#sodium phenoxide
Explore tagged Tumblr posts
Text
Water-insoluble phenols react quantitatively with strong bases to form water-soluble salts.
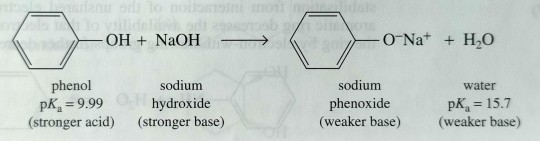
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#solubility#phenol#salt#sodium hydroxide#sodium phenoxide#chemical reactions
1 note
·
View note
Text
Crafting Clear Skin: The Precision of Salicylic Acid Manufacturing
Salicylic acid has long been a cornerstone in skincare and pharmaceutical formulations, celebrated for its remarkable efficacy in treating acne, exfoliating the skin, and managing various dermatological conditions. As a leading ingredient in numerous products, the demand for high-quality salicylic acid is unwavering. Salicylic acid manufacturers play a crucial role in meeting this demand, employing advanced technologies, stringent quality control measures, and innovative processes to produce this essential compound. In this blog, we explore the world of salicylic acid manufacturing, highlighting its significance, processes, benefits, and why it’s a cornerstone of modern skincare and pharmaceutical solutions.

The Importance of Salicylic Acid
Salicylic acid is a beta-hydroxy acid (BHA) derived from natural sources like willow bark and wintergreen leaves or synthesized in laboratories. It is renowned for its ability to penetrate pores, exfoliate dead skin cells, and reduce inflammation, making it a powerful ingredient in acne treatments, chemical peels, and dandruff shampoos. Its keratolytic properties help to soften and shed the outer layer of skin, promoting cell turnover and revealing a smoother, clearer complexion.
For more information salicylic acid manufacturer
Advanced Manufacturing Processes
Manufacturing salicylic acid involves sophisticated chemical processes to ensure purity, potency, and safety. The most common method is the Kolbe-Schmitt reaction, which synthesizes salicylic acid from sodium phenoxide and carbon dioxide under high pressure and temperature. This method yields high-purity salicylic acid, suitable for both pharmaceutical and cosmetic applications. Manufacturers utilize advanced equipment and precise control systems to maintain optimal reaction conditions, ensuring consistent quality and yield.
Quality Control and Assurance
Quality control is paramount in salicylic acid manufacturing. Rigorous testing protocols are implemented at every stage of production, from raw material selection to final product packaging. Analytical techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry (MS) are used to verify the purity, potency, and stability of salicylic acid. These tests ensure that the final product meets stringent industry standards and regulatory requirements, guaranteeing safety and efficacy for consumers.
Customization and Innovation
Salicylic acid manufacturers often work closely with cosmetic and pharmaceutical companies to develop customized formulations tailored to specific product needs. Whether creating a potent acne treatment, a gentle exfoliating cleanser, or an effective dandruff shampoo, manufacturers provide expertise in optimizing salicylic acid concentrations and formulations for maximum benefit. This collaborative approach fosters innovation, resulting in new and improved products that address evolving consumer demands and dermatological advancements.
Sustainability and Ethical Practices
In response to growing environmental concerns, many salicylic acid manufacturers are adopting sustainable and ethical practices. This includes sourcing raw materials from renewable resources, minimizing waste and emissions, and implementing energy-efficient technologies. Some manufacturers are also exploring greener synthesis methods that reduce environmental impact while maintaining high-quality production standards. These efforts align with the broader industry trend toward sustainability and responsible manufacturing.
Meeting Regulatory Standards
Compliance with regulatory standards is a critical aspect of salicylic acid manufacturing. Regulatory bodies such as the FDA (Food and Drug Administration) and EMA (European Medicines Agency) set stringent guidelines for the production and use of salicylic acid in cosmetic and pharmaceutical products. Manufacturers must adhere to Good Manufacturing Practices (GMP) and ensure their products are free from contaminants, properly labeled, and safe for consumer use. Regular audits and inspections by regulatory authorities help maintain compliance and uphold product integrity.
Future Trends and Innovations
The future of salicylic acid manufacturing is marked by continuous innovation and adaptation to emerging trends. Advances in green chemistry, biotechnology, and nanotechnology are poised to revolutionize production methods, enhancing efficiency and sustainability. Additionally, research into new applications and formulations of salicylic acid promises to expand its role in skincare and healthcare, offering consumers even more effective and versatile solutions.

Conclusion
Salicylic acid manufacturers are at the forefront of producing one of the most versatile and effective ingredients in skincare and pharmaceuticals. Through advanced manufacturing processes, stringent quality control, and a commitment to innovation and sustainability, these manufacturers ensure the consistent supply of high-quality salicylic acid. As consumer demand for effective skincare solutions continues to grow, the role of salicylic acid manufacturers remains vital, driving the development of products that promote healthier, clearer skin and improved well-being. Embrace the power of precision and discover the transformative benefits of expertly crafted salicylic acid.
2 notes
·
View notes
Text
Anisole
The global Anisole market is projected to reach a valuation exceeding USD 83 million in 2019, showcasing a steady CAGR of 4.3% over the forecast period. Anisole, scientifically known as methoxybenzene, is a colorless organic liquid compound represented by the chemical formula CH3OC6H5. Prepared through the methylation of sodium phenoxide with dimethyl sulfate or methyl chloride, Anisole is a vital derivative in both natural and artificial fragrances. Beyond its use in perfumes, fragrances, and cosmetics, Anisole plays a crucial role in the pharmaceutical industry, experiencing significant demand across various end-use sectors. Notably, Anisole production, particularly in China, has seen substantial expansion by engaged midsize companies, contributing to a considerable trade balance.
To read more about the topic please visit site: https://bekryl.com/industry-trends/anisole-market-share-analysis
The revenue growth in the Anisole market is primarily linked to the escalating sales in the personal care industry, with the global cosmetic market reaching USD 577 billion in 2018. This robust market, coupled with increasing demand, particularly in Asia Pacific and Western Europe, is expected to drive global Anisole sales. Macroeconomic factors, such as higher per capita income, augmented personal care spending, a surge in young demographics, and increased urbanization rates, are positively impacting the Anisole market. The industry has experienced steady growth, overcoming challenges like fluctuating raw material prices, crude oil price fluctuations, and changes in government policies. The growth trajectory is anticipated to persist throughout the forecast period.
Global Anisole Market Size and Forecast: By Grade Type
The global Anisole market is segmented by grade into 85%-90%, 90%-98%, and >98%. The 85%-90% grade, extensively used for industrial purposes, is anticipated to dominate the market, exhibiting the fastest CAGR of 5.7% during the forecast period. The >98% purity level Anisole is utilized for R&D purposes and in specific pharmaceutical products.
Global Anisole Market Size and Forecast: By Application Type
Segmented by application, the global Anisole market includes personal care, homecare, pharmaceuticals, research, and others. Personal care applications accounted for 47% of Anisole revenue, with notable demand from the cosmetic industry.
Global Anisole Market Size and Forecast: Regional Analysis
Asia Pacific contributed to 39% of the global market share in 2018, led by robust sales in China, India, and Japan. High consumer base, numerous small and midsize personal care and pharmaceutical industries, and favorable macroeconomic factors have created substantial business opportunities in the region. China accounted for 43% of total revenue in Asia, followed by India and Japan.
Western Europe stands as the second-largest market after Asia Pacific, commanding a population of 438 million. Germany, with 27% of regional Anisole sales, leads the market, followed by the UK and France. The region exhibits per capita cosmetic consumption almost 2.8 times higher than that in Asia.
Middle East and Latin America have a relatively smaller market share but are expected to register stable growth. The potential in these regions is, however, influenced by macroeconomic and political instability.
Global Anisole Market Size and Forecast: Competition Landscape
Key players in the Anisole market include:
Solvay SA
Huaian Depon Chemical Co., Ltd.
Merck
Westman Chemicals Pvt. Ltd.
Industry Segmentation
By Grade:
85%-90%
90%-98%
98%
By Application:
Pharmaceutical
Food & Beverage
Personal Care
Research and Others
0 notes
Text

Phenol reacts with sodium hydroxide to give sodium phenoxide which then reacts with carbon dioxide in acidic medium to give 2-hydroxybenzoic acid (Salicylic acid).
0 notes
Text


The Kolbe–Schmitt reaction or Kolbe process is a carboxylation chemical reaction that proceeds by heating sodium phenoxide with carbon dioxide under pressure, then treating the product with sulfuric acid. The final product is an aromatic hydroxy acid, also known as salicylic acid
1 note
·
View note
Text
Preparation of Phenol:
From haloarenes:
Chlorobenzene is fused with NaOH at 623K and 320 atmospheric pressure. Phenol is obtained by acidification of sodium phenoxide so produced.
From benzene sulphonic acid:
Benzene is sulphonated with oleum and benzene sulphonic acid so formed is converted to sodium phenoxide on heating with molten sodium hydroxide. Acidification of sodium salt gives phenol.
From Diazonium salts:
A diazonium salt is formed by treating an aromatic primary amine with nitrous acid (NaNO2 + HCl) at 273-278K. Diazonium salts are hydrolysed to Phenols by warming with water or by treating with dilute acids.
From Cumene:
Phenol is manufactured from the hydrocarbon, Cumene. Cumene is oxidized in the presence of air to cumene hydroperoxide. It is converted to phenol and acetone by treating with dilute acid. Acetone, a by-product of this reaction, is also obtained in large quantities by this method.
0 notes