal of Biological Rhythms 31:194-204.
Saraux, Claire; Chiaradia, Andre; Salton, Marcus; Dann, Peter; Viblanc, Vincent A (2016). "Negative effects of wind speed on individual foraging performance and breeding success in little penguins". Ecological Monographs. 86 (1): 61–77.
Simpson, G. G. 1946. Fossil Penguins. Bulletin of the American Museum of Natural History 87(1):1-100.
Tennyson, A. J. D., and P. R. Millener. 1994. Bird extinctions and fossil bones from Mangere Island, Chatham Islands. Notornis, Supplement 41:165-178.
Tennyson, A. J. D., J. H. Cooper, and L. D. Shepherd. 2015. A new species of extinct Pterodroma petrel (Procellariiformes: Procellariidae) from the Chatham Islands, New Zealand. Bulletin of the British Ornithologists' Club 135:267-277.
van Tets, G. F., and S. O'Connor. 1983. The Hunter Island Penguin, and extinct new genus and species form a Tasmanian midden. Records of the Queen Victoria Museum, Launceston 81:1-13.
Wallis, Robert; King, Kristie; Wallis, Anne (2017). "The Little Penguin'Eudyptula minor'on Middle Island, Warrnambool, Victoria: An update on population size and predator management". Victorian Naturalist. 134 (2): 48–51.
Wiebkin, A. S. (2011) Conservation management priorities for little penguin populations in Gulf St Vincent. Report to Adelaide and Mount Lofty Ranges Natural Resources Management Board. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. SARDI Publication No. F2011/000188-1. SARDI Research Report Series No.588. 97pp.
Williams, Tony D. (1995). The Penguins. Oxford, England: Oxford University Press.
Williams, M., A. J. D. Tennyson, and D. Sim. 2014. Island differentiation of New Zealand’s extinct mergansers (Anatidae: Mergini), with description of a new species from Chatham Island. Wildfowl 6:3-34.
Wood, J. R., K. J. Mitchell, R. P. Scofield, A. J. D. Tennyson, A. E. Fidler, J. M. Wilmshurst, B. Llamas and A. Cooper. 2014. An extinct nestorid parrot (Aves, Psittaciformes, Nestoridae) from the Chatham Islands, New Zealand. Zoological Journal of the Linnean Society 172:185-199.
Worthy, T. H. 1998. A remarkable fossil and archaeological avifauna from Marfells Beach, Lake Grassmere, South Island, New Zealand. Records of the Canterbury Museum 12:79-176.
Worthy, T. H., and J. A. Grant-Mackie. 2003. Late-Pleistocene avifaunas from Cape Wanbrow, Otago, South Island, New Zealand. Journal of the Royal Society of New Zealand 33(1):427-485.
#Eudyptula minor#Eudyptula#Penguin#Little Penguin#Dinosaur#Dinosaurs#Bird#Birds#Birblr#Palaeoblr#Factfile#Little Blue Penguin#Fairy Penguin#Aequorlitornithian#Ardeaen#Piscivore#Water Wednesday#Quaternary#Australia & Oceania#Blue Penguin#paleontology#prehistory#prehistoric life#biology#a dinosaur a day#a-dinosaur-a-day#dinosaur of the day#dinosaur-of-the-day#science#nature
231 notes
·
View notes
Text
Heracles inexpectatus

By Scott Reid
Etymology: For the Greek Demigod Heracles
First Described By: Worthy et al., 2019
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes, Neognathae, Neoaves, Inopinaves, Telluraves, Australaves, Eufalconimorphae, Psittacopasserae, Psittaciformes, Strigopoidea
Status: Extinct
Time and Place: Between 19 and 16 million years ago, in the Burdigalian of the Miocene
Heracles is known from the Bannockburn Formation of the South Island of New Zealand
Physical Description: Heracles is an utterly fascinating recent dinosaur discovery, both for its inherent qualities and those due to the circumstances of its discovery. This was a large, Kākāpō-like parrot, about the height of a shorter adult or a child. It is the largest known parrot, and it would have been about one meter tall and weighing about seven kilograms. Given this size, it was flightless, and probably mostly terrestrial. It had a very strong beak, and probably resembled in many ways a giant version of the modern-day Kākāpō, Kaka, and Kea. As such, it would have been quite fat looking, probably greenish in color, and very fluffy as well.

By Brian Choo, Press Release Image
Diet: As a parrot, Heracles is most likely to have fed on seeds and nuts, though the fact that it was closely related to the living Kea means there is a non insignificant chance it was carnivorous. For now, we’ll say it was most likely an omnivore.
Behavior: It is logical to presume that Heracles resembled its modern relatives, which means it would have been a loosely social animal, spending most of its time on the ground in groups of about a dozen animals. It would have been able to use tools to get at sources of food, especially difficult to reach ones. It possibly would have also used its sharp beak to attack other dinosaurs on the island, chasing them with a hopping gait until they were isolated and then killed. An intelligent animal, it would have been able to solve puzzles and work together to get at sources of food or shelter. As a member of the New Zealand Parrot Group, it probably would have been polygamous, with the males having multiple mates at a time. They would have made nests on the ground, and since they never came across with mammalian predators, they probably would have been fine in terms of reproduction rate.

By Ripley Cook
Ecosystem: The Saint Bathans Fauna was a unique ecosystem filled with almost entirely birds, and other creatures that could float or fly over to New Zealand after it emerged from having flooded. This meant that birds were filling niches that, in the rest of the world, were being taken up by mammals. In short, this was a weird sort of Jurassic Park - with dinosaurs wreaking havoc as echoes of their former reign. Dinosaurs of the Saint Bathans Fauna included another New Zealand Parrot, Nelepsittacus, a bittern Pikaihao, herons like Matuku and Pikaihao, the swimming flamingo Palaelodus, flightless rails such as Priscaweka and Litorallus, the early Adzebill Aptornis proasciarostratus, the pigeon Rupephaps, the stiff-tailed duck Dunstanneta, the early Kiwi Proapteryx, the small Manuherikia duck, and the early New Zealand Wren Kuiornis - just to name a few! Given that only sparse remains are known from some unnamed birds of prey, this points to Heracles being at least somewhat carnivorous and fulfilling that role in its ecosystem. This was a series of extensive lakes, filled with cycad and palm trees, and there were also a wide variety of geckos, skinks, crocodilians, turtles, tuatara, and bats. There was one other mammal present - but, for now, we have no idea what it was.

By José Carlos Cortés
Other: The Saint Bathans Fauna is one of the most fascinating ecosystems of fossil birds of the Cenozoic Era. The unique ecosystem of New Zealand, with its almost complete lack of mammals before human interference. Heracles is an extremely important fossil find, as it may help us to piece together how the weird New Zealand Parrots evolved - previously, little was known in the way of fossil members of this group beyond recent history. The more we research it, the more we will be able to understand one small piece of the evolutionary puzzle that is this unique island. Also, it’s common name is Squawkzilla.
~ By Meig Dickson
Sources Under the Cut
Mather, Ellen K.; Tennyson, Alan J. D.; Scofield, R. Paul; Pietri, Vanesa L. De; Hand, Suzanne J.; Archer, Michael; Handley, Warren D.; Worthy, Trevor H. (2019-03-04). “Flightless rails (Aves: Rallidae) from the early Miocene St Bathans Fauna, Otago, New Zealand”. Journal of Systematic Palaeontology. 17 (5): 423–449.
Scofield, R. Paul; Worthy, Trevor H. & Tennyson, Alan J.D. (2010). “A heron (Aves: Ardeidae) from the Early Miocene St Bathans Fauna of southern New Zealand.” (PDF). In W.E. Boles & T.H. Worthy. (eds.). Proceedings of the VII International Meeting of the Society of Avian Paleontology and Evolution. Records of the Australian Museum. 62. pp. 89–104.
Tennyson, Alan J. D.; Worthy, Trevor H.; Scofield, R. Paul (2010). “A heron (Aves: Ardeidae) from the Early Miocene St Bathans fauna of southern New Zealand. In Proceedings of the VII International Meeting of the Society of Avian Paleontology and Evolution, ed. W.E. Boles and T.H. Worthy”. Records of the Australian Museum. 62: 89–104.
Worthy, Trevor H.; Lee, Michael S. Y. (2008). “Affinities of Miocene Waterfowl (anatidae: Manuherikia, Dunstanetta and Miotadorna) from the St Bathans Fauna, New Zealand”. Palaeontology. 51 (3): 677–708.
Worthy, Trevor H.; Tennyson, Alan J. D.; Hand, Suzanne J.; Scofield, R. Paul (2008-06-01). “A new species of the diving duck Manuherikia and evidence for geese (Aves: Anatidae: Anserinae) in the St Bathans Fauna (Early Miocene), New Zealand”. Journal of the Royal Society of New Zealand. 38 (2): 97–114.
Worthy, T. H., S. J. Hand, M. Archer, R. P. Scofield, and V. L. De Pietri. 2019. Evidence for a giant parrot from the Early Miocene of New Zealand. Biology Letters 15:20190467
#Heracles#heracles inexpectatus#Parrot#New Zealand Parrot#Dinosaur#Dinosaurs#Birds#Bird#palaeoblr#birblr#psittaciformes#Australavian#factfile#Terrestrial Tuesday#Omnivore#Australia & Oceania#Neogene
248 notes
·
View notes
Text
Gymnorhina tibicen

By Pratyeka, CC BY-SA 4.0
Etymology: Bare Nostrils
First Described By: Gray, GR, 1840
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes, Neognathae, Neoaves, Inopinaves, Telluraves, Australaves, Eufalconimorphae, Psittacopasserae, Passeriformes, Eupasseres, Passeri, Euoscines, Corvides, Malaconotoidea, Artamidae, Cracticinae
Status: Extant, Least Concern
Time and Place: Since 126,000 years ago, from the Tarantian of the Pleistocene through the Holocene
Australian Magpies are known from throughout Australia and the closest point of New Guinea to Australia
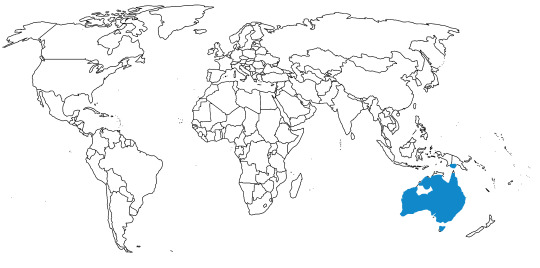
Physical Description: Australian Magpies are extremely distinctive and beautiful birds, despite their rather monstrous behavioral patterns! They range between 37 and 43 centimeters in length, though different subpopulations weigh more than others. They are black over most of their bodies, with distinctive white patterns that vary from population to population. Usually there’s a distinctive white spot on the back of the neck, that may or may not blend into the black of the back. There are white patches on the backs of their wings, and small ones on the undersides. Their rumps and under-tail feathers are also white, though often with a black tip on the very end. They have long dark legs, with noticeable claws. These birds have very large, triangular, sharp bills, and completely bright red eyes. In short - they are hard to miss! The juveniles seem to look rather scalloped compared to the ideas, and have more grey patches in general.

By J. J. Harrison, CC BY-SA 3.0
Diet: Australian Magpies are omnivores, eating an extremely wide variety of foods - invertebrates, including and especially insects and other arthropods; lizards, frogs, mice, other small animals, grains, tubers, figs, and walnuts are even features of the Australian Magpie diet. Interestingly enough, they can eat poisonous cane toads by flipping them and eating their underparts.

By Toby Hudson, CC BY-SA 3.0
Behavior: These notorious birds spend most of their time awake during the day, foraging on the ground for a wide variety of foods. They search very methodically, even listening to the slightest sounds and feeling the smallest vibrations in order to dig up beetles and other insects. They’ll overturn debris in their tireless search for food, and pull off dangerous parts of prey - such as insect singers - before eating. They also store their food for later times, coming back to the same cache sites with some regularity. They tend to walk around their environment, rather than hopping like their close relatives, and their legs are built to support this. They also can run, in very quick bursts, in order to catch prey.

By fir0002, GDFL 1.2
While Australian Magpies are social, living in groups together, they are extremely territorial as well - and these groups will defend their territories fiercely. They have sentinel birds, which watch for danger - they’ll make rallying calls, which lead to the other members of the group rallying together to attack the intruder, usually in a mob-like fashion. In particular, when attacking intruding birds of prey, the magpies will put themselves on either side of the bird, and then the attacker will come from behind while it tries to escape the defending birds. They will then harass the raptor until ti leaves the territory. When it comes to other Australian Magpies, they’ll make carolling calls to signal that they own this territory, and the intruders need to leave! When negotiating the boundaries of the territory, the dominant members of the group will parade around the border, while the other members watch; they then puff up their feathers to look bigger to intruders, or even make carols repeatedly. The group will also signal their strength to other groups that have about even numbers, all flying and forming rows at the territory border - and then the defending group will swoop and dive, while calling, to display and warn the intruding group.

By Alexis Hilton Hope, CC BY-SA 3.0
In general, Australian Magpies have more aggressive behaviors than friendly ones, even within the group. Birds displaying submission will crouch low and make quiet begging calls, sometimes fluffing up their primary feathers to indicate their submission further. Juveniles will also fall, roll, and expose their bellies to indicate their submission. When attacking, the Australian Magpie will fluff up their tail feathers as a sign of their aggression. The young birds will play with each other, while the older birds may egg on or start the playing - includingn picking up objects, manipulating and tugging at them, and handing things to each other. Sometimes, the birds will pick up objects and fly away with them, in order to give chase, with other birds trying to bring down the thief by grabbing onto tail feathers. They will also jump on each other, in mock-fights - even with other birds such as Blue-Faced Honeyeaters and Australasian Pipits! These are extremely vocal birds, with varied and complex songs - their carols are often melodious, flute-like yodels; they will make warble songs as well, and can incorporate mimicry of other calls heard. They have a wide range of harsh, “ka”-ing alarm calls, and their rallying calls tend to be loud, descending whistles.

By Sterry2607, CC BY-SA 3.0
Australian Magpies begin breeding in winter, though they can extend the breeding into the spring - and females will attempt to raise two broods often, if they start early enough in the season. They make nests out of sticks, grass, and bark, shaped into a bowl that are placed high up in tree forks. They will put nests in any sort of tree, though eucalypts are common choices. Other birds will often nest in the same tree, underneath the magpies, to use the protection of the aggressive magpies to their own advantage - since the magpies tolerate the other birds. Two to five eggs are laid in a clutch, which hatch around three weeks after they start incubating. They have fully open eyes ten days after hatching, and then start to develop feathers - the females are exclusively feeding the young throughout this process, though the male magpies give food to their partners. Helper birds do aid in feeding and raising the young - depending on the size of the group. In small groups, helper behavior is rare to nonexistent. After leaving the nest at around three weeks, the young will forage on their own after another three weeks, and can mostly feed themselves by six months. At this point, any begging for food is ignored. They reach adult size by one year of age, and tend to disperse from the group depending on their sex and the social customs of their home group - males are usually kicked out of the group before females. The latest time they leave their families is at four years of age. They then either make their own groups with other young birds, or join existing ones.

By Toby Hudson, CC BY-SA 3.0
These aggressive birds are most notable in Australia for their swooping. Ubiquitous all over Australia, including urban areas, they become extremely aggressive during the breeding season. As the eggs begin to hatch, these birds will swoop at passing humans, with some populations being more aggressive than others. That being said, by and large Australian Magpies are used to people, and fewer than 9% engage in swooping behavior. Most of the attacking birds are male, and will dive from up to 100 meters high in their nests and in the air. They tend to favor pedestrians and cyclists, and do not appreciate humans attempting to help fallen chicks. They start this behavior with alarm calls and mock-swoops, then closer swoops and snapping their beaks near the human’s body, and finally escalating to dive-bombing the intruder’s head. They may even land on the ground and lurch up to peck at the face and eyes of the intruder. In general, they cause very severe wounds to the head - and if they’re attacking a cyclist, their favorite sort of prey, they can even cause fatal accidents as the individual loses control of the bike. It is possible that the aggression can be curtailed by hand-feeding the birds, though that causes its own problems.

By J. J. Harrison, CC BY-SA 3.0
Ecosystem: Australian Magpies live in open habitats with grasses and other cover, in order to forage safely. They usually stick to eucalypt woodlands, but now are found frequently within urban areas. They enjoy heavily vegetated areas with open woodland (aka, the vegetation isn’t dense forest). They are also often found in open farmland. These birds are often victims of nest parasitism by cuckoos. They also associate greatly with blue-faced honeyeaters and Australaisan pipits, and allow the presence of other birds nesting below them in trees. They tend to be preyed upon by Australian Ravens. When they eat house sparrows, mice, rats, and rabbits that were targeted with baiting, they can end up poisoned.

By fir0002, GFDL 1.2
Other: Australian Magpies are not threatened with extinction at this time, which is a blessing, as they are important figures in their ecosystems. That being said, they are hurt often by power lines and angry humans. Their ability to thrive in towns and cities, however, means that this hasn’t had a huge effect on their general population. They are a protected species, so it is illegal to hurt or kill them, but if they attack a human too aggressively they may be controlled by authorities. Usually, these aggressive birds are caught and relocated rather than killed. They are featured in indigenous folklore, especially those of the Yindjibarndi. Sports teams often use the colors of the Australian Magpie to indicate strength and ferocity. Interestingly enough, though called magpies and greatly resembling them, these birds are most closely related to other Australian and Southern Asian birds such as Currawongs, Butcherbirds, and Woodswallows - rather than those magpies in the family Corvidae, aka the Eurasian Magpies.

An individual getting swooped; face covered for privacy. By Sarahlyeah, CC BY-SA 4.0
~ By Meig Dickson
Sources Under the Cut
Carrick, Robert (1972). "Population ecology of the Australian Black-backed Magpie, Royal Penguin, and Silver Gull". U S Dept Interior Res Report. 2: 41–99.
Clements, J. F., T. S. Schulenberg, M. J. Iliff, D. Roberson, T. A. Fredericks, B. L. Sullivan, and C. L. Wood. 2017. The eBird/Clements checklist of birds of the world: v2017
Higgins, Peter Jeffrey; Peter, John M.; Cowling, S. J., eds. (2006). "Gymnorhina tibicen Australian Magpie" (PDF). Handbook of Australian, New Zealand and Antarctic Birds. Vol. 7: Boatbill to Starlings. Melbourne, Victoria: Oxford University Press. ISBN 978-0-19-553996-7. Linked pdf is 118 MB and contains Vol. 5 pages 51–55; Vol. 7A pages 396-399, 579-629; plate 18
Hope, J. H., R. J. Lampert, E. Edmondson, M. J. Smith, and G. F. Van Tets. 1977. Late Pleistocene Faunal Remains from Seton Rock Shelter, Kangaroo Island, South Australia. Journal of Biogeography 4(4):363-385
Jones, Darryl (2002). Magpie Alert: Learning to Live with a Wild Neighbour. Randwick, New South Wales: University of New South Wales Press.
Jones, Darryl N.; Thomas Nealson (2003). "Management of aggressive Australian magpies by translocation". Wildlife Research. 30 (2): 167–77.
Kaplan, Gisela (2004). Australian Magpie: Biology and Behaviour of an Unusual Songbird. Melbourne, Victoria: CSIRO Publishing.
Marchant, Gillian (26 November 2007). "Birds learn to eat cane toads safely". Southern Cross University website. Southern Cross University.
Prideaux, G. J. 2004. Systematics and evolution of the sthenurine kangaroos. In S. W. Awramik, A. Barnosky, J. A. Doyle, M. L. Droser, P. M. Sadler (eds.), UC Publications in Geological Sciences, University of California Press 146:1-623
Russell, E., Rowley, I. & Christie, D.A. (2019). Australian Magpie (Gymnorhina tibicen). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Veltman CJ, Hickson RE (1989). "Predation by Australian magpies (Gymnorhina tibicen) on pasture invertebrates: are non-territorial birds less successful?". Australian Journal of Ecology. 14 (3): 319–26.
Warne, R. M.; Jones, D. N. (2003). "Evidence of target specificity in attacks by Australian magpies on humans". Wildlife Research. 30 (3): 265–267.
#Gymnorhina tibicen#Gymnorhina#Australian Magpie#Bird#Dinosaur#Magpie#Birds#Passeriform#Songbird#Perching Bird#Quaternary#Australia & Oceania#Songbird Saturday & Sunday#Omnivore#Factfile#Birblr#Palaeoblr#paleontology#prehistory#prehistoric life#dinosaurs#biology#a dinosaur a day#a-dinosaur-a-day#dinosaur of the day#dinosaur-of-the-day#science#nature
243 notes
·
View notes
Text
Chiappeavis magnapremaxillo

By José Carlos Cortés
Etymology: Chiappe’s Bird
First Described By: O’Connor et al, 2016
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Enantiornithes, Cathayornithiformes
Status: Extinct
Time and Place: 120 million years ago, in the Aptian age of the Early Cretaceous
Chiappeavis is known from the Jiufotang Formation of China, specifically in the Shangheshou Beds

Physical Description: Chiappeavis was an Opposite Bird, ie the group of bird-like dinosaurs that were extremely diverse and widespread during the Cretaceous period. Chiappeavis is known from a nearly complete skeleton, including some feather impressions. It was a fairly large bird, probably around 20 or so centimeters (though this is a very rough estimate). It had a small snout, with small pointed teeth inside of it, and a fairly large head. Its body was long, and it had large wings - good for more powerful flying as opposed to tighter maneuvering in between trees. Interestingly enough, Chiappeavis had a giant tail fan, which was not actually universal among Opposite BIrds as it is in modern birds. It also had fairly thick, strong feet.
Diet: It is probable that Chiappeavis fed mainly on arthropods and other hard invertebrates.

By Ripley Cook
Behavior: It is uncertain what the behavior of Chiappeavis was, given that we do not have extensive skeletons of this dinosaur. Still, it probably wouldn’t have flitted about the trees as much as birds with wings better built for maneuvering. The tail fan of Chiappeavis probably would have been extremely useful in sexual display, as well as other forms of communication - especially since it does not appear to have been very good at generating lift during flight (hence it not being widespread in other Opposite Birds). As such, it is more likely than not that Chiappeavis would have been fairly social, living in groups of multiple birds which communicated and recognized each other with feather displays. This, therefore, leads us to yet another likely hypothesis: that it took care of its young, at least to some extent. Beyond that, the behavior of Chiappeavis is a bit of a question - though it may have been able to dig out insects and other grubs with its strong feet, and then bit into the tough exteriors of these animals with its many needle-like teeth.
Ecosystem: The Jiufotang Formation was one of the Jehol Biota ecosystems, aka a group of extremely diverse and lush environments that preserved birdie dinosaurs of the Early Cretaceous with great detail. In that, Chiappeavis is one of many dinosaurs found in this location with extensive feather preservation. TheJiufotang Environment was a dense forest, surrounding an extensive number of lakes, and near volcanically active mountains. Still, it isn’t quite as well known as the earlier Yixian formation, and in fact doesn’t seem to have as many plants preserved to inform the exact environment and temperature. Still, it’s reasonable to suppose it may have also been a temperate ecosystem, like the earlier Yixian Formation, potentially even with snow.

By Jack Wood
In this environment, there were an extremely wide variety of animals. There was a decent diversity of fish, quite a few kinds of mammals, and the weird, unclassifiable Choristoderes were represented by Philydrosaurus, Ikechosaurus, and Liaoxisaurus. This ecosystem was lousy with pterosaurs, featuring a variety of Chaoynagopterids - like Chaoyangopterus itself, Eoazhdarcho, Jidapterus, and Shenzhoupterus; Pteranodonts like Guidraco, Ikrandraco, Liaoningopterus, Nurhachius, Liaoxipterus, and Linlongopterus; Tapejarids like “Huaxiapterus”, (probably) Nemicolopterus, and Sinopterus; and the weirdly late-surviving Anurognathid Vesperopterylus.
As for dinosaurs, there were many, and most were bird like! There was of course the Ankylosaur Chuanqilong, and the early Ceratopsian Psittacosaurus; there was also an unnamed titanosaur. There was a Tyrannosauroid, SInotyrannus, the Chickenparrot Similicaudipteryx, the raptor Microraptor, and tons of early Avialans like Confuciusornis, Dalianraptor, Jeholornis, Omnivoropteryx, Sapeornis, Shenshiornis, and Zhongjianornis. There were also “true” birds (ie, the line of dinosaurs that would evolve into those we see today) such as Bellulornis, Piscivoravis, Archaeorhynchus, Chaoyangia, Jianchangornis, Parahongshanornis, Schizooura, Songlingornis, Yanornis, and Yixianornis. However, the most diverse group of dinosaurs were the Opposite Birds, of which Chiappeavis was only one of many. There was Alethoalaornis, Boluochia, Bohaiornis, Cathayornis, Cuspirostrisornis, Dapingfangornis, Eocathayornis, Piscivorenantiornis, Pengornis, Gracilornis, Huoshanornis, Largirostrornis, Longchengornis, Longipteryx, Rapaxavis, Shangyang, Sinornis, and Xiangornis - just to name a few! As such the Jiufotange remains as a rich ecosystem in which to study the evolution of this fantastic group of Cretaceous dinosaurs.

By Scott Reid
Other: Chiappeavis is probably not its own thing - it is one of a number of Opposite Birds described without substantial evidence that it was a distinct genus and, indeed, many researchers consider them to be members of other genera. In this case, Chiappeavis is probably the same as Pengornis. Still, until it is officially lumped in, it must be treated as its own genus. It had a lot of similarities to Pengornis, regardless, indicating the two may belong to a larger clade of Opposite Birds. In short, Opposite Bird Phylogeny is kind of a mess, and needs a lot more intensive work than has currently been done.
~ By Meig Dickson
Sources Under the Cut
Czerkas, S. A., D. Zhang, J. Li and Y. Li. 2002. Flying dromaeosaurs. In S. J. Czerkas (ed.), Feathered Dinosaurs and the Origin of Flight. The Dinosaur Museum Journal 1. The Dinosaur Museum, Blanding, UT 96-126
Czerkas, S. A., and Q. Ji. 2002. A preliminary report on an omnivorous volant bird from northeast China. In S. J. Czerkas (ed.), Feathered Dinosaurs and the Origin of Flight. The Dinosaur Museum Journal 1. The Dinosaur Museum, Blanding, UT 127-135
Dong, Z.-M., Y.-W. Sun, and S.-Y. Wu. 2003. On a new pterosaur from the Lower Cretaceous of Chaoyang Basin, Western Liaoning, China. Global Geology 22:1-7
Dong, Z., and J. Lü. 2005. A new ctenochasmatid pterosaur from the Early Cretaceous of Liaoning Province. Acta Geologica Sinica 79(2):164-167
Dong, L., Y. Wang, and S. E. Evans. 2017. A new lizard (Reptilia: Squamata) from the Lower Cretaceous Yixian Formation of China, with a taxonomic revision of Yabeinosaurus. Cretaceous Research
Evans, S. E., and Y. Wang. 2011. A gravid lizard from the Cretaceous of China and the early history of squamate viviparity. Naturwissenschaften 98:739-743
Evans, S. E., and Y. Wang. 2012. New material of the Early Cretaceous lizard Yabeinosaurus from China. Cretaceous Research 34:48-60
Gao, K.-Q., and R. C. Fox. 2005. A new choristodere (Reptilia: Diapsida) from the Lower Cretaceous of western Liaoning Province, CHina, and phylogenetic relationships of Monjurosuchidae. Zoological Journal of the Linnean Society of London 145:427-444
Gao, K.-Q., D. T. Ksepka, L. Hou, Y. Duan, and D. Hu. 2007. Cranial morphology of an Early Cretaceous monjurosuchid (Reptilia: Diapsida) from Liaoning Province of China and evolution of the choristoderan palate. Historical Biology 19(3):215-224
Han, G., and J. Meng. 2016. A new spalacolestine mammal from the Early Cretaceous Jehol Biota and implications for the morphology, phylogeny, and palaeobiology of Laurasian ‘symmetrodontans’. Zoological Journal of the Linnean Society 178:343-380
He, H.Y.; Wang, X.L.; Zhou, Z.H.; Wang, F.; Boven, A.; Shi, G.H.; Zhu, R.X. (2004). "Timing of the Jiufotang Formation (Jehol Group) in Liaoning, northeastern China, and its implications". Geophysical Research Letters. 31 (13): 1709.
Hou, L., and J. Zhang. 1993. [A new fossil bird from Lower Cretaceous of Liaoning]. Vertebrata PalAsiatica 31(3):217-224
Hou, L. H. 1997. Mesozoic Birds of China.
Hu, D., L. Li, L. Hou and X. Xing. 2010. A new sapeornithid bird from China and its implication for early avian evolution. Acta Geologica Sinica 84(3):472-482
Hu, L. Li, L. Hou and D., X. Xu. 2011. A new enantiornithine bird from the Lower Cretaceous of western Liaoning, China. Journal of Vertebrate Paleontology 31(1):154-161.
Hu, D.-Y., X. Xu, L. Hou and C. Sullivan. 2012. A new enantiornithine bird from the Lower Cretaceous of Western Liaoning, China, and its implications for early avian evolution. Journal of Vertebrate Paleontology 32(3):639-645
Hu, Zhou and O'Connor, 2014. A subadult specimen of Pengornis and character evolution in Enantiornithes. Vertebrata PalAsiatica. 52(1), 77-97.
Hu, D., Y. Liu, J. Li, X. Xu, and L. Hou. 2015. Yuanjiawaornis viriosus, gen. et sp. nov., a large enantiornithine bird from the Lower Cretaceous of western Liaoning, China. Cretaceous Research 55:210-219
Hu, O'Connor and Zhou, 2015. A new species of Pengornithidae (Aves: Enantiornithes) from the Lower Cretaceous of China suggests a specialized scansorial habitat previously unknown in early birds. PLoS ONE. 10(6), e0126791.
Ji, Q., S.-a. Ji, and L.-j. Zhang. 2009. First large tyrannosauroid theropod from the Early Cretaceous Jehol Biota in northeastern China. Geological Bulletin of China 28(10):1369-1374
Li, J.-J., J.-C. Lü, and B.-K. Zhang. 2003. A new Lower Cretaceous sinopteroid pterosaur from western Liaoning, China. Acta Palaeontologica Sinica 42(3):442-447
Li, L., Y. Duan, D. Hu, L. Wang, S. Cheng and L. Hou. 2006. New Eoenantiornithid Bird from the Early Cretaceous Jiufotang Formation of Western Liaoning, China. Acta Geologica Sinica (English Edition) 80(1):38-41
Li, Q., J. A. Clarke, K.-Q. Gao, C.-F. Zhou, Q. Meng, D. Li, L. D'Alba and M. D. Shawkey. 2014. Melanosome evolution indicates a key physiological shift within feathered dinosaurs. Nature 507:350-353.
Li, Z., Z. Zhou, M. Wang and J. A. Clarke. 2014. A new specimen of large-bodied basal enantiornithine Bohaiornis from the Early Cretaceous of China and the inference of feeding ecology in Mesozoic birds. Journal of Paleontology 88(1):99-108
Lu, J.-C., Q.-J. Meng, B.-P. Wang, DLiu, C.-Z. Shen and Y.-G. Zhang. 2017. Short note on a new anurognathid pterosaur with evidence of perching behaviour from Jianchang of Liaoning Province, China. In D. W. E. Hone, M. P. Witton, D. M. Martill (eds.), Geological Society of London Special Publications 455:95-104.
Mortimer, M. 2016. Chiappeavis is just another Pengornis. Theropod Database Blog.
O'Connor and Chiappe, 2011. A revision of enantiornithine (Aves: Ornithothoraces) skull morphology. Journal of Systematic Palaeontology. 9(1), 135-157.
O'Connor, J. K., Y. -G. Zhang, L. M. Chiappe, Q. Meng, Q. Li and D. Liu. 2013. A new enantiornithine from the Yixian Formation with the first recognized avian enamel specialization. Journal of Vertebrate Paleontology 33(1):1-12.
O'Connor, Zheng, Sullivan, Chuong, Wang, Li, Wang, Zhang and Zhou, 2015. Evolution and functional significance of derived sternal ossification patterns in ornithothoracine birds. Journal of Evolutionary Biology. 28(8), 1550-1567.
O’Connor, J. K., X. Wang, X. Zheng, H. Hu, X. Zhang, Z. Hou. 2016. An Enantiornithine with a Fan-Shaped Tail, and the Evolution of the Rectricial Complex in Early Birds. Current Biology 26: 114 - 119.
O’Connor, J. K., X.-T. Zheng, H. Hu. X. Wang, Z.-H. Zhou, 2017. The morphology of Chiappeavis magnapremaxillo (Pengornithida: enantiornithes) and a comparison of aerodynamic function in Early Cretaceous avian tail fans. Vertebrata Palasiatica 55: 41 - 58.
Wang, X., T. Rodrigues, S. Jiang, X. Cheng, and A. W. A. Kellner. 2014. An Early Cretaceous pterosaur with an unusual mandibular crest from China and a potential novel feeding strategy. Scientific Reports 4(6329).
Wang, M., Z.-H. Zhou, and S. Zhou. 2016. A new basal ornithuromorph bird (Aves: Ornithothoraces) from the Early Cretaceous of China with implication for morphology of early Ornithuromorpha. 176(1):207-223.
Zhou, S., Z. Zhou, J. K. O’Connor. 2013. A new piscivorous ornithuromorph from the Jehol Biota. Historical Biology 26 (5): 608 - 618.
Zhou, Z.-h., F. Jin, and J.-y. Zhang. 1992. [Preliminary report on a Mesozoic bird from Liaoning, China]. Kexue Tongbao 1992(5):435-437
Zhou, Z. 1995. Discovery of a new enantiornithine bird from the Early Cretaceous of Liaoning, China. Vertebrata PalAsiatica 33(2):99-113
Zhou, X., and F. Zhang. 2001. Two new ornithurine birds from the Early Cretaceous of western Liaoning, China. Chinese Science Bulletin 46(15):1258-1264
Zhou, Z. 2002. A new and primitive enantiornithine bird from the Early Cretaceous of China. Journal of Vertebrate Paleontology 22(1):49-57
Zhou, Z., and F. Zhang. 2002. A long-tailed, seed-eating bird from the Early Cretaceous of China. Nature 418:405-409
Zhou, Z., and F. Zhang. 2002. Largest bird from the Early Cretaceous and its implications for the earliest avian ecological diversification. Naturwissenschaften 89(1):34-38
Zhou, X., and F. Zhang. 2003. Anatomy of the primitive bird Sapeornis chaoyangensis from the Early Cretaceous of Liaoning, China. Canadian Journal of Earth Sciences 40:731-747
Zhou, Z., J. Clarke, F. Zhang and O. Wings. 2004. Gastroliths in Yanornis: an indication of the earliest radical diet-switching and gizzard plasticity in the lineage leading to living birds?. Naturwissenschaften 91(12):571-574
Zhou, Clarke and Zhang, 2008. Insight into diversity, body size and morphological evolution from the largest Early Cretaceous enantiornithine bird. Journal of Anatomy. 212, 565-577.
Zhou, Z.-H., F. -C. Zhang, and Z. Li. 2009. A new basal ornithurine bird (Jianchangornis microdonta gen. et sp. nov.) from the Lower Cretaceous of China. Vertebrata PalAsiatica 10:299-310
Zhou, Z., F. Zhang, and Z. Li. 2010. A new Lower Cretaceous bird from China and tooth reduction in early avian evolution. Proceedings of the Royal Society B 277:219-227
#Chiappeavis magnapremaxillo#Chiappeavis#Opposite Bird#Bird#Dinosaur#Enantiornithine#Birblr#Palaeoblr#Factfile#Dinosaurs#Birds#Cretaceous#Eurasia#Insectivore#Mesozoic Monday#paleontology#prehistory#prehistoric life#biology#a dinosaur a day#a-dinosaur-a-day#dinosaur of the day#dinosaur-of-the-day#science#nature
174 notes
·
View notes
Text
Pitohui

Hooded Pitohui by Berichard, CC BY-SA 2.0
Etymology: Inedible (Rubbish) Bird
First Described By: Lesson, 1831
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes, Neognathae, Neoaves, Inopinaves, Telluraves, Australaves, Eufalconimorphae, Psittacopasserae, Passeriformes, Eupasseres, Passeri, Euoscines, Corvides, Orioloidea, Oriolidae
Referred Species: P. kirhocephalus (Northern Variable Pitohui), P. cerviniventris (Raja Ampat Pitohui), P. uropygialis (Southern Variable Pitohui), P. dichrous (Hooded Pitohui)
Status: Extant, Least Concern
Time and Place: Since 10,000 years ago, in the Holocene of the Quaternary
Pitohuis are endemic to New Guinea
Physical Description: The Pitohuis look like your average Corvids - large passerines with dark coloration, thick bills, and high intelligence - but there’s more to these strange birds than meets the eye. Pitohuis are some of the rare examples of poisonous birds! Pitohuis tend to range in size between 20 and 26 centimeters long, and they are mostly black all over with brown to bright orange patches on their bellies or backs - though there is, of course, one species that is primarily brown. They have large, thick black bills, short wings, and short tails. The sexes tend to be very alike in these birds, though some species have subpopulations with different colorations. The juveniles, in general, are more brown in regions that are black in the adults. These birds are extremely toxic, with a neurotoxin called homobatrachotoxin in their skin, feathers, and other tissues. The least toxic parts of these birds are their bones. The toxins are secreted into their feathers on purpose; though some is left behind in muscle and organ tissue, which indicates a lack of sensitivity to the toxins by the birds. Some individuals do not have the toxins, probably due to a lack of the toxin-making beetles in their diets; but this is a variable occurrence and the vast majority of Pitohuis have the toxins in their feathers and skin.

Southern Variable Pitohui by Markus Lilje
Diet: Pitohuis primarily feed on insects, though at least a few species also supplement their diets with fruit and seeds. Most importantly, they feed on beetles of the genus Choresine, which contain the toxin they incorporate into their feathers.

Raja Ampat Pitohui, by Carlos N. G. Bocos
Behavior: Pitohuis tend to forage at most levels of the forest, oftentimes in mixed-species flocks, though they are the ones that feed more on poisonous beetles than others in these flocks. They will hide in dense vegetation in order to avoid letting their prey know they’re there, but since they don’t have a lot of predators they don’t have to worry much about them. They do not migrate, but they are extremely vocal and social - making a variety of whistling and calls, which vary in word from species to species. Some are more leisurely than others, and they go between a variety of high and low pitches. Usually, they are quite musical and loud. Pitohuis begin breeding in early spring, and start nesting in the summer - though Hooded Pitohuis tend to lay more in the autumn. Multiple mated pairs probably work together to take care of the nest, which is a cup of vines and tendrils suspended on slender branches a few meters up from the ground. They typically lay one to two eggs a season.

Hooded Pitohui by Markus Lilje
Ecosystem: Pitohuis live mainly in tropical rainforest and mangrove forests, as well as mountainous forests. They live at a variety of elevations, up to 2000 meters high; many species can also be found at forest edges and swamp forests, as well as lowlands. They do overlap with many other Pitohuis - including each other - and share the poisonous beetles among one another. Potential predators, including a variety of snakes, show marked sensitivity and irritation to even small amounts of the toxins found in the feathers of these birds - and as such, predators of the Pitohuis are not known at this time, at least of the adults. The young, who might not have as much of these toxins in their feathers and skin, are still vulnerable to nest raiders. Humans also do not feed on Pitohuis - leading to their name!

Northern Variable Pitohui by Paul van Giersbergen
Other: Toxicity in birds is an extremely rare trait, but it seems to have evolved multiple times in birds in New Guinea. In fact, Pitohui is a general name often used for any such poisonous bird, but recent scientific studies has showcased they’re not actually closely related at all! So, many species of Pitohui actually belong to other genera in not closely related groups of songbirds. While the ones we discussed today are Orioles, some are Bellbirds and others are Whistlers/Shrikethrushes. They are all, however, in the general Corvides group. The reasons as to why toxicity evolves in birds is debated - it may be a defensive measure (and is used as such), but it also might just be a consequence of their diet. They had to do something with the toxins from the beetles - so why not shed it out into their feathers? And then, BONUS! They aren’t eaten by a lot of predators! Regardless of how this odd trait began, they aren’t hunted or eaten very much as a result! Though having a limited range, the toxicity of these birds means that they are rarely fed upon by predators - including humans - and as such, they are common in their ranges.

Hooded Pitohui by Frédéric Pelsy
Species Differences: The Pitohuis mainly differ based on their coloration from species to species, though there are some range differences (the Hooded and Northern Pitohuis live on the northern half of New Guinea, the Southern Pitohui on the Southern Half, and the Raja Ampat Pitohui only on the island of Waigeo. Hooded Pitohuis have black heads and wings, with black tails; the rest of their bodies are utterly black orange. Raja Ampat Pitohuis are brown almost everywhere, except for having a lighter orange on their bellies and black tips to their wings and tails. Northern Variable Pitohuis can be one of three color schemes: grey heads with brown backs black tails and wings and bright orange bellies; black heads with dark orange backs black wings black tails and lighter orange bellies; or dark orange on top all over and a lighter orange on the belly. Southern Variable Pitohuis can be black on their heads wings and tails with a dark red-orange on their backs and bellies; or black on their heads and tails and wings with dark orange on top and lighter orange on bottom in only the males, while the females have more brown heads. So much variation!
~ By Meig Dickson
Sources Under the Cut
Beadell, J.; Gering, E.; Austin, J.; Dumbacher, J.; Peirce, M.; Pratt, T.; Atkinson, C.; Fleischer, R. (2004). "Prevalence and differential host-specificity of two avian blood parasite genera in the Australo-Papuan region". Molecular Ecology. 13 (12): 3829–3844.
Boles, W. (2019). Hooded Pitohui (Pitohui dichrous). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Boles, W. & Kirwan, G.M. (2019). Northern Variable Pitohui (Pitohui kirhocephalus). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
del Hoyo, J., Collar, N., Kirwan, G.M. & Boesman, P. (2019). Southern Variable Pitohui (Pitohui uropygialis). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
del Hoyo, J., Collar, N., Kirwan, G.M. & Boesman, P. (2019). Waigeo Pitohui (Pitohui cerviniventris). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Diamond, J. (1987). "Flocks of brown and black New Guinean bird: a bicolored mixed-species foraging association". Emu. 87 (4): 201–211.
Dumbacher, J.; Beehler, B.; Spande, T.; Garraffo, H.; Daly, J. (1992). "Homobatrachotoxin in the genus Pitohui: chemical defense in birds?". Science. 258 (5083): 799–801.
Dumbacher, John P. (1999). "Evolution of toxicity in Pitohuis: I. Effects of homobatrachotoxin on chewing lice (Order Phthiraptera)". The Auk. 116 (4): 957–963.
Dumbacher, J. P.; Spande, T. F.; Daly, J. W. (2000). "Batrachotoxin alkaloids from passerine birds: A second toxic bird genus (Ifrita kowaldi) from New Guinea". Proceedings of the National Academy of Sciences. 97 (24): 12970–12975.
Dumbacher, J. P.; Fleischer, R. C. (2001). "Phylogenetic evidence for colour pattern convergence in toxic pitohuis: Mullerian mimicry in birds?". Proceedings of the Royal Society B: Biological Sciences. 268 (1480): 1971–1976.
Dumbacher, J. P.; Wako, A.; Derrickson, S. R.; Samuelson, A.; Spande, T. F.; Daly, J. W. (2004). "Melyrid beetles (Choresine): A putative source for the batrachotoxin alkaloids found in poison-dart frogs and toxic passerine birds". Proceedings of the National Academy of Sciences. 101 (45): 15857–15860.
Dumbacher, J; Deiner, K; Thompson, L; Fleischer, R (2008). "Phylogeny of the avian genus Pitohui and the evolution of toxicity in birds". Molecular Phylogenetics and Evolution. 49 (3): 774–781.
Dumbacher, John P.; Menon, Gopinathan K.; Daly, John W. (2009). "Skin as a toxin storage organ in the endemic New Guinean genus Pitohui". The Auk. 126 (3): 520–530.
Glendinning, J. (1993). "Pitohui: how toxic and to whom?". Science. 259 (5095): 582–583.
Jønsson, K. A; Bowie, R. C.K; Norman, J. A; Christidis, L.; Fjeldsa, J. (2008). "Polyphyletic origin of toxic Pitohui birds suggests widespread occurrence of toxicity in corvoid birds". Biology Letters. 4 (1): 71–74.
Jønsson, Knud A.; Bowie, Rauri C. K.; Moyle, Robert G.; Irestedt, Martin; Christidis, Les; Norman, Janette A.; Fjeldså, Jon (2010). "Phylogeny and biogeography of Oriolidae (Aves: Passeriformes)". Ecography. 33 (2): 232–241.
Lamothe, L. (1979). "Diet of some birds in Araucaria and Pinus forests in Papua New Guinea". Emu. 79 (1): 36–37.
Legge, S.; Heinsohn, R. (1996). "Cooperative breeding in Hooded Pitohuis (Pitohui dichrous)". Emu. 96 (2): 139–140.
Ligabue-Braun, Rodrigo; Carlini, Célia Regina (2015). "Poisonous birds: A timely review". Toxicon. 99: 102–108.
Menon, Gopinathan K.; Dumbacher, John P. (2014). "A "toxin mantle" as defensive barrier in a tropical bird: evolutionary exploitation of the basic permeability barrier forming organelles". Experimental Dermatology. 23 (4): 288–290.
Mouritsen, Kim N.; Madsen, Jørn (1994). "Toxic birds: defence against parasites?". Oikos. 69 (2): 357.
Poulsen, B. O. (1994). "Poison in birds: against predators or ectoparasites?". Emu. 94 (2): 128–129.
Sam, Katerina; Koane, Bonny; Jeppy, Samuel; Sykorova, Jana; Novotny, Vojtech (2017). "Diet of land birds along an elevational gradient in Papua New Guinea". Scientific Reports. 7 (44018): 44018.
#Pitohui#bird#Dinosaur#Songbird#Poisonous Bird#Passeriform#Perching Bird#Songbird Saturday & Sunday#Quaternary#Australia & Oceania#Insectivore#Northern Variable Pitohui#Raja Ampat Pitohui#Southern Variable Pitohui#Hooded Pitohui#Pitohui kirhocephalus#Pitohui cerviniventris#Pitohui uropygialis#Pitohuis dichrous#Birds#Dinosaurs#Factfile#Birblr#Waigeo Pitohui#biology#a dinosaur a day#a-dinosaur-a-day#dinosaur of the day#dinosaur-of-the-day#science
161 notes
·
View notes
Text
Pica

Black-Billed Magpie by USFWS, in the Public Domain
Etymology: Magpie
First Described By: Brisson, 1760
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes, Neognathae, Neoaves, Inopinaves, Telluraves, Australaves, Eufalconimorphae, Psittacopasserae, Passeriformes, Eupasseres, Passeri, Euoscines, Corvides, Corvoidea, Corvidae, Corvinae
Referred Species: P. mourerae, P. pica (Eurasian Magpie), P. serica (Oriental Magpie), P. bottanensis (Black-Rumped Magpie), P. asirensis (Asir Magpie), P. mauritanica (Maghreb Magpie), P. nuttalli (Yellow-Billed Magpie), P. hudsonia (Black-Billed Magpie)
Status: Extinct - Extant, Endangered - Least Concern
Time and Place: From 3.6 million years ago until today, from the Piacenzian of the Pliocene through the Holocene
Magpies are known from all around the Northern Hemisphere
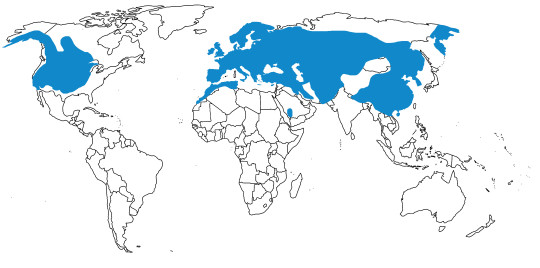
Physical Description: Magpies are beautiful, if fairly recognizable Corvids, famed from all over the Northern Hemisphere for their cleverness and beautiful plumage. They can range in size from 43 to 60 centimeters long, with the Yellow-Billed Magpie reaching the smallest sizes and the Black-Billed reaching the largest. This makes them rather large as far as songbirds are concerned, though they are still significantly smaller than the Ravens and Crows that they’re close cousins to. Magpies tend to have black backs, heads, and necks, with varying levels of black on their bodies; they then have white bellies and white tops to their wings. The rest of their wings, and tails, can be black - or an iridescent mixture of colors on a black background. These colors vary from species to species, but can be blue, green, and purple-ish tinted - at least one species can even blend into the yellow-brown range. They have thick, strongly clawed toes; and they have very large, thick beaks, like other Corvids. They have short to medium sized wings as well, indicating their adapted ability for flying between and among thick trees.

Oriental Magpie by Yoo Chung, CC BY-SA 2.5
Diet: Magpies are creative, opportunistic omnivores - they literally will eat anything. Insects, small vertebrates, eggs, carrion, leaves, fruit, seeds, your leftover pizza, that hamburger it found on the street, falafel, rice, a heaping load of spaghetti - literally, anything. It will eat anything. Hide your food.
Behavior: Magpies are clever little buggers, with complex behaviors and extensive social communication. They tend to feed on the ground, usually with other Magpies, and even in mixed-species flocks depending on the abundance of food. They’ll Pick food up from the ground and dig into soil and litter, flipping over all sorts of things to look for food - including trash and poop. They’ll also hunt live food from perches in trees, or make traps to catch flies and other insects. Some will also stick around with predators and larger scavengers, looking for roadkill and other sources of meat that could be easily gotten from. They often will also store the food, in crevices and trees and the like, though usually they don’t leave the food for long and go to pick it up in a few days. The walk around and strut, usually fearlessly, attempting to catch whatever food they can; thousands of them can often be found foraging together, and using their unique cleverness to track down food.

Black-Billed Magpie by the USFWS, in the Public Domain
Magpies are some of the smartest known animals - with large brain to body mass ratios, similar to those of cetaceans and primates; the region of their brains that works on cognitive tasks and higher thought processes is of a similar size to those found in chimpanzees and even close to those of people. As such, Magpies - much like their cousins the ravens - are some of the smartest animals alive today, probably holding second place after humans. They have high levels of social cognition, reasoning, flexibility, imagination, and ability to evaluate and predict the future. They also have very elaborate social rituals - they are able to recognize themselves, even in the mirror; and they show grief and rituals around the deaths of family members and friends. They also use tools, and use their experiences to predict the behavior of others. This knowledge of tool use is passed on from generation to generation, and modified as well - so they also have culture and rituals. They can count, imitate people, recognize words, and use tools to clean their own cages. They tend to form gangs in the wild, and use complex strategies in order to gather food and stick together. They also will ant - ie, apply ants to their plumage in order to prevent parasites and irritation - and sun-bathe to stay warm.

Maghreb Magpie by Charles J. Sharp, CC BY-SA 4.0
These birds roost communally and fly quite slowly over their ranges, usually interspersing their flaps with gliding to conserve energy. They make calls to flock members with regular frequency, mainly chattering and squeaking calls, as well as warbling songs and mimicry. Some species tend to have larger vocabularies than others, though they all make similar calls. Begging from the young sounds about as high pitched and peeping as one would expect. They have dominance hierarchies within the flocks formed during the non-breeding season, and dominant individuals in the flocks can and do steal food from the subordinate individuals. Interestingly enough, the younger males tend to dominate the older ones - though that may be more of a tolerance thing than anything else. These birds don’t tend to migrate, though they do move from place to place in response to climate and food availability.

Black-Rumped Magpie
Magpies are monogamous, possibly staying with the same partner for their entire lives, and multiple sets of parents will work together to tend for the nests and care for the young. They start laying eggs in the winter, though usually most eggs are laid in the early spring. The parents will build the nests together, with the male bringing materials and the female doing the building. This usually takes a couple of weeks, and at the end the pair have a large domed structure made of sticks and twigs - a very deep cup, lined with soft wool, fur, grasses, and feathers. A fresh nest is built every year, even though the pairs stay together; they’re usually built in trees, usually near to the top, though buildings are sometimes used. Old nests are sometimes reused, if they were particularly good and lasted that whole time. They lay between two and nine eggs, though of course four to six is the most common number; and they are incubated mostly by the female for about two weeks. Both sexes will then feed the chicks, while the female does most of the watching and caring for them at the nest - leaving the male in charge of gathering the food and helping to fend off predators. Other pairs may come to help - usually relatives or friends. The young will leave the nest after a month to two months, and young in the nests may come together with other young - especially if there was a communal brooding situation - to form a creche of juveniles their own age. Honestly, it’s almost like a school class in some ways, with how they behave with each other and socialize and learn from the adults. They reach sexual maturity in about one to two years, and live for six years in the wild.

Juvenile Eurasian Magpie by Cyberolm, CC BY-SA 4.0
Speaking of friends - yes, magpies have been known to befriend humans, or at least have interactions with them. They are tame and friendly in areas where they’re left undisturbed, and in areas where they are shot at or bothered, they are defensive against humans coming into their areas. This can vary wildly, as they used to be considered viable game birds, but today they aren’t hunted as much and tend to be a little less on guard. They defend their nests violently against humans, and do not abandon them except as a last resort. Parents will mob people looking in on the nests - even scientists - especially if they are repeat offenders. But, since they recognize people’s faces, they are able to tell friend from foe (or perceived foe) and will seek out humans who give them treats or protection.

Asir Magpie by Mansur Al Fahad
Ecosystem: Magpies stick to woodland and forests, though they do venture into more suburban and developed areas depending on the availability of trees. They will also inhabit open country, so long as there are some scattered trees available. Magpies can be found in all sorts of elevations, including in the mountains and as high as 4400 meters up into them. Some species, such as the Yellow-Billed, can tolerate warmer temperatures than others. Those that encroach on human habitat are often considered pests. While they do have predators, most are killed by West-Nile virus, to which they are particularly susceptible.

Eurasian Magpie by Andreas Eichler, CC BY-SA 4.0
Other: Magpies are Corvids, so they’re cousins with all the other ridiculously smart passerine birds, making the question of “are members of the genus Corvus or the genus Pica smarter” rather one of splitting hairs. In the end, both genera showcase extreme intelligence, and are either the second smartest animals or close to it. Honestly, if they were smarter than humans I wouldn’t be surprised - they show culture and the ability to pass down learned things from generation to generation, and they might just be smart enough to not fucking destroy the planet (unlike us). Anyways, Magpies are often thought of as pests for their ability to get at sources of human food and also steal the eggs of birds with more pretty songs, so birders actually hate them. However, they don’t actually have a negative impact on the song-bird population. Most Magpies aren’t threatened with extinction and have populations in the thousands, though the Yellow-Billed species is vulnerable due to poisons used to take care of squirrels, and the Asir species is endangered due to a restricted range and decreasing suitable habitat.

Yellow-Billed Magpie by Bill Bouton, CC BY-SA 2.0
Species Differences: The different species of Magpie differ primarily on coloration and range, as their sizes tend to be very similar. The Eurasian Magpie is often synonymized with the Oriental and Black-Rumped Magpies; whether or not these are three different species of bird is a bit of a taxonomical question. These birds range all over Eurasia - as the name suggests - with the “Oriental” subspecies (species?) being found more in Eastern Asia, and the Black-Rumped subspecies (species?) also found in Eastern Asia. At least a few varieties have a purple tail and dark blue wings; while others are more green on both the wing and tail, with only the tail tip coming out as purple. Asir Magpies are the only Magpies known from Saudi Arabia; they have dark blue wings and brownish tails, which end in a purple tip. The Maghreb Magpie is known from Northwestern Africa, and has a complete rainbow-colored tail and blue wings. The Yellow-Billed Magpie is especially distinct from the rest in having a yellow bill and yellow patches around the eyes (while the other species have black bills and black patches); they have blue wings and blueish-purple tails, and are found in California. Finally, the Black-Billed Magpie is found in the rest of North America (though not the eastern portion of the continent, or the southern), and has blue wings and a rainbow tail. There is one extinct species, P. mourerae, from the Pliocene of Spain; it seems to be very similar to living species except for that it wasn't as good of a flier - and it may have even be flightless, due to the fact that it lived on an island!
~ By Meig Dickson
Sources Under the Cut
Bekoff, M. (2009). "Animal emotions, wild justice and why they matter: Grieving magpies, a pissy baboon, and empathic elephants". Emotion, Space and Society. 2 (2): 1–4.
Clements, J. F., T. S. Schulenberg, M. J. Iliff, D. Roberson, T. A. Fredericks, B. L. Sullivan, and C. L. Wood. 2017. The eBird/Clements checklist of birds of the world: v2017
del Hoyo, J., Collar, N. & Christie, D.A. (2019). Maghreb Magpie (Pica mauritanica). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Emery, Nathan J.; Clayton, Nicola S. (Dec 2004). "The mentality of crows: convergent evolution of intelligence in corvids and apes". Science. 306 (5703): 1903–1907.
Madge, S. & Kirwan, G.M. (2019). Asir Magpie (Pica asirensis). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Madge, S., Christie, D.A. & Kirwan, G.M. (2019). Eurasian Magpie (Pica pica). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Marzluff, J. & de Juana, E. (2019). Black-billed Magpie (Pica hudsonia). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Marzluff, J. & Sharpe, C.J. (2019). Yellow-billed Magpie (Pica nutalli). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Mourer-Chauvire, C., J. A. Alcover, S. Moya and J. Pons. 1980. Une nouvelle forme insulaire d'effraie geante, Tyto balearica n. sp. (Aves, Strigiformes), du Plio-Pleistocene des Baleares. Geobios 13(5):803-811.
Parmalee, P. W. 1992. A late Pleistocene avifauna from northwestern Alabama. Science Series, Los Angeles Museum of Natural History, Science, and Art 36:307-318.
Segui, B. 2001. A new species of Pica (Aves: Corvidae) from the Plio-Pleistocene of Mallorca, Balearic Islands (Western Mediterranean). Geobios 34(3):339-347.
Wetmore, A. 1962. Notes on fossil and subfossil birds. Smithsonian Miscellaneous Collections 145(2):1-17.
#Pica#Magpie#Corvid#Passeriform#Dinosaur#Songbird#Perching Bird#Dinosaurs#Birds#Bird#Facfile#Songbird Saturday & Sunday#Birblr#Palaeoblr#Factfile#Omnivore#Neogene#Quaternary#North America#Eurasia#Africa#Prehistoric Life#Paleontology#Prehistory#Pica mourerae#Eurasian Magpie#Asir Magpie#Maghreb Magpie#Yellow-Billed Magpie#Black-Billed Magpie
166 notes
·
View notes
Text
Upupa

Eurasian Hoopoe by Jaiprakashsingh, CC BY-SA 3.0
Etymology: Hoopoe
First Described By: Linnaeus, 1758
Classification: Dinosauromorpha, Dinosauriformes, Dracohors, Dinosauria, Saurischia, Eusaurischia, Theropoda, Neotheropoda, Averostra, Tetanurae, Orionides, Avetheropoda, Coelurosauria, Tyrannoraptora, Maniraptoromorpha, Maniraptoriformes, Maniraptora, Pennaraptora, Paraves, Eumaniraptora, Averaptora, Avialae, Euavialae, Avebrevicauda, Pygostaylia, Ornithothoraces, Euornithes, Ornithuromorpha, Ornithurae, Neornithes, Neognathae, Neoaves, Inopinaves, Telluraves, Afroaves, Coraciimorphae, Cavitaves, Eucavitaves, Picocoraciae, Bucerotiformes, Phoeniculidae, Upupidae
Referred Species: U. africana (African Hoopoe), U. antaios (Saint Helena Hoopoe), U. epops (Eurasian Hoopoe), U. marginata (Madagascan Hoopoe)
Status: Extinct - Extant, Least Concern
Time and Place: Between 12,000 years ago and today, in the Holocene of the Quaternary

Hoopoes are known from all over the Eastern Hemisphere
Physical Description: Hoopoes are extremely distinctive birds! They have very long, thin, and curved bills that extend out greatly from their heads, and huge crests on their heads that are easily spotted. They have long, thin bodies, and feet built for perching. THeir wings are very square-ish, and they have shorter tails than other birds. However, their coloration is decidedly where they are most distinctive of all. They have bright orange heads, with orange crests - but the crests end in very slight white bandings and then black tips. Their bodies are orange, but their wings and rumps and tails are black and white striped all over! They are such beautiful, distinctive birds. The shades of orange can differ in brightness or redness based on species (for example, the African Hoopoe tends to be redder than the Eurasian Hoopoe), but they do tend to be overall similar to one another in appearance. Living species range between 19 and 32 centimeters long; the extinct Saint Helena Hoopoe, though it had smaller wings, probably could have reached 36 centimeters long.

Madagascan Hoopoe by Charles J. Sharp, CC BY-SA 4.0
Diet: Hoopoes primarily feed on insects, especially larvae, though some larger animals are also fed upon by these animals.

Common Hoopoe by Charles J. Sharp, CC BY-SA 4.0
Behavior: Hoopoes are very curious, adventurous birds, spending a lot of their time foraginging on the ground - they’ll dig with their bills into soft earth, using them to turn over leaves and probing into the mud and dung for insects and other invertebrates. They’ll even use their bills to prise off the bark from trees, or forage for insects in lichen! Sometimes, these birds also smash their food against the ground to They’ll usually forage in pairs or alone, spending a lot of their days looking for food. Some Hoopoes - especially the Madagascan Hoopoe - will forage in even slightly larger groups, of up to six individuals. Fascinatingly, Hoopoes have their own version of Penicillin - Anting! They’ll find piles of ants and roll around in them, allowing the ants to cover their feathers. The ants then secret substances that will kill bacteria, fungi, and other insects - protecting the Hoopoe (and other birds that Ant) from illness! These birds also take dust and sand baths to clean themselves; they’ll also sunbathe by spreading out their wings and tail low to the ground and tilting their heads up!

Madagascan Hoopoe by Charles J. Sharp, CC By-SA 4.0
Hoopoes are distinctive in one very special way that lead to its name - their voice! They literally make calls that sounds like “hoo-poo-poo” and “hoop-oop hoop-oop” - leading to the name, Hoopoe, as well as the genus name, Upupa, and the species name of the Eurasian species, epops. Interestingly enough, the Madagascan Hoopoe does not make this sound - but rather, more cooing sounds, like doves. These birds will also make harsh, scolding calls, trills, and hisses, depending on the situation. The females and males will communicate primarily in trilling sounds while watching out for their nests. These birds are often sedentary, not migrating over long distance, but northern populations usually do come south in the winter to avoid colder climates, creating a variety of populations with very distinctive seasons and migrational patterns from one another within the species.

Saint Helena Hoopoe by Apokryltaros, CC BY 2.5
Hoopoes are monogamous each breeding season (which varies throughout the year as Hoopoes live all over the Eastern Hemisphere), forming strong pair bonds (that only last for that period of time). Males make very frequent calls to establish their territories, and they often fight with each other very brutally - including stabbings that can leave their opponents blinded. Females will then mate with the winners of these contests, and together they make nests out of holes in trees and walls with very narrow entrances. They usually aren’t lined with much. The female then incubates the egg, while the male defends her and the nest. Clutch size tends to depend on location, varying between 4 and 12 eggs per nest. They are incubated for nearly three weeks. At hatching, the chicks are very white and fluffy after a few days, and the crest develops after two weeks. The chicks are able to leave the nest after about a month, though they still stick with their families for a little while. Sometimes, when males defeat each other and replace each other in the mated pair, they will kill the offspring of the replaced male. Females can produce foul-smelling liquid, as do the babies, to protect themselves from predators - since they smell like rotting meat, they can fend off meat-eaters and parasites, and potentially fend off bacteria. Chicks in the nests also are able to literally poop at intruders, helping them to protect themselves! After leaving the nest, they stay with the parents for another week as they gain their bearings; they then become sexually mature between ages one and two.

Eurasian Hoopoe by Frank Vassen, CC By 2.0
Ecosystem: Hoopoes live mainly in open country - pastures, orchards, steppe, dry savanna, wooded savanna, short grassland, and bare ground. They congregate near scattered, isolated trees for their roosting and nesting. They do need perches and shade, but they want the trees they get these services from to be rare in the environments - so they can go down to the ground to get their food! They are fed upon by herons, falcons, and many other birds of prey.

African Hoopoe by Derek Keats, CC BY 2.0
Other: Most hoopoes are not currently threatened with extinction - they are extremely common, widespread birds, that are even protected in many localities (being highly venerated in many cultures - it’s even mentioned extensively in the Quaran - and made the national bird of Israel; it is also considered a pest controller and thus is protected on that front also. Some local populations, such as those in Morocco, are more threatened due to local practices (such as selling them for medicine), but overall they seem to be doing well. In fact, there are probably as many as 10 million Hoopoe around today, if not more. Still, in more northern countries such as Germany they are more endangered, primarily due to changes in habitat, hunting, and human activity giving pressure to the populations. The numbers in Madagascar are slightly vulnerable too, given forest clearance. Hoopoes are closely related to the Hornbills!

Saint Helena Hoopoe by Scott Reid
Species Differences: The four species primarily differ based on location: The African Hoopoe is found in Africa; the Eurasian Hoopoe is found in Eurasia; the Madagascan Hoopoe is known from Madgascar: and the late Saint Helena Hoopoe - now extinct - was known from the island of Saint Helena off the coast of Africa! The Saint Helena Hoopoe differed from the other species in other ways, too - it had smaller wings, was somewhat larger, and was probably flightless! A giant flightless Hoopoe! And, like most large flightless birds of the recent past, it went extinct due to human activity on the island - this time, sometime in the 1500s.
~ By Meig Dickson
Sources Under the Cut
Ashmole, N. P. 1963. The extinct avifauna of St. Helena Island. Ibis 103b:390-408
Burney, D. A., N. Vasey, L. R. Godfrey, Ramilisonina, W. L. Jungers, M. Ramarolahy, and L. Raharivony. 2008. New findings at Andrahomana Cave, southeastern Madagascar. Journal of Cave and Karst Studies 70(1):13-24
Carroll, R. L. 1988. Vertebrate Paleontology and Evolution 1-698
Clements, J. F., T. S. Schulenberg, M. J. Iliff, D. Roberson, T. A. Fredericks, B. L. Sullivan, and C. L. Wood. 2017. The eBird/Clements checklist of birds of the world: v2017
del Hoyo, J., Collar, N. & Kirwan, G.M. (2019). Madagascar Hoopoe (Upupa marginata). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Goodman, S. M., M. J. Raherilalao, and K. Muldoon. 2013. Bird fossils from Ankilitelo Cave: inference about Holocene environmental changes in southwestern Madagascar. Zootaxa 3750:534-548
Kri?tín, A. & Kirwan, G.M. (2019). Common Hoopoe (Upupa epops). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona.
Linnaeus, C. 1758. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Editio Decima 1:1-824
Olson, S. L. 1975. Paleornithology of St. Helena Island, South Atlantic Ocean. Smithsonian Contributions to Paleobiology 23:1-49
Sinclair, Ian; Ryan, Peter (2009). Complete Photographic Field Guide: Birds of Southern Africa. Struik Nature.
#Upupa#Hoopoe#Bird#Dinosaur#Upupa epops#Afroavian#Factfile#Insectivore#Flying Friday#Quaternary#Africa#India & Madagascar#Eurasia#Birds#Palaeoblr#Birblr#Dinosaurs#Upupa africana#Upupa antaios#Upupa marginata#Madagascan Hoopoe#Common Hoopoe#Eurasian Hoopoe#African Hoopoe#Saint Helena Hoopoe#paleontology#prehistory#prehistoric life#biology#a dinosaur a day
207 notes
·
View notes