#Aliphatic Carbon
Explore tagged Tumblr posts
Text
Polyethyleneimine: An Important Building Block in Various Applications In Industry
What is Polyethyleneimine?
Polyethyleneimine (PEI) is a polymeric compound composed of repeating units of ethyleneimine monomers. It is a white solid polymer that is soluble in water. PEI is classified as a branched or linear cationic polymer that is formulated through the polymerization of aziridine. The polymer's molecular weight can range from 300 to over 10,000 Daltons depending on its intended use. Properties and Structure of PEI
PEI is a polymer that contains secondary nitrogen atoms along its backbone that provide cationic charge centers. These nitrogen atoms can interact and form electrostatic bonds with negatively charged macromolecules or surfaces. The cationic nature of PEI allows it to act as a polycation and complex or condense with negatively charged substances like DNA or RNA. PEI is also very hygroscopic due to its numerous amine groups that attract water molecules. Its branched structure provides greater buffering capacity and DNA condensation ability compared to the linear form of PEI. Uses in Gene Delivery
One of the most important uses of Polyethyleneimine is in non-viral gene delivery applications. Its cationic charge allows it to efficiently bind and compact negatively charged nucleic acids like plasmids, forming nano-sized polyplexes that can transfect cells. The polyplexes are effectively endocytosed by cells and the low pH of endosomes triggers PEI to buffer the compartment, releasing the DNA from the complex. This "proton sponge" mechanism of PEI facilitates effective intracellular delivery and expression of the gene of interest. It is commonly used as a transfection agent for in vitro studies as well as in vivo gene therapies and vaccines. Role in Biomaterials and Tissue Engineering
Given its favorable properties, PEI also finds extensive use as biomaterials for tissue engineering applications. Its cationic nature permits binding of PEI to negatively charged components of the extracellular matrix like glycosaminoglycans. This helps direct cell adhesion, growth, and function. PEI can act as a coating onto implants and scaffolds, promoting their integration with surrounding tissues. It allows bioconjugation of signaling cues to influence cell behavior in regenerative therapies as well. The tunable properties of PEI through controlling its molecular weight or branching also expand its utility in the design of biomaterials.
In addition to the major uses mentioned above, PEI has applications in many other fields due to its versatile characteristics. It acts as a flocculating agent in wastewater treatment processes to remove pollutants and purify water. PEI finds increasing importance as a hardener in epoxy resins used in manufacturing infrastructure and electronics. Its buffering ability makes it valuable as a non-corrosive alternative to replace hazardous chemicals in industrial cleaning agents as well. Overall, the unique polymeric structure and properties of PEI designate it as an essential building block material with myriad applications.
Get more insights on Polyethyleneimine
Unlock More Insights—Explore the Report in the Language You Prefer
French
German
Italian
Russian
Japanese
Chinese
Korean
Portuguese
Alice Mutum is a seasoned senior content editor at Coherent Market Insights, leveraging extensive expertise gained from her previous role as a content writer. With seven years in content development, Alice masterfully employs SEO best practices and cutting-edge digital marketing strategies to craft high-ranking, impactful content. As an editor, she meticulously ensures flawless grammar and punctuation, precise data accuracy, and perfect alignment with audience needs in every research report. Alice's dedication to excellence and her strategic approach to content make her an invaluable asset in the world of market insights.
(LinkedIn: www.linkedin.com/in/alice-mutum-3b247b137 )
#Polyethyleneimine#Aliphatic Carbon#Versatile Polymer#PEI#Linear Polymer#Cyclic Amine#Cationic Polymer#Biopolymer#Flocculant#Emulsifier#Nanoparticle Synthesis
0 notes
Text
Figure 12.3 shows a portion of a hypothetical structure of a generic humate molecule.

It is, of course, essential to realize that Fig. 12.3 does not depict a specific humate molecule and, in fact, there is no such thing as a single specific humate species in any of the three classes.
"Environmental Chemistry: A Global Perspective", 4e - Gary W. VanLoon & Stephen J. Duffy
#book quotes#environmental chemistry#nonfiction#textbook#humic substance#humate#hypothetical#aliphatic#aromatic#functionality#polymer#generic#hydrogen#carbon#oxygen#nitrogen
0 notes
Text
Some Spider Silk Facts
The strongest spider silk is produced by Darwin’s Bark Spider, which is twice as strong as any gossamer recorded before. It has a tensile strength of up to 520 megajoules per cubic metre.
Gossamer is stronger than steel and kevlar, and it has been suggested that a single pencil-width strand of the stuff could stop a Boeing 747 in its tracks.
The reason we can break such a strong material is because it is 20 times thinner than a human hair, usually measuring just 0.003mm across.
There are seven types of silk produced by a spider of Araneus Diadematus: dragline/major ampullate silk (which forms the basic structure of a web and also the web the spider itself dangles from), minor ampullate silk (which forms the auxiliary spiral in the centre of a web), flagelliform silk (which forms the core fibres of the ‘capture spiral’) , aggregate silk (forming the aqueous coating on a web), cylindriform silk (tough outer silk of an egg sac), aciniform silk (soft inner silk of an egg sac also used for swathing prey) and pyriform silk (which is used as a sort of cement for joining and attaching different parts of the web).
These little architects have seven different silk glands, as a result, all of which are employed by the spinnerets at the spider’s rear end.
Gossamer is made up of a blend of different proteins linked together in a chain: it consists of proteins rich in nonpolar (example; fats, oils, gasoline and petrol) and hydrophobic (example; oils, waxes and steroids) amino acids like glycine (C₂H₅NO₂ - white solid) and alanine (C3H7NO2 - white powder) but no (or very little) tryptophan (C11H12N2O2).
Glycine is a compound our bodies use to make protein. It is an antioxidant, anti-inflammatory, cryoprotective and immunomodulatory in peripheral and nervous tissues.
Alanine is an alpha amino acid also used to make proteins. It is a hydrocarbon. Hydrocarbons are divided into two classes in biochemistry: aromatic compounds and aliphatic compounds (from the Greek word ‘aleiphar’ - fat/oil). Alanine falls into the latter category. Another example of an aliphatic compound is squalene, which is found in shark livers and the stomach oil of birds.
So spidersilk seems to be mainly made up of carbon, hydrogen, nitrogen and oxygen, with more hydrogen and carbon than any other element, making it an aliphatic hydrocarbon based substance. (I think. I’m not a scientist, I’m just making an educated guess.)
So why have we not spun our own clothes / harvested spidersilk? Multiple reasons.
The main reason being that spiders can’t be farmed like silkworms due to the fact that they will cannibalise each other in close proximity. The silk is so fine that it would take harvesting from 400+ spiders to make a single yard of silk. And the silk also hardens when exposed to air which makes it difficult to work with.
This silk hardens as it passes through the spider’s spinnerets. Also, the problem with trying to genetically engineer spidersilk ourselves is that we can only partially replicate its chemical makeup.

Also here’s the heckin chungus of a spider in question, with the world’s strongest web:

He’s buff and he knows it. Proud chonky fella. He’s cute. 😭🥺
15 notes
·
View notes
Photo

New zirconia-based catalyst can make plastics upcycling more sustainable
A new type of catalyst breaks down polyolefin plastics into new, useful products. This project is part of a new strategy to reduce the amount of plastic waste and its impact on our environment, as well as recover value that is lost when plastics are thrown away. The catalyst was developed by a team from the Institute for Cooperative Upcycling of Plastic (iCOUP), a U.S. Department of Energy, Energy Frontier Research Center. The effort was led by Aaron Sadow, the director of iCOUP, scientist at Ames National Laboratory, and professor at Iowa State University; Andreas Heyden, professor at the University of South Carolina; and Wenyu Huang, scientist at Ames Lab and professor at Iowa State. The new catalyst is made only of earth-abundant materials, which they demonstrated can break carbon-carbon (CC) bonds in aliphatic hydrocarbons.
Aliphatic hydrocarbons are organic compounds made up of only hydrogen and carbon. Polyolefin plastics are aliphatic hydrocarbon materials composed of long chains of carbon atoms linked together to form strong materials. These materials are a big part of the plastic waste crisis. Wenyu Huang said, "More than half of produced plastics so far are polyolefin based."
Read more.
#Materials Science#Science#Catalysts#Polymers#Plastics#Recycling#Polyolefins#Zirconium#Iowa State University#University of South Carolina
32 notes
·
View notes
Text

Dwarf planet Ceres: Building blocks of life delivered from space
The dwarf planet is a bizarre, cryovolcanic world. However, the organic deposits discovered on its surface so far are unlikely to originate from its interior.
Organic molecules are among the necessary inventory of life-friendly worlds. On Earth, the compounds of carbon, hydrogen and – in smaller quantities – other elements form the basic building blocks of all life. In recent years, researchers have found such molecules at great distances from the Sun: on trans-Neptunian objects, comets, and far-away asteroids. These bodies are thought to be largely unaltered remnants from the early days of the Solar System. The building blocks of life may therefore have been part of their “basic configuration” from the very beginning and possibly reached the inner Solar System only later.
For the current study, the researchers looked for previously unknown deposits of organic material on dwarf planet Ceres. With its location in the middle of the asteroid belt between the orbits of Mars and Jupiter, the body is neither clearly native to the inner nor the outer Solar System. According to earlier studies, this location could even be its birthplace. Scientists are therefore interested in the origin of Ceres’s organic components. Did they originate locally in the asteroid belt? Or did they arrive later?
Searching for Organics from afar
Evidence of deposits of organic material was already found during the early stages of the Dawn mission. The Dawn spacecraft reached Ceres in March 2015 and accompanied it for about three and a half years. During this time, the scientific camera system and the spectrometer on board scanned the entire surface of the dwarf planet. Potential patches of organic material can be detected from the camera data: the brightness of the light reflected from these areas increases noticeably with increasing wavelength. The spectrometer splits the light into many more wavelengths than the camera and can therefore prove or disprove the presence of organics. Unfortunately, remote data is not sufficient to identify individual types of molecules beyond doubt. However, it is certain that the discovered deposits consist of organic compounds that have a chain-like structure. Researchers refer to such molecules as aliphatic hydrocarbons.
The authors of the current study have now used artificial intelligence to comb the entire surface of the dwarf planet for traces of aliphatic organic molecules. “Sites of such organic molecules are actually rare on Ceres, and devoid of any cryovolcanic signatures” says first author Ranjan Sarkar from the MPS, summarizing the results. The vast majority of deposits can be found along the edge or near the large Ernutet crater in the northern hemisphere of the dwarf planet. Only three are located at a greater distance from it. Two patches were not previously known. A closer look at the geological structures at the locations of the organic material allows further conclusions. “At none of the deposits do we find evidence of current or past volcanic or tectonic activity: no trenches, canyons, volcanic domes or vents. Furthermore, there are no deep impact craters nearby,” says Martin Hoffmann from MPS.
Impacts from distant neighbors
During the Dawn mission, Ceres had turned out to be an extraordinary, cryovolcanic world. Under its surface, a watery brine is hidden, which in some places has been seeping to the surface until recently. “Of course, the first assumption is that Ceres' unique cryovolcanism has transported the organic material from the interior of the body to the surface,” says Andreas Nathues from MPS, head of the camera team. “But our results show otherwise,” he adds. At the sites of cryovolcanic activity, there is no proof of organic matter. And where organic compounds have been reliably detected, there is no evidence of deep or surface activity.
The researchers therefore argue that the impact of one or more asteroids from the outer asteroid belt introduced the organic material. Computer simulations show that these bodies are among the ones that most frequently collided with Ceres. Since the not-too-distant neighbors do not pick up much speed, only little heat is generated upon impact. Organic compounds can survive these temperatures.
“Unfortunately, Dawn can't detect all types of organic compounds,” Andreas Nathues points out. It is quite likely that building blocks of life were also formed in Ceres' underground ocean and perhaps even reached the surface – or are still doing so. “However, the organic deposits that have been reliably detected with Dawn so far likely do not originate Ceres itself,” he explains. Nathues continues by saying that a future lander mission would be needed to detect organic material from the interior of Ceres.
About the mission
NASA’s Dawn mission studied two bodies in the asteroid belt up close: the protoplanet Vesta from 2011 to 2012, and the dwarf planet Ceres from 2015 to 2018. The mission’s scientific camera system, the Dawn Framing Cameras, were developed, built, and operated during the mission under the leadership of MPS. The VIR spectrometer was provided by the Italian Space Agency ASI.
IMAGE: Surface of dwarf planet Ceres. The sites of organic material are shown as or in red boxes. The vast majority of sites are found near the Ernutet crater in the northern hemisphere. Credit MPS
1 note
·
View note
Text
Curing Agents Market
Curing Agents Market Size, Share, Trends: Huntsman Corporation Leads
Growing demand for waterborne and eco-friendly curing agents
Market Overview:
The global Curing Agents market is projected to grow at a CAGR of 6.2% from 2024 to 2031. The market value is expected to increase significantly during this period. Asia-Pacific currently dominates the market, accounting for the largest share of global revenue. Key metrics include increasing demand from the construction and automotive industries, growing adoption of eco-friendly curing agents, and rising investments in research and development for innovative products.
The Curing Agents market is growing steadily, driven by expanding end-use industries and an increasing need for high-performance materials in a variety of applications. Technological improvements in curing agent formulations are driving market growth, with a focus on increasing curing efficiency, lowering environmental impact, and improving overall product quality.
DOWNLOAD FREE SAMPLE
Market Trends:
The curing agents market is undergoing a considerable change towards waterborne and eco-friendly formulations, owing to rigorous environmental regulations and rising consumer awareness. This trend is especially noticeable in the coatings and adhesives markets, where low-VOC (Volatile Organic Compound) and solvent-free solutions are gaining popularity. Manufacturers are investing in bio-based curing agents made from renewable resources like plant oils and natural amino acids. These ecologically friendly options perform similarly to standard curing chemicals while lowering carbon emissions and enhancing workplace safety. Waterborne epoxy curing chemicals, for example, are becoming increasingly popular in industrial flooring applications due to their high chemical resistance and durability without the environmental concerns associated with solvent-based systems.
Market Segmentation:
The Epoxy curing agents segment dominates the worldwide Curing Agents market. This dominance is due to the widespread usage of epoxy resins in a variety of applications, including paints, adhesives, composites, and electrical laminates. Epoxy curing agents have an important role in establishing the final attributes of cured epoxy systems, including strength, chemical resistance, and durability.
Epoxy curing agents' market leadership is due in large part to their versatility. Amine-based curing agents, including as aliphatic and aromatic amines, are particularly popular due to their superior curing qualities and ability to impart high performance attributes to the finished product. Cycloaliphatic amine curing agents, for example, are commonly employed in marine coatings due to their higher water resistance and corrosion prevention.
Market Key Players:
Huntsman Corporation
Evonik Industries
BASF SE
Hexion Inc.
Dow Chemical Company
Contact Us:
Name: Hari Krishna
Email us: [email protected]
Website: https://aurorawaveintellects.com/
0 notes
Text
Cas No: 109-72-8 | n-butyllithium
Understanding n-Butyllithium (CAS No: 109-72-8): A Vital Reagent in Organic Synthesis
In the world of chemistry, certain compounds play pivotal roles in a wide array of reactions, and n-butyllithium (CAS No: 109-72-8) is one such compound that stands out due to its versatility and importance in organic synthesis. It is a powerful, highly reactive chemical used primarily in laboratories and industrial settings, where it plays an essential role in forming carbon-carbon bonds and facilitating other reactions that are fundamental to the creation of complex organic molecules.
This blog will explore the chemical properties, uses, safety measures, and environmental concerns related to n-butyllithium.

What is n-Butyllithium?
This compound exists as a colorless to pale yellow liquid or as a solution in hydrocarbons, such as hexane or heptane, and is highly flammable. It is widely regarded as one of the most important organolithium reagents used in organic chemistry due to its strong nucleophilic properties.
It is a highly reactive substance, often used in a variety of reactions that require a strong base or nucleophile. One of its most important characteristics is that it readily reacts with a wide range of electrophiles, making it invaluable in synthetic chemistry.
Chemical Properties of n-Butyllithium
n-Butyllithium is a potent organometallic reagent that behaves as a strong nucleophile and base. Its key properties include:
Reactivity: n-Butyllithium reacts quickly with a variety of electrophiles and is a strong base. This allows it to initiate polymerization reactions, form carbon-carbon bonds, and deprotonate weak acids, making it an essential tool in synthetic chemistry.
Solubility: It is typically dissolved in aliphatic hydrocarbons like hexane, heptane, or diethyl ether. This solubility allows n-butyllithium to be easily handled and integrated into chemical reactions in the lab.
Electrophilic Reactivity: The lithium cation (Li+) in n-butyllithium can readily engage with electrophilic species, allowing it to participate in reactions like nucleophilic substitution, addition to carbonyl compounds, and other crucial transformations.
High Reactivity with Water and Air: n-Butyllithium is highly sensitive to moisture and air. It reacts with water to release hydrogen gas, a highly flammable product, and it is also reactive with carbon dioxide. These reactions are so vigorous that they must be handled under an inert atmosphere like nitrogen or argon.
Uses of n-Butyllithium in Organic Chemistry
n-Butyllithium has a broad range of applications in both academic research and industrial processes.
Preparation of Organometallic Compounds: n-Butyllithium is used to synthesize other organolithium reagents. By reacting n-butyllithium with different electrophilic compounds, a variety of functionalized lithium derivatives can be generated, which are crucial for creating more complex organic molecules.
Deprotonation Reactions: Due to its strong basicity, n-butyllithium is commonly used to deprotonate compounds, especially those with acidic protons such as alkynes, aromatic compounds, and certain heterocycles. This leads to the formation of nucleophilic organolithium intermediates, which can then participate in various addition and substitution reactions.
Polymerization of Olefins: n-Butyllithium is used in anionic polymerization, particularly in the production of synthetic rubbers and plastics. It can initiate the polymerization of dienes and other monomers, leading to the creation of polymers with specific properties. This makes n-butyllithium a critical component in the synthetic rubber industry.
Lithiation of Arenes: One of the hallmark reactions involving n-butyllithium is the lithiation of aromatic compounds, where the butyllithium reagent is used to introduce a lithium atom into the aromatic ring. This reaction is often a key step in the synthesis of a wide range of organic molecules, including pharmaceuticals and agrochemicals.
Formation of Carbon-Carbon Bonds: n-Butyllithium is widely used in coupling reactions, where it helps in the formation of carbon-carbon bonds, crucial for constructing complex molecules. The formation of these bonds is vital for the synthesis of various chemicals, from basic organic compounds to more complex pharmaceutical agents.
Safety Considerations and Handling of n-Butyllithium
Due to its extreme reactivity, handling n-butyllithium requires significant caution and adherence to strict safety protocols. Below are some of the critical safety considerations:
Storage and Handling: n-Butyllithium must be stored in tightly sealed containers, away from air and moisture. Typically, it is kept in containers filled with inert gases like nitrogen or argon to minimize the risk of reaction with oxygen and water. It is often stored under a solvent such as hexane to ensure stability.
Reaction with Water: One of the most hazardous reactions of n-butyllithium is its reaction with water. This results in the production of flammable hydrogen gas and the formation of a lithium hydroxide compound. Therefore, it is essential to keep n-butyllithium away from any water source, including humidity.
Fire Hazards: n-Butyllithium is highly flammable. In case of fire, it must be extinguished with appropriate fire suppression agents, such as Class D fire extinguishers designed for metal fires. Water should never be used to put out a fire involving n-butyllithium.
Protective Equipment: Laboratory personnel working with n-butyllithium must wear appropriate personal protective equipment (PPE), including flame-resistant lab coats, gloves, and goggles. Additionally, a well-ventilated fume hood is essential to avoid inhalation of any vapors or fumes released during handling.
Spill and Disposal: In the case of a spill, n-butyllithium should be treated with extreme care. All reactions involving spills should be carried out under a fume hood, and the area should be evacuated until appropriate cleanup can be performed. Disposal must be done following hazardous waste protocols, as n-butyllithium can react with both water and air.
Environmental Impact and Regulations
Due to its reactivity, environmental concerns regarding n-butyllithium are significant. Uncontrolled release of n-butyllithium into the environment can cause dangerous chemical reactions and pollution. To mitigate these risks, stringent regulations govern its transportation, storage, and disposal, and all users must adhere to relevant safety guidelines and disposal methods.
In industrial applications, n-butyllithium is used in closed systems to minimize exposure to the environment. Careful monitoring of chemical handling and disposal is necessary to ensure minimal environmental impact.
Conclusion
n-Butyllithium is a crucial reagent in modern organic chemistry, known for its reactivity and versatility in facilitating complex reactions. Whether it's for polymerization, deprotonation, or the formation of key carbon-carbon bonds, this compound remains indispensable to chemists around the world. However, due to its highly reactive nature, appropriate safety precautions must always be taken when working with n-butyllithium. As research continues and new synthetic methods evolve, the role of this vital compound in chemical and industrial processes is likely to expand further.
URL: For more information, visit Vasista Pharma : n-butyllithium (CAS No: 109-72-8)
1 note
·
View note
Text
How C11 Solvent Manufacturers Are Meeting Industry Demands for Eco-Friendly Solutions
In recent years, the industrial landscape has witnessed a significant shift toward sustainability, prompting businesses to seek eco-friendly solutions across various sectors. Among these solutions is the adoption of C11 solvents, a category of aromatic solvents that have gained popularity due to their effectiveness and reduced environmental impact. C11 solvent aromatic solvent manufacturers are at the forefront of this transition, developing innovative processes and products that align with both regulatory standards and consumer preferences for sustainability. This blog explores how these manufacturers are meeting industry demands for eco-friendly solutions while maintaining product efficacy.
1. Understanding C11 Solvent
C11 solvents are medium-chain aliphatic hydrocarbons primarily composed of 11 carbon atoms. They are known for their excellent solvency power and are commonly used in industrial applications such as paints, coatings, adhesives, and cleaning agents. Unlike traditional aromatic solvents, C11 solvents are considered less harmful to the environment and human health, making them a favorable alternative for industries looking to comply with stringent regulations and consumer expectations.
2. Embracing Green Chemistry Principles
One of the fundamental ways C11 solvent aromatic solvent manufacturers are addressing eco-friendly demands is through the application of green chemistry principles. Green chemistry focuses on designing chemical products and processes that reduce or eliminate the use and generation of hazardous substances. Manufacturers are increasingly incorporating these principles in the following ways:
a. Safer Raw Materials
To produce C11 solvents, manufacturers are prioritizing the use of renewable and non-toxic raw materials. This not only minimizes the environmental footprint of production but also ensures that the final product is safer for both the environment and end-users. By sourcing materials that have a lower environmental impact, manufacturers can produce solvents that align with sustainability goals.
b. Reducing Waste and Emissions
Many manufacturers are adopting closed-loop systems in their production processes. These systems minimize waste generation and reduce emissions by recycling solvents and other materials. By implementing such practices, C11 solvent manufacturers contribute to a circular economy, wherein resources are reused, and environmental impact is significantly lowered.
3. Developing Biodegradable Formulations
The push for sustainability has led many C11 solvent aromatic solvent manufacturers to invest in R&D for biodegradable formulations. These formulations break down naturally in the environment, reducing the risk of long-term pollution associated with traditional solvents. Biodegradable C11 solvents are designed to retain the same effectiveness as their conventional counterparts while being more environmentally friendly.
4. Meeting Regulatory Standards
As governments around the world impose stricter environmental regulations, C11 solvent manufacturers are proactively ensuring their products comply with these requirements. Many manufacturers are:
a. Adhering to REACH Regulations
In Europe, the Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH) regulation requires manufacturers to provide information on the properties and hazards of chemical substances. C11 solvent manufacturers are ensuring that their products meet these standards, enhancing their marketability and demonstrating their commitment to safety and sustainability.
b. Following VOC Emission Guidelines
Volatile organic compounds (VOCs) are regulated due to their harmful effects on air quality and human health. C11 solvent manufacturers are formulating their products to have lower VOC content, helping businesses meet emissions guidelines while providing effective solutions.
5. Investing in Sustainable Technologies
To enhance their eco-friendly credentials, C11 solvent manufacturers are investing in advanced technologies that reduce environmental impact throughout the production process. Some of these technologies include:
a. Energy-Efficient Production Methods
Manufacturers are exploring energy-efficient technologies that reduce the energy consumption associated with solvent production. This includes optimizing reaction conditions, employing waste heat recovery systems, and utilizing renewable energy sources. By reducing energy consumption, manufacturers not only lower their operational costs but also minimize their carbon footprint.
b. Innovative Extraction Techniques
Advanced extraction techniques, such as supercritical fluid extraction, allow manufacturers to obtain C11 solvents with minimal environmental impact. These methods reduce the need for hazardous solvents and enable the extraction of high-purity products while using less energy.
6. Collaboration and Industry Partnerships
To further enhance their eco-friendly initiatives, C11 solvent manufacturers are increasingly collaborating with industry stakeholders, including research institutions, NGOs, and other businesses. These collaborations aim to share knowledge, improve best practices, and drive innovation in sustainable solvent production.
a. Research and Development Initiatives
Collaborating with research institutions allows manufacturers to stay at the forefront of sustainable technologies. Joint R&D efforts focus on developing new formulations, exploring alternative raw materials, and optimizing production processes. This collaboration accelerates the development of eco-friendly solutions that meet evolving industry demands.
b. Industry Associations
Participation in industry associations helps manufacturers advocate for sustainable practices and regulatory compliance. By joining forces with other companies, manufacturers can influence policy changes, share resources, and promote the benefits of eco-friendly solutions within the industry.
7. Customer Education and Awareness
Educating customers about the benefits of C11 solvents and their environmentally friendly properties is crucial for driving demand. C11 solvent aromatic solvent manufacturers are engaging in awareness campaigns to inform consumers about the advantages of using C11 solvents over traditional aromatic solvents.
a. Technical Support and Resources
Many manufacturers provide technical support and resources to help customers understand how to effectively use C11 solvents in their applications. This includes detailed product information, usage guidelines, and case studies demonstrating successful applications. By equipping customers with knowledge, manufacturers foster loyalty and promote the use of sustainable solutions.
b. Sustainability Certifications
Manufacturers are also seeking sustainability certifications for their products, which can enhance credibility and appeal to environmentally conscious customers. Certifications like Green Seal or EcoLogo demonstrate a commitment to sustainable practices and help consumers make informed purchasing decisions.
8. Future Outlook
As the demand for eco-friendly solutions continues to grow, C11 solvent manufacturers are well-positioned to lead the way in sustainable solvent production. With ongoing investments in research, technology, and collaboration, these manufacturers are paving the path toward a more sustainable future.
9. Conclusion
The efforts of C11 solvent aromatic solvent manufacturers in meeting industry demands for eco-friendly solutions are commendable. Through the adoption of green chemistry principles, development of biodegradable formulations, and commitment to sustainability, these manufacturers are not only producing effective solvents but also contributing to a healthier planet.
As industries evolve and consumers become more environmentally conscious, the importance of sustainable practices will only increase. By continuing to innovate and invest in eco-friendly solutions, C11 solvent manufacturers are poised to play a pivotal role in shaping the future of solvent production, ensuring they meet both industry demands and environmental responsibilities. This commitment to sustainability not only benefits the environment but also positions manufacturers as leaders in a rapidly changing market.
0 notes
Text
https://www.maximizemarketresearch.com/market-report/global-polyethyleneimine-market/116628/
Polyethyleneimine (PEI), also known as poly-aziridine. It is a polymer made up of an amine group and a two-carbon aliphatic CH2CH2 monomer. In addition to branched PEIs, which contain primary, secondary, and tertiary amino groups, linear PEIs contain all secondary amines. At room temperature, linear PEIs are solids, whereas branched PEIs are liquids at all molecular weights.
0 notes
Text
Activated Carbon Market Trends, Business Growth, Opportunities and Forecast 2024-2030
The global activated carbon market size was estimated at USD 4.92 billion in 2023 and is projected to grow at a CAGR of 6.0% from 2024 to 2030. Activated carbon is used to purify liquids and gases in various end-use applications including municipal drinking water, food & beverage processing, and automotive among others. This is attributable to its beneficial properties such as cost effectiveness, easy removal of bad taste, color stability, and quick removal of bad odor.
Stringent regulations related to the maintenance of air quality, coupled with the rise in investments to set up municipal and industrial wastewater treatment plants worldwide, is likely to positively impact the market. They are commonly used in water treatment chemicals due to its ability to remove impurities and contaminants from water. The process of using the product in water treatment involves adsorption, which is the binding of contaminants to the surface of the carbon material.
Gather more insights about the market drivers, restrains and growth of the Activated Carbon Market
Activated Carbon Market Segmentation
Grand View Research has segmented the global activated carbon market report based on form, application, end use, and region.
Type Outlook (Volume, Kilotons; Revenue, USD Million; 2018 - 2030)
• Powdered
• Granular
• Others Application Outlook (Volume, Kilotons; Revenue, USD Million; 2018 - 2030)
• Liquid Phase
• Gas Phase
End Use Outlook (Volume, Kilotons; Revenue, USD Million; 2018 - 2030)
• Water Treatment
• Food & Beverage Processing
• Pharmaceutical & Medical
• Automotive
• Air Purification
• Other End Use
Regional Outlook (Volume, Kilotons; Revenue, USD Million; 2018 - 2030)
• North America
• Europe
• Asia Pacific
• Central & South America
• Middle East and Africa
Browse through Grand View Research's Petrochemicals Industry Research Reports.
• The global ceramic opacifiers market was estimated at USD 1.75 billion in 2023 and is forecasted to grow at a CAGR of 5.0% from 2024 to 2030.
• The global aliphatic hydrocarbon market size was estimated at USD 3,960 million in 2023 and is expected to grow at a CAGR of 4.8% from 2024 to 2030.
Key Activated Carbon Company Insights
Some of the key players operating in the market include Kuraray Co., Jacobi Carbons Group, and Osaka Gas Chemicals Co., Ltd. among others.
• Kuraray Co., Ltd. produces and sells activated carbon, along with other products. Its products are categorized under plastics & polymers, fibers & textiles, chemicals/elastomers & rubber, new businesses, engineering, and medical & environment related categories. The company also has a research and development department, which comprises two research and development centers in Kurashiki and Tsukuba. The company has a significant global presence with offices in countries such as the U.S., Germany, Belgium, China, Korea, Hong Kong, and India.
• Osaka Gas Chemicals Co., Ltd. is a Japan-based company, which operates through two major business segments, namely, advanced material solutions and absorption & separation solutions. The products of the company are categorized as fine chemical materials, surface processing, resin additives, wood preservatives, industrial preservatives, and activated carbon and its products. Activated carbon products of Osaka Gas Chemicals Co., Ltd. are marketed under the brand, Shirasagi. The company has a product development center and a distribution center in Osaka, Japan. In addition, it also has a technology center in Nara, Japan.
• CarbPure Technologies, a part of Advanced Emission Solutions, Inc., is a manufacturer and supplier of high-quality activated carbon products. Its products are majorly used in water treatment applications. The company is vertically integrated to ensure a constant and reliable supply of quality products to its customer base. CarbPure Technologies also has research and development centers to introduce new instrumentation required in unique product development techniques. The products of the company are regulated and tested under the American Society for Testing and Materials (ASTM) standards. It also has various supply agreements and partnerships to ensure a constant and quick supply of its products throughout various regions.
• Carbotech is one of the leading manufacturers and suppliers of granulated, powdered, and extruded activated carbon products. Its products are used in the food & beverages industry, as well as in fluid treatment, hydrogen purification, air & gas purification, water & wastewater treatment, and carbon molecular sieve applications. The production facility of the company is located in the industrial Ruhr conurbation of Germany. Under its sustainability initiatives, Carbotech is shifting its focus from producing powdered activated carbon to granular activated carbon owing to the highly sustainable nature of the latter. The products of the company are Halal, Kosher, and ISO certified and fulfill the REACH requirements.
Key Activated Carbon Companies:
• CarbPure Technologies
• Boyce Carbon
• Cabot Corporation
• Kuraray Co.
• CarboTech AC GmbH
• Donau Chemie AG
• Haycarb (Pvt) Ltd.
• Jacobi Carbons Group
• Kureha Corporation
• Osaka Gas Chemicals Co., Ltd.
• Evoqua Water Technologies LLC
• Carbon Activated Corporation
• Hangzhou Nature Technology Co., Ltd.
• CarbUSA
• Sorbent JSC
Recent Developments
• In January 2023, Ningbo Juhua Chemical & Science Co., Ltd. awarded a contract to Technip Energies for a Activated Carbon plant with an annual capacity of 72 kilo tons in Ningbo, Zhejiang, China. This is part of the company’s initiative to expand its petrochemical new material business.
• In January 2024, Germany-based chemical manufacturer Nordmann acquired Italy-based SD Chemicals S.r.l., a distributor of raw materials catering to the cosmetics industry catering to skin care, hair care and makeup applications. This acquisition will enable Nordmann to expand its presence and enhance customer reach.
Order a free sample PDF of the Activated Carbon Market Intelligence Study, published by Grand View Research.
#Activated Carbon Market#Activated Carbon Industry#Activated Carbon Market size#Activated Carbon Market share
0 notes
Text
Aliphatic Compounds Versus Aromatic
Aliphatic compounds are a class of organic molecules characterized by their non-aromatic structure[1]. These compounds are composed of carbon and hydrogen atoms connected by single, double, or triple bonds to form open chains or non-aromatic rings[1][2]. Key characteristics: Structure: Can be saturated (alkanes) or unsaturated (alkenes and alkynes)[1] Bonding: Single, double, or triple bonds…
0 notes
Text
Articles about 42900-54-9
"Ruthenium-Catalyzed Selective Hydrogenation of Epoxides to Secondary Alcohols Thiyagarajan, Subramanian,Gunanathan, Chidambaram supporting information, p. 9774 - 9778 (2019/12/02) A ruthenium(II)-catalyzed highly selective Markovnikov hydrogenation of terminal epoxides to secondary alcohols is reported. Diverse substitutions on the aryl ring of styrene oxides are tolerated. Benzylic, glycidyl, and aliphatic epoxides as well as diepoxides also underwent facile hydrogenation to provide secondary alcohols with exclusive selectivity. Metal-ligand cooperation-mediated ruthenium trans-dihydride formation and its reaction involving oxygen and the less substituted terminal carbon of the epoxide is envisaged for the origin of the observed selectivity." https://www.lookchem.com/CASDataBase_42900-54-9.htm
0 notes
Text
Carbon-13 NMR spectra for the same Kenyan humate samples are shown in Fig. 12.2. (...) Nevertheless, useful information is still obtainable and chemical shift assignments have been made as in Table 12.4.


"Environmental Chemistry: A Global Perspective", 4e - Gary W. VanLoon & Stephen J. Duffy
#book quotes#environmental chemistry#nonfiction#textbook#carbon 13#nmr spectra#nmr spectroscopy#nuclear magnetic resonance#humic acid#fulvic acid#aliphatic#methyl#methylene#alkyl#methylene c#ester#ether#methoxy#ethoxy#polysaccharide#acetal#ketal#aromatic#aryl#phenol#amide#carboxyl#carbonyl#kenya#soil
0 notes
Text
What is the Difference Between PC and PVC in Thermoforming?
Thermoforming is a versatile manufacturing process allowing manufacturers to mold thin and heavy gauge materials.The main principle is to heat and soften the flat hard plastic sheet, then use vacuum adsorption on the surface of the mold, and then form it after cooling. It is widely used in plastic packaging, lighting, advertising, decoration and other industries. Generally divided into heavy gauge thermoforming and thin gauge thermoforming.
Thermoformed products are processed with thermoplastic materials. The product production principle is to heat and soften the flat plastic hard sheet material, then adsorb it to the surface of the mold with vacuum, and then cool it into shape.
Thermoformed products are widely used in the electronics, electrical appliances, and food industries. , hardware tools, cosmetics industry, toy industry, daily necessities industry, medicine, health care products, automobiles, stationery, cultural and sports supplies and other categories of industries.
Plastic thermoforming materials usually use thermoplastic plastics, and common ones include polystyrene (PS), polypropylene (PP), polyethylene (PE), polycarbonate (PC), polyvinyl chloride (PVC), thermoformed acrylic(PMMA), polyethylene terephthalate(PET), polyethylene terephthalate glycol(PET-G), high molecular weight polyethylene etc. Different plastic materials have different properties and application areas.
This article will delve into the complexity and artistry of vacuum forming, pressure forming, focusing on a comparison of two common thermoplastic sheets, PC (polycarbonate) and PVC (polyvinyl chloride).
The Difference between PC and PVC Material
Raw Material
The components of PVC material are mostly polyvinyl chloride, which is a general-purpose plastic.
The components of PC material are mostly carbonate-based, which are engineering plastics.
Property
PC material has the advantages of high strength, good transparency, and good impact resistance; relatively speaking.
PVC material is softer, has average transparency, and has inferior mechanical properties to PC material.
Prices
PVC materials are cheaper, typically only half to a quarter of the price of PC materials.
Application
PC can be used as door and window glass, and PC laminates are widely used in protective windows in banks, embassies, detention centers and public places, as well as in aircraft cabin covers, lighting equipment, industrial safety baffles and bulletproof glass.
PVC materials are mostly used in the construction industry, packaging and other industries. For example, PVC materials can be used to make PVC pipes. PVC pipes have the advantages of easy construction, good corrosion resistance, low fluid resistance, good compressive strength, and long service life. We also It can be subdivided into different types such as drainage pipes, wiring pipes, medical pipes, etc. according to their uses.
Transparency
PC has high transparency, similar to glass, and can be used to make transparent plastic parts such as eyeglass frames and bottles.
In contrast, PVC has poor transparency and is usually translucent or milky white. Many plasticizers can be added to PVC, and after adding them, soft PVC can be added.
Chemical Stability
PVC has stable chemical resistant properties and is highly resistant to oxidants, reducing agents and strong acids. However, it can be corroded by concentrated oxidizing acids such as concentrated sulfuric acid and concentrated nitric acid.
It is also soluble in ethers, ketones, chlorinated aliphatic hydrocarbons and aromatic hydrocarbons. Organic solvents
PC has certain chemical corrosion resistance, resistance to weak acids and neutral oils, and is not resistant to strong acids and alkali. Dilute sodium hydroxide aqueous solution can slowly destroy it, and ammonia, amine or its 10% aqueous solution can cause it to rapidly saponify and degrade.
Mechanical Properties
PC has high mechanical strength, the highest impact strength among plastics, and can even be used as bulletproof material. This clear material forms well and is impact resistant. Its bending and tensile strength is equivalent to that of nylon, and it has high elongation and elastic modulus, but its fatigue resistance is low (less than nylon 66 ), lower compressive strength, better wear resistance (better than ABS), and small creep.
PVC has high mechanical strength, toughness and impact resistance. Its wear resistance at room temperature exceeds vulcanized rubber, and its hardness and rigidity are better than polyethylene.
Heat Resistance
PC Good heat resistance and cold resistance, wide temperature range, can be used for a long time at temperatures of -100℃-140℃, and still has certain toughness at -180℃.
PVC: Thermal stability and light resistance are poor. When it is above 100℃ or exposed to sunlight for a long time, it will decompose to produce hydrogen chloride, which will further auto-catalytically decompose and change color, resulting in a rapid decline in physical and mechanical properties and heat deflection . The use temperature is low (below 60℃)
Characteristics of PC Sheet Thermoforming
It is an amorphous plastic, and the temperatures that need to be controlled are different at different stages.
2) The thermal stability of PC sheet blister is relatively good and can be improved as the relative molecular weight increases.
3) PC sheet blister has good resistance to degeneration and good dimensional stability; but its internal stress is not easy to eliminate.
4) PC sheet blister is easily degraded when exposed to water at high temperatures, and the moisture content is required to be below 0.02% during molding.
5) Cracking may easily occur if care is not taken.
6) During the production process, the apparent viscosity of PC sheets is greatly affected by temperature and less affected by shear rate, and increases with the increase of relative average molecular weight.
7) PC sheet blister has no obvious melting point, the melt viscosity is high, and there are benzene rings in the PC molecular chain, so it is relatively rigid.
Special Processing Method for PC Sheet Thermoforming
Since the blister industry is a relatively new industry, there is currently no textbook-style process solution. We can only summarize relatively suitable processing methods through continuous exploration. In view of the process difficulties of PC material blister, including cracking due to stress, insufficient heating temperature and vacuum strength, and deformation caused by poor cooling, the industry has successively explored some special processing methods.
1) Sheet drying
In view of the fact that PC easily absorbs water, it is generally best to dry PC sheets before heating to absorb the internal moisture.
2) Control mold temperature
We must ensure that the anti-cracking ability and internal stress are balanced so that the cracking phenomenon of PC endurance boards will not occur.
3) Pay attention to molding and cooling time
It is necessary to strictly control the heating forming time not to be too long and ensure sufficient cooling time to prevent the sheet from deforming and curling.
4) Selection of blister machine
For the complex PC blister process, choosing a good blister machine is the first priority.
Characteristics of PVC Thermoforming
PVC sheet has high toughness and is not easy to burn. It will produce chlorine gas when burned, which will have a certain impact on the environment.
PVC is easy to heat seal and can be sealed with a sealing machine and high-frequency edge sealing. It is the main raw material for the production of transparent blister products. PVC sheet is a widely used and popular material.
PVC film can be divided into two types: environmentally friendly and non-environmental. It can be made into various blister packaging products such as transparent, colorful, anti-static, gold-plated, and flocked.
The main features are high transparency, good surface gloss, few crystal points, small water marks, wide use, strong impact resistance, and easy to form.
The products are widely used in toys, food, electronic products, medicine, electrical appliances, gifts, cosmetics, stationery, etc. Product outer packaging.
Conclusion
In conclusion, PC sheets and PVC sheets each have their own advantages and their disadvantages, and which one is better mainly depends on the specific use occasions and customerneeds. If you are more care about plastic part's performance and quality (such as temperature resistance, toughness, transparency), you may want to choose PC sheets. If you pay more attention to no pollution or low-cost needs during production process, then PVC may be a better choice.
0 notes
Photo
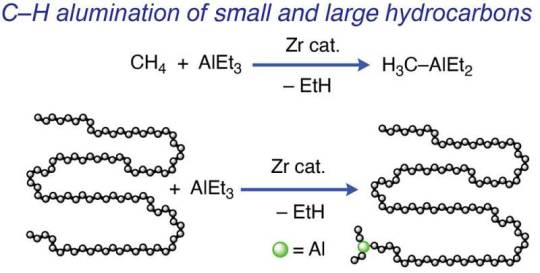
A newly developed catalyst makes single-use plastics easier to upcycle, recycle and biodegrade
Researchers created a new catalyst that transforms hydrocarbons into chemicals and materials that are higher value, easier to recycle, and biodegrade in the environment. This catalyst transforms materials such as motor oil, plastics in single-use grocery bags, water or milk bottles, and their caps, and even natural gas. It was developed by a team of scientists led by Aaron Sadow, a scientist at Ames National Laboratory, director of the Institute for Cooperative Upcycling of Plastic (iCOUP), and professor of chemistry at Iowa State University.
The new catalyst is designed to introduce functional groups into aliphatic hydrocarbons. Aliphatic hydrocarbons are organic compounds made up of only hydrogen and carbon. They typically do not mix with water, instead creating distinct layers, partly because they do not contain functional groups. Functional groups are specific groupings of atoms within molecules that have unique characteristics. Adding functional groups to these hydrocarbon chains can drastically affect their properties and make the materials recyclable.
"Methane in natural gas is the simplest of hydrocarbons with nothing but carbon-hydrogen (CH) bonds. Oils and polymers have chains of carbon atoms, linked by carbon-carbon (CC) bonds," Sadow explained.
Read more.
19 notes
·
View notes
Text
Development history and modification methods of polyketone resin (POK)!
Aliphatic polyketone (POK) resin is a high-performance thermoplastic polymer. It is a linear crystalline polymer obtained by alternating copolymerization of carbon monoxide and ethylene. By adding propylene during copolymerization process, a terpolymer polyketone material (POK-ep) with a lower melting point and easier processing can be obtained. Its structural diagram is shown in figure…

View On WordPress
#Application of POK#Chemical modification of POK#modified POK#Nature of POK#POK modification#POK modification method#POK thermoplastic crystalline plastics#polyketone resin
0 notes