#Alpha glucose
Explore tagged Tumblr posts
Text

Hey, haven't posted in a while cause IB is hectic and I care more about my sanity than my pride for keeping up daily art challenge sooo. I'm just gonna be posting when I can, as you see here it will probably be school related drawings like this cause studying "yayy". ANYWAYS hope you're having a good day and enjoy these Biology Blorbos
#digital art#character#original character#biology#Glucose#Alpha glucose#Beta glucose#DISCLAIMER this is not an omegaverse thingy#These things actually exist in biology#Just saying this cause I typed the alpha in alpha glucose and now I want to wash my eyes#<3
2 notes
·
View notes
Text
i’ve finally mapped out the plot for this damn slowburn now i can finally start writing!!
#shoutout to my amazing irl who is going to beta for me#i’ve known her for 3 weeks but i knew from day one where i said ‘alpha and beta glucose wheres omega glucose’ and she got the joke#that’s how i knew she was a fandom kid#and was equally traumatised by ABO growing up#anyway hope you like my fake dating fic#serennedy naturally
7 notes
·
View notes
Text
me in biochem only drawing things in their alpha configurations cuz im no beta
15 notes
·
View notes
Text
my beautiful son Ethenol

2 notes
·
View notes
Text
Mitochondrial Dysfunction in Type 2 Diabetes
Introduction
Mitochondria, essential for cellular energy metabolism, play a crucial role in bioenergetics and metabolic homeostasis. Mitochondrial dysfunction has been implicated as a key pathophysiological factor in Type 2 Diabetes Mellitus (T2DM), contributing to insulin resistance, metabolic inflexibility, and beta-cell dysfunction. This review explores the intricate mechanisms underlying mitochondrial impairments in T2DM, including defective oxidative phosphorylation, disrupted mitochondrial dynamics, impaired mitophagy, and excessive reactive oxygen species (ROS) generation, with a focus on potential therapeutic interventions targeting mitochondrial pathways.
Mechanistic Insights into Mitochondrial Dysfunction in T2DM
1. Defective Oxidative Phosphorylation and ATP Synthesis
Mitochondrial oxidative phosphorylation (OXPHOS) occurs through the electron transport chain (ETC), comprising Complexes I-IV and ATP synthase (Complex V). In T2DM, evidence suggests a downregulation of mitochondrial ETC activity, particularly in Complex I (NADH:ubiquinone oxidoreductase) and Complex III (cytochrome bc1 complex), leading to reduced ATP synthesis. This dysfunction is often linked to compromised NADH oxidation and inefficient proton gradient formation, resulting in cellular energy deficits and impaired insulin-stimulated glucose uptake.
2. Elevated Reactive Oxygen Species (ROS) and Oxidative Stress
Mitochondria are a primary source of ROS, predominantly generated at Complex I and Complex III during electron leakage. In T2DM, excess substrate influx due to hyperglycemia leads to mitochondrial overactivation, driving excessive ROS production. Elevated ROS induces oxidative damage to mitochondrial DNA (mtDNA), lipids, and proteins, disrupting mitochondrial integrity and function. Oxidative stress further impairs insulin signaling by activating stress-responsive kinases such as c-Jun N-terminal kinase (JNK) and IκB kinase (IKK), contributing to systemic insulin resistance.
3. Mitochondrial Biogenesis and Transcriptional Dysregulation
Mitochondrial biogenesis is regulated by the transcriptional coactivator Peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1α), which modulates downstream transcription factors such as Nuclear Respiratory Factors (NRF-1/NRF-2) and Mitochondrial Transcription Factor A (TFAM). In T2DM, PGC-1α expression is downregulated, impairing mitochondrial biogenesis and reducing mitochondrial density, leading to decreased oxidative capacity in metabolically active tissues like skeletal muscle and liver.
4. Disrupted Mitochondrial Dynamics and Mitophagy
Mitochondrial quality control is maintained through dynamic fission and fusion processes. Fission, mediated by Dynamin-related protein 1 (Drp1), is necessary for mitochondrial fragmentation and mitophagy, while fusion, regulated by Mitofusin 1/2 (Mfn1/2) and Optic Atrophy 1 (OPA1), maintains mitochondrial integrity. In T2DM, an imbalance favoring excessive fission leads to mitochondrial fragmentation, impairing energy metabolism and exacerbating insulin resistance. Moreover, defective mitophagy, regulated by PTEN-induced kinase 1 (PINK1) and Parkin, results in the accumulation of dysfunctional mitochondria, further aggravating metabolic dysfunction.
Implications of Mitochondrial Dysfunction in T2DM Pathophysiology
1. Skeletal Muscle Insulin Resistance
Skeletal muscle accounts for ~80% of postprandial glucose uptake, relying on mitochondrial ATP production for insulin-mediated glucose transport. Impaired mitochondrial function in muscle cells reduces oxidative phosphorylation efficiency, promoting a shift towards glycolysis and lipid accumulation, ultimately leading to insulin resistance.
2. Pancreatic Beta-Cell Dysfunction
Mitochondrial ATP production is essential for insulin secretion in pancreatic beta cells. ATP-sensitive potassium channels (K_ATP) regulate glucose-stimulated insulin secretion (GSIS), with ATP/ADP ratios dictating channel closure and depolarization-induced insulin exocytosis. In T2DM, mitochondrial dysfunction leads to inadequate ATP generation, impairing GSIS and reducing insulin secretion capacity. Additionally, oxidative stress-induced beta-cell apoptosis contributes to progressive loss of beta-cell mass.
3. Hepatic Mitochondrial Dysfunction and Lipid Dysregulation
Mitochondrial dysfunction in hepatocytes contributes to hepatic insulin resistance and non-alcoholic fatty liver disease (NAFLD). Impaired fatty acid oxidation due to dysfunctional mitochondria leads to lipid accumulation, exacerbating hepatic insulin resistance and systemic metabolic dysregulation.
Therapeutic Strategies Targeting Mitochondrial Dysfunction
1. Exercise-Induced Mitochondrial Adaptation
Physical activity upregulates PGC-1α expression, enhancing mitochondrial biogenesis and oxidative metabolism. High-intensity interval training (HIIT) and endurance exercise improve mitochondrial efficiency and reduce oxidative stress, mitigating insulin resistance in T2DM patients.
2. Pharmacological Modulation of Mitochondrial Function
Metformin: Enhances mitochondrial complex I activity, reducing hepatic gluconeogenesis and oxidative stress.
Thiazolidinediones (TZDs): Activate PPAR-γ, promoting mitochondrial biogenesis and improving insulin sensitivity.
Mitochondria-targeted Antioxidants: Agents like MitoQ, SkQ1, and SS-31 reduce mitochondrial ROS, preventing oxidative damage and preserving mitochondrial function.
3. Nutritional and Metabolic Interventions
Ketogenic and Low-Carb Diets: Enhance mitochondrial fatty acid oxidation, reducing lipid accumulation and improving metabolic flexibility.
Intermittent Fasting: Induces mitochondrial biogenesis and autophagy, improving metabolic homeostasis.
Nutraceuticals: Coenzyme Q10, resveratrol, and nicotinamide riboside (NR) enhance mitochondrial function and energy metabolism.
4. Emerging Gene and Cellular Therapies
Gene Therapy: Targeted upregulation of PGC-1α and TFAM to restore mitochondrial function.
Mitochondrial Transplantation: Direct transfer of healthy mitochondria to replace dysfunctional ones, an emerging frontier in metabolic disease management.
Conclusion
Mitochondrial dysfunction is a central determinant in the pathogenesis of T2DM, affecting insulin signaling, glucose metabolism, and lipid homeostasis. Targeting mitochondrial pathways through exercise, pharmacological agents, dietary modifications, and emerging gene therapies offers promising avenues for improving metabolic health in T2DM.

#Mitochondrial Dysfunction#Type 2 Diabetes Mellitus (T2DM)#Oxidative Phosphorylation (OXPHOS)#Electron Transport Chain (ETC)#ATP Synthesis#Reactive Oxygen Species (ROS)#Oxidative Stress#Mitochondrial DNA (mtDNA) Damage#Peroxisome Proliferator-Activated Receptor-Gamma Coactivator-1 Alpha (PGC-1α)#Nuclear Respiratory Factors (NRF-1/NRF-2)#Mitochondrial Transcription Factor A (TFAM)#Mitochondrial Biogenesis#Mitochondrial Dynamics (Fission & Fusion)#Dynamin-related protein 1 (Drp1)#Mitofusin 1/2 (Mfn1/2)#Optic Atrophy 1 (OPA1)#Mitophagy#PTEN-Induced Kinase 1 (PINK1)#Parkin#Insulin Resistance#Skeletal Muscle Metabolism#Pancreatic Beta-Cell Dysfunction#Glucose-Stimulated Insulin Secretion (GSIS)#ATP-Sensitive Potassium Channels (K_ATP)#Lipid Dysregulation#Non-Alcoholic Fatty Liver Disease (NAFLD)#Exercise-Induced Mitochondrial Adaptation#High-Intensity Interval Training (HIIT)#Metformin#Thiazolidinediones (TZDs)
0 notes
Text

TSRNOSS, p 755.
#soil formation#oxygen content of water#diffusion rate of oxygen in lipid#diffusion rate of glucose#wood frogs#antifreeze#glucose#alpha particle#dielectric constant of water#antigen-antibody complex
0 notes
Text
Alpha-D-Glucose
#Alpha-D-Glucose#Alpha and beta-D-Glucose#chemical#chemicalsupplies#chemicalsolutions#chemical industry#chemical engineering
0 notes
Text
UNDERSTANDING THE LINK BETWEEN DIABETES AND FATIGUE
Diabetes, a long-term metabolic condition marked by elevated blood sugar levels, impacts millions globally. One common yet often overlooked symptom of diabetes is fatigue. This article delves into the relationship between diabetes and tiredness, exploring the underlying causes, potential complications, and management strategies.
The Connection Between Diabetes and Fatigue
Fatigue is a prevalent symptom among individuals with diabetes. It can be caused by various factors directly or indirectly related to the disease. Understanding these factors is crucial for managing fatigue effectively.
1. Blood Sugar Imbalances
One of the primary reasons diabetes can cause fatigue is due to fluctuations in blood sugar levels. Both hyperglycemia (high blood sugar) and hypoglycemia (low blood sugar) can lead to feelings of tiredness.
Hyperglycemia: When blood sugar levels are too high, the body's cells cannot efficiently absorb glucose, the primary energy source. This lack of energy can cause persistent fatigue.
Hypoglycemia: Conversely, low blood sugar levels can deprive the body of necessary fuel, leading to weakness and exhaustion.
2. Insulin Resistance
In type 2 diabetes, the body becomes resistant to insulin, the hormone responsible for regulating blood sugar levels. This resistance means that even though insulin is present, glucose cannot enter the cells effectively, resulting in a lack of energy and subsequent fatigue.
3. Sleep Disruptions
Diabetes can also interfere with sleep, contributing to tiredness. Conditions such as sleep apnea, restless legs syndrome, and frequent urination at night (nocturia) are common among people with diabetes and can disrupt sleep patterns, leading to daytime fatigue.
4. Dehydration
Elevated blood sugar levels can increase urination as the kidneys work harder to expel excess glucose. This process can result in dehydration, which can cause tiredness and weakness.
5. Psychological Factors
The mental and emotional toll of managing diabetes can be significant. The constant monitoring of blood sugar levels, managing dietary restrictions, and coping with the potential complications of diabetes can lead to mental fatigue. Depression and anxiety, which are more prevalent among individuals with diabetes, can also contribute to feelings of tiredness.
Managing Fatigue in Diabetes
Effective management of diabetes-related fatigue involves addressing its underlying causes. Here are some strategies to consider:
1. Blood Sugar Management
Maintaining stable blood sugar levels is crucial for reducing fatigue. Regular monitoring, adhering to prescribed medication, and following a balanced diet can help keep blood sugar levels within the target range. Incorporating complex carbohydrates, lean proteins, and healthy fats into meals can provide sustained energy.
Simple Technique To Help Manage Blood Sugar

2. Physical Activity
Engaging in regular physical activity can improve energy levels and overall well-being. Exercise helps regulate blood sugar levels, enhances insulin sensitivity, and promotes better sleep. Always seek advice from a healthcare professional before beginning any exercise program to ensure it is safe and suitable for your condition.
3. Hydration
Staying well-hydrated is vital for preventing fatigue. Drinking adequate water throughout the day can help mitigate the effects of dehydration caused by high blood sugar levels.
4. Sleep Hygiene
Improving sleep quality is another critical aspect of managing diabetes-related fatigue. Establishing a consistent sleep schedule, creating a relaxing bedtime routine, and addressing any sleep disorders can contribute to better rest and reduced tiredness.
5. Psychological Support
Seeking psychological support can help manage the emotional burden of diabetes. Counselling, support groups, and stress-reduction techniques such as mindfulness and meditation can be beneficial in reducing mental fatigue.
Conclusion
Fatigue is a common symptom experienced by individuals with diabetes, stemming from various physiological and psychological factors. Understanding the connection between diabetes and tiredness is essential for effective management. By maintaining stable blood sugar levels, engaging in regular physical activity, staying hydrated, improving sleep quality, and seeking psychological support, individuals with diabetes can alleviate fatigue and enhance their overall quality of life.
#Diabetes supplement#Blood sugar supplement#Glucose support#Diabetic support supplement#Insulin resistance supplement#Blood sugar control supplement#Natural diabetes supplement#Pancreas support supplement#Diabetes management supplement#Metabolic health supplement#Chromium supplement for diabetes#Gymnema sylvestre supplement#Berberine supplement for diabetes#Cinnamon supplement for blood sugar#Alpha-lipoic acid supplement for diabetes#Magnesium supplement for diabetes#Vitamin D supplement for diabetes#Vitamin B12 supplement for diabetes#Zinc supplement for diabetes#Probiotic supplement for diabetes#Prebiotic supplement for diabetes#Fiber supplement for diabetes#Omega-3 supplement for diabetes#Curcumin supplement for diabetes#Milk thistle supplement for diabetes#Ginkgo biloba supplement for diabetes#Fenugreek supplement for diabetes#Aloe vera supplement for diabetes#N-acetyl cysteine supplement for diabetes#Resveratrol supplement for diabetes
1 note
·
View note
Text
At branch points, new chains of 24 to 30 units start by α-1,6-glycosidic bonds (figure 22.11).

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#amylopectin#polymer#glucose#alpha#glycoside#chemical bonding
0 notes
Text
Figure 22.3 shows structural formulae for α-D-glucopyranose and β-D-glucopyranose, both drawn as chair conformations.
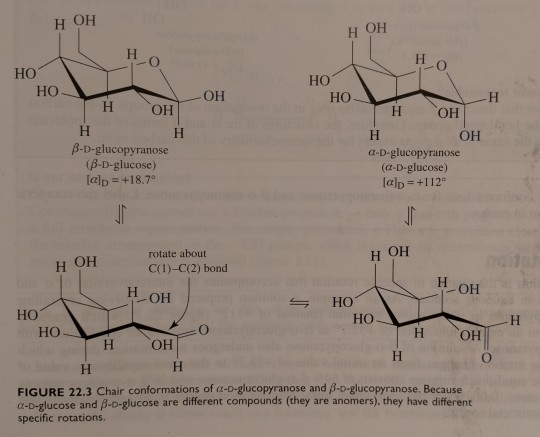
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
0 notes
Link
0 notes
Text


09.08.2024
.
Currently studying: Cell Biology - alpha and beta glucose structures, photosynthesis, and protein!
I did my first attempt of my post lab quiz and got an 83% which isn’t bad considering I missed my previous class because I was sick.
My midterm break is next week so I have a week of just studying planned (will I stick to my plan? Probably not).
126 notes
·
View notes
Text
🔬🌀Demystifying the Krebs Cycle: A Deep Dive into Cellular Respiration! 🌀🔬
Prepare for a thrilling journey into the heart of cellular metabolism! 🌟✨ Today, we unravel the intricacies of the Krebs Cycle, also known as the Citric Acid Cycle or Tricarboxylic Acid Cycle, a cornerstone of energy production in our cells. 💡🤯
The Krebs Cycle: Named after its discoverer, Sir Hans Krebs, this metabolic pathway occurs within the mitochondria and is a central hub in cellular respiration.
🔍Step 1: Acetyl-CoA Entry
Acetyl-CoA, derived from the breakdown of glucose or fatty acids, enters the cycle.
It combines with oxaloacetate to form citrate, a six-carbon compound.
🔍Step 2: Isocitrate Formation
A rearrangement converts citrate into isocitrate.
The enzyme aconitase facilitates this transformation.
🔍Step 3: Alpha-Ketoglutarate Production
Isocitrate undergoes oxidative decarboxylation, shedding a CO2 molecule and yielding alpha-ketoglutarate.
NAD+ is reduced to NADH in this step.
🔍Step 4: Succinyl-CoA Synthesis
Alpha-ketoglutarate loses CO2 and acquires a CoA group to form succinyl-CoA.
Another NAD+ is reduced to NADH.
This step is catalyzed by alpha-ketoglutarate dehydrogenase.
🔍Step 5: Succinate Formation
Succinyl-CoA releases CoA, becoming succinate.
A molecule of GTP (guanosine triphosphate) is generated as a high-energy phosphate bond.
Succinate dehydrogenase is pivotal, transferring electrons to the electron transport chain (ETC).
🔍Step 6: Fumarate Generation
Succinate is oxidized to fumarate with the help of the enzyme succinate dehydrogenase.
FADH2 (flavin adenine dinucleotide) is formed and transfers electrons to the ETC.
🔍Step 7: Malate Formation
Fumarate undergoes hydration to form malate, catalyzed by fumarase.
🔍Step 8: Regeneration of Oxaloacetate
Malate is oxidized back to oxaloacetate.
NAD+ is reduced to NADH.
Oxaloacetate is ready to initiate another round of the Krebs Cycle.
The Krebs Cycle - an intricate dance of chemical transformations fueling the cellular machinery of life. 🕺💃 Dive deeper into cellular respiration, where molecules tango to generate ATP, our cellular energy currency!

📚References for In-Depth Exploration📚
Berg, J. M., Tymoczko, J. L., & Stryer, L. (2002). Biochemistry (5th ed.). W. H. Freeman. Chapter 17.
Voet, D., Voet, J. G., & Pratt, C. W. (2008). Fundamentals of Biochemistry (3rd ed.). John Wiley & Sons. Chapter 17.
Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2008). Lehninger Principles of Biochemistry (5th ed.). W. H. Freeman. Chapter 17.
#science#biology#college#education#school#student#medicine#doctors#health#healthcare#biochemistry#cell#science nerds
116 notes
·
View notes
Text
Omegaverse Wordbuiling part2
scenting someone without their consent is considered as a harassment
Prision system is divided by the first AND the second gender. So female alpha and male alpha have different structures, as like male alpha and male beta, ecc ecc
For straight couples is meant (Alpha x Omega, indiffent the first gender, male beta x female beta)
All the other combinations are condidered as queer
Alpha x alpha couple usually doesn't work well, but omega x omega is a thing in modern times
In different parts of the word has diffrent point of view on queer people
Same for abort (in some cultures is accepted by only if the alpha makes the decion for example)
Poligamy is a thing in most of the cultures
Betas have pack too, but they simply call it family
Usually blood close related people (so like biological sibilings, mother and father ecc) are the primal pack of someone, but is not always said.
Breaking a bond is always cause emotional distress and hormonal unbalancing.
Hormonal problems are a big thing for omegas and alphas. It can be caused by big stress state, or despression/corellated disorders or for the malfuctioning of the glands. The cure can go from some simple pills for a short/long/forever periods of times.
In more seriouse cases, exists the equivalent of the CGM (constant glucose monitor) of diabetis, but for the Omega/Alpha hormons.
So is a little white thing you have on your back arm that bibs super high during a drop.
Like for anaphylactic shock, the people affected of this condiciotion has a autoinjector with the medicine.
Asexually/ Armomanticism is known, but accepted for betas than the others two.
13 notes
·
View notes
Text
Study on the storage stability of phycocyanin from Spirulina obtusususiae
Abstract: The effects of temperature, sunlight and different additives on the stability of aqueous solutions of phycocyanin were studied. It was concluded that phycocyanin should be stored at 40 ℃ and protected from light, and should be stored under neutral conditions; glucose, sodium chloride and sorbitol could effectively improve the stability of phycocyanin, and the pigment preservation rate of phycocyanin increased from 50.90% to 78.10%, 67.02% and 69.08% after 72 h at room temperature, respectively; the stabilizers of phycocyanin were compounded with glucose, sodium chloride and sorbitol in the mass ratio of 1 : 1 : 0.3 and left at 4 ℃ for 14 days. After adding glucose, sodium chloride and sorbitol as stabilizers in the mass ratio of 1:1:0.3, the pigment retention rate of the alginate was increased by 54.4% compared with that of the unadded alginate after being placed at 4 ℃ for 14 d. The pigment retention rate of the alginate added with the additive was increased by 16.1% compared with that of the unadded one after being placed at 25 ℃.

Spirulina (English name spirulina), also known as "spirulina", belongs to the family of Cyanobacteria, Chlamydomonas; at present, there are three types of large-scale cultivation at home and abroad, namely, Spirulina major, Spirulina obtususus and Spirulina indica. Spirulina obtususus is a blue-green seaweed (cyanobacteria) belonging to the Candida family.
It is a non-branched, multicellular spiral mycelium with a length of about 200 μm~300 μm and a width of about 5 μm~10 μm [1]. The amino acid composition of the proteins contained in Spirulina obtusususiformis is very uniform and reasonable, which suggests that it can be used as a potential health food for human beings [2].
Phycocyanin is one of the photosynthesizing proteins in the phycobilins, which are chromophore polypeptides consisting of α and β subunits with a molecular weight of about 20,000 daltons [3]. The phycobilisome in the cyanobacterium Spirulina obtususus is composed of an alpha and beta subunit in the center and a phycocyanin in the periphery. Phycocyanin is the most important bile protein in Spirulina, accounting for about 20 % of the dry weight [4-6]. It has a blue color in aqueous solution and fluoresces in purple. The UV-Vis spectra of phycocyanin in Spirulina obtusususiformis show characteristic absorption peaks at 278, 360 and 620 nm [7]. It has also been shown that the maximum absorption peak of L. obtususus is at 620 nm and its fluorescence emission peak at room temperature is at 645 nm [8].
Natural pigments are very rich in variety and are classified according to a variety of bases. According to solubility can be divided into fat-soluble pigments, water-soluble pigments; according to the source can be divided into animal pigments, microbial pigments and phytochromes; in order to classify the different chemical structures for anthocyanins, carotenoids and other five categories [9-10].
Alginin is a natural blue pigment with high application value. It has been shown to be anticancer[11-12] and can be used as a health food for patients with enteritis[13] . It is highly water-soluble and can be easily extracted from Spirulina. In the process of extraction and purification, the control of pH value and ionic strength is very crucial for the stability of algal blue protein. The discoloration and denaturation of phycocyanin is determined by the grade of protein polymers, and its polymer form is mainly affected by light intensity, light time, temperature, pH value, irradiation and protein concentration [14-17].
It has been studied that the higher concentration of sodium chloride can protect the stability of alginate, and the appropriate amount of sodium benzoate can protect the color and preservation of alginate to a certain extent [18-19], but the stability of alginate is still low. Therefore, on the basis of previous studies, this experiment was carried out to investigate the effects of different food-grade additives as well as glucose, sodium chloride and sorbitol additives on the stability of alginate.
1 Materials and Methods
1.1 Materials and Main Instruments
Spirulina obtususifolia powder: Inner Mongolia Wuxingzhao Ecological Industry Development Co.
FD-10 Freeze Dryer: Beijing DTY Technology Development Co., Ltd; 756PC UV Spectrophotometer: Tianjin Prius Instrument Co., Ltd; DK-98-II Electric Thermostatic Water Bath: Tianjin Taiste Instruments Co.
1.2 Extraction and purification of algal blue protein
1.2.1 Extraction of algal blue proteins[19]
Appropriate amount of spirulina powder was dissolved in distilled water according to the material-liquid ratio of 1:40 (mass ratio), and then stirred with a stirring rotor at a speed of 1,000 r/min for 1.5 h. It was frozen at -18 ℃, and then thawed rapidly in a 37 ℃ water bath for 24 h. After repeating this procedure for four times, it was centrifuged at a high speed for 10 min at 10,000 r/min, and the absorbance at 620 and 280 nm was measured after taking the supernatant and diluting it with appropriate multiplicity.
1.2.2 Purification of algal blue proteins[17]
Take the crude extract of algal blue protein with the concentration of 5 mg/mL, slowly add ammonium sulfate solid to the saturation degree of 40%, and at the same time, carry out magnetic stirring until complete dissolution, stand at 4 ℃ for 2 h, then centrifuged at 10 000 r/min for 15 min, collect the precipitate, dissolve it in an appropriate amount of distilled water, and then freeze-dried after dialysis and set aside.
1.3 Research on storage stability of algal blue protein
1.3.1 Effect of temperature on the stability of phycocyanin[19]
30 mg of alginate was dissolved in 30 mL of citrate phosphate buffer at pH 5.0, 6.0 and 7.0, and incubated in 6 temperature gradients (20, 30, 40, 50, 60 and 70 ℃) for 30 min. The absorbance was measured at 620 nm after appropriate dilution, and the pigment retention rate was calculated. The pigment retention rate was calculated according to equation (1):
Pigment retention rate/% = ×100 Equation (1)
1.3.2 Effect of daylight illumination on the stability of phycocyanin [19]
Two groups of 1 mg/mL aqueous phycocyanin solution were taken, one group was irradiated under a single light source (sunlight) and the other group was stored away from light, and then diluted appropriately after 12, 24, 36, 48, 60, and 72 hours, respectively, and the absorbance value was measured at 620 nm to compare the changes in the retention rate of phycocyanin pigments.
1.3.3 Effect of pH on the stability of algal blue protein
Take 0.1 g of alginate powder and dissolve it in 100 mL of citrate phosphate buffer with pH value of 5.0, 5.5, 6.0, 6.5 and 7.0 respectively, there are 5 groups in total, and take samples at 30 min intervals to dilute appropriately, and measure the absorbance value at the wavelength of 620 nm, and then compare the changes of the preservation rate of the alginate pigment.
1.3.4 Effect of food additives on the stability of algal cyanoproteins [20-21]
Take 100 mL of algal blue protein solution with a concentration of 1 mg/mL, and add the following additives in order according to the maximum additive amount of food additives stipulated in GB 2760-2011 Standard for the Use of Food Additives: glucose (5 g), sucrose (5 g), sodium chloride (5 g), sorbitol (0.003 g), sodium benzoate (0.000 2 g), ascorbic acid (0.002 g), and sodium benzoate (0.000 2 g), and the following additives are added to the solution. 0.002 g). After 24, 48 and 72 hours of exposure to sunlight at room temperature and appropriate dilution, the absorbance value at 620 nm was measured to compare the changes in the retention rate of phycocyanin pigments. The effects of different concentrations of glucose and sodium chloride on the stability of algal blue protein were measured according to the above method. Select appropriate concentrations of glucose, sodium chloride and sorbitol and add them into the aqueous solution of phaeocyanin, and carry out the test according to the above method to observe the change of pigment retention rate.
2 Results and analysis
2.1 Effect of temperature on the stability of phycocyanin
The effect of temperature on the stability of phycocyanin is shown in Fig. 1.
As can be seen from Fig. 1, the pigment retention rate of algal blue protein decreased with the increase of temperature when it was placed at different temperatures for 30 min. When the temperature was 20 ℃
The pigment retention rate of alginate stored at 40 ℃ was almost unchanged; the pigment retention rate of alginate stored at 50 ℃ and 60 ℃ decreased by 11.68% and 20.71%, respectively, compared with that of the initial one after 30 min, and the pigment retention rate of alginate stored at 70 ℃ showed the greatest decrease, which was 58.58% lower than that of the initial one.
High temperature will destroy the structure of algal blue protein and cause its denaturation, resulting in a decrease in the pigment retention rate of algal blue protein. It can be seen from the results that phycocyanin has the highest and most stable pigment retention rate between 20 ℃ and 40 ℃. Therefore, high temperature storage should be avoided below 40 ℃.
2.2 The effect of light on the stability of phycocyanin
The effect of sunlight illumination on the stability of phycocyanin is shown in Fig. 2.
As can be seen from Fig. 2, under the irradiation of room temperature and single sunlight source, the pigment retention rate of the algal blue protein solution decreased greatly from 48 h. At the same time, the color fading was obvious, and the color gradually changed from sapphire blue to light blue from 48 h, and became almost colorless and transparent at 60 h. The pigment retention rate decreased by 59.31% compared with that at 0 h, and the rate of the pigment retention rate was only 29.26% of the initial one at 72 h. The color retention rate of the solution decreased from 0 h to 60 h, and the color retention rate of the solution was only 29.26% of the original one at 72 h. After 72 h, the pigment retention rate was only 29.26%. The pigment retention rate of phycocyanin stored at room temperature under the condition of light protection was higher than that of sunlight, but the effect was not great, and the pigment retention rate of phycocyanin at 72 h was 13.51% higher than that of sunlight. It can be concluded that the sensitivity of phycocyanin to heat is greater than that to light, but light also has a certain effect on the pigment stability of phycocyanin. Therefore, phycocyanin should be stored under light-proof conditions.
2.3 Effect of pH value on the stability of algal blue protein
The effect of pH on the stability of phycocyanin is shown in Fig. 3.
Figure 3 shows that the pigment retention rate of phycocyanin solution at pH 5.0, 5.5, 6.0, 6.5 was small, and the pigment retention rate was kept in the range of 95.49%~102.19%; and it can be seen that the phycocyanin was the most stable and the highest pigment retention rate was found at pH 6.0. At pH 7.0, the pigment retention rate decreased greatly, from 100 % to 87.46 % gradually. This may be due to the fact that the alkaline condition damaged the structure of phycocyanin, so it should be preserved in neutral condition instead of alkaline condition.
2.4 Effect of additives on the stability of algal blue proteins
The effect of food additives on the stability of algal blue proteins is shown in Fig. 4.
Additive type
Fig. 4 Effect of food additives on the stability of algal blue proteins
Fig.4 The influence of food additives on stability of phycocyanin
Figure 4 shows that the retention rate of phycocyanin pigments in phycocyanin solutions with different additives increased and then decreased during 72 h of storage at room temperature under sunlight. This may be due to the incomplete dissolution of phycocyanin at the beginning. The highest pigment retention was observed in the alginate with glucose, sorbitol and ascorbic acid, which decreased from the initial 100 % to 78.10 %, 69.08 % and 67.24 %, respectively, which was significantly higher than that of the blank group (50.90 %). This may be attributed to the fact that the additives can protect the color of the algal blue protein and increase its pigment retention rate. However, the solution of phycocyanin with ascorbic acid produced a large amount of precipitation. Therefore, glucose, sodium chloride and sorbitol were selected for further study.
2.5 Effect of glucose concentration on the stability of algal blue proteins
The effect of glucose concentration on the stability of phycocyanin is shown in Fig. 5.
As shown in Fig. 5, the color retention rate of glucose-added phaeocyanin increased after 24 h, and then decreased with time. This may be due to the color protection effect of glucose on phycocyanin. The pigment retention rate of the alginate without glucose did not change much after 24 h at room temperature. When the concentration of glucose was 10 mg/mL, the absorbance value of phycocyanin increased greatly after 24 h, and the pigment retention rate of phycocyanin increased by 16.15%, which was 12.62% higher than that of phycocyanin without added glucose; the pigment retention rate of phycocyanin with added glucose at 10 mg/mL reached 78.09%, which was 27.19% higher than that of phycocyanin without glucose. After 72 h, the color retention rate of the solution with 10 mg/mL glucose reached 78.09%, which was 27.19% higher than that of the solution without glucose, and then the retention rate of alginate color tended to slow down as the concentration of glucose solution increased. Therefore, for the purpose of cost saving, 10 mg/mL of glucose was chosen for the next study.
2.6 Effect of sodium chloride concentration on the stability of algal blue proteins
The effect of NaCl concentration on the stability of algal blue protein is shown in Fig. 6.
Fig. 6 Effect of sodium chloride concentration on the stability of algal blue protein
As can be seen from Fig. 6, the pigment retention rate of the alginate without NaCl remained almost unchanged after 24 h, while the absorbance values of the alginate with NaCl increased, which was attributed to the protective effect of NaCl on the color of the alginate to inhibit the denaturation of the alginate. The color retention rate of the solution with 10 mg/mL NaCl was significantly higher than that of the blank group after 72 h, reaching 75.90%, and then leveled off. Therefore, in order to save the cost of the experiment, 10 mg/mL NaCl was chosen for the next study.
2.7 Effects of sorbitol, sodium chloride and glucose on the stability of phycocyanin
The effects of sorbitol, NaCl and glucose on the stability of phycocyanin are shown in Figure 7.
Figure 7 shows the complex color protection effect of the three additives on phycocyanin. The pigment retention rate of the alginate solutions increased to different degrees after 24 h at room temperature under sunlight, which was attributed to the color protection effect of the additives. In the blank group, the pigment content of the alginate solution remained almost unchanged after 24 h, and then decreased rapidly; the absorbance value of the alginate solution with the addition of sorbitol, dextrose and sodium chloride increased the most obviously, which was 41.29% higher than that at 0 h, and 38.38% higher than that of the alginate solution without the addition of the additives; and the color preservation was 23.01% higher than that of the blank group at 72 h. The effect of color preservation was very obvious. After 72 h, the color preservation rate was higher than that of the blank control group by 23.01%, and the color preservation effect was obvious. The stability of sorbitol-added phycocyanin was second, and its pigment preservation rate was 19.09% higher than that of the blank control group after 72 h at room temperature under sunlight. This is due to the compound effect of sorbitol, glucose and sodium chloride on alginate to play a good role in color protection and preservation, which is better than several other combinations of additives. Therefore, sorbitol, dextrose and sodium chloride can be added as compound stabilizers in alginate at a mass ratio of 1 : 1 : 0.3.
2.8 Effect of three additives on the stability of algal blue proteins
The initial pictures of phycocyanin (without additive) and phycocyanin (with additive) at (4±5)°C and (25±5)°C are shown in Fig. 8, and the pictures of phycocyanin (without additive) and phycocyanin (with additive) at (4±5)°C and (25±5)°C after 14 d are shown in Fig. 9, and the effects of three additives on the stability of phycocyanin are shown in Fig. 10.
Figures 8, 9 and 10 show the changes in pigment content of phycocyanin after the addition of glucose, sodium chloride and sorbitol as stabilizers for 14 d. The pigment retention rate of phycocyanin solutions decreased with the increase of storage days and varied under different conditions. The pigment retention of phycocyanin solutions decreased with the increase of storage days, and the pigment retention varied under different storage conditions. The most suitable storage condition for phycocyanin solution was 4 ℃ with preservative, and its pigment retention rate only decreased by 30.21% after 14 d of storage, which was 54.5% higher than that of phycocyanin stored at 4 ℃ without additive. However, the pigment retention rate of the unadditive alginate solution was almost zero after 14 d of storage at 25 ℃, with almost total loss of phycocyanin, and the pigment retention rate of the additive solution was 16.1% higher than that of the unadditive one. The pigment retention rate of the additive solution was significantly higher than that of the unadditive one at 25 ℃ and 4 ℃, which was attributed to the excellent color protection and anticorrosive effect of the three additives on the phycocyanin. This is due to the fact that the combination of the three additives has a good effect on the color protection and preservation of phycocyanin. Therefore, alginate is suitable for storage at low temperature with additives.
3 Conclusion
Differences in temperature, sunlight and pH all affect the storage stability of phycocyanin, with temperature having the most pronounced effect on the stability of phycocyanin and sunlight having a lesser effect on the stability of phycocyanin.
Appropriate concentrations of sorbitol, dextrose and sodium chloride can obviously protect the color of alginate and preserve it, and do not affect its properties. In this experiment, the three additives were added into the aqueous solution of phycocyanin, and it was found that they had obvious improvement effects on the storage stability of phycocyanin pigments. The compound additives added to phycocyanin can be widely used in food, cosmetics and other fields, and has high application value.
References:
[1] Hedenskog G, Hofsten A V. The Ultrastructure of Spirulina platensis -A New Source of Microbial Protein[J].Physiologia Plantarum, 1970, 23(1):209- 216
[2] Belay A, Ota Y, Miyakawa K, et al. Current knowledge on potential health benefits of Spirulina[J]. Journal of Applied Phycology, 1993, 5(2):235-241
[3] Serena Benedetti, Sara Rinalducci, Francesca Benvenuti, et al. Pu - rification and characterization of phycocyanin from the blue-green alga Aphanizomenon flos-aquae [J]. Journal of Chromatography B, 2006, 833(1):12-8
[4] Jaouen P, Lépine B, Rossignol N, et al. Clarification and concentra- tion with membrane technology of a phycocyanin solution extracted from Spirulina platensis[J]. Biochemical Society Transactions, 1999, 13(12):877-881
[5] Cohen Z. Spirulina platensis (Arthrospira), Physiology, Cell-Biology and Biotechnology [J]. Quarterly Review of Biology, 1997 (3):353 - 354
[6] Jespersen L, Stromdahl L D, Olsen K, et al. Heat and light stability of three natural blue colorants for use in confectionery and bever- ages[J]. European Food Research & Technology, 2005, 220 (3/4): 261-266
[7] Yin Gang, Li Chen. Separation and purification of algal bile proteins and polysaccharides from Spirulina and product characterization [J]. Fine Chemical Industry, 1999, 16(2):10-13
[8] PENG Weimin, SHANG Shutian, FU Youlan, et al. Studies on the nature of bile protein in Spirulina obtususus[J]. Journal of China Agricultural University, 1999, 4(C00):35-38
[9] Hui Qiusha. Research overview of natural pigments[J]. Northern Pharmacology, 2011, 8(5):3-4
[10] GUO Fenghua,WANG Hui . Research on the extraction and application of natural pigments[J]. Shandong Food Fermentation , 2007, 36(4):36-38
[11] Ch R,González R,Ledón N,et al. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects[J]. Cur- rent Protein & Peptide Science, 2003, 4(3):207-216
[12] Eriksen N T. Production of phycocyanin--a pigment with applica - tions in biology, biotechnology, foods and medicine[J]. Applied Mi- crobiology & Biotechnology, 2008, 80(1):1-14
[13] Fretland D J, Djuric S W, Gaginella T S. Eicosanoids and inflamma DOI: 10.3969/j.issn.1005-6521.2017.12.008
#phycocyanin #Spirulinaobtusususiae #phycocyaninpowder
9 notes
·
View notes
Text
Awarded 10% from the VA for tinnitus gets you nothing. No healthcare. Nothing. Funny thing happened after the audiology department where I’d been a chronic patient cut me loose. I called the audiology department at the VA and was told to come in. I did. I don’t have a primary provider - everything that happens in the VA system starts with your primary. So, now a patient of VA Audiology for about four years, I went to Alpha Desk (Registration) and fessed up that I am a patient without a primary. That was back in March after the Branson Tour. Alpha set me up an appointment to see a primary (24July), but first, they need lab work on me (yesterday). I showed up nearly a full hour early for that 1:10 appointment. Checked in with the desk and was told to have a seat. I watched the waiting area fill, then empty and fill again. Others who had also been told to have a seat got terse and asked to know when they were going to be seen. Most ended up leaving without having been seen. Forty five minutes passed my scheduled appointment I too asked. New woman at the desk. The prior was a lunchtime fill in who had clearly screwed up everything. Within five minutes I was called to the back. The lab tech.s were all sitting around bored. And the manager? No where to be found. Welcome to government healthcare at its finest.
Why put up with this? Because in another year, my government insurance for having retired from the military is going to expire and then transform into MediCare. At present, my medication comes by way of Express Scripts which may or may NOT get things right. I’m diabetic with high blood pressure. All of which increases my likelihood of another stroke. I’ve been out of glucose meter test strips for a month. They finally arrived today. The VA has an in house pharmacy. I need to see an Ear, Nose, Throat specialist. I can see one at the civilian hospital but their audiology cut me loose. I’m trying to get all of my healthcare under one roof.
3 notes
·
View notes