#pharmaceutical product development
Explore tagged Tumblr posts
Text
Explore India's Pharmaceutical Powerhouse with Chemxpert Database
Discover the list of pharmaceutical export companies in India and the extensive list of pharmaceutical products exported from India, contributing significantly to the booming pharma global market size. With Chemxpert Database, gain insights into the top API pharma database company in India and access comprehensive data on the top pharma company in the world. Your gateway to India's pharmaceutical excellence starts here – Chemxpert empowers you with actionable intelligence for strategic growth.
#new rules for clinical trials in India#top 10 pharmaceutical companies#biggest pharmaceutical companies#types of data in pharmaceutical industry#pharmaceutical company datasets#pharmaceutical product development#pharmaceutical biotechnology
1 note
·
View note
Text
Advancing Healthcare through Innovative AYUSH Formulations
Frontro Pharma is at the forefront of pharmaceutical product development, dedicated to transforming healthcare with cutting-edge solutions. Our specialization in developing innovative formulations for AYUSH products leverages advanced technologies such as liposomal and nano-formulations. Our team of experts focuses on efficiency, safety, and market differentiation, ensuring that your product achieves success in the competitive pharmaceutical landscape.
0 notes
Text
Looking For best Product Development Services In USA?
Looking For best Product Development Services In USA? Renejix Pharma Solutions Offers best services like contract research organization, distribution, supply-chain services many more services. Begin your search today! Feel free to contact us; we are here for you 24/7. Call us at +1 (631) 210-5235.
For more information, visit: https://renejix.com/product-development/
#pharmaceutical product development#pharma product manufacturing#preclinical formulation development#product development services
0 notes
Text
Vapor Patches for Kids | Agenix Pharmaceuticals | Rhino Patch
Vapor patches can be a safe and effective way to relieve nasal congestion in kids. These patches contain natural essential oils, like eucalyptus and peppermint, that can help to open up the airways and reduce congestion.
Vapor patches are easy to use and can be applied to the chest, back, or clothing of a child. They work by releasing soothing vapors that can provide relief from congestion and coughing.

Unlike traditional vapor rubs, vapor patches are mess-free and don't require any rubbing or massaging. They are also designed to be safe for kids and are free from harmful chemicals or additives.
When using vapor patches for kids, it's important to follow the manufacturer's instructions and to make sure the patch is applied securely to avoid any accidental ingestion. It's also important to monitor your child for any signs of allergic reactions or skin irritation.
While vapor patches can provide temporary relief from nasal congestion, it's important to address the underlying cause of the congestion and to consult with a healthcare professional if your child's symptoms persist or worsen.
Contact Us
721 W Tarrant Rd, Suite 120 Grand Prairie, TX 75050
+1 844–4-AGENIX
Related Searches
aromatherapy oils
nasal congestion relief
pharmaceutical product development
nasal vapor inhaler
#essential oil for chest congestion#vapor patch for kids#pharmaceutical product development#essential oils for stuffy nose
0 notes
Text
Clinical Development Solutions
In the rapidly evolving field of healthcare, clinical development plays a crucial role in bringing novel treatments and therapies to patients worldwide. Clinical Development Solutions (CDS) is at the forefront of this exciting journey, pioneering innovative approaches to accelerate the development and approval of life-saving drugs and medical devices. With a dedicated team of experts and cutting-edge technologies, CDS is committed to transforming the landscape of clinical research and improving patient outcomes.
At CDS, we understand the challenges and complexities of clinical development. Our comprehensive suite of solutions is designed to address these challenges head-on, providing tailored strategies and support throughout the entire drug development lifecycle. From early-phase clinical trials to post-marketing studies, we offer a wide range of services that enable pharmaceutical and biotech companies to navigate the regulatory landscape efficiently and effectively.
One of the key strengths of CDS lies in our expertise in clinical trial design and optimization. We work closely with our clients to design robust and scientifically rigorous trials that generate high-quality data while minimizing risks. By leveraging our extensive knowledge and experience, we can identify the most appropriate patient populations, endpoints, and study designs to maximize the chances of success. Our statistical and data management teams ensure that the collected data is accurate, reliable, and compliant with regulatory requirements.
In addition to trial design, CDS also excels in patient recruitment and retention strategies. We understand the importance of enrolling a diverse and representative patient population to ensure the generalizability of study results. Through our innovative patient-centric approaches, such as digital recruitment platforms and targeted engagement campaigns, we connect with potential study participants and enhance their overall trial experience. By fostering strong relationships with patients and investigators, we improve retention rates and reduce dropout rates, ultimately leading to faster and more reliable study results.
CDS is at the forefront of adopting emerging technologies to drive efficiency and innovation in clinical development. We harness the power of big data analytics, artificial intelligence, and machine learning to uncover valuable insights from complex datasets. These advanced analytics enable us to identify trends, predict outcomes, and optimize trial protocols, thus accelerating the development timeline and reducing costs. Our investment in digital health technologies and wearable devices further enhances data collection and remote monitoring capabilities, enabling more flexible and patient-friendly trial designs.
In the realm of regulatory affairs, CDS provides comprehensive support to ensure compliance with global regulations and standards. Our regulatory experts have in-depth knowledge of regional requirements, including those of the FDA, EMA, and other regulatory authorities worldwide. From preparing regulatory submissions to managing post-marketing safety surveillance, we guide our clients through every step of the regulatory process, ensuring timely approvals and post-approval compliance.
CDS is also committed to fostering collaboration and knowledge sharing within the clinical research community. We organize scientific symposia, webinars, and training programs to facilitate the exchange of ideas and best practices. By promoting interdisciplinary collaboration and staying up to date with the latest industry advancements, we continuously enhance our capabilities and stay at the forefront of clinical development.
In conclusion, Clinical Development Solutions is a leading provider of innovative solutions in clinical development. Through our expertise, technology-driven approaches, and commitment to patient-centricity, we strive to transform the drug development landscape and improve patient outcomes. By partnering with CDS, pharmaceutical and biotech companies can navigate the complexities of clinical research with confidence, bringing new therapies to patients faster and more efficiently. Together, let us shape the future of healthcare through innovation and collaboration.
#clinical development#development solutions#biometric solution providers#clinical development consultant#clinical development service#drug development solutions#clinical product development#clinical development solution company#clinical development specialist#Clinical Development Services in Hyderabad#Clinical Services in Hyderabad#clinical development services agency in hyderabad india#best clinical development agency in india#project management solutions provider#project management service provider#biometric service provider near me#Clinical Trial Services In Hyderabad#specialized clinical pharmacist#clinical pharmaceutical company
2 notes
·
View notes
Text
OP: fair. Everybody else: uhh...there's definitely more nuance than you're saying
mental illness hasn’t been destigmatised but commercialised
#hmm#while there's a point here i don't know if it's as extreme or new as all that#medical quacks have been around since curatives existed. sham sales bogus products overblown/exaggerated effects advertised#and yet real helpful therapies are also in use and others in development#one of my mental health adjacent illnesses (not itself a mental illness but has a huge impact on my mental welfare) is making a ton of $$#for pharmaceutical companies. even so without it i'd really be struggling to stay alive. it's bad they're able to charge my insurance $$$$$#but the drug is legitimately life saving#like...yes call out the shit that's wrong/broken/predatory but let's not act like the entire industry does zero good
98K notes
·
View notes
Text
How Zuventus Healthcare Brings Communities Closer Through CSR Initiatives
How Zuventus Healthcare Brings Communities Closer Through CSR Initiatives
Corporate social responsibility (CSR) is a cornerstone of Zuventus Healthcare's commitment to fostering societal well-being. As one of the pharmaceutical companies in India, Zuventus goes beyond developing innovative pharmaceutical products to actively contribute to the betterment of communities. Let’s explore how the objective of CSR activities at Zuventus supports the goal ofcorporate social responsibility and promotes sustainable development.
Focusing on Health and Wellness
As a trusted name in the pharmaceutical industry in India, Zuventus Healthcare aligns its CSR and sustainable development initiatives with its mission to improve healthcare access. By organizing health camps, blood donation drives, and medical outreach programs, the company ensures that individuals in underserved areas receive critical care. These efforts not only address health disparities but also build trust in the company as one of the best medicine companies in India.
Supporting Education and Community Empowerment
Zuventus Healthcare believes in education as a vital tool for growth. Through scholarships, school funding, and skill development programs, the company equips individuals to build better futures. These objective of CSR activities foster community empowerment, which is an integral part of the goal of corporate social responsibility.
Advocating for Sustainability and Environmental Protection
A key focus of Zuventus Healthcare's corporate social responsibility is environmental conservation. By engaging in initiatives such as tree plantations and promoting eco-friendly practices, the company demonstrates its commitment to csr and sustainable development. This not only protects natural resources but also brings communities together around shared environmental goals.
Empowering Women and Marginalized Groups
As one of the leading pharmaceutical manufacturing companies, Zuventus extends its support to women and marginalized communities. Vocational training, health awareness campaigns, and employment initiatives empower these groups, enabling them to contribute to society and achieve economic independence.
Supporting Disaster Relief and Rehabilitation
Zuventus Healthcare stands as a reliable partner during emergencies. Whether through providing medicines, relief materials, or logistical support, the company’s quick action during disasters highlights its role as a socially responsible leader in the pharmaceutical industry in India.
Collaborating for Broader Impact
Zuventus Pharma Company actively collaborates with NGOs, local authorities, and healthcare institutions to amplify the impact of its corporate social responsibility efforts. These partnerships enable the company to address pressing social challenges while fostering meaningful connections across communities.
About Zuventus Healthcare: A Legacy of Care
About Zuventus Healthcare: Known for its wide range of pharmaceutical products, Zuventus has carved a niche as one of the most trusted pharmaceutical companies in India. The company's consistent contributions to CSR and sustainable development reflect its deep commitment to society.
By addressing healthcare needs, promoting education, and supporting sustainability, Zuventus proves that businesses can achieve the goal of corporate social responsibility while remaining leaders in the pharmaceutical manufacturing sector. Through these efforts, the company not only transforms lives but also strengthens its reputation as the best medicine company in India.
#corporate social responsibility#goal of corporate social responsibility#csr and sustainable development#objective of csr activities#zuventus pharma company#best medicine company in india#about zuventus healthcare#pharmaceutical companies#pharmaceutical products#pharmaceutical companies in india#pharmaceutical industry in india#CSR
0 notes
Text
In today’s rapidly evolving technological landscape, Metaverse technology is ushering in new dimensions for industries worldwide. As businesses adopt immersive digital environments for training, simulations, and customer experiences, one metaverse company in India is truly making its mark — Simulanis. This company specializes in creating cutting-edge metaverse development solutions and virtual reality (VR) simulators, transforming industries from fire safety to pharmaceutical training.
#Virtual Reality Safety Training#Immersive VR Simulators#VR Training for Firefighters#Metaverse Training Solutions#Virtual Reality Fire Training#Industrial VR Simulators#VR Training for Paint Spraying#VR Fire Extinguisher Simulator#Virtual Reality Emergency Training#VR-based Skill Training#Metaverse for Workforce Development#3D VR Simulators#Fire Safety VR Training Programs#Virtual Reality for Hazardous Training#Immersive VR Training for Manufacturing#Augmented Reality Productivity Tools#Metaverse Learning Experiences#Virtual Fire Safety Training India#VR Training for Healthcare#Simulation Training in Metaverse#Realistic VR Fire Training Simulators#Advanced VR Training Solutions#Virtual Reality Training for Engineers#Industrial Fire Extinguisher Simulator#Pharmaceutical VR Training Simulators#VR Training for High-Risk Jobs#Fire Safety Simulation Tools#Remote Assistance in VR Training#Metaverse Development for Enterprises#Immersive VR Training for Safety Procedures
0 notes
Text
"The Biden Administration last week [early December, 2023] announced it would be seizing patents for drugs and drug manufacturing procedures developed using government money.
A draft of the new law, seen by Reuters, said that the government will consider various factors including whether a medical situation is leading to increased prices of the drug at any given time, or whether only a small section of Americans can afford it.
The new executive order is the first exercise in what is called “march-in-rights” which allows relevant government agencies to redistribute patents if they were generated under government funding. The NIH has long maintained march-in-rights, but previous directors have been unwilling to use them, fearing consequences.
“We’ll make it clear that when drug companies won’t sell taxpayer funded drugs at reasonable prices, we will be prepared to allow other companies to provide those drugs for less,” White House adviser Lael Brainard said on a press call.
But just how much taxpayer money is going toward funding drugs? A research paper from the Insitute for New Economic Thought showed that “NIH funding contributed to research associated with every new drug approved from 2010-2019, totaling $230 billion.”
The authors of the paper continue, writing “NIH funding also produced 22 thousand patents, which provided marketing exclusivity for 27 (8.6%) of the drugs approved [between] 2010-2019.”
How we do drug discovery and production in America has a number of fundamental flaws that have created problems in the health service industry.
It costs billions of dollars and sometimes as many as 5 to 10 years to bring a drug to market in the US, which means that only companies with massive financial muscle can do so with any regularity, and that smaller, more innovative companies can’t compete with these pharma giants.
This also means that if a company can’t recoup that loss, a single failed drug can result in massive disruptions to business. To protect themselves, pharmaceutical companies establish piles of patents on drugs and drug manufacturing procedures. Especially if the drug in question treats a rare or obscure disease, these patents essentially ensure the company has monoselective pricing regimes.
However, if a company can convince the NIH that a particular drug should be considered a public health priority, they can be almost entirely funded by the government, as the research paper showed.
Some market participants, in this case the famous billionaire investor Mark Cuban, have attempted to remedy the issue of drug costs in America by manufacturing generic versions of patented drugs sold for common diseases."
-via Good News Network, December 11, 2023
#united states#us politics#biden administration#executive order#prescription drugs#medical news#healthcare#healthcare access#biden#big pharma#drug prices#public health#nih#national institutes of health#good news#hope
10K notes
·
View notes
Text
Top Pharma Companies in India by Revenue | A Comprehensive Overview
The pharmaceutical industry in India is one of the most rapidly growing sectors in the country, known for its significant contributions to both domestic and international markets. India’s pharma companies are not only major players in drug manufacturing but also play a crucial role in clinical trials, generic drug production, and innovation in medical treatments. In this blog, we will explore the top pharma companies in India by revenue, discuss the landscape of the world pharma market size, and highlight some of the emerging trends in drug manufacturing and pharma clinical trials in India. We will also delve into the new rules for clinical trials in India that have been introduced to enhance regulatory oversight and patient safety.
#biggest pharmaceutical companies#types of data in pharmaceutical industry#pharmaceutical company datasets#pharmaceutical product development#pharmaceutical biotechnology#largest pharmaceutical companies
1 note
·
View note
Text
Expert Regulatory and Scientific Writing Services by FrontroPharma
Navigating the complex world of regulatory requirements is made simple with FrontroPharma. Our experts specialize in preparing and reviewing regulatory documentation, including narratives, systematic reviews, meta-analyses, and grant writing. We provide clear and effective articulation of your scientific endeavors, ensuring compliance and facilitating smooth regulatory approvals. Partner with us for seamless and successful regulatory navigation.
#pharma regulatory services#complianceconsulting#drugapprovalstrategies#pharmaceutical regulatory affairs services#regulatoryaffairsexperts#commercialization in new product development#regulatorynavigators#pharmaregulationguidance#regulatory compliance in pharmaceutical industry#cdmo
0 notes
Text
Pharma Formulation Company in USA
Renejix Pharma Solutions is a respectable American-based Pharmaceutical Formulation Company. We are experts in creating and producing an extensive array of pharmaceutical products, with a strong emphasis on quality and innovation. we provide best service like Amorphous Solid, Solubility enhancement, Micro encapsulation, Dispersions, and many more services. we are here for you 24/7. Call us at +1 (631) 210-5235.
For more information, visit:-https://renejix.com/formulation-technologies/
#formulation development pharmaceutical#formulation of pharmaceutical products#pharma formulation company
0 notes
Text
Essential oils for Stuffy Nose | Agenix Pharmaceuticals | Rhino Patch
Essential oils can be an effective natural remedy for a stuffy nose. When used correctly, certain essential oils can help open up the airways and reduce congestion, making it easier to breathe.
Some of the best essential oils for a stuffy nose include eucalyptus, peppermint, tea tree, rosemary, and lavender. Eucalyptus oil is particularly useful for respiratory issues because it has anti-inflammatory and decongestant properties, which can help open up the airways and reduce congestion.
Peppermint oil is also great for reducing nasal congestion and relieving sinus pressure. Tea tree oil has natural antimicrobial properties and can help clear out bacteria or viruses that may be causing congestion.
Rosemary oil has a warming effect that can help to increase circulation and reduce inflammation, while lavender oil can help to soothe inflammation in the respiratory system.
To use essential oils for a stuffy nose, they can be diffused into the air using a diffuser or added to a bowl of hot water for steam inhalation. It's important to dilute essential oils properly before using them, as they can be very potent and may cause skin irritation or allergic reactions.
If you have a pre-existing medical condition or are pregnant, it's recommended to consult with a healthcare professional before using essential oils.
Contact Us
721 W Tarrant Rd, Suite 120 Grand Prairie, TX 75050
+1 844–4-AGENIX
Related Searches
best essential oil for congestion
essential oils for nasal congestion
best aromatherapy oils
vapopatch
#aromatherapy oils#vapor patch for kids#pharmaceutical product development#essential oils for stuffy nose
0 notes
Text
ontract Manufacturing in the Generic Pharma Sector: Trends and Analysis
The global generic pharmaceuticals contract manufacturing market size is expected to reach USD 106.9 billion by 2030, registering a CAGR of 5.8% over the forecast period, according to a new report by Grand View Research, Inc. Cost-saving and time-saving benefits associated with the implementation of outsourcing is responsible for driving the industry. A significant number of people globally suffer from chronic diseases. For instance, the CDC states that 6 in 10 adults in the U.S. suffer from at least one chronic disease and 4 in 10 adults suffer from two or more chronic diseases. Chronic diseases are required to be treated for a long time. The high cost of medicines is increasing the demand for cost-effective generic drugs for the treatment of chronic diseases.
Generic Pharmaceuticals Contract Manufacturing Market Report Highlights
The branded generics segment held the largest share in 2021due to the preference for branded generics among physicians. Some branded generic manufacturers offer benefits and gifts to physicians for boosting their product sales. This further contributes to the demand for branded generic manufacturing in the market
The API product segment held the largest share in 2021. The growing demand for generic drugs is supporting the demand for generic API contract manufacturing
The parenteral route of administration segment is expected to grow at the fastest CAGR over the forecast period due to the bioavailability of parenteral drugs over other formulations
The oncology segment is expected to register the fastest CAGRfrom 2022 to 2030 owing to the high cost of cancer drugs contributing to the demand for cost-effective generic medicines
Asia Pacific is expected to record the highest CAGR over the forecast period mainly due to the low cost of generic drug manufacturing
Gain deeper insights on the market and receive your free copy with TOC now @: Generic Pharmaceuticals Contract Manufacturing Market Report
This is expected to support the industry's growth post-pandemic. There is an improvement in the regulatory approval of generic drugs. For instance, in 2021, the FDA approved 93 generic drugs, and by October 2022, the regulatory authority approved over 95 generic drugs. Such improvements are expected to have a positive impact on the manufacturing of generic drugs and; thus, support the industry growth. The Japanese government is constantly trying to improve the generic pharmaceuticals market in the country. The government is also taking measures to improve the supply of generics in the country and is also encouraging medical institutes to promote the use of generic drugs.
This is expected to improve CMO activities for generics in the coming years. Global spending on medicines is also on the rise. According to the data provided in a report published by IQVIA in April 2021, global spending on medicine is expected to increase in the next 4-5 years. The report states that global spending on medicine accounted for USD 1, 265 billion in 2020 and is going to reach USD 1,580-1,610 billion by 2025. This is also expected to improve the demand for generic drugs owing to their cost efficiency, thereby supporting the industry in growth.
#Pharma Contract Manufacturing#Generic Pharmaceuticals#Pharma Industry#Drug Manufacturing#Pharmaceutical Supply Chain#Outsourcing#Supply Chain Management#Pharmaceutical Partnerships#Pharmaceutical sourcing#Healthcare Manufacturing#Drug Development#API Production#CRO#CMO#Pharmaceutical Trends
0 notes
Text
The Best News of Last Week
1. ‘We are just getting started’: the plastic-eating bacteria that could change the world

In 2016, Japanese scientists Oda and Hiraga published their discovery of Ideonella sakaiensis, a bacterium capable of breaking down PET plastic into basic nutrients. This finding marked a shift in microbiology's perception, recognizing the potential of microbes to solve pressing environmental issues.
France's Carbios has successfully applied bacterial enzyme technology to recycle PET plastic waste into new plastic products, aligning with the French government's goal of fully recycling plastic packaging by 2025.
2. HIV cases in Amsterdam drop to almost zero after PrEP scheme

According to Dutch AIDS Fund, there were only nine new cases of the virus in Amsterdam in 2022, down from 66 people diagnosed in 2021. The organisation claimed that 128 people were diagnosed with HIV in Amsterdam in 2019, and since 2010, the number of new infections in the Dutch capital has fallen by 95 per cent.
3. Cheap and drinkable water from desalination is finally a reality

In a groundbreaking endeavor, engineers from MIT and China have designed a passive solar desalination system aimed at converting seawater into drinkable water.
The concept, articulated in a study published in the journal Joule, harnesses the dual powers of the sun and the inherent properties of seawater, emulating the ocean’s “thermohaline” circulation on a smaller scale, to evaporate water and leave salt behind.
4. World’s 1st drug to regrow teeth enters clinical trials

The ability to regrow your own teeth could be just around the corner. A team of scientists, led by a Japanese pharmaceutical startup, are getting set to start human trials on a new drug that has successfully grown new teeth in animal test subjects.
Toregem Biopharma is slated to begin clinical trials in July of next year after it succeeded growing new teeth in mice five years ago, the Japan Times reports.
5. After Decades of Pressure, US Drugmaker J&J Gives Up Patent on Life-Saving TB Drug
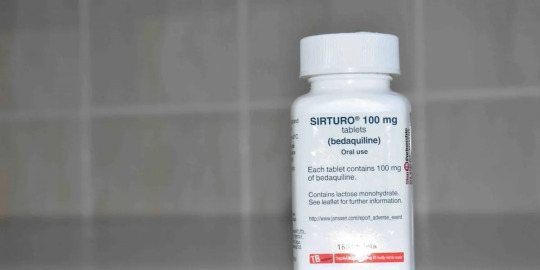
In what can be termed a huge development for drug-resistant TB (DR-TB) patients across large parts of the world, bedaquiline maker Johnson and Johnson said on September 30 (Saturday) that it would drop its patent over the drug in 134 low- and middle-income countries (LMICs).
6. Stranded dolphins rescued from shallow river in Massachusetts
youtube
7. ‘Staggering’ green growth gives hope for 1.5C, says global energy chief

The prospects of the world staying within the 1.5C limit on global heating have brightened owing to the “staggering” growth of renewable energy and green investment in the past two years, the chief of the world’s energy watchdog has said.
Fatih Birol, the executive director of the International Energy Agency, and the world’s foremost energy economist, said much more needed to be done but that the rapid uptake of solar power and electric vehicles were encouraging.
---
That's it for this week :)
This newsletter will always be free. If you liked this post you can support me with a small kofi donation here:
Buy me a coffee ❤️
Also don’t forget to reblog this post with your friends.
11K notes
·
View notes
Text

#clinical development#development solutions#biometric solution providers#clinical development consultant#clinical development service#drug development solutions#clinical product development#clinical development solution company#clinical development specialist#Clinical Development Services in Hyderabad#Clinical Services in Hyderabad#clinical development services agency in hyderabad india#best clinical development agency in india#project management solutions provider#project management service provider#biometric service provider near me#Clinical Trial Services In Hyderabad#specialized clinical pharmacist#clinical pharmaceutical company
1 note
·
View note