#rmgx
Explore tagged Tumblr posts
Text
It is important to realise that it is not possible to use RMgX and an ester to prepare an anhydride or a ketone; the intermediate aldehyde or ketone is more reactive than the ester and reacts immediately with the Grignard reagent to give an alcohol.
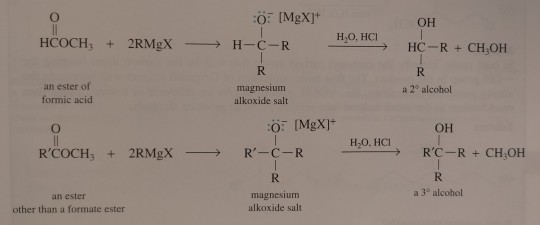
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#important#magnesium#ester#aldehyde#ketone#intermediate#reactivity#grignard reagent#chemical reactions
0 notes
Text
I've got a few comments on this image.
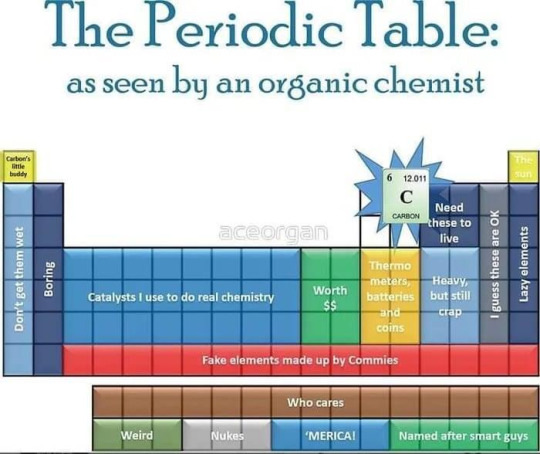
This is pretty much spot on perfect. My only correction is the first two cols are more "useful, boring counterions" or "disturbingly strong reducing agents", halogens are extremely useful but all vaguely the same thing with varying oxidizing strength/softness. Group 1 (alkali) and 17 (halogen) are often lumped together as just M and X respectively, with increasing ionic strength and size varying for M.
I guess carbon's missing neighbors are pretty important still, Boron and silicon aren't just catalysts but aren't the normal heteroatoms NOPS. They're more like "NOPS, but only used as stable reaction intermediates", like I'm not gonna find phenylboronic acid in the wild nor in an over the counter product but I am ABSOLUTELY gonna have shelf stable phenylboronic acid in the lab for a Suzuki coupling or an anti-markovnikov oxidation. Or borohydride is the BEST reducing agent, ranging from BH4 for the strongest, to HB(OAc)3 or H3BCN. Aluminum hydrides are comparable but stronger, although you won't really find organoalanes just sitting on the shelf. Aluminum kinda falls into the catalyst only area outside of LiAlH4.
Or, organosilicon chemistry is something I poke fun at often because it's FAR more like organotin or organolead than classic organics, just without the electronegativity difference of carbon group metals. But aside from organic silicones like poly(dimethylsiloxane), trimethylsilyl [Si(CH3)3 or TMS] groups are indispensable when you need something that's bulky, relatively easy to move around while still being mostly inert.
Heavy pnictogens (As, Sb, Bi) and chalcogens (Se, Te) are pretty similar to their parent P and S (the periodic table starts at period 3 imo, only period 2 cares about the octet rule), just more toxic, more stinky, and generally more useless. Organoarsenic was the first ever branch of "organometallics" but hasn't been relevant in over a century.
The d block is all random catalysts and the f block is nearly useless tho that's nearly SPOT on. Cu, Zn, Hg, Pd, Pt are all useful but in the "$" category. It feels like it takes as long to learn each transition metal's uses as it does to learn all of organic 1, they just break the rules and I won't go into organometallics right now I promise (even though I'm foaming at the mouth over ferrocene two years later)
Actually I've literally never seen a group 2 element but magnesium in an organic recipe, ever. MAYBE calcium as a counterion, because calcium salts are more often insoluble while calcium ion itself is completely inert. Sr and Ba are like calcium but way more expensive and equally useless. Be is... actually just alien tech, I'm pretty sure, maybe we could use it but we don't bc its so rare and toxic. If anyone wants I'll post more about metals from an organic chemist's perspective.
Magnesium is EVERYWHERE bc it can form carbon bonds (grignard reagent RMgX is used to make dreaded carbon carbon bonds and is super easy to make, it's the first metal you learn) and the reduced metal doesn't immediately self destruct (like all other group 1 and 2 save Be) despite still being such a reducing agent it burns in pure nitrogen. Noble gases come in here, for being perfectly inert (save Xe) and Ar is the go-to inert atmosphere (similar weight and density to N2 or O2).
But, yeah, C H O N S P X M are the only REAL elements (in order), B and Si are Secret Elements, and everything else is a catalyst.
#biochem#biochemistry#chemistry#studyblr#learning#nerd#organic chemistry#science#chem#late night#infographic#periodic table of elements#learning periodic table
3 notes
·
View notes
Photo

Preparation of several Grignard reagents used for a specific reaction.
Grignard reagents form via the reaction of an alkyl or aryl halide with magnesium metal. The reaction is conducted by adding the organic halide to a suspension of magnesium in an etherial solvent, which provides ligands required to stabilize the organomagnesium compound. On the above picture the Grignard reagents were prepared under nitrogen gas atmosphere and both the solvent and the halide was added to the magnesium with a syringe to prevent the contact with air and moisture.
Something interesting: François Auguste Victor Grignard (May 6, 1871 - December 13, 1935) discovered in 1900, that organic halides react with metallic magnesium forming a highly reactive compound what even reacts with carbon dioxide from air forming carboxylic acids. The reaction between the halide (RX) and magnesium (Mg) gives the Gringard reagent (RMgX) what is an organometallic compound, still widely used by chemists in a lot reactions. Victor Grignard was awarded the Nobel Prize in Chemistry in 1912 for his work.
156 notes
·
View notes
Text
Best mobile app development company in Gurgaon
Enuke:
It is the best mobile app development company in Delhi NCR, and Gurgaon, offering services for android app development. This business has a wealth of experience across numerous sectors and verticals.
With the aid of their technological expertise, professional and trained individuals implement high-end solutions for mobile app development. Their team meticulously develops business solutions that are cost-effective and safe.
You can produce excellent Android apps for a range of industries, including social networking, education, health and fitness, travel, entertainment, lifestyle, and more. This business has produced more than 30 internal Android and iPhone apps that are currently dominating the market.
Orangemantra:
It began as a web development firm in 2001 and has now evolved into the top mobile app development company in Delhi, NCR, and Gurgaon, providing mobile app development services. Their team is made up of subject matter specialists with a wide range of technical knowledge as well as interpersonal, problem-solving, trust, and leadership skills.
This organisation is well-known in the field of creating Android applications and has more than 16 years of experience. Travel apps, agency apps, influencer marketing apps, on-demand service apps, etc. are typically available from companies that offer application solutions. They regularly surpass clients' expectations and have worked with over 900 clients globally.
RMgX Technologies LLP:
Another best mobile app development company in Delhi, NCR, and Gurgaon. The designers and technologists employed by this organisation are extremely motivated and skilled at developing digital goods. For almost 7 years, this company has offered digital solutions, and they also help their customers get the most out of their technical investments.
Mobibiz:
A rapidly growing top mobile app development firm in Delhi, NCR, and Gurgaon. This company specializes in creating apps for Android, iPhone, and iPad and has an accomplished and creative team of developers committed to assisting you in reaching your professional objectives.
This organization develops mobile applications for your eCommerce goals and corporate requirements across all major platforms. Additionally, they now offer cross-platform app development services using Ionic, Xamarin, Apache, and web apps. This company's development team is an expert at developing mobile app strategies, UI designs, and app engineering to deliver the best outcomes in line with the value of your business.
Stellar Digital:
In 2015, Stellar Digital, the best mobile app development company in Delhi, the NCR and Gurgaon, was founded. Web design and development, digital marketing, and application development are the company's primary business sectors. The company's main objective is to offer solutions to all growing businesses and organizations through their extensive array of digital services. They generally specialize in web design and development, programming for Android and iOS, and digital marketing services.
They want to make their consumers as happy as possible by providing premium mobile, web app, and digital solutions at affordable prices. Their objective is to continuously upgrade and develop cutting-edge services and apps to improve business possibilities.
They put a high priority on providing the best services on time and cherish complete client satisfaction.
Conclusion: Check out some of the top mobile app development companies in Delhi NCR, and Gurgaon. There are many additional businesses, but we chose the ones on the list based on their customer service and best-in-class solutions. The best mobile app development company, Stellar Digital, offers services in mobile app development, digital marketing, web design, and development, but if you're still perplexed and want a comprehensive business partner, welcome. Send us an email at [email protected] to get in touch with us.
#App Development Company#App Development Company in India#App Development Company in Gurgaon#App Development Company In Delhi NCR
0 notes
Photo

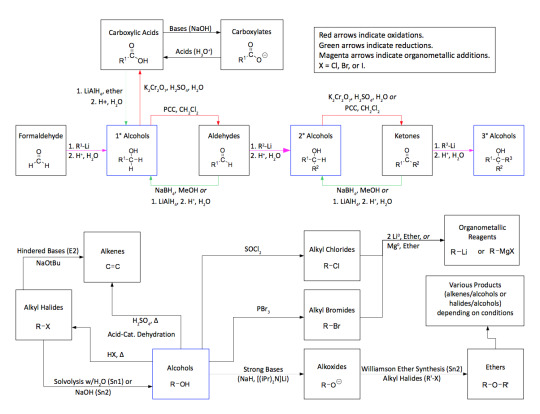
As promised my master post of all general organic chemistry reactions. Please credit me if needed and understand this took a very long time to complete. So respect my post and don’t be afraid to ask me any questions!
Disclaimer: Not every reaction contains practice problems or full details of the mechanism. The purpose of this master post is to help students start somewhere with synthesis reactions. Also, to have a list of the possible reactions that will be covered in organic chemistry. Some universities might cover more than what I’ve posted so message me if you need any other help or I should add anything to this master post!
First Semester of Organic Chemistry
Radical Halogenation - [x] [x] [x]
Initiation // Propagation // Termination
Substitution Nucleophilic Reactions - [x] [x] [x]* [x]
Walden Inversion // “umbrella flip” that occurs with SN2 reactions No stereospecificity in SN1 reactions Watch out for hydride shifts and degree of carbocations Solvolysis // solvent is the nucleophile Electrophile // Nucleophile // Leaving Group // Solvent
Elimination Reactions/Dehydrohalogenation - [x] [x] [x] [x]
Zaitsev’s Rule // non-bulky base favors more sub. alkene Hoffmann’s Rule // bulky base removes less-hindered protons Beta Hydrogens // anti to leaving group
*Contains information for radical halogenation, SN and E reactions
Alkene Reactions - [x]
Syn // same side Anti // opposite side Hydrogenation Palladium // catalyst of platinum on carbon Markovnikov’s Rule // regiospecific to the more sub. carbon Acid-Catalyzed Hydration - [x] Hydrofluoric Acid // not a strong enough acid for this reaction Kinetic Product // Thermodynamic Product Oxymercuration Mercury // allows no rearrangement Hydroboration Ozonolysis - [x]
Alkene Practice Problems - [x] [x] Alkene Roadmap
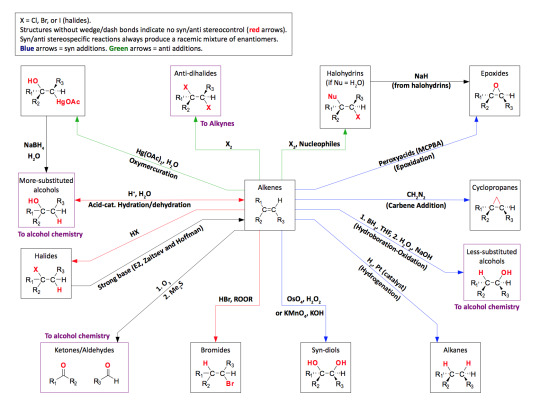
Alkyne Reactions - [x]
Hydrogenation Lindlar’s Catalyst // alkyne to alkene only! Hydration Alkylation of Acetylides - [x] Double Dehydrohalogenation
Alkyne Practice Problems - [x] [x] [x] Alkyne Roadmap
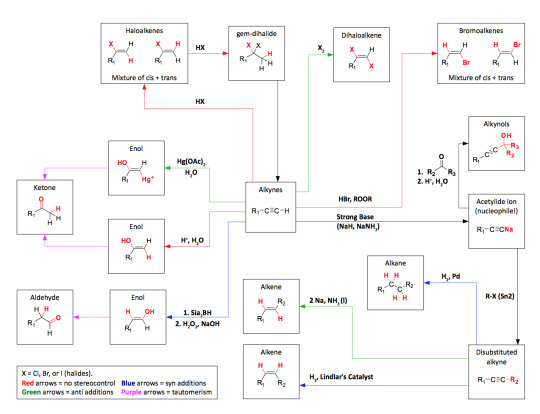
Alcohol Reactions - [x]
Primary // Seondary // Tertiary Sodium Borohydride // doesn’t work with esters Lithium Alummunum Hydride // more reactive and does work with esters
Alcohol Practice Problems - [x] [x] [x]* *Also contains epoxide practice problems! Alcohol Roadmap
Epoxide Reactions - [x]
Pretty straightforward reactions Weak Nucleophile // more sub. side Strong Nucleophile // less hindered side
Epoxide Practice Problems - [x] Epoxide Roadmap

Second Semester of Organic Chemistry
Ether Reactions - [x]
Reaction with HX Breaks the bond at the oxygen connection to form two alkyl halides Williamson Ether Synthesis - [x] Alkoxy Mercuration Demeruration
Sulfide Reactions - [x]
Diels-Alder Reactions - [x] [x] [x]
Diene // nucleophilic with an electron donating group Dienophile // electrophilic with an electron withdrawing group Watch out for regiochemistry and endo product is preferred
Electrophilic Aromatic Substitution - [x] [x] [x]
Halogenation Halogens are deactivators but ortho/para directors Alkylation Rearrangement // a 2+ carbon chain can undergo rearrangement for a more stable carbocation Limitation // installing one alkyl group is difficult due to a reactive ring Acylation Clemmensen Reduction // removes the carbonyl and is preferred to overcome rearrangement Limitations // 1) unable to install more than one acyl group because the ring becomes less reactive 2) no reaction if the ring has a nitro group present Sulfonation Can be used to make ortho position more active Nitration Reduction Reaction // converts the nitro group into an amine Carboxylic Group Addition Gatterman-Koch Reaction The addition of an aldehyde, not a ketone!
Nucleophilic Aromatic Substitution - [x] [x]
Criteria // 1) must have strong electron withdrawing group 2) must contain an LG 3) the LG must be ortho or para to the electron withdrawing group Addition-Elimination Meisenheimer Complex // the intermediate where the negative charge is being shifted around the ring for stability Reservoir // negative charge is pushed onto an atom when the ring gets attacked by a nucleophile Halogens // F > Cl > Br > I, due to bond length and electron density Elimination-Addition - [x] Benzyne // funky intermediate where a triple bond is located on the ring
**Please note that I won’t go into detail about activators versus deactivators.
Aldehyde and Ketone Reactions - [x] [x] [x]
Oxygen Nucleophiles Dean-Stark // removal of water to create the two ethers Diol // forms a cyclic acetal Hydration // addition of two -OH groups Sulfur Nucleophiles Raney Ni // neutral conditions Thiols // react similar to diols Clemmensen // acidic conditions Nitrogen Nucleophiles Preparation // jones reagent, PCC, ozonolysis Imine // primary amine that forms a double bond between N - C Enamine // secondary amine Oxime // the nitrogen is bonded to an -OH group Hydrazone // the nitrogen is bonded to another amine group Wolff-Kishner Reduction // basic conditions Carbon Nucleophiles Grignard Reagent - [x] // Wittig Reagent - [x] Addition of CN Oxidation of Aldehydes Tollen’s Test // works in the presence of aldehydes Acid Chlorides
Amine Reactions - [x] [x]
Synthesis of Amines - [x] [x] SN2 Reaction // alkylation of ammonia and doesn’t stop at mono- Reductive Amination // formation of an amine from a carbonyl Gabriel Synthesis Note // only works with primary amines Acylation of Amines Acids Hoffmann Rearrangement Nitrene // Isocyanate Hoffmann Elimination Also, forms a terminal alkene in the process Nitrosation - [x] In Situ // formation that immediately react with the amine before anything happens Sandmayer Reaction Aromatic Diazonium Salts // CuCl, CuBr, CuCN...
Carboxylic Acid and Derivative Reactions - [x] [x] [x] [x]
Golden Rule // never expel H or C Proton Transfer // may or may not occue Fisher-Sterification - [x] Amides from Carboxylic Acids Acid Halides Water // Alcohol // Amine // LAH // RMgX // R2CuLi Acid Chlorides Water // forms a carboxylic acid Alcohol // forms an ester Formation of Anhydrides - [x] Ester Synthesis Hydrolysis // acidic conditions in water Saponification // basic conditions in water Alcoholysis // formation of a new ester from another ester Reaction with RM // adds R group twice Reaction with Hydride // adds H twice Aminolysis Reaction of Amides Hydrolysis // Alcoholysis // Aminolysis Synthesis of Dicarboxylic Acids - [x] Polycarbonates - [x] Polyurethanes Ring Opening Polymerization - [x] Remember // Acid Halides > Anhydrides > Esters > Amides Can only travel from left to right in synthesis
Enol and Enolate Reactions - [x] [x]
Aldol Condensation - [x] Ketones // yields are small Crossed Aldol - [x] Note // watch out what product needs to be formed, the compound being added dropwise is important Alpha Halogenation Haloform Reaction Iodoform Reaction Hell-Volhard-Zelinsky Reaction - [x] Claisen Condensation - [x] Acetoacetic Ester Synthesis - [x] Malonic Ester Synthesis - [x] Conjugate Addition Robinson Annulation Kinetic Enolates
An Amazing Secondary Aid Book(s)
Organic Chemistry As a Second Language First Semester // Second Semester
#Mine#Chemistry#Master Post#Synthesis#Reaction#Organic Chemistry#Help#Studyblr#Student#Study#Amine#Halogens#Carbon#Enol#Enolate#Carbonyl#Ester#Ketones
274 notes
·
View notes
Text
アンペグガール / Ampeg Girl (English Lyrics)
“ ----Ampeg and bass, Sansamp, dismal girl.”
Song Title: アンペグガール / Ampeg Girl Music: テヅカ / Teduka Vocals: Kagamine Rin
NND Link: http://www.nicovideo.jp/watch/sm31745119 Original Lyrics (Official): http://piapro.jp/t/Rmgx
Comments: "Ampeg” and “Sansamp (Sans)” are brands that sell amp and other electronic guitar/bass equipment.
In the end, There's not much meaning to lyrics, is there? Yeah, it's better when there's just noise there
Fool me with the roar from an Ampeg Roar out, bass!
1, 2, 3, there! Downtown, I can't breathe well Staring at the ground, searching for oxygen, Let's go walking
Yeah, whether at the edge of the roof, or even the studio, it's all full of unhappiness Because each person's thoughts set the mood off-key
The routine of life continues on without fail With the exception of you, nothing was supposed to change, but Because I lost you, My voice is nowhere to be found Since everything's become awful, I won't sing Saying things like that, I'm a meaningless girl Every walk back-- (Still, I'm scared), --at the same intersection I miss my chance to die
In the end, There's not much meaning to lyrics, is there? No, that's not right at all It wasn't that way at all, but...
Oh yeah, It's your fault that I've started to like carbonated water The same old, familiar studio, at nighttime I still feel you here A lot of different things all connect, huh And also, end Like that memory, and this memory, too, And also, us being together
From now on, With this voice, I'll kill All the things about me and you, who changed into an entirely different person
Even if this feeling is drowned out by loud noise, Even though that can disappear too, Bass, answer me At least until the morning, when I have to sleep alone
I set my aim, And smash through the Sans onstage, And now I'll sing, so For right now, let's forget it all!
2 notes
·
View notes
Text
Organomagnesiums Market Profound Impact on the Market over 2021
Organomagnesiums belong to class of organometallic compounds which contain carbon-magnesium and other halide bonds. Organomagnesiums are one of the major reagents used in organic synthesis, mostly used to transfer organic group through nucleophilic addition. Organomagnesium compounds should be handled only under inert gas environment for safety purposes. Inert gases like nitrogen or argon are usually used for this purpose. Addition of acids, water or any oxiding materials to organomagnesium compounds mainly due their high reactivity. Organomagnesiums find large number of industrial applications primarily due to their nature to bind with halogens and other halides through single electron transfer process. Organomagnesiums are commonly found as Grignard reagents like ethylmagnesium bromide phenylmagnesium bromide and several others. Phenylmagnesium bromide is a strong nucleophile as well as a strong base. It reacts with carbon di-oxide to give benzoic acid which has numerous applications such as precursor for plasticizers and sodium benzoate (for preservatives).The market for organomagnesium is largely dominated by the U.S, followed by Europe and then Asia-Pacific region. The organomagnesium market is largely affected by increased prices of raw materials and cheaper substitutes. The application in form of catalysts acts as a prime driver for organomagnesium market.
Request For Report Sample@ https://www.persistencemarketresearch.com/samples/4251
The use of organomagnesium as an effective reagent was first pioneered by François Auguste Victor Grignard and hence these compounds find their utmost application as a Grignard Reagent. The Grignard reagent is basically an organomagnesium halide (RMgX) having a positively polarized metal, which makes the alkyl group act as a carbanion. Organomagnesiums are used as agents to prepare alcohols, ketones, keto-esters, terpene and nitriles compounds. Organomagnesiums are also used to induce a substitution at carbon, which helps in carbenoid and arynoid reactions. Organomagnesiums can also be utilized for the formation of carbon-nitrogen bonds, carbon-sulfur, carbon-oxygen bonds, carbon-selenium, carbon-halogen bonds, and other organometallic compounds. Organomagnesium compounds reactions with pyridinium salts are more useful than those of organolithium compounds and hence can act substitution to organolithiums. Organomagnesium compounds play an imperative role as catalysts and are widely used for this purpose. Organomagnesium are used in synthesis of polyolefins. Polyolefins find range of industrial applications in production of thermoplastic polyolefin and polyolefin elastomers. Thermoplastic polyolefin such as polyethylene find a range of applications in packaging (plastic bags or plastic films), while polypropylene is used in packaging, labeling, stationery, plastic parts and reusable containers. Polyolefin elastomers such as polyisobutylene, ethylene propylene rubber can also be produced through organomagnesiums. Organomagnesiums to a certain extent are also used in synthesis of pharmaceutical and fine chemicals.
Request For Report Table of Content (TOC)@ https://www.persistencemarketresearch.com/toc/4251
Organomagnesium belong to the family of organometalics which are used across the globe for varied applications including those of stabilizers, catalysts for chemical reactions and for synthesis of pharmaceuticals. The market for organomagnesium as Grignard reagents is largely dominated by the U.S. and European region. However the increased environmental regulations can lead to shift of production of these to developing countries in Asia. Also the availability of better substitutes makes the market for organomagnesiums vunerable to these substitutes. The market for organomagnesium is expected to be steady across Europe and African countries. Organomagnesium market is expected to grow at a rapid pace across Middle East owing to increase in number of production facilities. Asia Pacific and especially China is also expected to be the upcoming market for organomagnesium in the next six years.
The major players operating in the global organomagnesium market are Rockwood Lithium GmbH and Rieke Corporation among others.
Know More About Report@ https://www.persistencemarketresearch.com/market-research/organomagnesiums-market.asp
ABOUT US:
Persistence Market Research (PMR) is a third-platform research firm. Our research model is a unique collaboration of data analytics and market research methodology to help businesses achieve optimal performance. To support companies in overcoming complex business challenges, we follow a multi-disciplinary approach. At PMR, we unite various data streams from multi-dimensional sources. By deploying real-time data collection, big data, and customer experience analytics, we deliver business intelligence for organizations of all sizes.
CONTACT:
Persistence Market Research
305 Broadway
7th Floor, New York City,
NY 10007, United States,
USA – Canada Toll Free: 800-961-0353
Email: [email protected]
Web: http://www.persistencemarketresearch.com
0 notes
Text
12th class notes in hindi
MENU  अल्कोहल और फिनोल बनाने की विधियां Methods of making alcohol and phenols अल्कोहल बनाने की विधियाँ (Alcohol forming methods):
1.ग्रिन्यार अभिकर्मक से :
इस विधि द्वारा 10 , 20 , 30 एल्कोहल बनाये जाते है।
जब RMgX की क्रिया HCHO से की जाती है तो बने पदार्थ के जल अपघट्न से 10 एल्कोहल बनते है। जब RMgX की क्रिया R-CHO से की जाती है बने पदार्थ के जल अपघट्न से द्वितीयक…
View On WordPress
0 notes
Text
Customized research for $RMGX vs. $LVHD!
http://speculatingstocks.com/stockcomparison/versus.php?stock1=15409&stock2=21125
0 notes
Text
My two favorite orgo reactions!
FISCHER RXN
The Fischer esterification is a reaction as essential to the beginner organic chemistry student as it is to the layman observing his fruit ripen. Esters are a weakly polar, organic compound of chemical characterized by the dehydration of an acid (generally carboxylic) and an alcohol; as such, they are named X-yl Y-oate for the alcohol X-ol and the acid Y-oic acid. As characterized by the carbonyl and the carbon-oxygen sigma bond, the compound is in between the polarity of a comparably sized ketone above (such as ethyl acetate and acetone) and ether below (such as ethyl acetate and diethyl ether), making them miscible with each but sparingly soluble in a highly polar compound such as water or glycerol [# pubchem etoac].
Esters are highly significant in the natural world and are even known among chemistry laymen, such as wine enthusiasts, for their generally pleasant odor reminiscent of fruit (aliphatic esters) or mint (aromatic esters). This stands in stark contrast to the acrid smell and taste of most carboxylic acids and alcohols. For example, butyric acid, a carboxylic acid, is named for being the primary component of rancid butter. This response is for a good reason, when bacteria grow in over-ripe fruit, they hydrolyze the esters into their constituents (enzymatically or with increasing acidity). Over many generations, animals evolved to detect the constituents with an unpleasant smell and/or taste and associate them with rotten fruit and therefore illness.
With a clever knowledge of chemistry, this natural process of hydrolysis can be directly reversed. An extreme pH is generally required to catalyze the hydrolysis of an ester, and the reaction equilibrium in nature is driven by an excess of water. Therefore, by le Chatelier’s principle an absence of water and an excess of alcohol or acid should drive the equilibrium in the opposite direction. Indeed this is the case: Fischer and Speier noted in 1895 that in dehydrating conditions (especially over 100 C) methanol or ethanol condensed with organic acids in the presence of mineral acids [#german source]. The ester can then be readily extracted from the reaction bath of alcohol and water with a weakly polar organic solvent like diethyl ether.
In this particular experiment, benzoic acid will be esterified with excess methanol under sulfuric acid catalysis to form methyl benzoate, a compound similar to the natural product wintergreen (methyl salicylate, or methyl (o-OH)benzoate. One should note that as well as aliphatic polar solvents (most commonly ethyl acetate is used as a non-toxic alternative to diethyl ether or acetone), esters are useful as protected precursors to other compounds. For example, the Grignard reagent RMgX is an extremely strong base and would deprotonate a carboxylic acid R'COOH rather than react with the carbonyl to form a carbon-carbon bond (as Grignard reagents are intended for); however, the ester R’COOR” reacts in the way one would otherwise expect of a carboxylic acid in forming carbon-carbon bonds with a Grignard reagent.
https://pubchem.ncbi.nlm.nih.gov/compound/ethyl-acetate#section=Solubility
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cber.189502803176
GRIGNARD RXN
While the basics of organic chemistry had been known since the 1800s, and inorganic chemistry for much longer, the bridge between the two (rather directly named “organometallics”) was not understood until much more recently. The Grignard reaction is one of the first such organometallic reactions; Victor Grignard was the first to publish on the chemistry of alkylmagnesium halides RMgX (named Grignard reagents) in 1900 and was awarded a Nobel prize for this groundbreaking discovery in 1912. This discovery was so revolutionary that the field of organometallic chemistry was not even approached again until the 1930s, when organolithium reagents RLi were developed as a less sterically hindered alternative to Grignard reagents. The phenomenon of bridging two seemingly unrelated fields is significant on its own, but the discovery of Grignard reagents opened the door to modern chemistry by providing a simple and reliable way of forming carbon-carbon sigma bonds.
Carbon is a relatively neutral element with regard to electronegativity, and as such it does not readily react with other carbon atoms in molecules and preferentially reacts with more electronegative compounds. In the case of Grignard reagents, the imbalance is in the opposite direction, disrupting an already polarized carbon-halogen bond with the highly electropositive and reducing magnesium metal (via a radical intermediate). This C-Mg bond is extremely unstable (so much so that the Grignard reagent is almost exclusively generated in situ), with a strong ionic character almost resembling a persistent carbanion coordinated to an oxidized Mg2+ ion. A carbanion is extremely nucleophilic and will readily attack any electrophile (such as a carbonyl) by donating its lone pair to form a sigma bond. In the case of a carbonyl a prized carbon-carbon bond is formed, transferring the spectator MgX+ ion from the carbanion to the newly formed alkoxide ion. However, carbanions are also extremely strong bases (arguably stronger than they are nucleophiles), and any protic solvent such as water or an alcohol will act as an acid and donate a proton to form the corresponding alkane and a magnesium-solvent salt. Therefore, it is prerequisite in any Grignard reaction to keep the reaction completely dry of moisture and any protic solvent.
The Grignard reagent was first explored with carbonyl chemistry, and when named without clarification the “Grignard reaction” implies reaction of an alkyl- or aryl-magnesium halide (generally chloride or bromide) with a carbonyl of some sort. The traditional introductory example is the reaction of a Grignard reagent with a ketone to form a tertiary alcohol, where the addition is relatively straightforward and the resulting alkoxide is neutralized with acidic aqueous workup (generally with a HCl, leaving a byproduct of MgCl2 or the mixed MgClBr). The reaction with aldehydes follows similarly, forming a secondary alcohol instead, and the Grignard reagent will react with electrophiles including nitriles, alkenes, and epoxides, and (more unusually) esters.
The reaction of Grignard reagents with esters behaves most regularly in at least a 2:1 excess to the ester, where the carboxylate group is turned into a tertiary alcohol. The first equivalent of carbanion attacks the ester carbonyl to form a hemiketal, which is unstable with respect to the alkoxide leaving group from the ester and forms a ketone between the (formerly) carboxyl and the carbanion. By preserving the carbonyl from the ester carboxyl group, the intermediate molecule is open to a second attack by the carbanion to form a tertiary alcohol as described previously. However, when only one equivalent of Grignard reagent is available, the reaction will not proceed uniformly and a mix of the ester, tertiary alcohol, and intermediate ketone will be present in the product. In this experiment, phenylmagnesium bromide (itself formed from phenyl bromide and magnesium metal in situ) is reacted with methyl benzoate to form triphenylmethanol, via the intermediate benzophenone.
#chemistry#biochemistry#organic chemistry#studyblr#study blog#science#grignard#fischer#ester#perfume
1 note
·
View note
Text
Organomagnesiums Market Intelligence with Competitive Landscape 2021
Organomagnesiums belong to class of organometallic compounds which contain carbon-magnesium and other halide bonds. Organomagnesiums are one of the major reagents used in organic synthesis, mostly used to transfer organic group through nucleophilic addition. Organomagnesium compounds should be handled only under inert gas environment for safety purposes. Inert gases like nitrogen or argon are usually used for this purpose. Addition of acids, water or any oxiding materials to organomagnesium compounds mainly due their high reactivity. Organomagnesiums find large number of industrial applications primarily due to their nature to bind with halogens and other halides through single electron transfer process. Organomagnesiums are commonly found as Grignard reagents like ethylmagnesium bromide phenylmagnesium bromide and several others. Phenylmagnesium bromide is a strong nucleophile as well as a strong base. It reacts with carbon di-oxide to give benzoic acid which has numerous applications such as precursor for plasticizers and sodium benzoate (for preservatives).The market for organomagnesium is largely dominated by the U.S, followed by Europe and then Asia-Pacific region. The organomagnesium market is largely affected by increased prices of raw materials and cheaper substitutes. The application in form of catalysts acts as a prime driver for organomagnesium market.
Request Sample Copy of the Report @ https://www.persistencemarketresearch.com/samples/4251
The use of organomagnesium as an effective reagent was first pioneered by François Auguste Victor Grignard and hence these compounds find their utmost application as a Grignard Reagent. The Grignard reagent is basically an organomagnesium halide (RMgX) having a positively polarized metal, which makes the alkyl group act as a carbanion. Organomagnesiums are used as agents to prepare alcohols, ketones, keto-esters, terpene and nitriles compounds. Organomagnesiums are also used to induce a substitution at carbon, which helps in carbenoid and arynoid reactions. Organomagnesiums can also be utilized for the formation of carbon-nitrogen bonds, carbon-sulfur, carbon-oxygen bonds, carbon-selenium, carbon-halogen bonds, and other organometallic compounds. Organomagnesium compounds reactions with pyridinium salts are more useful than those of organolithium compounds and hence can act substitution to organolithiums. Organomagnesium compounds play an imperative role as catalysts and are widely used for this purpose. Organomagnesium are used in synthesis of polyolefins. Polyolefins find range of industrial applications in production of thermoplastic polyolefin and polyolefin elastomers. Thermoplastic polyolefin such as polyethylene find a range of applications in packaging (plastic bags or plastic films), while polypropylene is used in packaging, labeling, stationery, plastic parts and reusable containers. Polyolefin elastomers such as polyisobutylene, ethylene propylene rubber can also be produced through organomagnesiums. Organomagnesiums to a certain extent are also used in synthesis of pharmaceutical and fine chemicals.
Organomagnesium belong to the family of organometalics which are used across the globe for varied applications including those of stabilizers, catalysts for chemical reactions and for synthesis of pharmaceuticals. The market for organomagnesium as Grignard reagents is largely dominated by the U.S. and European region. However the increased environmental regulations can lead to shift of production of these to developing countries in Asia. Also the availability of better substitutes makes the market for organomagnesiums vunerable to these substitutes. The market for organomagnesium is expected to be steady across Europe and African countries. Organomagnesium market is expected to grow at a rapid pace across Middle East owing to increase in number of production facilities. Asia Pacific and especially China is also expected to be the upcoming market for organomagnesium in the next six years.
The major players operating in the global organomagnesium market are Rockwood Lithium GmbH and Rieke Corporation among others.
Request TOC of the Report @ https://www.persistencemarketresearch.com/toc/4251
0 notes
Text
Customized research for $RMGX vs. $AAXJ!
http://speculatingstocks.com/stockcomparison/versus.php?stock1=15409&stock2=64
0 notes
Text
#SOCIALMEDIABUZZ 2-23-17
$IJJP $SIML $LPPI $MDCN $DRNK $RSII $GDSI $GOSY $SFOR $ICNB $CTIX $HJOE $WHEN $SPRV $SDVI $TPAC $SGBY $NNLX $GIGL $EVLI $MFST $GEQU $NTRP $DBMM $PKGM $MYEC $GTRQ $BIEI $NTEK $AHIX $NWAV $MJTK $UAPC $BLTA $OPMZ $COTE $MNGG $KGET $USMJ $BMXC $URBF $MKAU $TXTM $DECN $GEGI $UAMM $ICNM $ELTP $TTCM $POETF $TALK $PNOW $MCOA $NYXO $NEIK $ISBG $QTMM $IBRC $NEWC $SNMN $NECA $LBTG $OWCP $DCAC $RMHB $LWLG $SHOM $STCC $FTNW $DKGR $TRTC $VDRM $CATV $INCC $FNHI $WSGP $OXDG $HEMP $MOXC $RMGX $GLLK $BIEL $QLTS $FFFC #SOCIALMEDIABUZZ 2-23-17 http://dlvr.it/NSXy42
0 notes
Text
Stock Analysis Videos: $TRMR $AMMA $RJETQ $MCOA $ADVT $RMGX $LIGA $MJTK $DRYS $SINO https://t.co/UbIlJ2s1BS
Stock Analysis Videos: $TRMR $AMMA $RJETQ $MCOA $ADVT $RMGX $LIGA $MJTK $DRYS $SINO https://t.co/UbIlJ2s1BS http://dlvr.it/NMvhCg
0 notes
Text
Digital Innovation Unleashed: UI/UX Mastery and Consulting in Gurugram
Discover the magic of RMgX, where mobile application development meets exceptional digital consulting. Immerse your audience in stunning UI/UX designs that captivate and engage. With us as your creative ui ux design agency, elevate your digital journey with visually striking and user-friendly interfaces that reflect your brand's essence. Explore the possibilities of mobile application development with RMgX digital consulting at https://www.rmgx.in/services. Transform your brand's digital experience with RMgX today!
To Know mor visit https://www.rmgx.in/services
#rmgx#digital consulting#ui ux agency#website design and development company#wegetitdone#digital transformation solutions#web developer for startups#mobile application development#software product development company
0 notes
Text
Crafting Digital Elegance: The Essence of Mobile App Development & Product Design
RMgX, a premier mobile application development service company, excels in delivering top-notch solutions, setting the benchmark for industry excellence. Specializing in mobile app development, RMgX is renowned for tailoring exceptional applications to meet client needs. Recognized as one of the best product design companies in the field, RMgX stands out through its unwavering commitment to quality, innovation, and client satisfaction. Their adept team of developers harnesses the latest technologies and employs a robust development process, ensuring the creation of feature-rich and user-friendly mobile apps. Whether initiating a new app or enhancing an existing one, RMgX is the trusted partner for reliable and high-quality app development services in the realm of digital consulting.
#rmgx#digital consulting#ui ux agency#best website development services#mobile application development#web developer for startups#wegetitdone#digital transformation solutions
0 notes