#Antibiotic-resistant Microbes
Explore tagged Tumblr posts
Text
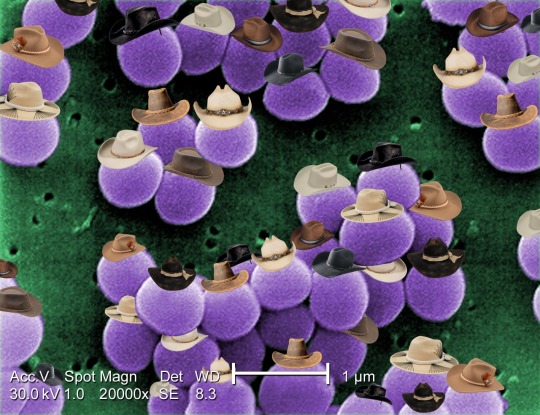
Staphylococcus aureus
Staphylococcus aureus is a type of bacteria with many known strains that have become resistant to antibiotics. Methicillin resistance (MRSA), vancomycin intermediate resistance (VISA), and vancomycin resistance (VRSA) are some examples of strains that are partially or fully resistant to antibiotics, making them major concerns in hospitals. It's estimated that only 2% of S. aureus strains are sensitive to penicillin antibiotics.
#staphylococcus#staph#staphylococcus aureus#cowboy#cowboy hat#microbiology#microbes#biology#hat#hats#microbes in hats#microorganisms#bacteria#protozoa#microscopy#antibiotics#antibiotic resistance#MRSA
135 notes
·
View notes
Text
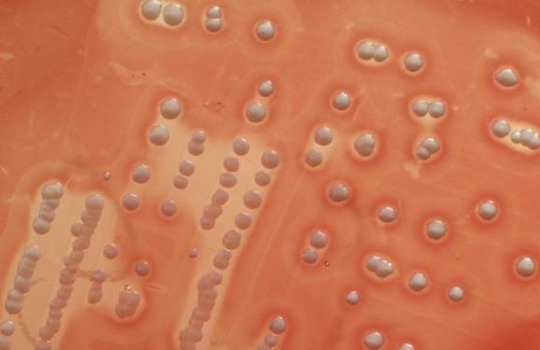
Vancomycin-resistant Staphylococcus aureus
“Staphylococcus aureus colonies on blood agar. Note the golden yellow pigment and beta hemolysis around it.” - via Wikimedia Commons
#staphylococcus aureus#vrsa#mrsa#wikipedia#wikipedia pictures#wikimedia commons#beta hemolysis#staph#staphylococcus#microbes#microbiology#vancomycin#antibiotics#antibiotic resistance#antimicrobial resistance#antimicrobial#blood agar#blood agar plate#microbio#medical microbiology#eskape pathogens#infectious diseases#medicine#bacteria#bacterial infections
29 notes
·
View notes
Text
https://www.canada.ca/en/public-health/services/publications/drugs-health-products/canadian-antimicrobial-resistance-surveillance-system-report-2021.html
watching a tiktok and someone just described the black/blue gold/yellow dress meme as “one of the first things to ever go viral”
#antibiotic resistant bacterial infections#death of humanity#TB is back#polio is back#gonorrhea is back#syphilis is back#chlamydia is back#E.coli#Salmonella#Streptococcus pneumoniae#Streptococcus pyogenes#medical research#microbes will reign supreme
115K notes
·
View notes
Text
"A new study evaluated a low-cost yet effective way to combat bacterial resistance using curcumin–the natural yellow plant compound in turmeric.
In 2017, a tragic death in a Nevada hospital was linked to a new strain of bacteria that had developed a resistance to 26 different antibiotics. Called ‘superbugs’, such antibiotic-resistant bacteria (including MRSA) remains a pressing public health threat.
Now researchers at Texas A&M University have shown that curcumin, the compound that gives turmeric its characteristic bright yellow color, can be used to reduce this antibiotic resistance.
They showed that when curcumin is intentionally given to bacteria as food, and then activated by light, it can trigger deleterious reactions within these microbes, eventually killing them. They demonstrated that this process reduces the number of antibiotic-resistant strains and renders conventional antibiotics effective again.
The results of the study were published this week in the journal Scientific Reports.
“We need alternative ways to either kill the superbugs or find a novel way to modify natural processes within the bacteria so that antibiotics start to act again,” said Dr. Vanderlei Bagnato, professor in the Department of Biomedical Engineering and senior author on the study.
Photodynamic inactivation, a technique that has shown promise in combating bacterial resistance, uses light and light-sensitive molecules, called photosensitizers, to produce reactive oxygen species that can kill microorganisms by disrupting their metabolic processes.
In the new experiments, the team used curcumin, which is also a natural food for bacteria. They tested this technique on strains of Staphylococcus aureus (MRSA) that are resistant to amoxicillin, erythromycin, and gentamicin.
The researchers exposed the bacteria to many cycles of light exposure and then compared the minimum concentration of antibiotics needed to kill the bacteria after light exposure versus those that did not get light exposure.
“When we have a mixed population of bacteria where some are resistant, we can use photodynamic inactivation to narrow the bacterial distribution, leaving behind strains that are more or less similar in their response to antibiotics,” Bagnato told Texas A&M News.
“It’s much easier now to predict the precise antibiotic dose needed to remove the infection.”
MORE PROGRESS ON SUPERBUGS: • The Humble Potato Could Hold Key to Beating Hospital Superbugs and Crop Diseases • Compounds in Amber Could Help Fight Drug-Resistant Bacteria Superbugs, Say Scientists • When Antibiotics Failed, She Found a Natural Enemy of the Superbug to Save Husband’s Life
The team noted that photodynamic inactivation using curcumin has tremendous potential as an adjuvant or additional therapy with antibiotics for diseases, like pneumonia, caused by antibiotic-resistant bacteria.
“Photodynamic inactivation offers a cost-effective treatment option, which is crucial for reducing medical expenses not only in developing countries but also in the United States,” said Dr. Vladislav Yakovlev, professor in the Department of Biomedical Engineering and author on the study..."
-via Good News Network, February 8, 2025
#superbugs#immunology#epidemiology#microbiology#turmeric#antibiotics#antibiotic resistance#public health#medical news#medical research#good news#hope
1K notes
·
View notes
Text
The industrially ravaged Gowanus Canal, long regarded as a symbol of urban environmental neglect, is being reimagined through the lens of scientific inquiry as a complex reservoir of microbial life shaped by intense selective pressures. Research led by NYU Tandon School of Engineering has discovered microbes in Brooklyn's Gowanus Canal that carry genes for breaking down industrial pollutants and neutralizing heavy metals. Genetic screening also uncovered resistance to multiple antibiotic classes and thousands of biosynthetic gene clusters with implications for developing new antibiotics, industrial enzymes, and bioactive compounds.
Continue Reading.
109 notes
·
View notes
Text

Aquae Sulis. Bath, England
Waters at Roman Bath may have super healing properties
A new study, published in the Microbe journal, has uncovered a diverse array of microorganisms in the geothermal waters at Roman Bath that may have super healing properties.
Bath, known as Aquae Sulis during the Roman period, is located in the modern-day city of Bath, England. The Romans constructed a bathing complex and temple at the site of three natural hot springs, which are heated by geothermal activity raising the water temperature to between 40 and 45°C.
The entire process spans 10,000 years, beginning with rainwater that penetrates through the carboniferous limestone rock in the Mendip Hills and the Avon Valley.
The water then percolates through aquifers to depths of 2,700 to 4,300 metres, where it is heated to temperatures close to boiling point, and then rises to the surface through fissures and faults in the limestone rock.
Following the collapse of Roman Britain, the baths were redeveloped over the following millennia. This includes the 12th century construction of a curative bath over the King’s Spring reservoir, the Queen’s Bath during the 16th century, and the more recent 18th century construction.
Throughout the centuries, the waters at Bath were thought to have restorative or healing properties. In a legend based on a story told by Geoffrey of Monmouth, King Lear’s father, Bladud, cured himself of leprosy by trying the waters.
In a recent study by the University of Plymouth, scientists collects samples of water, sediment, and biofilm from the King’s Spring and the Great Bath.
Metagenomic sequencing was performed on the samples for bacterial and archaeal community exploration, revealing 300 distinct types of bacteria, including Actinobacteria and Myxococcota which are known for antibiotic production.
Further tests revealed that 15 of these isolates – including examples of Proteobacteria and Firmicutes – showed varying levels of inhibition against human pathogens, including E.coli, Staphylococcus Aureus and Shigella flexneri.
According to the study authors: “From this data, there is clear potential for novel antimicrobial natural products from the Roman Baths, as has been demonstrated from other thermal hot springs globally.”
This discovery comes at a time when science is seeing a rise in the antimicrobial resistance of pathogenic bacteria to currently used antibiotics, responsible for the deaths of more than 1.25 million people globally each year.
Article by Mark Milligan (June 10, 2024) Heritage Daily
Image Credit : Shutterstock – Alexey Fedorenko
137 notes
·
View notes
Text
My money is on fungus. E. coli doesn't survive heat very well but many fungal spores do.
I've had an ongoing science project for a few years now that I'd like to share. I've been leaving raw chicken breasts—real cheap, unregulated shit from the local mom-and-pop grocery store—out on my counter long enough for E. coli bacteria to develop. Then I microwave the chicken breasts and try to produce genetic mutations in the bacteria of each batch through microwave radiation, just in short bursts of 45 seconds per "rep" and maybe 3 "reps" per "set". The bacteria that do survive get fresh, raw chicken mixed into their feed and left to rest at room temperature for a few more days to grow. Then I just rinse and repeat until I see weird shit under the microscope. Sometimes I don't even need a microscope! Pic related, the fuzzy red mound is the modified E. coli (more obvious under a microscope). It's one of my most successful batches, a relative newcomer at only ten generations. I don't really microwave it any more because I'm pretty happy with it. This batch actually still has chicken inside, it's just completely covered in the bacteria "fur". Each bacterium is about a quarter the length and width of an eyelash. They aren't as quick at eating the chicken breasts as some of my other batches. I'm not sure how it happens, but between the actual chicken and the bacteria layer is some nasty chicken glob, like they slowly dissolve it or something. But this batch is definitely my "ambassador species" since it's pretty flashy with its beautiful maroon color and marimo-like appeal, and it doesn't make me sick too much. Anyone else doing something similar?
#I have accidentally created Weirdly Long bacteria before#but it was because I was in a microbe research lab in Europe who just did not work with soil samples like we were using#and hadn't noticed that literally all of their agar was contaminated with antibiotics#which their antibiotic-resistant medical strains were apparently fine with and our soil samples did Not Like#so the samples weren't dividing properly because they were being poisoned
4K notes
·
View notes
Text
'How could it have been allowed to happen?': The threat of 'superbugs' was known from the first antibiotic, but we've failed to stop it. | Live Science
2 notes
·
View notes
Note
The blood thing is confusing, so they aren't related cause blood transfusions don't make the receptor and donor related but it's presented on the play as a bloodline/family tree thing? And do you still think el and Henry can be related outside of the blood transfusions?
It's presented very ambiguously!
Brenner says that Henry's as much a father to the children as he is in relation to the blood transfusions. I know in earlier showings they specified that Henry met the woman who was pregnant with 002, and that the mothers had been receiving transfusions. However, in the showings I saw, Brenner only says that 10 children had survived the transfusions. He never mentions mothers (the mentions of pregnant women only show up in one newspaper scrolling past at like. light-speed).
Which...You truly are preaching to the science choir here, Nonnie (and I say that lovingly). The blood thing has been fucking with me for a hot minute because, well...it straight up doesn't work like that, unless you start majorly stretching biology.
Brenner says Henry has a totally unique blood type, the same blood type Brenner Sr. had after his time in Dimension X in 1943. It caused Brenner Sr. to reject all blood types for transfusions. Which...this could make Henry a universal donor, I won't write that off entirely, but it's unlikely, imo, given that Brenner mentions survival rate. This means some of the babies rejected the blood transfusions and died (whether they received it via their mother or otherwise).
So it becomes more likely that they'd be looking for kids who have a predisposition to surviving the transfusions of the unique blood.
And that, my dearest Nonnie, is genetic.
So while there may be some wild-caught kids like Kali, it's likely there are also direct descendants.
On that note:
I feel like there's definitely still some confusion about the purpose of the transfusions, specifically in the way of correlation vs causation irt powers...so I thought I'd toss my 2¢ in.
Based on TFS canon so far, Brenner is intent on exploring Dimension X to further his father's work. But in order to do that, he needs subjects that can survive it. Imo, he's looking for what made Henry able to survive whatever happened to him. From what I've gathered, the transfusions seem to be less about giving children psionic powers and more about either collecting children who are predisposed to survival in Dimension X (akin to antibiotic resistance in microbes), or predisposing them to it (like a particularly harsh vaccine that only a select few survive, which is still a genetic/selective trait...just with an extra step: do they survive the vaccination?).
The fact that these children survived the blood transfusions and have psionic powers, many of which are shared abilities, makes me think there's some specific trait in these children's biological makeup that makes them predisposed to both characteristics. The children that survived the blood also have powers, in what seems to be all cases. Children who present these powers are more likely to tolerate transfusions, and vice versa. Correlation, not causation.
Thus, I firmly don't believe the blood transfusions gave the children abilities. I think it acts as a screening process, and the psionic abilities are a correlative marker of that ability to survive Henry's blood.
We do the same thing in microbiology all the time. Selective media. Grow the culture on a medium that's deadly to anything that doesn't have the desired trait, and whatever grows is the desired microbe (see: mannitol salt agar for pathogenic staphylococci). The medium didn't cause the microbe to change in order to survive, the microbe was just predisposed to surviving exposure to the selective agent. Correlation, not causation.
tl;dr: I think the transfusions are a selection technique, a screening process, to ensure Brenner gets the most important trait: Survival in Dimension X.
The children are all genetically similar in that trait, which leads to "you're a father to them irt your blood". It's a shared genetic trait between Henry and the children, whether it came from him via sex cells or if it evolved wild-caught. They all have his blood...blood like his. And what's the most efficient way to select for a genetic trait? A breeding program. So yes, I do still think some version of Henry and El are related.
(This isn't even getting into the music choices, with Henry singing TYBTM to baby El, which is the same song his blood-parent mother sang to him as a child, or the casting choices irt: Jamie and Millie. There's the blood stuff, and then there's the Everything Else about Henry and El.)
26 notes
·
View notes
Note
Hey Acti Have you heard the latest about H5N1 (bird flu) not only crossing from animals to humans, but also transmission in via handling/consuming of animals? https://twitter.com/Globalbiosec/status/1775709161738895706
It is worrying, but it is important to keep it in perspective. I have heard about the new human cases, but I think some of the coverage on it is a little bit misleading. I’ve seen lots of people using it as a way to conclude that eating chicken/eggs means you’re risking catching bird flu; but this is hyperbolic and the evidence that it carries a significant risk of contamination is just not there.
The World Health Organisation found that all the human cases were linked to close contact with infected animals or contaminated environments. The new case in the US is significant because it’s linked to handling cattle rather than chicken, but risk to the public is still very low - much lower than the likes of E. coli, Campylobacter, or Salmonella which chicken already carries a risk of.
The virus is not well adapted to infect humans, and even less so to transfer between humans. It is workers and non-human animals who we should be most concerned about when it comes to bird flu, rather than the wider public. No human or animal should have to endure being exploited in filthy, overcrowded, demoralising conditions where the risk of disease is so high.
Bird flu represents a major pandemic risk and it would be truly disastrous if transmission between humans and other animals become common. The conditions in factory farms and the overuse of antibiotics in animal agriculture has created the perfect environment for an antibiotic resistant microbe to emerge. However, as troubling as all such cases are, we are not there yet.
14 notes
·
View notes
Text



On 6th August 1881 Sir Alexander Fleming was born in Lochfield, near Darvel.
Well before Fleming accidentally discovered penicillin he tried to convince doctors in the field hospitals of World War One that treating wounds with antiseptic, not only cleaned the wounds but also lowered the soldier’s natural resistance to infection because they were killing white blood cells. His mentor Almroth Wright believed that a saline solution – salt water – should be used to clean deep wounds, because this did not interfere with the body’s own defenses and in fact attracted white cells. Fleming proved this result in the field.
Wright and Fleming published their results, but most army doctors refused to change their ways, resulting in many preventable deaths.
After the war Fleming discovered an enzyme called Lysozyme, an important role in the prevention of bacterial infections, while this was an important breakthrough in preventing infections in the first place it had little or no effect on many other microbes that infect humans. Lysozyme is present in the body, for instance it is i in tears, saliva, sweat, and other body fluids, It destroys bacteria that attempts to enter our body through these passageways. In the case of tears, they protect our eyes from bacterial invaders.
Fleming had left a jar of mould unattended during a holiday. On returning to work he noticed that a jar of Staphylococcus bacteria – a green yellow mould – had covered the dish except one area which was clear of the bacteria – rather like a halo effect.
This was Fleming’s great Eureka moment – the moment he correctly deduced that some antibacterial agent had crept in and successfully stopped the bacteria. He later identified this antibacterial agent as a rare form of Penicillium notatum which had drifted in from a mycology lab nearby. He later talked about the importance of chance in this discovery.
"I have been trying to point out that in our lives chance may have an astonishing influence and, if I may offer advice to the young laboratory worker, it would be this—never neglect an extraordinary appearance or happening. It may be—usually is, in fact—a false alarm that leads to nothing, but may on the other hand be the clue provided by fate to lead you to some important advance."
Fleming named this fungus penicillin and tried to grow it in his lab. He published his discovery in the “Journal of Experimental Pathology” in 1929 but did not receive much attention at the time as most of his clinical trials had been inconclusive and penicillin could not be produced in large enough quantities to be useful. Fleming was convinced that the fungus had limited usefulness but his research was taken up by two researchers from Oxford University named Ernst Chain and Howard Florey. These two were successful in producing purified penicillin which became indispensable during World War II, and helped to save thousands of lives through its use in antibiotics. In 1945, as a result of their combined efforts and discoveries, Fleming, Chain and Florey were jointly awarded the Nobel Prize in Medicine.
So the untidiness of a Scottish scientist ultimately led to countless many lives being saved, Fleming himself predicted that one day the antibiotics might be less effective, as is the case nowadays, we wait in hope of another "accident"
40 notes
·
View notes
Text
I don’t like most biological sciences because almost every innovation or breakthrough is either A. Seemingly a cure all and solution to some of our most pressing issues only to a be a Super Fuck You Chemical that makes 9 million% more at risk of every cancer ever or B. Not fully integrated or used for its clear and obvious benefits because of the risk of it being a Super Fuck You chemical
Antibiotics: “Wow it can basically nuke most otherwise untreatable diseases how useful!” Well jokes on you it makes microbes capable of immunity to antibiotics making them what are scientifically known as multidrug-resistant microbes, commonly known as superbugs, and known only by me as Fuck You Microbes that are basically untreatable with most modern medicine.
CRISPR: “Wow we can now edit genomes and manipulate life on a cellular level, meaning we can eliminate dangerous genes that cause hereditary diseases, and hypothetically grant us cool body modifications like wings how cool!” Too bad there is a very precedent risk of it causing long term genealogical damage which we have no virtual way to foresee, meaning we could damage your DNA permanently and not know till generations later.
Bacteriophages: “Wow an evolving bacteria killer that can remove illnesses without the risks that antibiotics bring how amazing!” Jokes on you again because a large part of the scientific community is worried that it could probably cause similar unforeseen consequences we won’t see till decades later.
2 notes
·
View notes
Text
"An international research team has found almost a million potential sources of antibiotics in the natural world.
Research published in the journal Cell by a team including Queensland University of Technology (QUT) computational biologist Associate Professor Luis Pedro Coelho has used machine learning to identify 863,498 promising antimicrobial peptides -- small molecules that can kill or inhibit the growth of infectious microbes.
The findings of the study come with a renewed global focus on combatting antimicrobial resistance (AMR) as humanity contends with the growing number of superbugs resistant to current drugs.
"There is an urgent need for new methods for antibiotic discovery," Professor Coelho, a researcher at the QUT Centre for Microbiome Research, said. The centre studies the structure and function of microbial communities from around the globe.
"It is one of the top public health threats, killing 1.27 million people each year." ...
"Using artificial intelligence to understand and harness the power of the global microbiome will hopefully drive innovative research for better public health outcomes," he said.
The team verified the machine predictions by testing 100 laboratory-made peptides against clinically significant pathogens. They found 79 disrupted bacterial membranes and 63 specifically targeted antibiotic-resistant bacteria such as Staphylococcus aureus and Escherichia coli.
"Moreover, some peptides helped to eliminate infections in mice; two in particular reduced bacteria by up to four orders of magnitude," Professor Coelho said.
In a preclinical model, tested on infected mice, treatment with these peptides produced results similar to the effects of polymyxin B -- a commercially available antibiotic which is used to treat meningitis, pneumonia, sepsis and urinary tract infections.
More than 60,000 metagenomes (a collection of genomes within a specific environment), which together contained the genetic makeup of over one million organisms, were analysed to get these results. They came from sources across the globe including marine and soil environments, and human and animal guts.
The resulting AMPSphere -- a comprehensive database comprising these novel peptides -- has been published as a publicly available, open-access resource for new antibiotic discovery.
[Note: !!! Love it. Open access research databases my beloved.]"
-via Science Daily, June 5, 2024
#superbugs#bacteria#viruses#microbiology#antibiotics#medicines#public health#peptides#medical news#antibiotic resistance#good news#hope#ai#artificial intelligence#pro ai#machine learning
188 notes
·
View notes
Text
PRINCE WILLIAM BECOMES PATRON OF THE APPEAL TO CREATE THE FLEMING CENTRE
Prince William has become Patron of a new appeal, launching today, to create The Fleming Centre which will drive a global movement to tackle antimicrobial resistance.
The Centre will be based at St. Mary's Hospital in Paddington, London, where Sir Alexander Fleming discovered penicillin, the first antibiotic, in 1928 and changed the course of medicine forever. This important programme of work is being led by Imperial College London and Imperial College Healthcare NHS Trust, which includes St Mary's Hospital.
Antimicrobial resistance occurs when the microbes that cause infections develop resistance to treatments such as antibiotics and antifungals and already kills over one million people around the world each year. It has been caused by the widespread misuse and overuse of antibiotics and other antimicrobials which has led to the global spread of drug-resistant microbes. If the problem is not resolved, it is estimated that by 2050, drug-resistant microbes will lead to around ten million deaths per year.
In order to effectively tackle antimicrobial resistance, global awareness and behaviour change is needed alongside scientific advances. The aim of The Fleming Initiative - the driving force behind the construction of an initial Centre in London - is to put society at the heart of solving this problem.
The Fleming Centre will deliver exhibitions and engagement activities to educate, inspire and catalyse action, convening diverse global voices and building consensus for change. At the Centre, scientists will work alongside patients, members of the public and policy makers to scope, test and scale solutions, so that antibiotics can continue to keep us all safe. It is hoped that this transformative approach at the London Centre will act as a blueprint which can be shared and adapted to local contexts around the globe.
In becoming Patron of the appeal to build the Centre, Prince William will support efforts over the next five years to make these ambitious plans to overcome global anti-microbial resistance a reality.
via Kensington Palace
29 notes
·
View notes
Text
Researchers have developed a new antibiotic that reduced or eliminated drug-resistant bacterial infections in mouse models of acute pneumonia and sepsis while sparing healthy microbes in the mouse gut. The drug, called lolamicin, also warded off secondary infections with Clostridioides difficile, a common and dangerous hospital-associated bacterial infection, and was effective against more than 130 multidrug-resistant bacterial strains in cell culture. The findings are detailed in the journal Nature. "People are starting to realize that the antibiotics we've all been taking—that are fighting infection and, in some instances, saving our lives—also are having these deleterious effects on us," said University of Illinois Urbana-Champaign chemistry professor Paul Hergenrother, who led the study with former doctoral student Kristen Muñoz.
Continue Reading.
203 notes
·
View notes
Text
River microbes near wastewater treatment plants express high levels of antibiotic resistance genes, study shows
2 notes
·
View notes