#protein enzymes
Explore tagged Tumblr posts
Note
How do you feel about aminoacyl-tRNA synthetase enzyme?
HOIUUOOUUOOUOHHH IM CATALYZING!!!!!!!!!!!!!!!!!!!!
#asks#anon#aminoacyl-tRNA synthetase enzyme#translation#protein translation#tRNA#RNA#enzymes#tRNA-ligase#tRNA ligase#synthetase#synthetases#amino acids#acids#proteins#catalysis#ribosomes#ribosome#1ASZ#1SET#codons#anticodons#serine#isoleucine#1FFY#valine#1GAX#glutamine#1EUQ#phenylalanine
3K notes
·
View notes
Note
Can you explain to me the chemistry behind the denaturation of enzymes and the process of allosteric and competitive inhibition?
First of all it's important to understand what an enzyme is. An enzyme is a protein, that catalyses reactions after binding to a substrate and then converts it or splits it. An enzyme has a very specific shape so it can bind to the substrate, it's like a key and a lock. The substrate binds to the binding site, and in the catalytic or active centre the reaction takes place.
This is important to understand denaturation: Proteins are made out of amino acids that are bound with peptide bonds. Proteins have several levels of structure: Their primary level is the sequence of amino acids, the secondary structures are folded structures due to interactions of the peptide backbones via hydrogen bonds, like alpha helices or beta sheets. The tertiary structure is folding of the peptides due to electrochemical interactions between different amino acids. Amino acids can have different charges due to their side chains, they can be positive, negative or neutral charged. So those charges will either attract or repel each other, putting the peptide chain in a certain three dimensional shape or conformation. In the quaternary structure several peptide chains come together to create a bigger functional unit (the enzyme) made out of subunits, they often also interact with ions (cofactors) as their catalytic centre where the catalysed reaction takes place. All those levels create the specific shape of the enzyme that is required to bind to their target substrate. So if those structures are changed in any way, it won't work anymore because it can't bind. Just reading it probably makes it difficult to understand, so here's a textbook graphic.
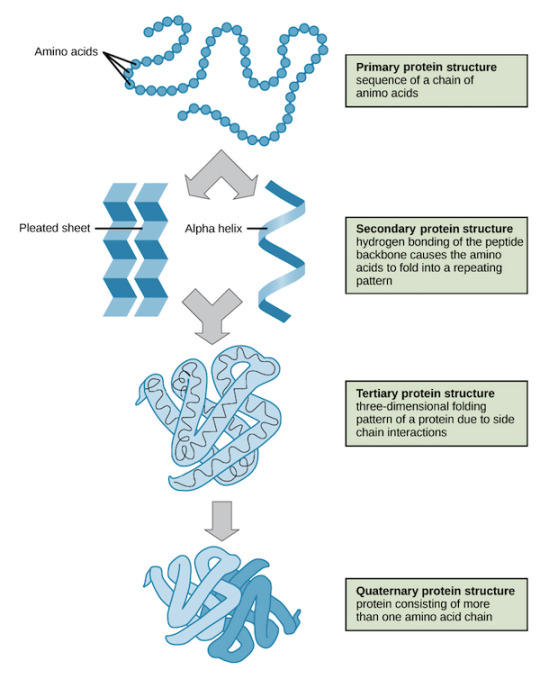
This shape can be changed by denaturation. A protein can be denaturated by heat, or changed pH or high salinity and other not optimal conditions. During those conditions like changed pH the interactions between the molecules and side chains do not work anymore because pH can change the charges of the sidechains, so secondary, tertiary and quarternary structures will be changed. When those structures are changed the binding site will change too and not resemble the lock anymore where the key substrate can bind, so now the enzyme is inactivated/inhibited.
Enzymes can also be inhibited (or activated) by regulatory molecules binding to the enzyme, inhibitors or activators. This can be a competitive inhibition, when the inhibitor binds at the same binding and active site and blocking it, making the actual substrate that should be processed unable to bind there. Like a lock that already has a key stuck in it, you can't put another key in there. Allosteric inhibition is when the inhibitor binds at another site, not directly at the active site where the substrate binds. But by binding to the allosteric site the conformation of the enzyme gets changed by chemical interactions, changing the binding site so the substrate doesn't get recognised anymore. A key can't be insterted into a lock that has been changed.
Inhibtion can be irreversible (making the enzyme dysfuntional for the rest of it's existence until it is degraded) or reversible.
#roleplay#rp#sherlock roleplay#sherlock rp#sherlock#sherlock holmes#biology#molecular biology#biochemistry#chemistry#a level chemistry#chemblr#bioblr#science#scientist#scienceblr#biology student#biology facts#a level biology#enzyme inhibitors#protein#molecular biology enzymes#science side of tumblr#studying#biology studyblr#chemistry major#biology major#college#university#sherlock replies
28 notes
·
View notes
Note
Ayo come get your Howdy soup while it’s…uncomfortably warm and acidic
prolongedslurpingsound.wav
#im picturing everyone gathering around The Chrysalis with silly straws#Partake In The Soup. Partake In The Soup.#howdy emerges and he's short now#they straight up drank half of him#rambles from the bog#yassified howdy <3#my first thought reading this ask was the COME GET YALL JUICE vine#actually wait now you have me wondering... Would it be warm and acidic#warm.. yeah probably there's a lot of activity goin on & energy being used#acidity... hold on im doing Research for this gimme a sec#well for yall reading this its instantaneous. for me ive had this ask open for fifteen minutes#alright so caterpillars have been described as tasting nutty / leafy / earthy / meaty#just saw one description say 'if trees were made of meat thats what they taste like'#and caterpillar Soup is very very Very protein-rich#now lets look up what chitin looks like since im 90% certain thats what chrysalises are made of#hmmm the only things im seeing is it would either be mostly tasteless or nutty#then lets look at the flavor of enzymes... im seeing sour notes...#this taking too long okay Basically caterpillar soup#would be protein-y maybe a little leafy or sour... hints of nuttiness... and of course the overwhelming flavor of Guilt & Disgust#thank you for listening to this ted talk. im getting nauseous thinking about what the texture must be like
58 notes
·
View notes
Text
the only thing my sister and i have to offer to the rise fandom is theories about how lou jitsu's dna recombined with the turtles
#i think about this a lot. like an embarrassing amount actually#i also think about what sorts of enzymes draxum mustve thrown into the ooze to trigger it all#i think there was probably a mystic factor as well#like a mystic catalyst#the turtles (and now splinter) would be considered transgenic organisms#and i dont have a whole lot of background in transgenic techniques so its a little harder for me#there are a lot of ppl at work who are experts on making humanized mouse models so i should ask them LOL#most of my research is on rna and protein expression and not dna modification#still compels me tho#megan we should try to find if anyone has sequence aligned turtle and human dna#im curious how similar the genomes are#and also what genes and consensus sequences are shared between reptiles and humans
6 notes
·
View notes
Photo

Wearable, printable, shapeable sensors detect pathogens and toxins in the environment
Researchers at Tufts University School of Engineering have developed a way to detect bacteria, toxins, and dangerous chemicals in our environment using a biopolymer sensor that can be printed like ink on a wide range of materials, including wearable items such as gloves, masks, or everyday clothing.
Using an enzyme similar to that found in fireflies, the sensor glows when it detects these otherwise invisible threats. The new technology is described in the journal Advanced Materials.
The biopolymer sensor, which is based on computationally designed proteins and silk fibroin extracted from the cocoons of the silk moth Bombyx Mori, can be embedded in films, sponges, filters, or molded like plastic to sample and detect airborne and waterborne dangers, or signal infections or even cancer in our bodies.
The researchers demonstrated how the sensor emits light within minutes as it detects the SARS-CoV-2 virus that causes COVID, anti-hepatitis B virus antibodies, the food-borne toxin botulinum neurotoxin B, or human epidermal growth factor receptor 2 (HER2), an indicator of the presence of breast cancer.
Read more.
#Materials Science#Science#Sensors#Biopolymers#Medical technology#Wearable technology#Enzymes#Proteins#Silk#Tufts University
26 notes
·
View notes
Text
not to be a stem nerd on main but like something about science makes me feel really good about myself. like being into science has actually increased my confidence. ik its not everyone’s cup of tea but WAH what a dear thing to me..
#its the knowing that ur simply just atoms existing. made up of elements which are made of protons and electrons and such.#that theres an electron cloud MULTIPLE in fact and orbitals and so much when ur broken down…it doesnt matter ur size or ur imperfections…#the fact that ur body is full of proteins and enzymes and cycles that keep u running.#the fact that ur blood has a buffer so no matter the pH (which is tech just abt the concentration) of stuff u eat#ur always relatively neutral so u SURVIVE and its like. yeah maybe im not stick thin skinny. but like my body does INCREDIBLE things…and#its full of incredible processes and who cares abt the way i look in this one dress when theres so much science in me :)#ive had this thought for a while and so idk if i said it properly…ugh i hate that bc i think its such a fascinating thought and a comfort t#me but#this kind of sounds weird like idk how to articulate it and its just how i feel but like. yeah im in love with science its bewitched#me mind body and soul…literally!!!#i love science so so so much every single one is incredible to me in every way..#found this in my drafts and i feel like posting it this is so so true i love it sm...yeah..science makes me feel good :] thats it
10 notes
·
View notes
Text

shes so right about everything ever
[ID: a screenshot of aoi asahina from danganronpa, saying "guess what i found! a pool! there's a pool here! a POOL! pool pool pool!" end ID.]
#im pretty sure ive said this#OK IM WATCHING THE KILL COUNT RN and actually loving it#the hosts like. little reference turns of phrase im so obsessed with#ok wait hold up. why did they call the little creatine thing from the lab a portmanteau of protein and enzyme.#enzymes are proteins this is how you can tell the characters are fuckin freshmen 👆#id added
2 notes
·
View notes
Text
Crazy how every single homestuck kid and troll with the minor exclusions of June, Rose, Jane and Equius have the potential to be lactose intolerant
#homestuck#by which i mean like#they never drank it ergo they don't have to enzymes to digest it#for dave there's a chance he's fine but idk if bro would buy milk for anything other than protein shakes#the trolls think equius is a freak for multiple reasons drinking milk included#the resf of the kids (that bring jade and the alphas -jane) wouldn't have acess to milk in their circumstances#maybe roxy could use her appearerfier to get somd but idk
5 notes
·
View notes
Text
Updated research on small cellular machine called TRiC controls the folding of tubulin, a human protein that is the foundation of microtubules.
This challenges the previous understanding that TRiC and other machines like it, known as chaperonins, only passively create a favorable environment for folding but do not actively take part in it.
Many of the proteins that fold with the aid of TRiC are intimately linked to human diseases, including certain cancers and neurodegenerative disorders like Parkinson’s, Huntington’s, and Alzheimer’s diseases.
3 notes
·
View notes
Text
the human enzyme galactokinase
6 notes
·
View notes
Text

Did you know? Drinking snake venom will do nothing but… 🐍💦
Drinking snake venom will do nothing, because snake venom is a type of protein, when it enters the stomach it will be broken down into protease and peptone by proteolytic enzymes into the last amino acid!
But if there is a wound in the mouth, esophagus or stomach, it's "game over".
Because then it can mix directly with the blood, through the capillary.
Don't do this. It can be very deadly!

#kemetic dreams#snake#snake venom#venom#proteolytic enzyme#amino acid#esophagus#stomach#mouth#game over#blood#capillary#protein
5 notes
·
View notes
Text
biochemistry is NOT for the weak🙏
#help me i'm actually gonna cry what is this#the architect is speaking about something else#biochemical#all that DNA RNA genes and proteins im so done#i wanna learn about ENZYMES AND METABOSUM NOT DNA
1 note
·
View note