#what is enthalpy of reaction?
Explore tagged Tumblr posts
Text
Feeling like a ∆S -ve ∆H +ve today.
Figure 8.23 summarises the effects of the signs of ∆H and ∆S on ∆G, and hence on the spontaneity of physical and chemical events.
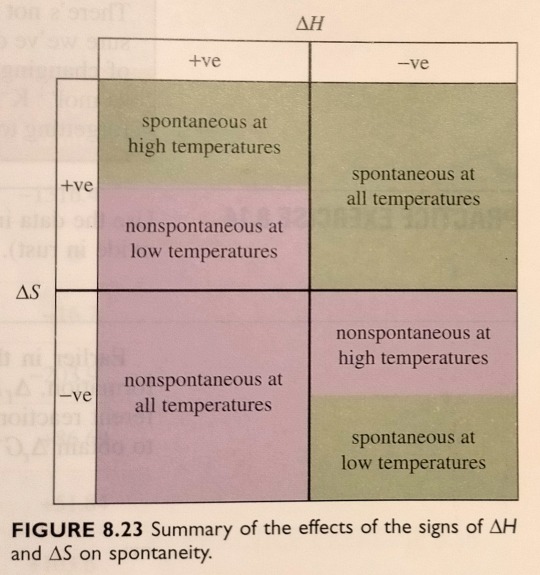
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#what are you today?#meme twist#book quotes#nonfiction#chemistry#textbook#entropy#enthalpy#thermodynamics#spontaneity#chemical reactions
15 notes
·
View notes
Note
Hello, sir! Apologies for being so formal and a little Piltov (haha), but I figured it appropriate since I wanted to give my regards to you.
For a while now, I have been working day and night to develop the proper technology to improve prosthetics, to provide neuron-to-muscle technology in said prosthetics. This requires both neuroscience and another discipline in which I’ve not yet found a name for. It is a study I’ve found little to no research in, leaving me to be the one to splice all the information together. It involves human biology as well as movement and mechanisms. Perhaps it would be called … bio … mechanics?— I’m rambling! Please excuse me, I go on when I am excited.
There are sparse opportunities in Zaun for heavy research and upper education — you and I both know how it is! As such, I have scoured Piltover’s archives (I even have a ‘library card,’ woohoo! Although I do think the attendant is breaking a rule or two by allowing that—), and I can feel a breakthrough coming soon! As I near the conclusion of my preliminary studies, I wish to thank and commemorate you for your contribution to a paper I reference heavily in my dissertation. Don’t be modest! You have no idea how much your research has aided in my efforts.
I rejoiced when I found an article dating around ten years ago from Heimerdinger’s eighth-semester internship. Does this sound familiar at all? The publication focused on ideal gas thermodynamics and a gaseous entity’s behavior due to– agh, I will write pages if I continue. You and I both know it was far more involved than what I am describing! Enthalpy, entropy, the like!
The study helped me immensely with choosing mediums, establishing measures for the average Zaunite’s mobility based on the density of the gas— I refer to it often, as my work relies heavily on how the remnants of the Grey impact Zaunites. It intrigued me especially because one of the prime controls were the fissures of Zaun’s upper end! Zaun being mentioned in one of the Academy’s published journals? I could hardly believe my eyes! I deduced that only a Zaunite would have such a hand in that! (Apologies to any Piltovs reading this, it’s just that many do not concern themselves with the air quality and the body’s –specifically a Zaunite’s– reaction to it. You understand!)
You were marked under ‘et. al,’ can you believe that? I spent ages researching the names further within the piece, every name traced back to Piltover save for one, which was only a letter … V. ‘Who is this V?’ I thought, and after a rigorous (VERY rigorous, you’re quite elusive!) search, my investigation has led me to you! Pardon my pertinacity. It took many conversations with Piltovs in which they referenced a ‘Vincent with the leg’ who worked for Heimerdinger years ago. I then found, or rather, cornered Councillor Heimerdinger, who is an easy Yordle to find if you pretend to drop papers around every corner of the upper-levels buildings! I asked him who Vincent was– sans the mention of ‘with the leg,’ as I couldn’t help but feel that comment was a bit mean-spirited. He responded that your name was in fact Viktor, that you have long surpassed what his courses had taught, and are now working on Hextech! Hextech! A Zaunite, working on Hextech! I couldn’t believe it. I said it out loud, to which he politely corrected himself and elaborated on the fact you are one of the founding members. I was ecstatic! Hextech! What serendipity that the man who contributed to a journal I hold in high regard and am using as a primary reference point for my thesis is such a prolific inventor. FOR HEXTECH! AND A ZAUNITE! Excuse the caps.
In my excitement, I told many back home of your position, and … ah, well, that ‘I’ll see it when I believe it’ viewpoint remains as strong as ever. However, it may be for the best that I fell on deaf ears. After much consideration, I came to understand why you would choose not to attach yourself to Hextech. Such is the blight of a Zaunite. Alas, we shall see where Zaunite scientists head, you and I. May we be more than ‘et. al!’
All in all, my kindest wishes and highest regards to you. Best of luck in your endeavors, you … oh, and Jayce, of course! How could I forget the Man of Progress? (No sarcasm intended!)
P.S. I say with the utmost respect, I hope to see your contributions come our way in Zaun!
My friend, I have received letters before. Pleas for funding. Petitions for approval. Veiled attempts at favor through flattery. But not this. Not like this.
What you describe, the vision, the fusion of mind and machine, of biology and movement, is biomechanics. The word itself may be young, but the discipline has always existed, hiding between the seams of anatomy and engineering. And if no one has yet named it properly, then perhaps you shall be the one to do so.
Your tenacity is... remarkable. I never thought anyone would dig that deeply into the old thermodynamics publication, let alone trace it back to me through half-scrubbed initials and whispers. You are correct: I was the “V.” And you are more correct still that no one else in that room gave much thought to the Grey.
You see, data gathered from the fissures? That was my contribution. They saw it as background noise. I saw it as the very condition of Zaunite life.
You say my work has helped yours. But now it is your work that helps me. To hear that someone, down in Zaun, is building, fighting, learning, not for glory, not for patents, not for approval, but for people… it is more than I thought possible.
Hextech… yes. It is a beacon. But it is also a fire, and fire consumes. You, on the other hand, are constructing something from the ashes.
May you never again be "et. al." May your name appear in full. And may it be one that others cite, years from now, with the same awe and determination you’ve shown here.
I pray that the environmental Hextech devices that Jayce and I have been developing reaches you and the rest of Zaun. You may have noticed construction of late on the ventilation system; it is being refitted with Hextech technology to better filter the toxicity.
I wish you all the best in your endeavors. Please, feel free to reach out for supplies, information, or any other support you may need. You know where to reach me.
V.
#arcane viktor#viktor lol#viktor arcane#viktor league of legends#askviktor#arcane#viktor#ask viktor#arcane roleplay#arcane rp#viktor rp#viktor roleplay#viktor rp arcane#viktor arcane rp#viktor arcane roleplay#arcane rp blog#arcane ask blog#arcane league of legends#arcane lol
20 notes
·
View notes
Note
Funk Branch: Hey, S-man! Can you do me a mad favor and look over my notes for me?
Synth: Oh sure, Dubstep! Just hand it over!
Funk Branch: Ok, so as you know, we have recently discovered anomalistic chemical kinetic behavior over at dimension RB08-v2tpt3 variety 208A [...] and the ongoing theory is that the root problem is the radiation spread of the entity V.R.C. C.D.: positive. [...] And so, to assist me on the confirmation of that theory, I will need you to help me find the number corresponding to the reaction rate constant on the Eyring equation, [...] so then we will be finally be able to run that over the error propagation formula for the enthalpy of activation and find the solution!
Synth: ......
Branch, mumbling: You're right, we may also need to do the error propagation formula for the entropy as well... But we can decide on that later!
Synth: Yeah, sure... But before that, can you remind me who Eyring is again?
Branch: Huh, what did you say?
Synth: Eyring, uhm, who is... she?
Branch: ...
Synth: ......
Branch: Synth, in all my years of interdimensional and intergalactical travel, you're by far the dumbest person I ever meet.
Synth: Hey!
Branch: *Pulls Synth in by the fins and kisses him on the mouth, with tongue*
Branch, holding Synth's face in his hands: Please, never change!
Funk Branch:

#sibblings qna#funk branch au#trolls branch#trolls synth#brynth#If anyone is curious I normally have Branch give Synth the petname Synthwave
56 notes
·
View notes
Note
Xeno :D
what's your favorite science experiment / reaction :3
Dear creatorbiaze,
This is admittedly a more difficult question to answer than I originally anticipated. However, after some thought, an experiment I find particularly elegant is simple in nature, yet produces fantastical results: the rapid oxidation of aluminum by iron (III) oxide, otherwise known as the thermite reaction.
The resulting reaction of the two compounds is so intensely exothermic - heat producing - that the pure iron product melts on contact. It's most commonly used for welding in situations where a torch is inapplicable.
If you would allow me to explain further:

Looking at the reaction itself, I've written the oxidation states of each component below the reaction. For brevity, I've stated the state of the atom individually; for example, in the first reactant there are two iron atoms, each with a 3+ oxidation state.
You can see that Aluminum metal has been oxidized from a 0 state to 3+, and inversely, Iron is reduced from 3+ to 0. This is a very dramatic change, and should give the first hint as to the violence of the reaction itself. Such a large change in oxidation state requires a very high amount of energy exchange.
We can then calculate the overall energy change of the reaction from the enthalpy of formation of each reactant and product.

The final result is a standard enthalpy of 851.3 kilojoules per mole of reactants. The value is negative because it is exothermic, meaning that energy is being released as opposed to being absorbed into the products. That is, in short, a large amount of energy being released.
The final question to be asked: how is this energy released?
Heat. Hot enough to smelt iron instantaneously. How absolutely elegant that such innocuous solids can react in such a manner that is both violent yet perfectly ordained by the laws of chemistry and thermodynamics.
I highly recommend viewing this experiment yourself given the chance; you are in for quite the show.
-Dr. X
#dr. x 🚀#// he will go on for hours without stanley to stop him yapping#//unfortunately i dont have a stanley and i am an enabler#//also this is genuinely one of the fave reactions ever
9 notes
·
View notes
Note
quastion. for the benzene ring post what makes him wrong about the structure (genuinely confused)
Great question! I'll try my best to answer it simply, but it is going to take a while to lay out the evidence so stick with me here. I dont know the extent of your chemistry knowledge so I will assume some basic background knowledge of chemistry (mainly that you know what a bond is). Kekulé was working with benzene in the 1860s. By this point the formula of C6H6 (tumblr why wont you let me use subscripts?) was known, but the struggle among chemists was explaining the 1:1 ratio of carbon to hydrogen atoms in terms of a structure. (There were some absolutely wild suggestions, you should look them up). I'm not going to go too into this though, I'm just going to discuss why Kekulé's benzene structure is not the actual structure of benzene.
Let's start by looking at what Kekulé proposed. Kekulé proposed that benzene was made of a ring of six carbons. Each of these carbons was bonded to one hydrogen each. Between each carbon in the ringthere were alternating single and double bonds. It looked like this:
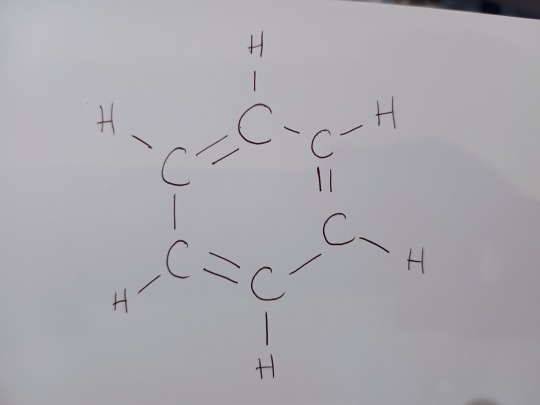
Or you may have seen the beloved skeletal structure:
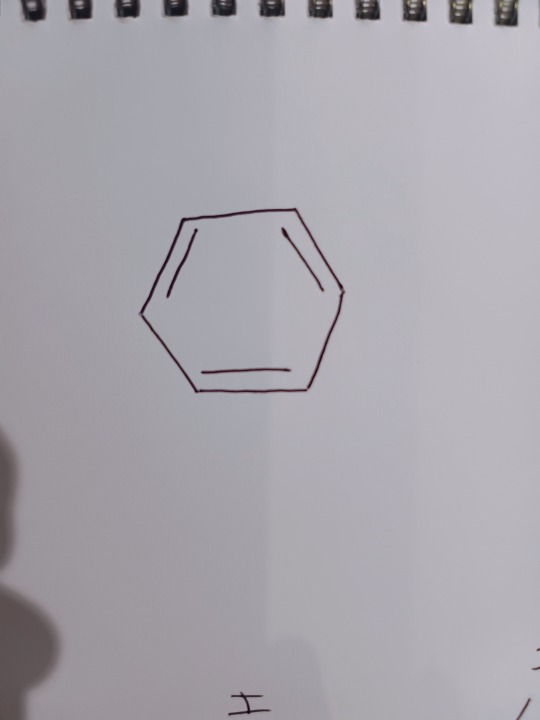
Now, this structure explains quite a lot about benzene. And Kekulé made some modifications to his theory that explained that the double and single bonds rapidly changed between carbons. Consider this diagram:
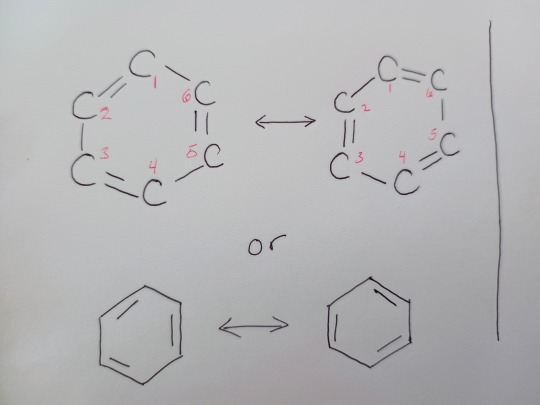
It might look like the ring has just been rotated, but remember, each carbon is its own carbon and these are technically slightly different (think about a set of sextuplets, even though they are very similar in appearance, are they actually each the same person?) What Kekulé proposed, the rapid switching of these bonds was good but it isn't wholly correct. Let's look at why with some thermochemical evidence and then some structural evidence.
The thermochemical evidence might be a bit difficult to explain since I'm not sure of your chemistry knowledge but I'll try. Let's look at a reaction. You can add hydrogens to benzene to make something called cyclohexane (C6H12). If you do this to Kekulé's proposed structure and use theoretical values (message me if you want more and I'll show this in more detail) you get a change in enthalpy value of roughly -360kJ/mol (if this makes no sense dont worry. Just worry about the number.) However if you carry out the experiment on actual benzene, you get a value of -207kJ/mol. Huh? That's a difference of 153kJ/mol! That means actual benzene is more stable than Kekulé benzene. That's one piece of proof showing something isn't quite right with his structure.
Now let's look at structural evidence. Most of this work was done via a technique called x-ray crystallography in the late 1920s by Kathleen Lonsdale. Each type of bond has a certain length. We determine this with x-ray crystallography. A carbon-carbon single bond is 0.154nm. A carbon-carbon double bond is 0.133nm. That means if you were to look at Kekulé's benzene using this technique, you might expect three of the carbon-carbon bonds to be 0.133nm and three carbon-carbon bonds to be 0.154nm. In reality, this isn't what is seen. Every carbon-carbon bond in Benzene is the same length (about 0.140nm). Something in between. So the bonds between the carbon also must be something in between. Not quite single, not quite double. This means it is better to describe the structure of benezene as having what's called a delocalised pi electron system. Instead of alternating double single bonds it's more like each carbon-carbon bond is a single bond and then nearly has a double bond. This diagram shows it a bit better than the description:
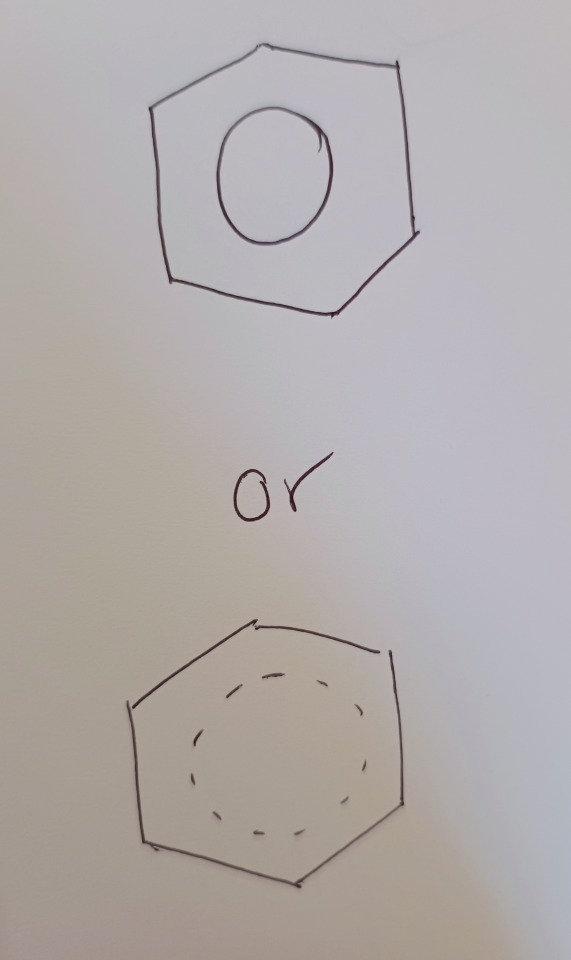
Further evidence for this delocalised system comes from looking at the number of isomers (same atoms but arranged in space differently) of a substituted benzene ring (we'll look at C6H4Cl2, but just think of it as two hydrogens being replaced by two chlorines). Let's see Kekulé's Benzene first:
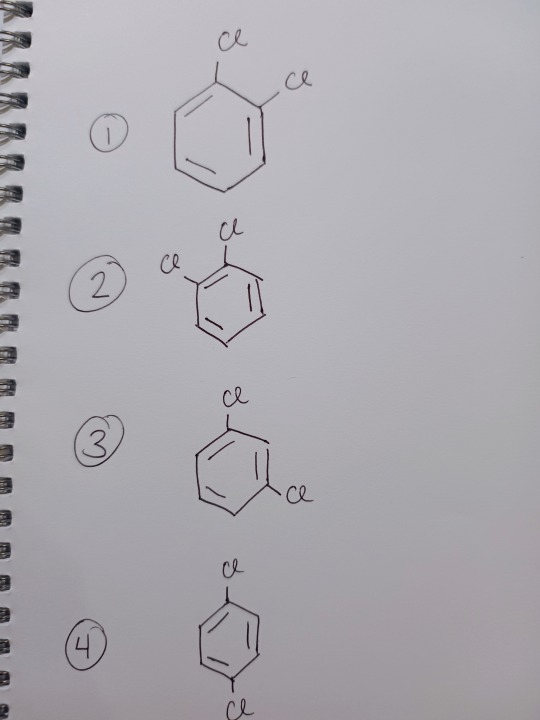
We see four separate isomers (remember, each carbon is it's own entity). Now if we look at actual benzene:
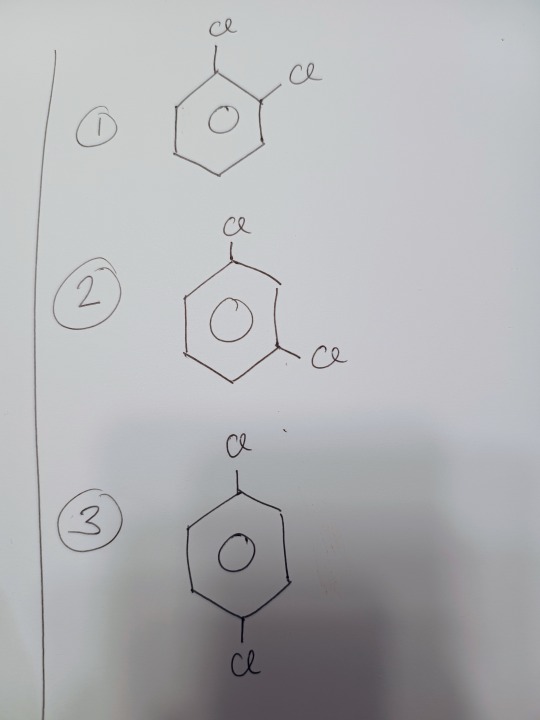
There are only three.
Another thing is that substances with carbon-carbon double bonds under go a type of reaction called an addition reaction so with Kekulé's structure you would expect it to do the same. But benzene does not do this (it undergoes substitution reactions though).
But yea! Kekulé's benzene (or cyclohexa-1,3,5-triene if you'd like) was a good enough structure for a while but it isn't quite right. I hope this was helpful anon and sorry for the essay. Let me know if you'd like something further explained
#Benzene#Kekulé#He also wrote a lot of his papers using his last name only#I also am pretty sure the snake dream was meant to be a joke of his
3 notes
·
View notes
Text
Conquer AP Chemistry with Confidence: Your 900‑Word Guide with Tychr
AP Chemistry is often seen as one of the most challenging high school Advanced Placement courses. Stoichiometry, equilibrium, kinetics, thermodynamics, and complex lab concepts—this course demands strong analytical skills and conceptual depth. However, with the right structure, support, and expert tutoring, mastering AP Chemistry isn’t just achievable—it’s empowering. Tychr is here to guide you every step of the way.

🌟 Why Choose AP Chemistry?
If you’re aiming for degrees in chemistry, biology, engineering, medicine, or environmental science, a strong AP Chemistry score (ideally a 4 or 5) can:
Earn you college credit or placement
Enhance your college applications to top universities
Provide a solid foundation before university labs and coursework
AP Chemistry pushes your critical thinking, quantitative reasoning, and scientific communication to new heights.
🎓 How Tychr's AP Chemistry Tutoring Transforms Learning
We offer a structured, student-centered approach designed to build confidence, clarity, and competence.
1. Expert Tutors You Can Trust
All our tutors have real-world AP expertise—many have scored a 5 themselves. They combine academic proficiency with teaching experience to simplify complex topics and align lessons with College Board expectations.
2. Personalized, Flexible Learning
We tailor each course to your background, pacing, and academic goals. Whether you’re a beginner struggling with balancing reactions or an advanced student seeking deep dives into thermodynamics, your Tychr tutor adjusts to your needs.
3. Robust Support Framework
Monthly Progress Reports (no anxiety, just clear feedback)
WhatsApp Support Groups for quick questions and motivation
AP‑Style Test Series to simulate real exam conditions
One‑on‑One Doubt Clearing Sessions to resolve every confusion
Toppers’ Talks & Examiner Insights to understand exam expectations from those who’ve aced it
🔬 The AP Chemistry Curriculum & Tychr’s Approach
We cover all key units while ensuring clarity, application, and exam readiness:
Atomic Structure & Periodicity Learn electron configurations, trends, and subatomic behavior with visual aids and quizzes.
Stoichiometry Balance reactions, calculate yields, and track molarity with guided problem-solving sessions.
Thermochemistry Understand enthalpy, calorimetry, and energy transfers through concept visuals and lab simulations.
Equilibrium Break down Le Chatelier’s principle and equilibrium constants (Kc, Kp) with real-world examples.
Kinetics Analyze reaction rates, rate laws, and activation energy through data tables and practice problems.
Acid‑Base Chemistry Tackle calculations, titrations, and pH logic with step-by-step breakdowns.
Thermodynamics & Electrochemistry Dive into spontaneity, Gibbs free energy, and galvanic cells using diagrams and labelled explanations.
Complex Equilibria & Lab Skills Build robust lab techniques—indicator selection, titration logic, error analysis—to reflect university standards.
🔁 Tychr’s T.E.S.T. Methodology
Our learning framework ensures holistic support:
T – Theoretical Foundation Build strong conceptual understanding before jumping into problem-solving.
E – Engagement Through Application Integrate visuals, analogies, and real-world experiments to cement learning.
S – Strategic Exam Training Learn question decoding, formula application, and time-saving tactics for both MCQs and FRQs.
T – Tracking Progress Use periodic assessments and feedback loops to stay on track and focused.
🧪 Practice That Produces Results
You don’t just learn—you perform. Our AP-Style Test Series replicates exam timing, format, and difficulty. Tutors deliver focused feedback on every MCQ and FRQ, highlighting what earned full points and where improvements are needed.
Regular monthly progress reports make your growth visible—without report-card stress.
🌐 Community & Motivation
Stay connected and motivated through WhatsApp groups, Toppers’ Talks, and sessions with AP examiners. Gain insider tips on what a scoring 5 essay looks like and how to approach complex problems effectively.
🎯 College & Career Impact
A strong AP Chemistry score opens doors to majors like:
Chemistry
Biochemistry
Chemical & Biomedical Engineering
Medicine & Health Sciences
Environmental Science
Forensic Science
Top universities such as MIT, Stanford, UC Berkeley, and Oxford prize high-achieving AP students in their admissions process.
📌 Final Takeaway
AP Chemistry demands discipline, deep understanding, and dedicated practice. With Tychr’s personalized tutoring, structured support, and data-driven strategy, achieving a top score is within reach. Let us help you not only conquer chemical equations but also build analytical skills that will last a lifetime.Please visit site for further queries: https://www.tychr.com/ap-chemistry-tutor/
0 notes
Text
HYDROCARBONS!! ALKENE EDITION!!
STRUCTURE OF A DOUBLE BOND
The double bond is shorter in bond length (134 pm) than the C–C single bond (154 pm).
The presence of the pi (π) bond makes alkenes behave as sources of loosely held mobile electrons. Therefore, alkenes are easily attacked by electrophilic reagents.
The presence of weaker π-bond makes alkenes unstable molecules in comparison to alkanes and thus, alkenes can be changed into single bond compounds by combining with the electrophilic reagents.
Strength of the double bond (bond enthalpy, 681 kJ mol–1) is greater than that of a carbon-carbon single bond in ethane (bond enthalpy, 348 kJ mol–1).
GEOMETRICAL ISOMERISM
The restricted rotation of atoms or groups around the doubly bonded carbon atoms gives rise to different geometries of such compounds. The stereoisomers of this type are called geometrical isomers (cis and trans).
Due to different arrangement of atoms or groups in space, these isomers differ in their properties like melting point, boiling point, dipole moment, solubility etc.
Cis form of alkene is found to be more polar than the trans form.
In the case of solids, it is observed that the trans isomer has higher melting point than the cis form.
PREPARATION
Lindlar's catalyst. We react an alkyne with H2 and use Pd/C (partially deactivated palladised charcoal) as a catalyst. This gives us a cis-alkene.
To obtain a trans-alkene, we react the alkyne with H2 in the presence of Na metal and liquified NH3.
Dehydrohalogenation is an example of a beta-elimination reaction as a beta hydrogen is lost from the alkyl halide (this is important to remember, i always remember everything but sometimes forget what the reactant is which is PRETTY DAMN STUPID IF I MAY SAY SO MYSELF. i am NOT failing chemistry again). Anyway, we react the alkyl halide with potash alcohol (A.K.A alcoholic KOH in the presence of heat) which gives us a trans-alkene as a major product because trans alkenes are more stable. and hence thermodynamically favoured. The rate of reaction in case of halogens is (I>Br>Cl>F) and alkyl groups (tertiary>secondary>primary).
Dehalogenation. We have a vicinal dihalide which we react with Zn metal which forms cis alkene as major product.
Acidic dehydration of alcohols. Beta elimination reaction. Very simple. You have an alcohol, you react it with conc. H2SO4 and heat, you get trans alkenes. Mostly. If the reaction is intramolecular or constrained by a ring, the product may be cis.
PHYSICAL PROPERTIES
C₂ to C₄ alkenes: Gases at room temperature (e.g., ethene, propene).
C₅ to C₁₇: Liquids.
C₁₈ and above: Solids.
Boiling points increase with molecular mass due to stronger van der Waals forces.
Trans-isomers usually have higher melting points than cis-isomers due to better packing in the solid state.
Cis-isomers have slightly higher boiling points than trans, due to greater polarity (they have a net dipole).
Insoluble in water (nonpolar doesn't dissolve in polar protic).
Soluble in nonpolar solvents like benzene, ether, and chloroform. (like dissolves like)
Alkenes are nonpolar or weakly polar.
Cis-isomers are more polar than trans due to the net dipole moment.
Lower alkenes are colorless and have a faint odor.
Some cyclic or substituted alkenes may have a stronger smell.
CHEMICAL PROPERTIES
catalytic hydrogenation to give alkanes. did that in prep of alkanes.
addition of halogens. F and I don't show this reaction on account of being explosive and reversible respectively. NOTE: The reddish orange colour of bromine solution in carbon tetrachloride is discharged when bromine adds up to an unsaturation site. This reaction is used as a test for unsaturation. ANYWAY, alkene reacts with X2 in CCl4 to give a vicinal dihalide.
addition of HX. order of reactivity: HI > HBr > HCl X can attach itself to any carbon (with a pi-electron) if the alkene is symmetrical. in case of unsymmetrical alkenes, markovnikov's rule is followed. this is VERY important. if you don't remember this, the organic chemistry tutor has a very helpful video. revise anti-markovnikov's while you're at it. also known as the kharash effect (anti-markovnikov).
addition of sulphuric acid to alkenes gives alkyl hydrogen sulphate. follows markovnikov
addition of water gives alcohols in acc. to markovnikov
oxidation of alkenes with baeyar's reagent (cold and dilute KMnO4). decolourisation of KMnO4 solution is used as a test for unsaturation. Acidic KMnO4 reacts with alkenes to form ketones. further oxidation with the same gives carb. acid.
ozonolysis. no explanation. im tired. i know this. O3 + Zn-H2O. gives aldehydes / ketones.
polymerisation. not very important (acc. to present syllabus. very very important irl)
0 notes
Text
Saying things outside the tags for the first time in 800 years because while the diet industry is a scam and calorie-counting is an L some of the science stuff in this post is super misleading.
"There's no solid evidence that this method is at all equivalent to how our bodies process food."
Our bodies process food by breaking molecules down into smaller molecules and harvesting the energy. This is totally right that measuring food's nutritional value by how well it burns would be silly! But we don't do that.
You don't really burn "food" so to speak in a bomb calorimeter, you burn a chemical compound. By measuring the energy released by a bunch of combustion reactions, you can set up a standard of how much energy is produced in the formation of those compounds, and then compare to figure out how much energy is produced by the entirely different chemical processes OP mentions.
When you combust something in a bomb calorimeter, you measure the "heat of combustion," or enthalpy change, of the reaction. Enthalpy (H) is a function that combines the internal energy (U) of a material, which is all its kinetic and potential energy summed up, with pressure and volume (H = U + pV). This is why it's done in a "bomb;" the structure of the "bomb" maintains constant volume so that we can calculate the enthalpy change. At constant pressure, H = q, q is the heat flow of the reaction. So using OP's example of dry sawdust, 4800 calories of energy would be released when you combined dry sawdust with oxygen gas to make water and carbon dioxide gas.
This is almost exactly what OP described! But it doesn't actually stop there. In doing a bunch of those, we can calculate the Enthalpy of Formation of a bunch of compounds, which measures the energy change that happens when those compounds are formed from the most stable forms of all the elements in the compound (for example oxygen is most stable as O2 gas). When we have the enthalpy of formation for a bunch of compounds, we can then calculate from that the enthalpy change of any reaction involving those compounds! (If you're curious about this google Hess's Law). Because we burned all that stuff in the bomb calorimeter, we can calculate how much energy is released by pretty much any reaction, including those involved in digestion! So even though the bomb calorimetry combustion is totally different from the reactions involved in how we process food, we can use it to calculate how much energy is released by those reactions.
Even though the chemical reactions occur in a different environment, they still release the same amount of energy. For example, when you break down a protein in food, a different protein in your body catalyzes (speeds up) a reaction in which the food protein is split in two parts, and a water molecule (H2O) splits into H and OH to cap off the ends of those two protein segments. This is called hydrolysis (hydro = water, lysis = cutting). When this happens, wherever that protein is cut, whether in or out of your body, that reaction will release the same amount of energy, because the same chemical bond within that protein is cut. That chemical bond is an attraction between charges which has a certain amount of energy (this amount of energy can be calculated, it's determined by how charged the charges are, how far apart they are, and a number related to what environment theyre in). When that bond is cut, the energy of that bond is released, and you can calculate exactly how much energy is released. To calculate how much energy is released in this digestive reaction, you use the standard enthalpies of formation which we calculated from the bomb calorimetry. So bomb calorimetry does actually allow us to calculate how much energy is released by specific reactions in digestion.
2. "calories don't measure nutritional value, just how well something burns"/"'calorie' measures what happens when you set food on fire, rather than when it's digested"
Calories don't measure nutritional value or how well something burns, they measure energy. If you've ever heard of a Joule, a calorie is just 4.184 Joules, the same way a foot is 12 inches. You can use calories to measure pretty much any energy transformation. So you can use it to measure the energy released when something burns (different from how quickly it burns/how easy it is to make it burn), how much energy is released from bonds broken in molecules of food/how strong those bonds are, how much potential energy something high off the ground has, how much kinetic energy something has, how much work it takes to throw a book, or anything else involving energy or work. You can measure how many calories of energy a lightbulb burns per second. The fact that a calorie can measure all these things is not a design flaw, it's just a consequence of how physics works. Energy can move from one thing to another, it can do work, and it can make things happen. The energy of a chemical bond is the same energy that makes things move, runs your toaster, etc, and it can be calculated and measured. We use the Joule or calorie to measure all these energies not because of oversight, but because energy is the same thing in all those cases.
So returning to the sawdust example, if the combustion of sawdust has an enthalpy of 4800 calories per some amount of sawdust burned, does that mean science says eating that amount can provide you with 4800 calories of energy? No! The combustion of sawdust is a different chemical reaction. The calories you see on a label don't measure How Much Energy Does This Granola Bar Release When You Set It On Fire, it sums the energy released by all the various digestive reactions that occur on all the granola bar's chemical ingredients (ex. proteins, lipids/fats, sugars, etc).
The diet industry is absolutely a massive scam and enormously harmful, but if you're trying to convince someone of that or learn about it please use tumblr user queeranarchism's links and facts. While digestion is more complicated than just Putting Energy From Your Food's Chemical Bonds Into Your Body, the argument that the calorie is meaningless and just measures what happens when you blow stuff up, while powerful, snappy, and easy to memorize, isn't actually true.
Just found out that the dietary calorie is still measured by burning food in a "bomb calorimeter" and then measuring the heat produced. There's no solid evidence that this method is at all equivalent to how our bodies process food (an entirely different chemical process from combustion), the accuracy of this system has been disputed for as long as it's existed, and there are no available alternatives
There are 4800 calories in a kilogram of dry sawdust even though wood is completely indigestible to humans, because calories don't measure nutritional value, just how well something burns
Nutritional "science" is pure bullshit
46K notes
·
View notes
Text
How does entropy relate to spontaneous reactions?

How Does Entropy Relate to Spontaneous Reactions?
As you're here, that probably means you already peeked at the textbook explanation and went, “Yeah no thanks.” So instead of all that science-speak, let’s go full casual mode and really get what’s going on with entropy and those “spontaneous reactions.” — First: What Even Is Entropy? Okay, imagine you clean your room. Everything’s in place. Tidy. Beautiful. Now give it a few days… and somehow your socks are in your bag, your notes are under your bed, and there’s a snack wrapper on your pillow. What happened? That, my friend, is entropy. In science terms, entropy is just a fancy way of measuring disorder. The higher the entropy, the more chaotic or messy something is. Nature has a habit — it loves to go from neat to messy. From organized to scattered. That’s why your room never magically cleans itself. —




So What Are Spontaneous Reactions? Spontaneous reactions are chemical reactions that just happen — no extra push needed. Like iron rusting. Or ice melting on a warm day. Or your banana turning brown when you forget it on the table for too long. They don’t need your help. They’re just gonna do their thing when conditions are right. But here’s the twist: “spontaneous” doesn’t always mean “fast.” Rusting takes time, but it’s still spontaneous. So don’t think of it like a sudden kaboom — think of it like a chill, inevitable process. — So How Does Entropy Tie Into This? Alright — picture this: When a chemical reaction happens, the particles (atoms and molecules) can get more spread out, more disordered, or more randomized. If that happens, the entropy of the system goes up. And guess what? Reactions that increase entropy (aka create more disorder) are more likely to be spontaneous. Let’s break it down with a few examples: ✅ Ice melting: Solid water (ice) has super organized molecules. But when it melts into liquid, those molecules move more freely = more disorder = entropy increases = spontaneous. ✅ Firecracker exploding: Before — one tiny, compact thing. After — boom, gas and heat everywhere = way more entropy = spontaneous reaction. But hold on — it’s not just about entropy. There’s a partner in this called enthalpy (which is about energy). Together, they help decide if a reaction happens on its own. And scientists put these two things together in something called the Gibbs Free Energy equation: ΔG = ΔH - TΔS Don’t worry — you don’t have to memorize it. Just know this: - If ΔG is negative → spontaneous reaction - ΔS is entropy (disorder) - ΔH is energy (heat released or absorbed) So more entropy (higher ΔS) = more likely ΔG is negative = boom, spontaneous! — Why It Matters in 2025 Understanding entropy helps us design better batteries, predict how pollution spreads, develop efficient chemical processes, and even explain why your cold coffee gets warm sitting out (and never the other way around — sorry!). In short, entropy tells us what’s gonna happen when we’re not looking. — TL;DR — Simple Version: - Entropy = disorder or randomness. - Nature likes things to be messy (aka high entropy). - Spontaneous reactions are more likely when they increase entropy. - Think melting ice, rusting metal, or mixing cream in coffee. - It’s not just about being messy — energy and temperature also play a role. — 📌 Disclaimer: This easy version is meant to help you understand the concept better. If your exam or teacher expects a textbook explanation and you write this one instead, we’re not responsible if it affects your marks. Use this for understanding, not copy-pasting. — 🔗 Related Articles from EdgyThoughts.com: What If Atoms Could Remember Past Lives 2025 https://edgythoughts.com/what-if-atoms-could-remember-past-lives-2025 How Quantum Particles Decide Their Path 2025 https://edgythoughts.com/how-quantum-particles-decide-their-path-2025 🌐 External Resource: Want the deep science behind it? Check out the Wikipedia page: https://en.wikipedia.org/wiki/Entropy — Read the full article
#chemicalreactionsandentropy2025#disorderinchemistryexplained#entropyandspontaneousreactions2025#entropychangesinreactions#entropyclassroomexplanation#entropyinnatureexamples#entropyincreaseandspontaneity#entropysimpleexplanationforstudents#entropyvsenthalpysimpleterms#examplesofentropyinreallife#gibbsfreeenergyandentropy2025#howentropydrivesreactions#meltingiceentropy#spontaneouschemicalreactionsexplained#spontaneousprocessandentropy#studentfriendlyentropynotes#thermodynamicseasyversion#whatisentropyinchemistryeasy#whyreactionsarespontaneous
0 notes
Text
You ever think about how the universe is already dead? Not in the poetic sense, not in some grand metaphor about human decay—literally dead. Just hasn’t finished twitching yet. See, entropy doesn’t stop. It doesn’t slow down. Everything that burns cools, everything that moves stops, and one day, every star that ever lit up the void is gonna gutter out like a cigarette in an ashtray. That’s heat death. The final silence. The end of everything.
And it’s not some distant, hypothetical horror. It’s happening right now. You feel it in your bones, in the way your body breaks down a little more every day. That ache in your joints, the way your memories slip through your fingers like sand—that’s entropy, man. That’s the same law that’s gonna tear the universe apart, working its way through you on a smaller scale. Every living thing is just a temporary structure, an arrangement of matter pretending it has permanence.
Heat death isn’t just an end. It’s the end. The final state of everything. People think of death as something with edges, something with borders—the moment your heart stops, the second the light leaves your eyes. But that’s a small death. That’s just biological failure. Heat death is bigger. More absolute. It’s not just the death of living things, or planets, or stars. It’s the death of difference.
Entropy, mathematically speaking, is a measure of disorder—more precisely, it’s the number of microstates that a system can occupy.
S = k_B ln(Ω)
Where S is entropy, k_B is Boltzmann’s constant (1.38 × 10⁻²³ joules per kelvin), and Ω is the number of possible microscopic configurations of a system. That’s the math of inevitability, right there. Because as a closed system progresses, it moves toward higher Ω, higher disorder. More ways to arrange itself. That’s why you can’t unburn a fire, why you can’t unscramble an egg—because those higher entropy states vastly outnumber the lower ones. The universe doesn’t ‘prefer’ chaos, it just follows probability. And the probability of the entire universe spontaneously reorganizing itself into something structured again? Functionally zero.
Now, extend that to a cosmic scale. The universe right now is a nonequilibrium system—it’s full of energy gradients. Hot stars, cold space. Galaxies spinning in the vast dark. But that won’t last. Every time a star burns, it’s not just producing heat and light. It’s spreading energy out, making it less usable. That’s what free energy is—energy that can still do work. Once energy spreads out evenly, once everything is the same temperature, there’s no gradient left. No work. No structure.
ΔG = ΔH - TΔS
Where G is free energy, H is enthalpy (total energy), T is temperature, and S is entropy. You see that negative sign? That’s the kicker. As entropy (S) increases, the ability to do work decreases. The universe isn’t just dying—it’s fading. Every action, every reaction, is just one more step toward equilibrium, which is just another way of saying ‘universal heat death.’
It’s not an explosion. It’s not fire or collapse. It’s just everything slowing down. Cooling. Spreading out. Until every last subatomic interaction ceases, not because something stopped it, but because there’s simply nothing left to move. No energy left to transfer. No gradients. No contrast.
And here’s the part that’ll keep you up at night: It’s irreversible. The moment the universe started, the moment that first asymmetry emerged, this was always the final destination. You can’t stop it. You can’t fight it. You can’t invent some last-minute technological miracle to turn back the thermodynamic clock. There’s no equation that undoes entropy. The only way to reset the system would be to violate the laws of physics themselves.
So when the last remnants of existence flicker out—when the black holes evaporate, when the last protons decay, when even fundamental particles stretch into meaningless diffusion—that’s it. No afterimage. No memory. Just perfect, absolute nothing.
Because everything, everything that’s ever happened, has only happened because of contrast. Hot and cold. Light and dark. Order and chaos. Without that, without imbalance, nothing can exist. No movement. No thoughts. No matter shifting from one state to another. Just a uniform, static void stretched so thin that reality itself stops functioning. You can’t even call it blackness, because blackness implies the possibility of light. You can’t even call it silence, because silence needs something to compare itself to. Heat death is worse than destruction. It’s the absence of destruction. No fire, no explosions, no final moment. Just an infinite suffocation.
No memory of what came before. No last observer to bear witness. No evidence that there was ever such a thing as ‘something.’ Just an infinite, frozen void stretching in all directions, unchanging, unbroken.
And yet, here we are. Waking up. Pouring coffee. Loving people. Building things. We pretend we matter, because the alternative is realizing we were ghosts the whole time—just flickers of heat burning themselves out in a universe that’s already gone cold.
Maybe that’s all we are. Just sparks flying off a dying flame, burning bright for a second before the darkness swallows us whole.
1 note
·
View note
Text
The dark magic of Thermodynamic functions
Abstract: You probably won't refer to "free enthalpy" as the "Gibbs Energy" anymore (and finally there will be sense for what an 'enthalpy' even is.)
If you stumbled upon this post by using the #thermodynamic tag on tumblr, well, please lord have mercy on you. Tho, if you truly did, he most likely had forsaken you long ago.
Internal energy 'U' and enthalpy 'H', as well as their more refined (or literally, "freer,") counterparts free energy 'F' and free enthalpy 'G', all share the same unit: energy (Joules).
Sharing the same unit implies some similitude, even between two very distinct stuff: the Eiffel tower's height is not the same as mine, but we can see how they *can* be compared.
Yet, when it gets more abstract, such as energy, the nuances aren't so clear. We're not even sure sometimes that what we define actually means anything: for what we know, it could just be a convenient way to handle the values.
What I'll tell next are analogies, not images. In analogies you can manipulate two object as being truly equivalent. Because the same rules apply for both, it's great if thinking with one of them makes it easier than with the other.
Enthalpy 'H', is to internal energy 'U', what some person's overall wealth are to their bank account.
Both 'total wealth' and 'bank account' are expressed in some currency, be it €, $, or whatever. But if we want to tell how wealthy someone is, the amount of cash on their bank account isn't enough. It could currently be at 1.000€, yet the guy in question is a CEO owning a mansion, a boat, etc. So, to get the whole wealth, we add the Value of all the Possessions (for now let's call it PV) to the bank account. If we take back our thermodynamic functions, this become:
H = U + PV
Honestly I just made the PV naming pun because I could. For coherence, I rather think it's both simpler and better to call V the "Volume (of possessions)" and P the average "Price (of possessions)." Volume analogy is straightforward. For Pressure-Price, if Price is cost per volume, then its units are €/m3, which in our analogy € act as Joules, so it also adds up.
So, an enthalpy, is a total wealth. It not only includes the core bank account (U), but also all the person's (or 'system's') belongings, via PV.
This also explain why, in chemical reactions, it's easier to talk in terms of enthalpy, and internal energy is seldom used. The same as you guess someone's wealth by looking at everything they owns rather than just their bank account (which probably you can't access anyway).
Let's get back to the thermodynamic story.
Having 1.000€ of wealth (not just bank account) mean I can afford stuff up to 1.000€ (taxes included). But if I'm being rather cautious, I would think a lot before spending my whole 1.000€, or even way less, on something. There is some kind of threshold, under which I will spontaneously spend the money, but above which I'd think twice before doing so. Out of all my wealth, I subtract some of it for savings, and the rest of the wealth is free for me to use. That's what free enthalpy (or free energy) is about.
G = H - TS
I set aside some of my wealth (-TS) to stay in a comfortable state of mind. My comfortable state of mind is an analogy for my thermodynamic equilibrium state.
Thus, if we look from the thermodynamic side of the analogy, the TS term means some kind of stored away energy. Which actually is exactly that. The TS term represents the energy the system get from how 'probable' it is. This energy cannot be retrieved in any way. So to correct a bit our analogy, rather than being about "savings", it's more like "comfort investment." You already spent this money to make your life easier, and you won't get it back. So I hope it was worth your money.
Same can go for free energy and bank account, if you only think about bank account-related purpose. (Which is why most "first principles" derivations, will use the free energy in formulas rather than the free enthalpy. They care more about the energy of the particle of the system rather than the energy of the whole system, which include the work it did at some point to occupy its volume.)
We got a grasp of what TS is, but are there analogies for T and S alone? When refining any analogy, before adding new axioms, it's better if we can let the analogy produce them itself.
TS is a product, representing some 'comfort value.' It is important to not say just 'comfort' but also precise 'value', as its units are Joules, i.e. currency € in the analogy. Very often in physics or whatever, when you have a product, you can always make the individual factors analogies of "level of something" and "the value of a level of that something." So, since TS is a comfort value, it means that either T or S is "comfort level" and the other is "the comfort value of a level." Looking at their -physical- units, K and J/K, we can infer that T is comfort level while S is comfort value of a level.
It implies that 'Kelvin' are our equivalent of 'comfort level.' Which I can kinda see how: the less comfortable it is, the more restricted we feel. And at absolute 0, total restriction, we can't move at all.
For entropy, by physical intuition we may be aware that entropy quantifies how statistically favourable a system is. Fortunately I think it's quite accurate to say that "how statistically favourable" is analogous to "how comfortable" a system is.
I like that about analogies, when you're checking the logic is self-consistent, you unveil insights simplifying the whole.
To recap:
U = Bank account
H = U + PV = Bank acc. + Possessions*price of possessions = Total wealth
G = H - TS = Total wealth - comfort level* value of comfort level = Free wealth to spend
I hope this will help you in your thermodynamic journey, but be aware that whatever path you take, they all end up in hell (Boltzmann wanted to get there faster, I guess)
#thermodynamics#enthalpy#entropy#temperature#gibbs#free enthalpy#free energy#gibbs energy#analogies#physics#internal energy#analogy
1 note
·
View note
Text
solar power… why it’s actually really cool and you should care about it more🌞✨
ok so let me learn you a thing. we all know the sun, right? as humans, we are incredibly privileged to exist as we are in relation to the sun. as the largest body in our solar system, it gives us our wonderful water and climate cycle; light itself–which beyond being the reason we can perceive literally anything is also the reason we have plants #photosynthesis; extending beyond that, the sun is the reason we have any form of life (Planas, 2020). it’s pretty essential if i do say so myself, the fact its energy has empowered us for billions of years—and what if we could use this power for power.
as a source of energy, sunlight is incredibly immense. on average, the sun shines down 120 000 terawatts of power to the earth, which–by 2025–is 4000 times the needed amount to flow throughout the globe (Herron, 2010). however, this energy cannot be weaponized on its own. this is where solar panels come in.

these panels are composed primarily of solar cells, made from silicon #semiconductor, which captures sunlight to produce an electrical current; this process is known as the photovoltaic effect.
function of the effect:
solar cells have two layers, a negative “n-type” layer with extra electrons and a positive “p-type” layer with missing electrons or “holes.” The space where these layers are in contact, leading to the formation of an electric field, is known as a “p-n junction.”
when sunlight hits the solar cell it transfers its energy to the electrons in the p-n junction, liberating them from their chemical bonds to conduct electricity. though, this transfer leaves behind holes, which can carry charge.
as a result of the aforementioned electric field these excited electrons and holes are induced to flow in opposite directions
this opposing flow creates an electric current
wiring and other conductive metals in the panels collect and route this current for later use (Donev, 2024; Walker, 2024).
another way to think of this process is that if it were a traditional chemical reaction, it would be akin to an endothermic reaction. The absorption of sunlight would necessitate a positive enthalpy gain!

though, despite the arduous set-up of this process to guarantee energy conversion, due to the nature of life, this conversion is not 100% efficient. despite common misconceptions about snow and darkness harming production, this simply isn’t the case. through storage facilities and angling of panels so snow slides off 😲–-many of these traditional problems have been circumvented (Office of Energy Efficiency & Renewable Energy, 2017).
it is instead numerous other factors limiting perfect function, such as being unable to account for all wavelengths of sunlight; the recombination of the electrical charge back to sunlight #reverse_reaction; higher temperatures messing with various properties of the panel; and sunlight simply being reflected back and not absorbing😞 (U.S. Department of Energy, n.d.). combined, this leads to an average conversion efficiency of 22% for modern solar panels. research is currently pushing this further with multi-junction and perovskite technologies (Elliott, 2024).

efficiency is not the be and end all of energy production, as “[a]n efficient solar panel is one that generates more electricity by occupying less space” (Enel X, n.d.). so, if the advantages of solar power outweigh the disadvantages of space requirements and initial costs for production, then this is virtually a non-issue.

the unique benefits of solar power make it a #game-changer🔥🔥 in energy production. its renewability, long-term cost-effectiveness, and low environmental impact show solar energy is worth investing in. solar power is more than just a sustainable energy source for underserved communities. once installed, solar panels offer free energy for decades; as long as the sun exists, so does solar power. with reliable electricity, clinics can store vaccines safely, surgeries aren’t conducted in darkness, and healthcare workers can serve remote areas more effectively. programs like UNDP’s Solar for Health have proven that solar energy doesn’t just save costs; it saves lives, empowering millions with access to essential services while lowering the health sector’s carbon footprint 👣🍃, unlike fossil fuels, solar power doesn’t emit greenhouse gases (Burton & Alers, 2019; Richardson, 2023).

circling back around to some of the negatives, as a #true comparison, while it is a bit challenging to get over the need for the significant land area as a result of the lower efficiency, innovative combined urban installations mitigate this through rooftop use (Khan & Anand, 2024). however, the other major placement for these solar farms is in the desert ecosystem. this may seem like a good use of space given the supposed bareness of these landscapes, yet in actuality, deserts are thriving fragile ecosystems, which the needed large solar installations harm (Courage, 2021). solar panels have been shown to have negative effects on wildlife, deterring common keystone species of the area from behaving and settling as they once were. this alteration in animal behaviour fundamentally changes how these ecosystems function; this change is for the worse (Chock et. al, 2020). the people living near these ecosystems are also harmed in the process as the heated climate produced from the unconverted solar energy would result in a reorganization of “global air and ocean circulation” leading to more frequent extreme weather occurrences and natural disasters in neighbouring countries, greatly impacting the health of their populations (Lu & Smith, 2021).

the intentionality of placement matters, this does not necessarily limit the implementation of solar panels completely. instead, it promotes better land surveying and research investment to increase solar panel efficiency.
compared to a fossil fuel like coal, this needed support of solar power is minimal. coal emits on average approximately 1kg of CO₂ per kWh of energy produced, and for the amount that this CO₂ and other dangerous gases contribute to air pollution, acid rain, and respiratory diseases the efficiency for this combustion process is not that great 👎 (U.S. Energy Information Adminstration, 2023; Union of Concerned Scientists, 2017). coal plants convert 33% of energy from combustion; solar’s 22% might seem lower, but it’s infinitely cleaner and improving (Farris, 2012).

solar power isn’t just an energy source; it’s a movement toward a cleaner, healthier, and more sustainable planet. many countries are adapting its usage around the world, and it is at the forefront of the renewable energy wave (Ritchie et. al, 2024).

it reduces climate change impacts, preserving ecosystems and biodiversity; is going to be around as long as we are; and promotes personal interaction with the energy of our future. it's also really cool.
1 note
·
View note
Text
What are the Basic Concepts of Chemical Thermodynamics?
Ever wondered why a cold drink feels warm after a while? Or why does a hot cup of tea cool down? These phenomena are explained by thermodynamics, a branch of science that deals with heat and its relationship with work. In simpler terms, it’s like understanding the rules of energy exchange in the universe.
Chemical thermodynamics focuses on the energy changes that occur during chemical reactions. Think of it as the accountant of the energy world, keeping track of how energy is spent and gained in chemical processes.
Key concepts in chemical thermodynamics include:
Internal energy: The total energy of a system.
Enthalpy: The heat absorbed or released during a reaction at constant pressure.
Entropy: A measure of disorder or randomness in a system.
Gibbs free energy: A measure of the spontaneity of a reaction.
Understanding the Basic Concepts of Chemical Thermodynamics
Chemical thermodynamics is a branch of chemistry that deals with the relationship between heat and work in chemical reactions. To excel in this subject, especially for competitive exams like JEE, it’s crucial to grasp the fundamental concepts. Let’s break down some of the key terms:
Internal Energy (U)
Imagine a system like a container filled with tiny particles. These particles possess kinetic energy (due to their motion) and potential energy (due to their position). The internal energy is the total of all this kinetic and potential energy. It’s like the total wealth of a country, considering both cash and assets.
Enthalpy (H):
Enthalpy measures the heat absorbed or released during a reaction at constant pressure. Think of it as the “energy currency” of a reaction. If a reaction releases heat (exothermic), the enthalpy decreases. If it absorbs heat (endothermic), the enthalpy increases.
Entropy (S):
Entropy is a measure of the disorder or randomness in a system. It’s like the messiness of your room. The more scattered and disorganized the particles are, the higher the entropy. A tidy room has low entropy.
Gibbs Free Energy (G):
Gibbs free energy is a combination of enthalpy and entropy. It’s like a decision-maker for a reaction. If the Gibbs free energy is negative, the reaction is spontaneous and will occur on its own. If it’s positive, the reaction is non-spontaneous and requires external energy to proceed.
Systems
Earlier we have asked you to imagine about the “system” to have an easy understanding of the basic concepts of Thermodynamics.
System, Surroundings, and State Functions of Thermodynamics: A Simplified Explanation
Imagine a box filled with air. This box is our system. Everything outside the box, like the room, the people, and the weather, is the surroundings.
Now, imagine you’re trying to understand the air inside the box. You’d want to know things like its temperature, pressure, and volume. These properties are called state functions. They only depend on the current state of the system, not on how it got there.
Think of it like this: The state of a system is like a snapshot. It doesn’t matter how you got to that snapshot; what matters is what’s happening right now.
Here’s a breakdown of state functions:
Temperature: How hot or cold the air is.
Pressure: How much force the air exerts on the walls of the box.
Volume: How much space the air takes up.
Every thermodynamic system in the universe can be classified into these three types:
Open System
Imagine you’re sipping a hot cup of tea. Have you noticed how steam escapes from the cup, and if you wait long enough, the tea cools down? This is a perfect example of an open system. In an open system, both energy (like heat) and matter (like water vapor) can move freely between the system (your tea) and the surroundings (the air around you). Just like how your body works: you eat food (matter), and your body uses it to generate energy. You also release heat and waste, constantly exchanging energy and matter with your environment.
Closed System
Now, think about a sealed water bottle. The water inside can’t escape because the cap prevents any matter from leaving or entering. But if you leave the bottle in the sun, the water inside will warm up. Here, only energy (heat) is being transferred through the bottle, while the water (matter) stays inside. That’s what a closed system is all about—only energy can move in or out, but it does not matter. The amount of matter remains the same, even if the temperature changes.
Isolated System
An isolated system is like a super-locked treasure chest that keeps everything inside, with no way for energy or matter to get in or out. Imagine a high-tech thermos that keeps your drink at the same temperature for hours. If it’s perfectly insulated, no heat escapes, and nothing gets in. That’s an isolated system. The best example? The universe itself! Nothing can come in or go out, and the total amount of energy stays constant.
In some cases, a system can change its type. Take a car engine, for example. When fuel is injected into the engine, it’s an open system because matter (fuel) is entering. But once the fuel is inside, the engine acts as a closed system, with only energy being transferred as the engine runs.
The Laws of Thermodynamics
To understand thermodynamics, let’s explore its fundamental laws. There are four laws of thermodynamics, but the first three are the most relevant for our study:
Zeroth Law of Thermodynamics: If two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. This law helps define the concept of temperature.
First Law of Thermodynamics (Law of Energy Conservation): Energy cannot be created or destroyed, only transferred or converted from one form to another. This law explains that the total energy of an isolated system remains constant.
Second Law of Thermodynamics: This law introduces the concept of entropy. In simple terms, entropy measures the disorder in a system. The second law states that in any energy transfer, the total entropy of a system and its surroundings will always increase over time.
Third Law of Thermodynamics: As the temperature approaches absolute zero, the entropy of a system approaches a constant minimum. This law implies that it’s impossible to reach absolute zero.
Thermodynamic Equilibrium
An essential concept in chemical thermodynamics is Thermodynamic Equilibrium. A system is in equilibrium when its macroscopic properties, like pressure, temperature, and concentration, do not change over time. For a system to reach equilibrium, the forward and reverse reactions must occur at the same rate.
For example, consider a closed bottle of soda. Initially, when you shake it, carbon dioxide gas escapes. After some time, the rate of gas escaping equals the rate at which it dissolves back into the liquid, achieving thermodynamic equilibrium.
Applications of Chemical Thermodynamics
Chemical thermodynamics has wide-ranging applications across various fields. Here are some examples:
Chemical Engineering: Thermodynamics helps engineers design reactors where energy transformations take place.
Biochemistry: Understanding how energy is used by cells in biochemical reactions is essential for advancing medical research.
Environmental Science: Thermodynamic principles are applied in energy conservation, understanding climate change, and predicting environmental impacts.
These applications demonstrate the importance of chemical thermodynamics in real-world scenarios.
Limitations of Chemical Thermodynamics
While chemical thermodynamics is powerful, it does have limitations. For instance:
Cannot Predict Reaction Rates: Thermodynamics can tell you if a reaction is possible, but not how fast it will occur. That’s the job of kinetics.
Only Applies to Bulk Properties: Thermodynamics deals with macroscopic properties and does not provide detailed information about molecular-level phenomena.
Despite these limitations, the importance of chemical thermodynamics in science and engineering remains immense.
Conclusion
Chemical Thermodynamics is more than just a chapter in your textbook—it’s a key to unlocking how energy behaves in chemical reactions. By understanding the laws of thermodynamics, thermodynamic equilibrium, and the applications of chemical thermodynamics, you’ll gain a deeper insight into the processes that govern the natural world.
So, as you prepare for either your exams or any competitive exams like JEE, remember that mastering chemical thermodynamics will not only help you ace your tests but also open doors to understanding some of the most fundamental concepts in science. Keep experimenting, keep learning, and let the laws of thermodynamics guide you!
If you’re looking for more simplified explanations like the ones above, visit the Tutoroot Blog for a wealth of learning resources. Enhance your understanding with Tutoroot’s expert Chemistry online Tuition. Ready to excel in your studies? Schedule a FREE DEMO session with Tutoroot’s online home tuition and experience personalised learning tailored to your needs.
0 notes
Text
CAS NO.103-69-5 N-Ethylaniline Manufacturer test report

Quick Details Product name:N-Ethylaniline CAS:103-69-5 Molecular formula:C8H11N Molecular weight:121.18 EINECS No.:203-135-5 Purity:≥99% Brand:MIT -IVY INDUSTRY CO.,LTD Other names:Ethylaniline;N-Ethylbenzenamine;N-ethyl-Benzenamine;p-Ethylaminobenzene;N-monoethylaniline;Anilinoethane;Aniline,N-ethyl- (8CI);Anilinoethane;Ethylphenylamine;N-Ethyl-N-phenylamine;N-Ethylaminobenzene;N-Ethylbenzenamine;NSC 8736; Packing: 250 kg drum Delivery: by air,by sea,by courier Storage:Stored in a cool dry place out of direct sunlight. Appearance:yellow liquid Port: any port in china Density:0.963 g/cm3 PSA:12.03000 LogP:2.19140 Solubility Water: 50 g/L (20 °C) Melting Point:- 63 °C Boiling Point:201.7 °C at 760 mmHg Molecular Weight:121.182 Flash Point:85 °C Safety:28-37-45-28A Risk Code:23/24/25-33 Packing:according to the clients requirement Storage: Store in dry, dark and ventilated place. Transportation: by sea or by air payment methods: L/C, T/T, D/A, D/P, O/A, paypal, western union etc.accept all payment.

CERTIFICATE OF ANALYSIS Product:N-乙基苯胺-N-Ethylaniline CAS:103-69-5 Inspect Date:2022.09.02 Production Date:2022.09.02 Molecular Formula:C8H11NMolecular Weight:121.18 Quantity:25T Batch No.:MITSC22090517 Shelf life:Five years 检测项目 Test Item And Results Item Specification Result Appearance Colorless liquid Colorless liquid N-Ethylaniline %≥ 99.15 99.27 Benzene amine %≤ 0.4 0.2 N,N-Diethylaniline %≤ 0.4 0.38 moisture capacity %≤ 0.005 0.004 Conclusion Qualified N-Ethylaniline Specification The N-Ethylaniline with CAS registry number of 103-69-5 is also known as Benzenamine,N-ethyl-. The IUPAC name and product name are the same. It belongs to product categories of Intermediates of Dyes and Pigments. Its EINECS registry number is 203-135-5. In addition, the formula is C8H11N and the molecular weight is 121.18. This chemical is a yellow liquid that miscible with alcohol, ether. It at low levels causes damage to health and should be sealed in ventilated, cool place away from fire, heat without light. What's more, this chemical can be used as pesticide and dye intermediates, rubber promoting agent and also used in organic synthesis. Physical properties about N-Ethylaniline are: (1)ACD/LogP: 2.13; (2)ACD/LogD (pH 5.5): 1.98; (3)ACD/LogD (pH 7.4): 2.12; (4)ACD/BCF (pH 5.5): 17.2; (5)ACD/BCF (pH 7.4): 24.21; (6)ACD/KOC (pH 5.5): 241.62; (7)ACD/KOC (pH 7.4): 340.12; (8)#H bond acceptors: 1; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 2; (11)Index of Refraction: 1.559; (12)Molar Refractivity: 40.49 cm3; (13)Molar Volume: 125.3 cm3; (14)Surface Tension: 35.4 dyne/cm; (15)Density: 0.966 g/cm3; (16)Flash Point: 85 °C; (17)Enthalpy of Vaporization: 43.79 kJ/mol; (18)Boiling Point: 201.7 °C at 760 mmHg; (19)Vapour Pressure: 0.304 mmHg at 25 °C. Preparation of N-Ethylaniline: it is prepared by reaction of aniline, ethanol, and phosphorus trichloride. The reaction occurs at the temperature of 300 °C with the reaction pressure of 9.84 MPa. Product is obtained by vacuum distillation. Uses of N-Ethylaniline: it is used to produce N-ethyl-N-benzyl-aniline by reaction with benzoic acid. The reaction occurs with reagent trimethylamine-borane and solvent xylene with other condition of heating for 7 hours. The yield is about 99%. When you are using this chemical, please be cautious about it. As a chemical, it is toxic by inhalation, in contact with skin and if swallowed. Besides, it has danger of cumulative effects. During using it, wear suitable gloves. After contact with skin, wash immediately. In case of accident or if you feel unwell seek medical advice immediately. You can still convert the following datas into molecular structure: 1. Canonical SMILES: CCNC1=CC=CC=C1 2. InChI: InChI=1S/C8H11N/c1-2-9-8-6-4-3-5-7-8/h3-7,9H,2H2,1H3 3. InChIKey: OJGMBLNIHDZDGS-UHFFFAOYSA- The toxicity data is as follows: Organism Test Type Route Reported Dose (Normalized Dose) Effect Source mammal (species unspecified) LD50 unreported 600mg/kg (600mg/kg) Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 48(6), Pg. 22, 1983. mouse LD50 intraperitoneal 242mg/kg (242mg/kg) Yakugaku Zasshi. Journal of Pharmacy. Vol. 97, Pg. 1117, 1977. rat LC50 inhalation > 1130mg/m3/4H (1130mg/m3) United States Environmental Protection Agency, Office of Pesticides and Toxic Substances. Vol. 8EHQ-0282-0429, rat LD50 intraperitoneal 180mg/kg (180mg/kg) Archiv fuer Gewerbepathologie und Gewerbehygiene. Vol. 15, Pg. 447, 1957. rat LD50 skin 4700mg/kg (4700mg/kg) Archiv fuer Gewerbepathologie und Gewerbehygiene. Vol. 15, Pg. 447, 1957. Application 1.This product is used in organic synthesis and is an important intermediate of azo dyes and triphenylmethane dyes. 2.It can also be used as an intermediate of fine chemicals such as rubber additives, explosives and photographic materials. N-Ethylaniline Consensus Reports Reported in EPA TSCA Inventory. Superiority 1.High quality with competitive price: We are manufacturer and can provide high quality products with factory price. 2.Fast and safe delivery ① Parcels can be sent out within 48 hours after payment. Tracking number is available. ②Secure and discreet shipment. You have various choices of transportation methods. 3.We have clients throughout the world. ① Professional service and rich experience make customers feel at ease, adequate stock and fast delivery meet your desire. ②Market feedback and goods feedback are appreciated, meeting customers's requirement is our responsibility. ③High quality, competitive Company Information MIT-IVY INDUSTRY CO.,LTD is a manufacturer and exporter of fine chemical dyes & pharmaceutical intermediates in China. Mainly produce aniline series products and chlorine series products. MIT -IVY Industry use advance d production technology and test methods to realize production, quality controlling to meet the standard. We have been approved by REACH CETIFICATION ,SGS, ISO9001, ISO140 01, GB/HS16949 and T28001. Technology is the first productive force. It uses science and technology to create a brand, constantly adapts and meets the diverse needs of the market and customers, in order to realize the highest value of the company. MIT -IVY Industry hold “Integrity as root, technology s foundation,quality superiority,and top service”to produce our goods in International standard,our main technology index all meet International standard. We always believe that technology is the first productive force to creat “first class “brand to make out company among the top in this line. So we also set up its own laboratory, hired excellent scientific and technical management personnel, give priority to the development of science and technology, and strive to be the best in the industry. The company has a group of energetic, well-trained employees and strong technical research and development capabilities. We specialize in the production, development and sales of API intermediates, fine chemicals and plant extracts. Relying on advanced equipment and strict management, adhere to the business philosophy of "openness, tolerance, innovation, and sharing" to create a win-win cooperationplatform.Everything comes from innovation, it is our philosophy ! If you are interested in getting more quotations, please add WHATSAPP:0086-17363307174 or E-MAIL:[email protected] Main products MIT-IVYINDUSTRYCO.,LTDMit-Ivy is a well-known fine chemicals and pharmaceutical intermediates manufacturer with strong R&D support in China. Mainly involved Aniline, Chlorine products. Payment:DA 60 DAYSTEL:008617363307174 E-MAIL:[email protected] http://www.mit-ivy.com 产品 Product CAS N,N-二甲基-1,4-苯二胺 N,N-Dimethyl-1,4-phenylenediamine DMPD 99-98-9 N,N-二甲基苄胺 N,N-Dimethylbenzylamine BDMA 103-83-3 N,N-二甲基甲酰胺 N,N-Dimethylformamide DMF .68-12-2 N,N-二甲基甲酰胺二甲缩醛 DMF-DMA N,N-Dimethylformamidedimethyl acetal (DMF-DMA) 4637-24-5 N,N-二甲基乙酰胺 N,N-Dimethylacetamide DMAC 127-19-5 N,N-二乙基间甲苯甲酰胺 避蚊胺 N,N-diethyl-m-toluamide DEET 134-62-3 N,N-二乙基羟胺 N,N-Diethylhydroxylamine DEHA 3710-84-7 N-甲基-N-羟乙基苯胺 2-(N-甲基苯胺)乙醇 2-(N-methylanilino)ethanol 93-90-3 N-甲基吡咯烷酮 N-methylpyrrolidone 872-50-4 N,N-二甲基苯胺 N,N-Dimethylaniline DMA 121-69-7 N,N-二甲基对甲苯胺 N,N-Dimethyl-p-toluidine DMPT 99-97-8 N,N-二甲基邻甲苯胺 N,N-Dimethyl-o-toluidine DMOT 609-72-3 N,N-二乙基苯胺 N,N-Diethylaniline 91-66-7 N,N-二乙基间甲苯胺 N,N-Diethyl-m-toluidine 91-67-8 N,N-二羟乙基苯胺 N,N-Dihydroxyethylaniline PDEA 120-07-0 N-乙基间甲苯胺 N-乙基-3-甲基苯胺 N-Ethyl-m-toluidine/N-Ethyl-3-methylaniline 102-27-2 N-乙基-N-氰乙基苯胺 3-(N-ethylanilino)propiononitrile 148-87-8 N-乙基-N-羟乙基苯胺 N-Ethyl-N-hydroxyethylaniline 92-50-2 N-乙基-N-苄基苯胺 乙基苄基苯胺; N-苄基-N-乙基苯胺 N-ethyl-N-phenylbenzenemethanamine 92-59-1 N-乙基-N-氰乙基间甲苯胺 N-2-cyanoethyl-N-ethyl-m-toluidine 148-69-6 N-乙基-N-苄基间甲苯胺 N-Benzyl-N-ethyl-m-toluidine 119-94-8 N-乙基邻甲苯胺 N-Ethyl-o-toluidine/2-Ethylaminotoluene 94-68-8 N-乙基苯胺 N-Ethylaniline 103-69-5 N-甲基苯胺 N-Methylaniline 100-61-8 N,N-二甲基-间甲基苯胺 N,N-DIMETHYL-M-TOLUIDINE 121-72-2 N-甲基二苯胺 N-Methyldiphenylamine 552-82-9 N-甲基-邻甲基苯胺 N-METHYL-O-TOLUIDINE 611-21-2 N-甲基-对甲基苯胺 N-METHYL-P-TOLUIDINE 623-08-5 4-甲基-N-苯基苯胺 N-PHENYL-P-TOLUIDINE 620-84-8 N-异丙基苯胺 N-ISOPROPYLANILINE 768-52-5 N,N-二氰乙基苯胺 N,N-Dicyanoethylaniline 1555-66-4 N,N-二羟乙基-对甲基苯胺 N,N-DIHYDROXYETHYL-P-TOLUIDINEDHEPT .3077-12-1 N-乙基-2-硝基苯胺 N-Ethyl-2-Nitro-Benzenamine 10112-15-9 2,4-二氯苯胺 2,4Dichloroaniline 554-00-7 N-(2-羟乙基)乙二胺 AEEA 111-41-1 1,3-二甲基-2-咪唑啉酮N,N-二甲基亚乙基脲1,3-二甲基-2-咪唑啉酮(DMI) 1,3-Dimethyl-2-imidazolidinone DMI N,N'-dimethylimidazolidinone 80-73-9 N,N-二苄基羟胺 N,N-Dibenzylhydroxylamine 621-07-8 对甲苯胺 P-Toluidine PT 106-49-0 邻甲苯胺 O-Toluidine OT 95-53-4 二乙基乙醇胺 DEEA;DEAE 100-37-8 甲萘胺 AlphaNaphthylamine 134-32-7 间二氯苯 1,3-Dichlorobenzene MDCB 541-73-1 间甲苯胺 M-Toluidine MT 108-44-1 间苯二胺 M-PHENYLENEDIAMINE MPDA 108-45-2 多乙烯多胺 PEPA 68131-73-7 二乙烯三胺(DETA) Diethylenetriamine DETA 111-40-0 三乙烯二胺 Triethylenediamine 280-57-9 三乙烯四胺 TriethylenetetramineTETA 112-24-3 四乙烯五胺 TEPA 112-57-2 Read the full article
#103-69-5#103-69-5N-Ethylaniline#Aniline#anilinoethane#anyport#Bestprice#BuyHighqualityofN-Ethylaniline#C8H11N#CAS:NO.103-69-5#Dyeintermediates#EINECSNo:203-135-5#Ethylaniline#Ethylphenylamine#FactorySupplyN-ethylaniline#Finechemicals#HighqualityN-EthylAnilinesupplierinChina#HotsaleN-Ethylaniline#instock#Manufacturer#N-ethyl-(8CI)#N-Ethyl-N-phenylamine#N-ethylaminobenzene#N-Ethylaniline#N-Ethylbenzenamine#NSC8736#pesticideanddyeintermediates#pharmaceuticalintermediates#rubberaccelerators#SupplyN-Ethylaniline#Topsale103-69-5N-Ethylanilinewithbestprice
0 notes
Text
*cries over chemistry*
#my braincells cant handle this anymore#who is Gibb and why does he have free energy#enthalpy this entropy that#reaction formation crustaceans#what why are these molecules activated#is that a bad thing
4 notes
·
View notes
Text
Omg I have 2 finals today I am going to DIE
#one for chemistry#and one for archaeology#chemistry is killing me rn#what is a redox reaction#idk#what is enthalpy???#once again idk
0 notes