#regulatory devices
Explore tagged Tumblr posts
Text

Get The Perfect Medical Consulting Service
We deliver professional medical device consulting services with in-depth attention to safety, reliability and compliance. Our consultant looks for ways to optimize a facility's ability to deliver high-quality care to the products. For more information, call us at 919-313-3960 or reach our website.
#regulatory devices#ce certification#medical device registration#medical device classification#medical device consultant#medical device regulation
0 notes
Text
Moral of Casanova (2005):
Don’t emotionally neglect your child with selective mutism or take them to public executions.
#Casanova (2005)#David Tennant#Like okay I’m a parapro and any time a child was onscreen I was SCREAMING directions at the parent#no he’s not the idiot; you are! GIVE HIM TO ME AND I WILL TAKE CARE OF HIM THE RIGHT WAY#Ughhh poor Giacomo Sr. and Jr.#That one old nurse was like “have you ever seen such a miserable child” like yeah you’re right but must you say it to his face like THAT#Give that kid some fun snacks and other kids to play with and a bin of wooden blocks (so he can dump it)#and also someone who can incorporate his interests into learning and OH MY GOD HIRE ME PLEASE FOR THE LOVE OF YOUR CHILD#That poor kid had absolutely zero regulatory skills and the only thing he could manage doing was stand there#like a starched 2x4 and expressionlessly knock over vases to watch them break. You don’t understand I am clawing at my EYES#I’d be like “Hey man… I don’t know if you’re angry or if you just enjoy watching things break; but you’ve been through a lot.#You seem tired. Am I correct?” [wait] “You don’t have to speak if it’s too much. It’s okay.#You’ve been dragged across the world without your consent by someone who doesn’t care about your life or his.#And you’ve just seen him get shot which — regardless of how you feel about him — is pretty scary. I’d be worn out too.”#Okay THAT is how you talk to that kind of kid. You don’t pressure him into being proud of you or call him “idiot” like what the fuck#communication devices weren’t a thing back then but by god I’d draw up the meanest flash cards you’ve ever seen#LET ME IN THE TELEVISION
5 notes
·
View notes
Text
Expert Medical Device Regulatory Consulting Services for Compliance Success | Trinity M Consulting

Trinity M Consulting provides expert medical device regulatory consulting services to ensure compliance success. Our team of experienced professionals will guide you through the complex regulatory landscape, helping you navigate requirements and achieve market clearance efficiently. Trust Trinity M Consulting for reliable and comprehensive regulatory support tailored to your specific needs.
#medical device regulatory consulting services#medical device consultants#medical device consulting services#medical device regulatory consulting
2 notes
·
View notes
Text
0 notes
Text
Why CDSCO Registration is Crucial for Your Medical Products
CDSCO registration is mandatory for all medical devices and pharmaceutical products being sold or imported into India. This process ensures your product meets the necessary health and safety standards, gaining approval for market access. Navigating the regulatory environment can be complex, but with the right guidance, it becomes a manageable task. Let us help you through every step of the CDSCO registration process, from documentation to certification. Get in touch to begin your registration today!

#CDSCO#CDSCO Registration#Regulatory Compliance#Medical Product Approval#Medical Devices#CDSCO Compliance
0 notes
Text
#medical device consulting services#medical writing regulatory documents#medical device consultancy in india
0 notes
Text
//healthcareeverything.com/navigating-regulatory-compliance-in-medical-device-manufacturing/
#BestHealthcareDigitalMagazine#TopHealthandWellnessMagazines#tophealthmagazines#Medical Device#Navigating Regulatory Compliance#Device Manufacturing
0 notes
Text
AfriSummit 2024: African Health Authorities and Industry Leaders Unite to Discuss Pharma Regulations and Innovations
PharmaReg and MedDevReg AfriSummit Host 80+ Regional and International Speakers Alongside Over 300 Pharmaceutical and Medical Device Professionals Cairo, Egypt — (AfricaNewswire.Net) – Prominent healthcare authorities and industry specialists came together for AfriSummit 2024, a vital 4-day hybrid event dedicated to advancing pharmaceutical and medical device regulations along with interactive…
#AfriSummit#AfriSummit 2024#distribution sectors#Grand Nile Tower#legal#manufacturing#MedDevReg#medical device#Pharma Regulations#Pharmaceutical#PharmaReg#regulatory
0 notes
Text
Unlocking Compliance: Essential Guide to the Non-Conviction Certificate for Medical Devices in India
In India, medical device registration is essential for manufacturers, importers, and distributors aiming to ensure regulatory compliance and public safety. Governed by the Central Drugs Standard Control Organization (CDSCO) under the Medical Device Rules 2017, the process requires various approvals, including CDSCO medical device registration, product approvals, import licenses, and the Non-Conviction Certificate (NCC). Among these, the NCC is a crucial document that reinforces a company’s compliance record. This guide delves into the Non-Conviction Certificate, highlighting its importance, eligibility, and the steps involved in obtaining it.
What is a Non-Conviction Certificate (NCC)?
A Non-Conviction Certificate (NCC) is issued by the CDSCO to Indian medical device companies. This certificate attests that a company has not been convicted of offenses related to safety, quality standards, or product malfunctions associated with its devices. Often essential in international tenders and product registration in foreign markets, the NCC demonstrates a company's adherence to high regulatory standards. This certification is vital in the medical device regulatory services landscape in India, underscoring the company’s commitment to ethical practices.
Importance of the Non-Conviction Certificate (NCC)
The Non-Conviction Certificate has numerous advantages for medical device companies, including:
- Building Trust: Demonstrates a company’s dedication to safety, quality, and regulatory compliance. - Unlocking Business Opportunities: Required in many tenders and pre-qualification applications, especially in CDSCO medical device registration. - Maintaining Market Reputation: Strengthens a company’s credibility and reputation in the medical devices India sector.
Eligibility Criteria for the Non-Conviction Certificate
To be eligible for the NCC, a company must: - Hold a valid medical device registration India license from the Central Licensing Authority (CLA) or State Licensing Authority (SLA). - Maintain a clean record with no prior convictions or violations under the Drugs and Cosmetics Act 1940 and related Rules.
Required Documentation for the Non-Conviction Certificate
Obtaining an NCC involves compiling the following documents: 1. A cover letter stating the purpose of the application. 2. A copy of the company's valid medical device registration or import license. 3. A list of products for which the NCC is being requested. 4. Prescribed government fees. 5. A Legal Undertaking on a ₹100 registered, notarized stamp paper, confirming no convictions related to product malfunctions or compliance violations.
Who Issues the Non-Conviction Certificate?
The licensing authority that initially issued the manufacturing or import license (either the CLA or SLA) is responsible for granting the NCC.
Validity of the Non-Conviction Certificate
The NCC is valid for one year from issuance or until the company's manufacturing license expires, whichever occurs first.
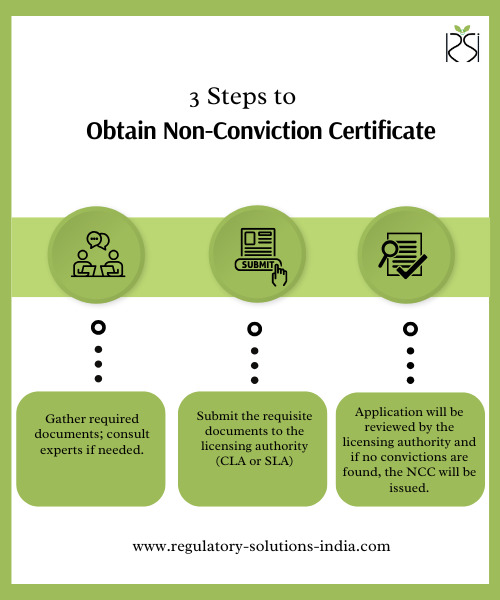
Steps to Obtain the Non-Conviction Certificate
1. Submit the application with the necessary documents to the relevant licensing authority. 2. For a smooth application process, consider engaging a regulatory consultant in India who specializes in medical device regulatory consultancy.
Benefits of Hiring a Regulatory Consultant for NCC Application
Engaging a medical device regulatory consultant in India can ease the application process, given their expertise in regulatory consultancy services. Consultants bring several advantages: - Requirement Clarity: Ensures the applicant meets eligibility criteria and provides complete documentation. - Efficient Navigation of Procedures: Streamlines applications and reduces potential delays. - Expert Advice: Consultants bring in-depth knowledge, particularly beneficial for medical device registration India requirements. - Peace of Mind: Consultants manage the regulatory process, allowing companies to focus on core operations.
Consult Regulatory Solutions India for Your NCC Needs
Regulatory Solutions India (RSI) offers expert regulatory consultancy services for medical devices and IVDs in India, providing end-to-end support for compliance, including CDSCO consultancy services. With RSI's guidance, you can ensure compliance with regulatory standards, obtain the Non-Conviction Certificate, and gain a competitive edge in India’s regulated markets. ContactRSI today to discuss your specific requirements. Our team of regulatory consultants is here to assist with your CDSCO medical device registration and NCC needs, making the compliance journey smoother and more efficient.
0 notes
Text
0 notes
Text
Navigating Medical Device Compliance: Expert Regulatory Consulting Services
In the highly regulated field of medical devices, ensuring compliance with industry standards and regulations is crucial for bringing products to market and maintaining their legality. Medical Device Regulatory Consulting Services are essential for companies looking to navigate complex regulatory landscapes and achieve successful product launches. Here’s how expert consulting services can help streamline the process and ensure your medical devices meet all necessary requirements.
Understanding Medical Device Regulations
1. Global Regulatory Landscape
Medical device regulations vary significantly across different regions, including the U.S., Europe, and other international markets. In the U.S., the FDA (Food and Drug Administration) sets rigorous standards for medical devices, while the European Union has its own set of regulations under the MDR (Medical Device Regulation) and IVDR (In Vitro Diagnostic Regulation). Understanding these regulations is essential for global market access.
2. Classification and Requirements
Medical devices are classified based on their risk levels, intended use, and technological characteristics. Each classification has specific regulatory requirements that must be met. Consulting services help determine the appropriate classification for your device and guide you through the corresponding regulatory pathways.
Key Benefits of Medical Device Regulatory Consulting Services
1. Expert Guidance on Compliance
Regulatory consultants provide expert advice on navigating the complex regulatory requirements for medical devices. They ensure that your device meets all necessary standards, including safety, effectiveness, and quality, helping you avoid costly delays and non-compliance issues.
2. Streamlined Submission Processes
Consultants assist in preparing and submitting regulatory documents, including 510(k) submissions, PMAs (Premarket Approvals), CE Mark applications, and other necessary paperwork. They help ensure that submissions are complete, accurate, and compliant with regulatory requirements, expediting the approval process.
3. Risk Management and Quality Assurance
Effective risk management and quality assurance are critical for medical device compliance. Consultants help implement robust risk management plans, conduct thorough documentation reviews, and establish quality management systems (QMS) that align with ISO 13485 standards, ensuring ongoing compliance throughout the product lifecycle.
4. Market Access and Strategy
Consultants provide strategic advice on market access, helping you understand and meet the specific requirements for different regions. They assist in developing regulatory strategies that align with your business goals and facilitate smoother entry into international markets.
5. Ongoing Support and Updates
Regulatory requirements are constantly evolving, and staying up-to-date is essential. Consulting services offer ongoing support and updates on regulatory changes, helping you adapt to new requirements and maintain compliance over time.
Choosing the Right Regulatory Consulting Partner
1. Experience and Expertise
Select a consulting firm with extensive experience and expertise in medical device regulations. Look for consultants who have a proven track record of successful submissions and regulatory approvals in your specific device category.
2. Understanding of Your Industry
Choose a consultant who understands your particular industry segment and the unique challenges associated with your device. Their industry knowledge will be invaluable in addressing specific regulatory concerns and ensuring compliance.
3. Personalized Service
Opt for a consulting firm that offers personalized service and is responsive to your needs. Effective communication and a tailored approach are crucial for addressing your specific regulatory challenges and achieving successful outcomes.
Conclusion
Medical device regulatory consulting services play a vital role in helping companies navigate the complex regulatory landscape and achieve compliance. By leveraging expert guidance, streamlined submission processes, and ongoing support, you can ensure that your medical devices meet all necessary requirements and successfully enter the market. Partnering with a reputable consulting firm will provide you with the expertise and resources needed to achieve regulatory success and maintain ongoing compliance.
0 notes
Text
Medical Device 510(k) Consulting Services
Navigating FDA regulations can be tough, but Sigma Biomedical makes it easier with Medical device 510(k) consulting. The FDA requires a 510(k) submission for many medical devices, and without proper guidance, it can be a time-consuming process. Sigma Biomedical offers comprehensive consulting to guide you through every step.
Complete submission guidance
Review of regulatory requirements
Expert assistance in avoiding delays
Choose Sigma Biomedical for expert 510(k) consulting to accelerate your medical device approval.
#510(k) preparation services#510(k) submission consulting#medical device 510(k) consulting#regulatory consulting for 510(k)
0 notes
Text
QMS & Risk Management for IVD Manufacturers
IVD manufacturers must comply with strict regulations to ensure product safety and quality. A well-implemented Quality Management System (QMS) and risk management strategy help manufacturers meet regulatory standards, minimize risks, and improve overall product reliability. This content offers insights on regulatory frameworks like ISO 13485 and FDA 21 CFR Part 820, designed to support manufacturers in the medical diagnostics industry.
For more info: https://www.makrocare.com/blog/developing-and-maintaining-a-qms-for-ivds/
0 notes
Text
Medical Device Labeling: The Important Role Of Medical Device Label In Patient Care

Medical devices sold in the United States must comply with labeling requirements set by the Food and Drug Administration (FDA). All labels must contain specific information about the device such as its intended use, any potential risks or side effects, and instructions for use. Device labels provide critical safety information that helps ensure devices are used properly. The FDA regulates labeling to protect patient health.
Labels Must Clearly Identify The Device
First and foremost, a Medical Device Labeling must clearly identify the specific device. This includes stating the product name and any applicable product codes or reference numbers. Having a clear device name and identification numbers helps providers and facilities properly select, use, and track the intended device. Any trade or brand names must also be included but must not overshadow the core product identity information.
Instructions For Use Must Be Clear And Complete
Detailed instructions for use are extremely important for medical devices to be operated safely and as intended. Labels must provide step-by-step directions on how the device is to be used, prepared, fitted, applied, implanted, or operated. Pictures, diagrams or other illustrative guides can help visualize proper technique when words alone may not fully explain the process. Instructions must account for all reasonably foreseeable uses. Omitting any necessary steps could result in misuse leading to patient harm.
Packaging Labels Provide Sterility And Shelf Life Assurances
For devices distributed in single-use sterile packaging, labels must affirm the method used to sterilize the contents as well as the expiration date or shelf life. This information guarantees the continued sterility and integrity of the device up until the noted expiration date. Devices like surgical tools and implants must remain free of contaminants when used. Packaging labels demonstrate the steps taken to achieve and maintain sterile conditions.
Potential Adverse Reactions And Hazards Must Be Disclosed
Device labels must include a complete list of known or reasonably foreseeable adverse health effects or hazards from use. This involves describing any potential allergic reactions, biological risks, toxicology concerns and interactions with other devices, drugs or substances. Precautions, contraindications and any use limitations due to patient risks or conditions should also be detailed. Making providers aware of safety issues enables them to properly assess risks and benefits for individual patients.
Symbols Standardize Hazard And Safety Communications
Pictograms and symbols play an important role in medical device labeling by allowing concepts to be recognized universally without language barriers. Common symbols indicate requirements such as “Do Not Reuse”, “Sterilized Using Irradiation”, “Keep Away From Heat or Flames”, and many others defined by International Standards Organization (ISO) regulations. Having standardized symbols helps labeling information be consistently interpreted.
Product Labels Remain With The Device
Device manufacturers must ensure all labeling remains affixed or adjacent to the medical device itself throughout distribution and use. Shipping labels serve to identify devices during transportation but do not replace the requirement for complete labeling on the finished able product. Device labels need to be available anytime and anywhere the device is utilized to provide critical use and safety guidance to providers.
As described, medical device labelingserves the fundamental purpose of guiding appropriate and safe use while communicating potential risks. Complying with comprehensive FDA labeling policies supports quality patient care.
Get more insights on this topic: https://www.pressreleasebulletin.com/medical-device-labeling-device-labeling-regulations-ensuring-patient-safety-and-compliance/
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
#Medical Devices#Regulatory Compliance#Product Labeling#FDA Regulations#UDI (Unique Device Identification)#MDR (Medical Device Regulation)#Labeling Standards#Risk Management#Health Canada Regulations#Clinical Labeling
0 notes
Text
1 note
·
View note
Text
Master Of Science In Regulatory Affairs: Advancing Your Career At Aleph University
Are you looking to take your career in the life sciences industry to the next level? A Master of Science in Regulatory Affairs from Aleph University could be the key to unlocking new opportunities and advancing your professional journey.
The rapidly evolving landscape of healthcare and pharmaceutical regulations demands skilled professionals who can navigate complex regulatory frameworks. Aleph University's Master of Science in Regulatory Affairs program is designed to meet this growing need, providing students with the knowledge and skills necessary to excel in this critical field.
Why Choose Aleph University's Master of Science in Regulatory Affairs?
Aleph University's program stands out for several reasons:
1. Comprehensive Curriculum: Our Master of Science in Regulatory Affairs covers a wide range of topics, including global regulatory strategies, clinical trial regulations, and quality management systems.
2. Industry-Experienced Faculty: Learn from professors with extensive experience in regulatory affairs, bringing real-world insights into the classroom.
3. Flexible Learning Options: Choose between on-campus and online formats to fit your busy schedule.
4. Networking Opportunities: Connect with industry professionals and fellow students, building a valuable network for your future career.
5. Hands-on Experience: Gain practical skills through internships and capstone projects, applying your knowledge to real-world scenarios.
Career Prospects for Graduates
Graduates of the Master of Science in Regulatory Affairs program at Aleph University are well-positioned for success in various roles, including:
- Regulatory Affairs Specialist
- Clinical Research Associate
- Quality Assurance Manager
- Compliance Officer
- Regulatory Policy Analyst
These positions are in high demand across pharmaceutical companies, medical device manufacturers, biotechnology firms, and government agencies.
Program Structure
The Master of Science in Regulatory Affairs at Aleph University is typically completed in two years of full-time study or three years part-time. The program consists of core courses, electives, and a capstone project or thesis.
Key courses include:
- Global Regulatory Strategy
- Clinical Trial Design and Management
- Pharmaceutical Quality Systems
- Medical Device Regulations
- Regulatory Submissions and Documentation
Admission Requirements
To be considered for the Master of Science in Regulatory Affairs program at Aleph University, applicants should have:
- A bachelor's degree in a related field (e.g., life sciences, pharmacy, engineering)
- Relevant work experience (preferred but not required)
- Strong analytical and communication skills
Why Regulatory Affairs Matter
The regulatory affairs field plays a crucial role in ensuring the safety and efficacy of medical products. Professionals in this area help companies navigate the complex regulatory landscape, facilitating the development and approval of innovative healthcare solutions.
By pursuing a Master of Science in Regulatory Affairs at Aleph University, you'll be positioning yourself at the forefront of this essential industry. You'll gain the expertise needed to guide products through the regulatory process, from initial development to market approval and beyond.
Take the Next Step
If you're ready to advance your career in regulatory affairs, consider applying to Aleph University's Master of Science in Regulatory Affairs program. Our expert faculty, comprehensive curriculum, and industry connections will provide you with the tools you need to succeed in this dynamic field.
Contact Aleph University's admissions office today to learn more about how our Master of Science in Regulatory Affairs can help you achieve your professional goals and make a lasting impact in the life sciences industry.
#master of science in regulatory affairs#regulatory affairs programs online#medical devices regulatory affairs courses#masters in quality assurance and regulatory affairs
0 notes