#single use bioreactor
Explore tagged Tumblr posts
Text
#biopharma#single use bioreactor#wavebioreactor#bioprocess#science#sciencelife#upstream#fermentation#processing#calibration#validation#sampling#upstreaming#industrialbioprocessing#pharmaceuticthings#pharmaceutics
1 note
·
View note
Text

The single-use bioreactors market, valued at USD 4.1 billion in 2024, is projected to reach USD 13.6 billion by 2030, growing at a compound annual rate of 22.0%. This surge is driven by the increasing demand for biologic and biosimilar drugs due to the rising prevalence of communicable and chronic diseases, coupled with the availability of innovative single-use bioreactors.
0 notes
Text
The Single Use Bioreactors Market in 2023 is US$ 5.05 billion, and is expected to reach US$ 24.47 billion by 2031 at a CAGR of 21.80%.
#Single Use Bioreactors Market#Single Use Bioreactors Market Growth#Single Use Bioreactors Market Forecast
0 notes
Text
Single-Use Bioprocessing Market in the Quest for Efficient Biopharmaceutical Production
The global single-use bioprocessing market size is projected to reach USD 80.13 million by 2030, registering a compound annual growth rate (CAGR) of 16.24% over the forecast period, according to a new report by Grand View Research, Inc. The demand for single-use bioprocessing offerings is driven by the commercial advantages offered, including a reduction in costs and time required for bioprocessing operations. Originally used for monoclonal antibody production, single-use technologies are also gaining traction for cell and gene therapy manufacturing. As a result, broadening the scope of applications in biomanufacturing operations is likely to drive industry growth.

Single-use Bioprocessing Market Report Highlights
The simple & peripheral elements segment held the largest share in 2023 due to the significant adoption of these products as a result of a variety of customizable options available for bioprocessing applications
The upstream bioprocessing workflow segment accounted for the largest share in 2023. Continuous developments and betterment in technologies for upstream bioprocessing are driving the segment growth
North America was the leading region in 2023 due to the high R&D spending and growth of the biopharmaceutical manufacturing sector in the region
Furthermore, the presence of key players, such as Thermo Fisher Scientific, Inc. and Danaher Corp., is driving the regional market
The biopharmaceutical manufacturers end-use segment dominated the industry in 2023 and accounted for the maximum revenue share. This was due to the high demand for biologics and heavy investments in cell & gene therapy manufacturing
For More Details or Sample Copy please visit link @: Single-use Bioprocessing Market Report
Furthermore, strategic initiatives from key players are expanding the industry's growth prospects. For instance, in July 2021, Cytiva and Pall Corp. announced investment plans for capacity expansion over two years. Among other key products, more than USD 300 million were invested in single-use technologies, such as bioreactor bags for cell expansion, used to make personalized therapies and syringe filters for scientific research. Similarly, the growing adoption of single-use equipment for in-house and contract manufacturing has opened new avenues for the flow of investments in this space. The industry is witnessing significant advancements in several product portfolios, including disposable probes and sensors, stirring systems, bioreactor designs, and filtration technologies, which are expected to contribute to strong revenue growth.
The benefits offered by single-use bioprocessing systems have enabled biopharmaceutical manufacturers to offer their products faster to the market by introducing multi-product facilities, entering into partnerships, or outsourcing pipeline products for contract development and manufacturing. For instance, in January 2021, Sartorius AG partnered with RoosterBio, a leading supplier of human Mesenchymal Stem/Stromal Cells (hMSC). This collaboration aimed at advancing cell & gene therapy manufacturing by leveraging the single-use manufacturing technologies from Sartorius AG. The COVID-19 pandemic has generated new growth opportunities for key stakeholders in the industry.
#Single-Use Bioprocessing#Biomanufacturing#Bioprocessing Technology#Biopharmaceuticals#Disposable Technology#Sustainable Bioprocessing#Flexible Manufacturing#Bioproduction#BioProcess Containers#Bioreactors#Downstream Processing
0 notes
Text
#Single Use Bioreactors Market#Single Use Bioreactors Market Trends#Single Use Bioreactors Market Growth#Single Use Bioreactors Market Industry#Single Use Bioreactors Market Research#Single Use Bioreactors Market Report
0 notes
Text
latest Freddie DeBoer seems odd. It's very focused on a sort of consumer-facing understanding of technology for a lot of its runtime. He's not wrong that the changes are smaller than they were in the 1800's but like. That's the low hanging fruit, we all know this, the jump from "not having trains" to "having trains" beats almost any improvement in "trains"
A major change technology has brought to the modern world imo is heavily streamlined manufacturing all across the industrial stack.
If you read like, Bunie Huang's Made in China blog series, in the early 2000's getting a piece of technology made required enormous in-person investment of time and effort working with your manufacturing teams across a pretty broad number of suppliers and industries, you had to get PCB's made, components sourced, moulds designed and set up for injection.
I know people manufacturing small to medium run commercial and industrial electronics, and I did that at my last job. You order machine populated PCB's from your favourite Chinese PCB solutions provider over a web form. If you need ten thousand buttons, you can get that delivered with three emails. Hell, if you want a custom genome to use for some experimental bioreactor, there's multiple competing suppliers who will mail you plasmids that you can customise from online templates and you don't even have to talk to anyone.
And that's just mass manufacturing. If you're making a few thousand of some high end medical equipment, or still in the development phase of design, you can order a titanium laser print to be delivered by the end of the week, or run off a dozen prototypes on your company's fleet of printers using body safe plastics.
Consumer needs don't change much because people are people, we have limited capacity to need things and do things. I've long said that no human can digest more than 50Mbps of media in real time, really. One home cook can only economise their movements so much. A food processor and a pressure cooker can save you some time but the solution to"I want to spend less time cooking at home" will eventually become "don't cook at home, lean on industrial manufacturing of food" and that's fine. There's only so much tech can improve your individual experience before you become the bottleneck.
Faster computers sure, means you can edit video on your phone a little quicker (also hey people ARE editing video on their phone, despite what this blog post says) but it also means Netflix can serve their 4 Petabytes of video library at 400+Gbps from a single server occupying less than 50 liters of space.
It seems disingenuous to act like consumer products feeling stagnant means technology is stagnant.
21 notes
·
View notes
Text
Apparently Serbia, and especially Belgrade, has a huge problem with air pollution.
Ms. Francine Pickup, Resident Representative of the United Nations Development Program (UNDP) in Serbia, explained that: “It is estimated that cities are the source of as much as 75% of total CO2 emissions in the world, of which the largest percentage comes from traffic and cooling and heating in buildings”. She later continued to explain that 59% of the Serbian population lives in urban areas and that the number is constantly increasing. Because the population density is so high, creating green areas and planting trees – which represent natural air purification in urban areas– is a complex goal to achieve, as there is a lack of free areas for landscaping.
The microalgae replace two 10-year-old trees or 200 square meters of lawn. The function of the LIQUID 3 is practically an imitation of it. Both trees and grass perform photosynthesis and bind carbon dioxide. However, the advantage of microalgae is that it is 10 to 50 times more efficient than trees. The team behind LIQUID 3 has stated that their goal is not to replace forests or tree planting plans but to use this system to fill those urban pockets where there is no space for planting trees. In conditions of intense pollution, such as Belgrade, many trees cannot survive, while algae do not have a problem with the great levels of pollution.
The project is designed to be multifunctional. LIQUID3 is also a bench, it has chargers for mobile phones, as well as a solar panel, thanks to which the bench has lighting during the night.
Dr. Ivan Spasojevic also explained that “the Institute used single-celled freshwater algae, which exist in ponds and lakes in Serbia and can grow in tap water, and are resistant to high and low temperatures. The system does not require special maintenance – it is enough to remove the biomass created by dividing algae, which can be used as an excellent fertilizer, in a month and a half, pour new water and minerals, and the algae continue to grow indefinitely. This project aims to popularize and expand the use of microalgae in Serbia, because they can be used in wastewater treatment, as compost for green areas, for the production of biomass and biofuels, as well as for air purification from exhaust gases from the factories”.
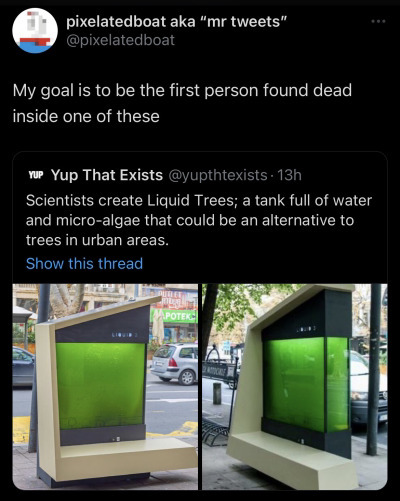
#seems like a cool solution but go off on how dumb it is I guess#my only question is: how much does a unit cost to build?#it also needs to run for a couple years to become carbon positive
32K notes
·
View notes
Text
Single-use Bioprocess Systems

What Are Single-Use Bioprocess Systems?
Single-use bioprocess systems are disposable technologies designed for one-time use in biologic production processes. Unlike traditional stainless-steel setups, SUS require no cleaning or sterilization, making them ideal for rapid setup and turnover.
Components of Single-Use Systems
1. Bioreactors
Single-use bioreactors (SUBs) replace traditional steel tanks with disposable plastic liners. They are used for culturing cells and are available in various sizes, supporting both small-scale research and large-scale production.
2. Mixing Systems
These systems facilitate the preparation of buffers, media, and other process solutions. The disposable mixing bags reduce contamination risks and simplify operations.
3. Storage and Transport Bags
Sterile storage bags provide a secure environment for intermediates or final products. Their capacity ranges from a few liters to several thousand liters.
4. Tubing and Connectors
Pre-sterilized tubing systems ensure seamless fluid transfer between components, maintaining sterility throughout the process.
Advantages of Single-Use Bioprocess Systems
1. Cost Savings
Traditional setups require significant investments in cleaning, sterilization, and maintenance. SUS eliminate these needs, reducing costs significantly.
2. Reduced Cross-Contamination Risk
Disposable components ensure a fresh, sterile setup for each batch, lowering the risk of contamination.
3. Increased Efficiency
With quick setup times and minimal downtime between batches, SUS boost production efficiency.
Challenges in Single-Use Systems Adoption
1. Waste Management
The disposable nature of SUS generates significant waste. However, recycling programs are emerging to address this issue.
2. Material Compatibility
Some biologics may interact with plastic materials, requiring careful selection of system components.
3. Scalability
While ideal for small to medium scales, SUS face challenges in ultra-large-scale production scenarios.
Applications of Single-Use Bioprocess Systems
1. Vaccine Production
SUS allow for rapid vaccine production, critical during public health emergencies.
2. Monoclonal Antibodies (mAbs)
These systems streamline the production of mAbs, widely used in treating cancers and autoimmune diseases.
3. Cell and Gene Therapy
SUS support the highly specialized requirements of cell and gene therapy manufacturing, enabling precise control over conditions.
Emerging Trends in SUS
1. Digital Integration
IoT and AI technologies are being integrated into SUS to enable real-time monitoring and predictive maintenance.
2. Sustainability Initiatives
Efforts to recycle components and reduce waste are gaining traction, making SUS more environmentally friendly.
How to Select the Right Single-Use System
Assess Your Needs: Understand your production scale and process requirements.
Evaluate Supplier Reliability: Choose suppliers with a strong track record.
Ensure Compatibility: Verify that materials and designs meet your specific process needs.
Case Studies: Success Stories with SUS
Company A leveraged SUS to scale vaccine production during a global health crisis, cutting setup time by 50%. Company B reduced cross-contamination risks in their mAb production line by switching to SUS, saving millions annually.
Future of Single-Use Bioprocess Systems
The future of SUS lies in their adaptability, integration with smart technologies, and advancements in sustainable materials. As the biopharmaceutical industry continues to innovate, SUS will play an increasingly vital role in meeting global health challenges.
Conclusion
Single-use bioprocess systems are a game-changer for biopharma companies. Their cost-efficiency, flexibility, and scalability make them indispensable for modern manufacturing. While challenges remain, innovations in material science and sustainability will further enhance their appeal.
Contact Us : [email protected]
https://www.linkedin.com/company/foxxlifesciences
0 notes
Text
https://introspectivemarketresearch.com/reports/single-use-bioreactor-market/

0 notes
Text
0 notes
Text
https://tannda.net/read-blog/68852_single-use-bioreactors-market-size-overview-share-and-forecast-2031.html
The Single Use Bioreactors Market in 2023 is US$ 5.05 billion, and is expected to reach US$ 24.47 billion by 2031 at a CAGR of 21.80%.
#Single Use Bioreactors Market#Single Use Bioreactors Market Trends#Single Use Bioreactors Market Growth
0 notes
Text
The Future of CDMOs: Key Trends Shaping the Biopharma Outsourcing Industry in 2024
In the fast-paced world of pharmaceuticals, the demand for speed, efficiency, and expertise has given rise to the prominence of Contract Manufacturing Organizations (CMOs). These organizations play a critical role in bringing innovative drugs to market while allowing pharmaceutical companies to focus on research, development, and innovation.
In this blog, we explore the evolving role of CMOs, the benefits they offer, and the latest trends shaping this dynamic industry.
What Are CMOs and Why Are They Essential?
A Contract Manufacturing Organization (CMO) is a company that provides manufacturing services to pharmaceutical and biotechnology firms. CMOs handle everything from small-scale development to large-scale commercial production. By outsourcing manufacturing to CMOs, pharmaceutical companies can save costs, enhance flexibility, and scale their operations without investing heavily in infrastructure.
Key Benefits of CMOs for Pharmaceutical Companies
Cost EfficiencySetting up and maintaining manufacturing facilities can be prohibitively expensive. CMOs offer a cost-effective solution, as pharmaceutical companies can avoid capital investment in equipment, facilities, and regulatory compliance processes.
Access to Specialized ExpertiseCMOs often possess expertise in areas like high-potency API (HPAPI) production, biologics, and sterile manufacturing that many pharmaceutical companies lack in-house.
Faster Time to MarketCMOs help accelerate production timelines, enabling quicker delivery of life-saving drugs to patients. Their established infrastructure and streamlined processes make it easier to scale production.
Regulatory CompliancePharmaceutical manufacturing requires adherence to stringent regulatory standards. CMOs invest heavily in maintaining compliance with FDA, EMA, and other global regulatory authorities, reducing the compliance burden for their clients.
Focus on Core CompetenciesBy outsourcing manufacturing, pharmaceutical companies can focus on their core strengths—such as drug discovery, R&D, and marketing—without getting bogged down by production challenges.
Emerging Trends in the Pharmaceutical CMO Industry
The pharmaceutical CMO landscape is evolving rapidly, driven by technological advancements, changing regulations, and market demands. Here are the key trends to watch:
1. Growth in Biologics Manufacturing
With the rise of biologics and biosimilars, CMOs are expanding their capabilities in areas like monoclonal antibodies, cell therapies, and gene therapies. Advanced manufacturing technologies, such as single-use bioreactors, are transforming biologics production.
2. Digital Transformation
CMOs are adopting cutting-edge technologies like Artificial Intelligence (AI), Machine Learning (ML), and Internet of Things (IoT) for process optimization, predictive maintenance, and enhanced quality control.
3. Flexible Manufacturing
The need for agile production systems is growing. CMOs are investing in modular and continuous manufacturing solutions to meet diverse client needs and improve cost-effectiveness.
4. Focus on Sustainability
Eco-friendly practices are becoming a priority, with CMOs adopting green chemistry, reducing carbon footprints, and using sustainable raw materials. This aligns with the global push toward Environmental, Social, and Governance (ESG) goals.
5. Expansion of Global Manufacturing Hubs
To ensure resilience and reduce supply chain disruptions, CMOs are setting up manufacturing facilities in emerging markets, particularly in Asia-Pacific, Eastern Europe, and Latin America.
How to Choose the Right CMO Partner
Selecting the right CMO partner is critical for a pharmaceutical company’s success. Here are some factors to consider:
Experience and Track Record: Evaluate the CMO’s experience with similar projects and their history of meeting client expectations.
Technical Expertise: Ensure the CMO has the technical capabilities to handle your product’s specific requirements.
Regulatory Compliance: Verify the CMO’s adherence to international regulatory standards.
Capacity and Scalability: Choose a partner that can scale production to meet your needs, whether for clinical trials or commercial-scale manufacturing.
Communication and Transparency: Strong communication ensures smoother collaboration and quicker resolution of issues.
Future of CMOs in the Pharmaceutical Industry
The pharmaceutical industry is evolving at an unprecedented pace, and CMOs are at the heart of this transformation. From developing advanced therapies to ensuring supply chain resilience, CMOs are becoming strategic partners rather than mere service providers. As outsourcing becomes more integral to the pharmaceutical value chain, CMOs will continue to innovate and adapt to meet the industry's demands.
Conclusion
Contract Manufacturing Organizations are not just manufacturers; they are enablers of innovation and efficiency in the pharmaceutical industry. Whether you are a pharmaceutical company seeking to optimize operations or an industry professional exploring the latest trends, understanding the role of CMOs is essential for navigating the future of healthcare.
0 notes
Text
Get More Insights On Single-Use Bioreactor https://hallbook.com.br/blogs/379830/Single-Use-Bioreactor-The-Growing-Popularity-of-Single-Use-fermentor
0 notes