#qms documents
Explore tagged Tumblr posts
Text
The need for ISO 9001 certification is rising across a wide range of industries, helping organizations' quality management systems to meet worldwide standards. It is the most extensively used standard for quality management systems globally. It assists companies in moving forward and making sure that the standard of the products or services they offer meets the demands of their customers. But who precisely benefits from an ISO 9001 certification, and why is it so crucial for these kinds of businesses?
#QMS#QMS certification#iso 9001#iso 9001 certification#iso 9001 qms certification#iso 9001 documents#qms documents
0 notes
Text
A quality management system must meet the requirements of ISO 9001:2015, the only standard in the family that can be certified. Any organization, no matter how big or little, can use it, regardless of what they do. Numerous quality management concepts, such as a strong customer focus, senior management's incentive and involvement, the process method, and continuous improvement, form the foundation of this standard.
#iso 9001 audit#iso 9001#iso 9001 certification#iso 9001 audit checklist#iso 9001 external audit#qms documents#iso 9001 documents
0 notes
Text
ISO 9001 Audit plays a critical role in self-check mechanisms. The last step for your business to become certified to ISO 9001 is an external audit. Your quality management system (QMS) will be evaluated by the auditor to make sure it satisfies ISO 9001 criteria. Although audits may be intimidating, your business should be recommended for certification if you are prepared and know what to expect. To help you prepare, we examine the primary processes of an external audit in this article.
#iso 9001 audit#iso 9001#iso 9001 certification#iso 9001 audit checklist#iso 9001 external audit#qms documents#iso 9001 documents
0 notes
Text
Streamlining ISO 13485 Documentation: Compliance and Efficiency in QMS

In the domain of medical device manufacturing, keeping a strong Quality Management System (QMS) is central to ensuring product security, viability, and regulatory ISO 13485 Compliance. ISO 13485, the global standard for QMS in the medical device industry, presents severe documentation requirements to accomplish these objectives.
The test lies in meeting these requirements, however, in doing so productively. Smoothing out ISO 13485 QMS documentation includes making clear, brief, and viable processes that work with consistency and understanding while at the same time staying away from the entanglements of administrative formality. This approach guarantees compliance as well as upgrades operational proficiency, permitting organizations to zero in on development and quality improvement.
This article dives into the significance of viable documentation practices, the design of ISO ISO 13485 Documentation, and reasonable systems for accomplishing a smoothed-out documentation process that upholds your organization’s targets.
The Importance of Documentation
The motivation behind the documentation in the QMS is to ensure that basic cycles, where you want to ensure that all employees reliably do exactly the same thing, are recognized and repeatable.
To make this work, it is savvy to have these processes as transparent as could be expected and introduced in the easiest way to make them transparent. Frequently, utilizing a graphical stream outline can do the trick to hand off all the significant data rapidly and without any problem.
The less muddled the process documentation, the simpler it will be to guarantee that all employees can deliver repeatable, quality results for the cycles. Over the long haul, the proverb is in many cases right: “The simpler, the better.” And this is the very thing that the significance of ISO 13485 good documentation practices is about.
The Most Effective Method to Structure QMS Documentation As Per ISO 13485
When you ponder QMS documentation, do you picture heaps of reports? Perhaps administrative formality and pointless strategies? For certain organizations, this is the appalling reality, since they erroneously accept that the more archives they make, the more compliant they will appear to be. Try not to allow your organization to fall into this snare.
Obviously, being the worldwide standard for QMSs in the medical device industry, ISO 13485 requires specific documentation. In any case, that documentation fills various needs and not one of them includes making your organization essentially seem, by all accounts, to be consistent.
The genuine purpose behind ISO 13485 QMS documentation is below:
To give a clear structure to the organization's tasks
To work with process consistency and a better understanding of the QMS
To show proof of the organization's accomplishment of its objectives and goals
In this way, when you set off to make your QMS documentation, your emphasis ought to be on proficiency, and on making just those processes and documents that will help your association.
ISO 13485 Documentation Requirements
At this point, you ought to be intimately acquainted with the maxim ...if it isn’t documented, then it didn’t happen.”
Indeed, documentation of QMS processes, quality occasions, and work processes is fundamentally significant. Once in a while, the thought of documentation can create tension inside an organization. Sometimes the possibility is considered excessively troublesome and generally pointless with little worth added.
I would say, that most organizations really do make many weights and disruptions in regard to documentation. Also, it doesn't need to be like this.
Documentation ought to be tied in with characterizing processes and keeping up with the documents expected to show these processes are being followed. It is key for genuine proof. Objective proof to help your workers through plan, advancement, manufacturing, and backing of medical devices. Objective proof to exhibit that requirements are being tended to.
Laying out intensive, yet useful, document management practices rehearses for your business is one of the most significant essential components of a QMS.
Mandatory Documents Required by ISO 13485
The new ISO 13485 depends on ISO 9001:2008, and that implies that the ISO 13485 2016 documentation requirements depend on the prerequisites of the past rendition of ISO 9001, with the expansion of reports well-defined for the medical device industry.
In this way, here is the checklist underneath, you will see the mandatory ISO 13485 reports, yet remember that the QMS documentation comprises the compulsory records, yet additionally other ISO 13485 documents as determined by pertinent regulatory necessities.
A portion of the key ISO 13485 documentation requirements are:
Quality manual
Obligations and authorities
Medical device file
Methodology for document control
Methodology for plan and improvement
Methodology for approval
Subsequent to meeting every one of the expected documents of ISO 134585 Medical Devices, you should
Fill up essential prerequisites about the product and the organization certified in the structure.
In the wake of applying for accreditation, the group of examiners will review your documents given, and nearby certification, and give any ideas to work on before another audit.
In the second audit, the auditor assesses any execution made against standard, execution, detailing, and customer reaction. Ensures that the internal audit system and management system have been actually performed.
Assuming that any documentation is against the standard, whether it is major or minor, the time span is given
For major non-conformity 60 days
For minor non-conformity 90 days
After being happy with all the certification processes. The endorsement is issued.
The certificate has been legitimate for a very long time and, like clockwork, it ought to be re-certified.
For re-certification, the audit will be prescribed as finished in the stage 2 audit.
Conclusion
All in all, smoothing out ISO 13485 Documentation is a basic undertaking for any medical device organization meaning to offset compliance with functional proficiency. By streamlining and obviously characterizing processes, associations can guarantee that all employees learn and stick to important methods, eventually prompting reliable and top-notch results.
Underscoring the utilization of succinct, all-around organized documentation helps with regulatory compliance as well as upgrades in general efficiency and diminishes the weight of superfluous desk work. Good documentation practices support seamless improvement, work with audits, and give fundamental proof of compliance.
As the medical device industry keeps on developing, keeping a smoothed-out way to deal with ISO 13485 document keeping will be vital to supporting quality and competitiveness in the market-sphere.
0 notes
Text
Medical device companies must hire professional ISO 13485 consultant or a consulting firm that is knowledgeable about all risk-class devices. The organization must manage and extract value from huge quantities of organized and unstructured information and data, while also staying up-to-date and in compliance with regulatory, regional, and specific laws. By ISO 13485 criteria, the organization should build effective systems to track and monitor safety and benefit-risk information throughout the life-cycle of their goods.
#iso 13485#iso 13485 documents#ISO 13485 consultant#ISO 13485 consultancy#iso 13485 certification#ISO 13485 Manual#QMS#Quality management#ISO#document consultancy
1 note
·
View note
Text
Which pharma Aqueous product give bring more water loss 60℅Rh OR 90℅ Rh
It’s 60℅ Rh because (100 – 60 = 40 ℅ loss water (100-90 = 10 ℅ loss water Here 100 is in side containers water Inside container concentration might be different but, it’s water remain 100 ℅
View On WordPress
#Analytical chemistry#CAPA#Chromatography#Deviation#Documente error Qa#Gas chromatography (gc)#glp#GMP Updates#Hplc#ICH Guideline#Incident#Internal standard#Interview preparation#IR#job#job alerts#Lab incident#Mental Health#QMS#sor#stability
0 notes
Text
When should you start a QMS?
“When do I need a Quality Management System (QMS)?” is the most common question that we get from new clients who are just entering the medical device field. The answer depends on your target market and your exit plan.
QMS is a quality management system as the name suggests this is essential for any simplest medical device too. There are two aspects of QMS. One initial is to build a system wherein you will document the system mostly as per ISO standard 13485. System build means, writing down the procedure for each section of ISO stating what, how, when and where you are going to do. At this stage, you are only developing a strategy or system but not doing anything in action.
In the second stage, you will carry out the action and create evidence of action through records of your system. This is an explanation of what QMS one must do.
This is just a basic need. Then comes regulatory requirements which are different for different countries. These are must requirements, and you have no choice to follow them or not.
In theory, a medical device is supposed to be developed using design control as described in ISO 13485 and 21 CFR 820. In the US once clearance or approval is granted for a device it can be legally placed on the market and the FDA may inspect the manufacturing facility to ensure the required quality system is in place. During an inspection, the auditor looks for evidence that the QMS is being obeyed and this most often comes in the form of records, or more specifically manufacturing records.
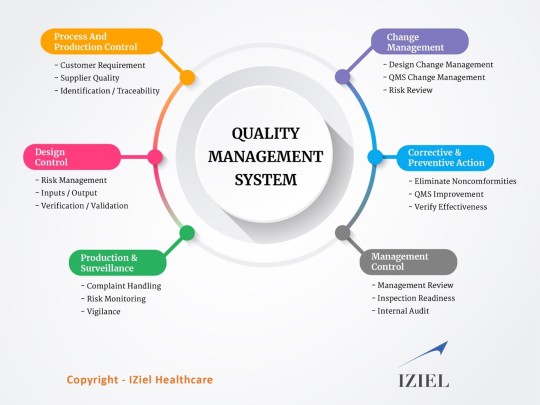
IZiel covers all the product-specific requirements for all components, manufacturing processes, verification & validation along with corrective and preventative actions (CAPA) for assessing customer satisfaction, product non-conformance, assessing and improving quality policies and procedures, carrying out and assessing the results of internal audits, and implementing systems for continuous improvement.
IZiel works with clients to make the QMS simple yet effective and flexible to allow changes to keep up with the changing regulatory requirements.
#Quality Management System#QMS Certificate#QMS Documentation#ISO 13485 Certification#ISO 13485 Consultants
0 notes
Text
this college au isn't getting out of my head so here y'all go, some headcanons
despite being the first in the world at age 15 LXY still ended up with crippling self-worth issues and imposter syndrome.. makes me think he'd be a burnt out fgli (first-generation low-income) student at a rich school.. university au (this has nothing to do at all with my experiences as a fgli student at an ivy league school lmaoo)
of course FDB is a shoo in legacy student and at first LLH is is incredibly annoyed bc FDB is clearly a rich kid who got in with his parents money
the name i picked for FDB is 择信 ("zéxìn" or "to choose faith"). his dad's name is 则仕 ("zéshì" literally "officer" the author is laugh at us so that zé didn't work, i picked another one to better fit my intention. same sound though.)
he's still called duobing as a nickname bc he was a sickly child, but fang zexin is what appears on official documents and his student id. of course his mom and his aunt still call him "xiaobao" and later on so does LLH
FDB abuses his "xiaohua" priviledges and when he extra wants something he'll pull out "xiaohua'er"
just them warming up to each other to deal with school stress. think of all the shenanigans!! i feel like 3am on a school campus is when you feel like you can do anything super well, even make empanadas when you've never made empanadas before (did i do this freshman year? yes.)
bonus: LXY has a phd in bio-med and is a famous researcher, but he's getting a herbology? nursing? (still deciding tbh) degree as LLH and passing it off as his first undergrad. he made a small fortune from his first go around so he can afford it, but i feel like he donated most of it and/or gave it to QMS + QP + SGD
yes i made LXY a genius progidy med student who got into college at 15 and wrangled a phd + patent at age 18. he's literally every asian parent's golden child wet dream. no wonder he disappeared and changed his name and only resurfaced 10 years later.
FDB is of course an engineering and business student (dual degree, our xiaobao is a champ) and he buys all the school swag cuz he has the spirit
bonus bonus: fdb is a professional college fencer lmaoo. do they have professional college martial arts?
bonus bonus li'er is a part of the family staff but the fang/he family pays handsomely so she's kinda uppity. she doesn't understand why her young master is hanging out with someone 1) obviously older and 2) obviously poorer
FDB of course falls in love at first sight bc LLH happens to be the most drop dead gorgeous person he's ever seen and reminds him vaguely of the researcher that helped with the cure to his childhood illness. surely there's no connection right..
FDB is 20 when he starts college bc he took a two year break to do an internship at his mother's company. it looks great on his resume and conveniently works well so i can line up canon ages
FDB finds out LLH is living in a van and immediately tries to move him into his dorm (freshman year). of course that doesn't work out, but i think around sophmore year, once they've started dating, he would've whittled down LLH enough for him to agree to live together in a house off-campus. LLH tries several times to convince the 20yo that he can do better than some old man who's just starting his undergrad at age 28 (my man breathes lies). FDB is literally signing their lease as he speaks.
bonus bonus bonus (and my fave): LLH makes those terrible tiktok recipes that never work but he keeps trying and the first couple of times FDB actually tries pretending that LLH's cooking is fantastic
bonus x4: DFS went to trade school and owns a handyman service (he makes bank doing it) and the jinyuan alliance is his crew of fixers. FDB is convinced DFS is a mob boss because LLH is always calling him Di 老大 (lǎo dà) when he comes around to fix LLH's rundown van. DFS gives LLH the i'm in love with u family discount but he'll never admit to it. the two of them have known each other since grade school.
bonus x5: DFS and LXY both did the same martial arts extracircular and were known rivals (affectionate) in those circles. they were constantly swapping between placing 1st and 2nd in tournaments.
bonus x5 extra bonus just for me: at one point LXY did call DFS "gege" before he grew out of it / they drifted a part. obvs they reconcillated bc LLH needed a van guy and DFS under all those scowls does care for LXY a lot.
bonus x6: LLH and JLQ absolutely know they are cousins (just bc it's hilarious for me if they do). they don't talk about it but JLQ hates him for "stealing" DFS's attention since their naptime days. he's her cousin timmy. LLH doesn't even know that he's seduced the love of her life, he's just vibing. DFS tries to explain to her that he's gay and has never been interested in women, but she doesn't believe in homosexuals exactly like in canon lmaoo
bonus x7: SGD and LXY were both in an orphanage before they got adopted by qi mushan and qin po. SGD protected LXY like an older brother, but got more bitter and jealous as LXY clearly displayed genius level academic excellence and as a by product got more attention (more care into selecting schools, more time dedicated to LXY's extracirculars, more time spent driving LXY to conferences and stuff as his research gained more traction)
can you imagine the pressure little xiangyi would've been put under to excel, and to excel bc he got this chance when all the other kids at the orphanage didn't? qi mushan and qin po weren't exacty rich, i imagine he must've felt so stressed being bombarded with scholarships and whatnot while his brother steadily closed himself off from xiangyi
nothing just imposter syndrome going off the charts when rumors started going around that a mistake in LXY's research cost someone their life. that no one should've trusted a teenager to be that smart. that some orphan kid just wanted attention and should've never been given a chance. it breaks him.
unintential pressure from qi mushan, qin po and SGD. why was he protected / saved / chosen if not to make their lives easier and to make them proud with his achivements. he's carrying his made-up expectations of their expectations and SGD's expectations
something something my dad was drunk one night and came on campus and told me i was the hope of our entire family bc i was the first to get into a good school and i could make something of myself. i was 18 at the time, same age as when LXY during his famous battle, and i just. feel some type of way. like. the man was carrying the expectations of the entire jianghu on his back. how was he not gonna be overwhelmed and break down?
bc this is a modern au i can make LXY go to therapy :) it takes a few years for him to be convinced to go (he is asian after all LOL), but he does go eventually and it helps him get the will to start again. FDB knows and actively encourages and praises LLH for taking care of himself.
LLH still carries the same self-hatred he has for his younger self bc he thinks his arrogance caused a mistake in his research and ended up causing ppl to die. he's working on it, okay, it's gonna take time
i'll end this with some crack: FDB accidentally hears DFS call LLH "xiangyi" and proceeds to give him an entire speech abt deadnames and such. it's bc he's seen LLH react to being called "xiangyi" before (come on, this guy was a prodigy and he's back at uni, some of the professors are bound to recognize him) and it's never pretty. he ends by saying "it's not like lianhua is running away from the law, he's not doing anything wrong" yes he thinks LLH is trans lmaoo. the entire time, DFS is giving him an incredious look. LXY was in fact running away from the law (or at least the press lmaoo). LLH is just standing there with an amused look on his face like "my xiaobao is a little confused, but he's got the spirit"
#sorry this is such a long post i got very excited#pls come talk to me abt the lotus man and his boyfriends#i love them#maybe this is spurred by my desire to see cy in a good modern drama#but my brain was like 'modern au modern au!!!'#also was i the only one that found out sgd was 10 years older than lxy two months after i finished the show#mysterious lotus casebook#mlc#mlcb#lianhua lou#lhl#li lianhua#li xiangyi#fang duobing#di feisheng#kitty rambles#my stuff
55 notes
·
View notes
Text
Benefits of ISO 9001 Certification Implementation

ISO 9001 Implementation : A Blueprint for Quality Excellence
What is ISO 9001?
ISO 9001 is an internationally recognized standard that provides a framework for quality management systems (QMS). It outlines the requirements for a company to ensure that its products and services consistently meet customer expectations. By implementing ISO 9001, organizations demonstrate their commitment to quality and customer satisfaction.
Why is ISO 9001 Important?
Enhanced Customer Satisfaction: ISO 9001 focuses on understanding and meeting customer needs. By implementing a QMS, organizations can improve their products and services, leading to increased customer satisfaction.
Improved Efficiency and Productivity: ISO 9001 promotes process efficiency and reduces waste. This can lead to increased productivity and cost savings.
Enhanced Reputation: Organizations certified to ISO 9001 demonstrate their commitment to quality. This can enhance their reputation and attract more customers and business partners.
Increased Market Access: Many industries require ISO 9001 certification for suppliers. By obtaining certification, organizations can expand their market reach.
Continuous Improvement: ISO 9001 emphasizes continuous improvement. This means that organizations are always looking for ways to enhance their processes and products.
Key Principles of ISO 9001
Customer Focus: Understanding and meeting customer requirements.
Leadership: Strong leadership is essential for implementing and maintaining a QMS.
Involvement of People: All employees should be involved in the QMS and empowered to contribute to its success.
Process Approach: Managing activities as interrelated processes.
System Approach to Management: Integrating and managing processes as a system.
Continuous Improvement: Constantly seeking to improve performance.
Evidence-Based Decision Making: Making decisions based on data and evidence.
Relationship Management: Managing relationships with external providers.
Benefits of ISO 9001 Certification
Increased Efficiency and Productivity: Streamlining processes and reducing waste.
Improved Quality: Ensuring products and services consistently meet customer expectations.
Enhanced Customer Satisfaction: Building stronger relationships with customers.
Boosted Reputation: Enhancing credibility and trust.
Increased Market Access: Expanding business opportunities.
Reduced Costs: Identifying and eliminating inefficiencies.
The Certification Process
Gap Assessment: Identifying the gap between current practices and ISO 9001 requirements.
Documentation: Developing and implementing a QMS that meets ISO 9001 standards.
Internal Audits: Conducting regular internal audits to ensure compliance.
Certification Audit: Undergoing an external audit by a certified certification body.
Maintenance: Continuously improving the QMS and maintaining certification.
Conclusion
ISO 9001 is a valuable tool for organizations that want to improve their quality, efficiency, and customer satisfaction. By implementing a QMS, organizations can gain a competitive advantage and achieve long-term success.
Would you like to know more about the specific requirements of ISO 9001 or the certification process?
#iso certificate management#iso management#iso design consultant#iso implementation pretoria#iso consultant pretoria
2 notes
·
View notes
Text
https://docs.google.com/document/d/1J2DfX0WTUHt5TZ87jJlbNEqlmJ6dU9ZbgRj_zmCjBfk/edit with the work of cjfs i have compiled it into one google doc. Jashometers.
24 notes
·
View notes
Text
Guide for Importers on Manufacturing Control
Efficient Manufacturing Control in China
1. Understand the Chinese Manufacturing Environment:
Diverse Ecosystem: China offers a vast range of manufacturers, from small workshops to large factories. This variety is beneficial but also poses challenges in ensuring consistent quality.
IP Concerns: Despite improvements, IP protection in China can be inconsistent, requiring robust measures to safeguard your innovations.
Regulatory Landscape: China’s complex and changing regulations make compliance crucial to avoid fines and reputational damage.
Quality Control: While quality has improved, some sectors still prioritize quantity over quality. Rigorous quality protocols are essential.
Labor & Costs: Labor costs are rising, pushing manufacturers toward automation. Infrastructure is robust, but supply chains can be disrupted by natural disasters or policy changes.
Government Policies: China’s government heavily influences manufacturing through policies and incentives, which can affect costs and market access.
2. Build a Strong Foundation:
Supplier Selection: Choose reliable suppliers through thorough due diligence, including factory visits and financial checks.
Clear Communication: Provide detailed specifications and maintain open communication to avoid misunderstandings.
Quality Management: Implement a Quality Management System (QMS) and conduct regular audits to ensure consistent product quality.
Strong Relationships: Develop long-term partnerships with suppliers to build trust and collaboration.
3. Implement Effective Control Strategies:
QA & QC: Establish a robust QA/QC framework to ensure consistent product quality.
Supplier Development: Invest in your suppliers’ capabilities to improve quality and efficiency.
Risk Management: Prepare for disruptions with backup plans and diversified suppliers.
Contractual Coverage: Clearly define product specifications and include IP protections in contracts.
Third-Party Verification: Use third-party inspections to ensure compliance and quality.
4. Manage Logistics & Supply Chain:
Transportation: Choose the best transport mode and routes to minimize costs and delays.
Warehousing & Inventory: Optimize warehouse locations and use management systems to track inventory.
Customs & Documentation: Ensure compliance with customs regulations and prepare accurate documentation.
Supply Chain Visibility: Use technology to monitor shipments and collaborate with suppliers.
Risk Assessment: Develop contingency plans for disruptions like natural disasters or strikes.
5. Overcome Common Challenges:
Language & Cultural Barriers: Hire experts to bridge gaps in communication and cultural understanding.
IP Protection: Secure your IP with patents, trademarks, and NDAs; monitor for infringements.
Regulatory Compliance: Stay updated on regulations and partner with local experts for compliance.
Supply Chain Disruptions: Diversify suppliers, maintain sufficient inventory, and use smart tools for monitoring.
6. Continuous Improvement:
Use data analytics to track trends and improve processes.
Regularly evaluate supplier performance and invest in employee training.
Embrace technology to enhance communication and efficiency.
7. Build a Culture of Quality:
Encourage employees to propose improvements.
Reward contributions to quality enhancement.
Focus on exceeding customer expectations.
Conclusion: Effective manufacturing control in China requires ongoing effort, attention, and adaptation. Invest in strong practices to improve product quality, reduce costs, protect your brand, and optimize your supply chain.
2 notes
·
View notes
Text
A quality management system (QMS) is required for ISO 9001 compliance. It is also critical to audit this system to ensure that it is having the desired effect. An internal quality audit reviews the QMS to verify that its goals are met and that the firm has ISO 9001 certification. ISO 9001 is the most widely accepted Quality Management System (QMS) standard in the world. Its goal is to assist enterprises in more successfully meeting the needs of their consumers and other stakeholders. This is accomplished by erecting a framework to assure constant quality in the provision of goods and/or services.
#ISO 9001#ISO 9001 Manual#ISO 9001 certification#ISO 9001 documents#ISO 9001 procedures#QMS#ISO 9001 Quality management system#certification consultancy
0 notes
Text
A guide to enhance your business growth
Running a business is akin to navigating a complex maze, and every entrepreneur dreams of not just surviving but thriving. In the Indian business landscape, the government has laid out a golden path for micro, small, and medium enterprises (MSMEs) through a simple yet powerful tool – MSME registration. In this guide, let's explore how this seemingly mundane registration process can be your ticket to unparalleled business growth.
Understanding the MSME Advantage
The Heartbeat of the Economy:
Micro, Small, and Medium Enterprises collectively form the heartbeat of the Indian economy. From local grocery stores to innovative startups, these businesses contribute not only to economic development but also to job creation, fostering a robust and inclusive growth environment.
Unlocking Financial Avenues:
One of the immediate perks of MSME registration is the access to financial assistance and credit facilities. Financial institutions offer tailored loans at favorable terms, recognizing the importance of these enterprises in driving economic progress.
The MSME Registration Journey
A Simpler Path Than You Think:
Contrary to popular belief, the MSME registration process is not a bureaucratic labyrinth. It's a straightforward journey that involves providing essential details about your business, such as PAN, Aadhaar, and other relevant information. Whether you choose the online portal or opt for the traditional route at District Industries Centres, the process is designed to be accessible.
Documents: Your Passport to Opportunities:
The importance of documentation in the registration process cannot be overstated. Your Aadhaar card, PAN card, business address proof, and details of your plant and machinery are the keys that unlock the door to a myriad of government schemes and subsidies.
The MSME Advantage Unveiled
Market Access and Procurement Preferences:
Once you've acquired your MSME registration, you find yourself in a prime position in government procurement. MSMEs are often given preference in government tenders, providing a golden opportunity to secure contracts and expand your market reach.
Technology Upgradation and Subsidies:
In the rapidly evolving business landscape, technology is the differentiator. MSME registration brings with it the chance to upgrade your technology with subsidies for adopting new and advanced processes. This not only boosts efficiency but also enhances your competitiveness.
Navigating the Schemes and Subsidies Landscape
Credit Linked Capital Subsidy Scheme (CLCSS):
At the forefront of government schemes is CLCSS, a game-changer for technology upgradation. It provides capital subsidies to MSMEs, facilitating access to credit for purchasing new machinery and equipment.
Pradhan Mantri Employment Generation Programme (PMEGP):
For those looking to embark on the entrepreneurial journey, PMEGP is the beacon. This credit-linked subsidy program promotes self-employment, creating not just businesses but livelihoods.
Credit Guarantee Fund Scheme for Micro and Small Enterprises (CGMSE):
The fear of collateral is a common hurdle for many small businesses. CGMSE eliminates this barrier by offering collateral-free credit facilities, making it easier for MSMEs to access the capital needed for growth.
Tailoring Your Approach
District Industries Centres (DIC) and National Small Industries Corporation (NSIC):
Think of DIC and NSIC as your business allies. DIC, as a local agency, offers guidance and support, while NSIC provides a range of services from marketing assistance to credit facilitation. Engaging with these institutions can significantly enhance your MSME journey.
Tech and Quality Upgradation Support:
The government's emphasis on quality is evident through schemes like Lean Manufacturing Competitiveness Scheme (LMCS) and Quality Management Standards & Quality Technology Tools (QMS/QTT). These initiatives not only boost competitiveness but also position your business as a paragon of quality in the market.
Export Promotion and Market Development:
Venturing into global markets can seem daunting, but the Market Development Assistance Scheme for MSMEs is a trustworthy companion. It provides financial support for participating in international trade fairs, opening doors to new business horizons.
Overcoming Challenges for Seamless Growth
Lack of Awareness:
One of the challenges MSMEs often face is the lack of awareness about available schemes. Entrepreneurs can overcome this by actively seeking information through government portals, industry associations, and local MSME support cells.
Complex Application Processes:
Cumbersome application procedures can be discouraging, but persistence pays off. Simplifying the application process and seeking assistance from dedicated facilitation services or MSME support agencies can make the journey smoother.
Continuous Evaluation and Adaptation
Performance and Credit Rating Scheme:
Enhancing your creditworthiness is an ongoing process. The Performance and Credit Rating Scheme allows MSMEs to undergo assessments, showcasing financial stability to potential investors and lenders.
Embracing Continuous Improvement:
The business landscape is dynamic, and your approach should be too. Regularly assess the impact of government schemes on your operations, adapt to changes, and stay informed about updates to maximize benefits continually.
Conclusion: Your Journey to Unprecedented Growth
In conclusion, MSME registration in India is not just a formality; it's your gateway to a realm of opportunities. By understanding the classifications, embracing government schemes, and overcoming challenges, you position your business for sustainable growth. The government's commitment to fostering MSMEs is a testament to the integral role these enterprises play in shaping the nation's economic future. So, don't just register – embark on a journey of growth, innovation, and success. The path is laid; it's time to walk it.
Learn more at : https://msme-registration.in/
#udyog aadhar free registration#msme free registration#msme registration free#print udyam certificate#free udyog aadhar registration#udyog aadhar update#msme registration online#msme loan#online business#msme
2 notes
·
View notes
Text
How to apply for ISO 9001 Certification in Canada

How to apply for ISO 9001 Certification in Canada
How does ISO 9001 Certification work?
There are four stages in the ISO 9001 audit process:
Pre-assessment meeting and audit preparation
Conducting the ISO 9001 Certification Audit
Meeting to discuss assessment results
Re-certification preparations
Certification Consultants in Canada
To implement ISO 9001 Certification in Canada, organizations need a process-oriented quality management system tailored to their specific requirements. ISO 9001 certification in Canada can improve communication, reduce costs, optimize processes, and more for your organization.
ISO 9001 Certification in Canada Requirements
When a company achieves ISO 9001 certification in Canada, they are expected to have established quality policies, processes, responsibility assignments, roles, and responsibilities. An International Organization for Standardization quality management system procedures are covered by ISO 9001 certification in Canada. Certification bodies also conduct ISO audits to ensure that companies comply with ISO standards. ISO 45001 certification in Canada
According to ISO, an ISO-approved auditor creates, implements, and audits a quality management system (QMS) as part of ISO 9001 certification. Getting ISO 9001 certification in Canada may require a lot of paperwork, and an auditor may make suggestions. The documentation for ISO audits should be in place for organizations ready to implement ISO 9001 in Canada.
ISO 9001 Certification in Canada Audits Online
Using an online web testing platform, IAS conducts ISO 9001 certification audits. Behind the audit, IAS issued a certificate proving your company has been certified to ISO 9001 in Canada based on several criteria, including the online test scenarios for your system documentation, the testing of all policies, and other related ISO 9001 certification procedures. You can implement ISO 9001 in Canada much more quickly with remote audits from IAS. ISO Certification Bodies in Canada
ISO 9001 Certification in Canada available ?
Any organization can achieve ISO 9001 certification in Canada, regardless of its size, type, or field of operation, if they want to improve their QMS performance systematically. Several businesses in Canada can obtain ISO 9001 certification, including:
Corporate Institutions
Construction Companies
Production/Manufacturing Companies
Hospitals
Banks
Institutions of higher learning
Governmental Organizations
For more information visit: ISO certification in Canada
3 notes
·
View notes
Text
The international standards organization (ISO) established ISO 13485 as the global standard for medical device quality management systems. How do you manage your QMS? If you're like the majority of the medical device business, your QMS is probably a mishmash of paper-based processes and general-purpose tools kept together by a group of individuals within your company--typically document control. It is significant since the last edition, released 13 years earlier in 2003, was long delayed. ISO 13485 is primarily a bridge standard. This bridge, in other words, expressly explains and defines contemporary QMS expectations for medical device firms. Before to the fully specified and documented modifications in the standard, many of the best practices recommended and carried out were haphazard and appeared to be based on auditor assessments.
#iso 13485#iso 13485 documents#iso 13485 certification#Manual#QMS#Quality management#ISO#document consultancy
0 notes
Text
How does ISO 9001 Certification In South Africa beneficial for company?

ISO 9001 certification in South Africa can be very beneficial for organizations. It can help them to improve their first-class control structures and to turn out to be extra green and effective. It can also help them improve their consumer satisfaction ranges and reduce their expenses.
Many blessings may be won from ISO 9001 certification in South Africa. The advantages consist of:
1. Improved quality management device
2. Increased efficiency and effectiveness
3. Improved consumer pride
4. Reduced prices
5. Improved communique
6. Improved organizational structure
7. Improved worker morale
8. Increased marketplace share
9. Improved product high-quality
10. Increased sales
Requirements for ISO 9001 Certification in South Africa
ISO 9001 certification in South Africa is. However optional, it may be helpful for organizations that choose to pursue it. The certificate presents a framework for first-rate control structures and may help corporations enhance their average overall performance.
There are two main requirements for ISO 9001 certification in South Africa:
1. Implement a quality management gadget
2. Demonstrate compliance with the excellent control machine requirements
Organizations that enforce a nice control machine can improve their performance and effectiveness, and ISO 9001 certification can provide third-birthday-party validation of these enhancements. The certification method can also assist companies in picking out areas wherein they need to make similar improvements.
To pursue ISO 9001 certification, organizations ought to first develop and implement a great control system that meets the standard’s requirements. Once the device is in the region, they should post an assessment using a certification frame.
If you’re interested in pursuing ISO 9001 certification for your enterprise, there are some things to keep in thoughts:
The certification manner may be costly and time-consuming.
You will want to keep your exceptional management machine to remain certified continuously.
Certification isn’t always a guarantee of fulfilment, but it lets you enhance your probability of success.
ISO 9001 Certification Benefits in South Africa
Benefits of ISO 9001 Certification in South Africa
There are many blessings that your organization can enjoy by turning into ISO 9001 licensed. Here are simply 3 of them:
1. Improved Customer Satisfaction
One of the primary desires of the ISO 9001 standard is to enhance client pride. By becoming licensed, you may show your customers that you are dedicated to imparting them with satisfactory products and services. This helps build acceptance and loyalty, leading to more commercial enterprise in the long run.
2. Increased Efficiency
Another benefit of ISO 9001 certification is that it can assist in increasing the efficiency of your corporation. The general presents a framework for imposing powerful methods and controls. This can cause less waste and fewer errors, increasing efficiency and productivity.
3. Enhanced reputation
Finally, ISO 9001 certification can also enhance the popularity of your business enterprise. Clients are much more likely to do commercial enterprise with a licensed corporation, as it reassures them they will get high-quality services and products. In addition, certified agencies are frequently seen as more professional and trustworthy. This can assist in drawing new clients and enterprise companions.
Process of ISO 9001 Certification in South Africa
ISO 9001 is a globally identified satisfactory management gadget (QMS) that provides a framework for companies to observe to ensure that they may offer their clients the best services and products. South Africa needs to be more specific, and many companies within the US have already executed ISO 9001 certification.
There are four predominant steps within the ISO 9001 certification process in South Africa:
1. Document your first-class control device
The first step is to report your first-class control system. This includes developing or updating your best manual, procedures and other relevant documentation.
2. Implement your great control gadget
The next step is to put in force your exemplary control device. This consists of educating your employees on the brand-new device and ensuring they follow the processes.
3. Get audited
The 1/3 step is to get audited using an authorized ISO 9001 certification frame. This will contain an online audit of your quality management device on-website to ensure it’s being accompanied efficiently.
4. Maintain your certification
The final step is to keep your certification. This includes ordinary audits and reviews of your pleasant management gadget to ensure it is updated and observed efficaciously.
How to Get ISO Certification in South Africa?
We provide the best ISO consultants in South Africa, Who are very knowledgeable and provide you with the best solution. And to know how to get ISO certification in South Africa? Kindly reach us at [email protected] ISO Certification consultants follow the guidelines set by the international organization for standardization and help the organization to implement ISO certification in South Africa in an easy way with proper documentation and audit.
For more information visit: ISO 9001 Certification in South Africa
Related Links
ISO Certification in South Africa
ISO 9001 Certification in South Africa
ISO 14001 Certification in South Africa
ISO 45001 Certification in South Africa
ISO 27001 Certification in South Africa
ISO 22000 Certification in South Africa
2 notes
·
View notes