#pharmaregulationguidance
Explore tagged Tumblr posts
Text
Expert Regulatory and Scientific Writing Services by FrontroPharma
Navigating the complex world of regulatory requirements is made simple with FrontroPharma. Our experts specialize in preparing and reviewing regulatory documentation, including narratives, systematic reviews, meta-analyses, and grant writing. We provide clear and effective articulation of your scientific endeavors, ensuring compliance and facilitating smooth regulatory approvals. Partner with us for seamless and successful regulatory navigation.
#pharma regulatory services#complianceconsulting#drugapprovalstrategies#pharmaceutical regulatory affairs services#regulatoryaffairsexperts#commercialization in new product development#regulatorynavigators#pharmaregulationguidance#regulatory compliance in pharmaceutical industry#cdmo
0 notes
Text
Comprehensive Analytical Techniques for Product Development
Discover FrontroPharma's comprehensive suite of analytical techniques essential for optimizing product development and evaluation. Our state-of-the-art facilities and expert analytical team offer a wide range of services, including dissolution studies, in vitro permeation testing, particle size analysis, zeta potential measurement, FTIR spectroscopy, DSC analysis, TEM imaging, NMR spectroscopy, texture analysis, confocal microscopy, HPLC analysis, GC-MS analysis, LC-MS analysis, X-ray diffraction (XRD), and more. These techniques provide critical insights into formulation characteristics, stability, and performance, enabling informed decision-making throughout the product development lifecycle. Whether you require routine analytical testing or specialized investigations, FrontroPharma delivers accurate, reliable data to support your regulatory submissions and market aspirations.
#pharmaregulationguidance#complianceconsulting#drugapprovalstrategies#regulatoryaffairsexperts#regulatorynavigators#pharmaceutical regulatory affairs services#commercialization in new product development#regulatory compliance in pharmaceutical industry
0 notes
Text
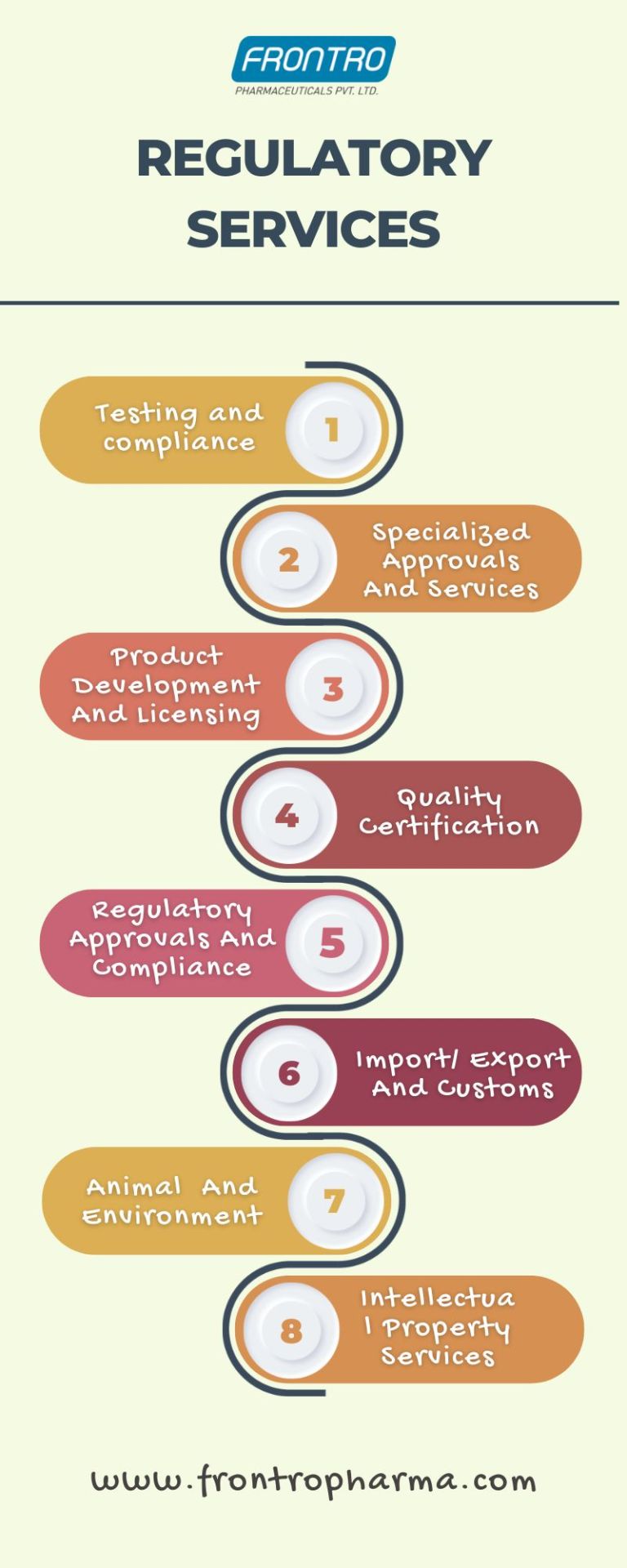
#pharma regulatory services#pharmaregulationguidance#regulatoryaffairsexperts#pharmaceutical regulatory affairs services#regulatory compliance in pharmaceutical industry
0 notes
Text
The Role of Regulatory Affairs Consultants in Indian Pharmaceutical Regulations
Ensuring regulatory compliance in the complex pharmaceutical sector can be difficult. Pharmaceutics regulatory affairs specialists help organizations negotiate this complicated terrain from product development to commercial launch. Here, we explain why pharmaceutical regulatory advisors are crucial to success in this dynamic industry.
Important Role of Pharmaceutical Regulatory Affairs Consultants
Consultants help pharmaceutical companies comply with strict requirements by providing expert guidance. These experts support the regulatory process from product safety and efficacy to legal and ethical compliance.
Key Regulatory Affairs Consultant Duties
Regulatory affairs specialists have many duties:
Compliance Guidance: Consultants help organizations navigate the complex regulatory landscape and comply with standards.
Consultants carefully analyze license and regulatory applications, compiling scientific data, clinical trial results, and production details to aid approval.
Consultants check labeling and packaging for appropriate dosage, usage instructions, precautions, and adverse effects.
Consultants build comprehensive quality processes to ensure product conformance and conduct audits and inspections to quickly correct regulatory breaches.
After-market surveillance: Consulting firms are essential for post-launch drug safety and efficacy monitoring, adverse event reporting, and post-market surveillance.
Pharmaceutical Regulatory Consultants: Why They Matter
Regulatory affairs consultants are crucial to the pharmaceutical sector. They're essential because:
Experience and Industry Knowledge: Companies may keep ahead of regulatory changes and reduce compliance risks with regulatory consultants' extensive understanding of changing legislation and best practices.
The cost-effectiveness Since regulatory consultants can provide project-specific services, they are generally cheaper than in-house regulatory teams.
Consultants simplify regulatory processes, product registration, certification, and international standard compliance to help expand markets.
Mitigating risk: By identifying and managing regulatory risks early in product development, consultants protect companies' interests and reputation from delays, rejections, and enforcement actions.
Regulatory Affairs Consultant Selection Considerations
Before hiring a regulatory affairs consultant, organizations should consider:
Track Record: Look for regulatory consultants with experience. Select experts with expertise in biologics, generics, or medical devices. Consultants need good interpersonal skills to work with internal and external stakeholders. Network: Consultants with regulatory authority contacts can speed approvals and improve compliance.
Conclusion: Expert Advice for Regulatory Complexities
In conclusion, pharmaceutical businesses use regulatory affairs consultants to manage the complex regulatory landscape. Companies may ensure compliance, innovate, and protect public health by using their expertise, supporting growth and sustainability in a competitive business.
0 notes
Text
Pharmaceutical Regulatory Affairs Consultants in India offers a comprehensive overview of the vital role played by regulatory affairs consultants in the pharmaceutical industry. From navigating complex regulatory frameworks to ensuring product safety and efficacy, these experts provide invaluable guidance to companies seeking to bring their medications to market. The article emphasizes the importance of consultants in maintaining compliance with evolving laws and industry standards, while also highlighting their role in mitigating risks and fostering innovation. With insights into consultant selection and the benefits they bring, this piece serves as a valuable resource for pharmaceutical companies operating in India and beyond.
#pharma regulatory services#complianceconsulting#regulatoryaffairsexperts#regulatorynavigators#drugapprovalstrategies#pharmaregulationguidance#pharmaceutical regulatory affairs services
0 notes
Text
Why Pharma Regulatory Consultants ?

Regulation consultants know evolving laws and industry best practices. They keep up with regulations and norms, enabling companies to respond fast and avoid compliance concerns.
Consulting may be cheaper than in-house regulation. Consultants provide project-specific services.
Consultants streamline regulations to expand global marketplaces. Their services include product registration, certification, and international standards.
Risk Mitigation: Consultants identify and manage regulatory risks early in product development to reduce delays, rejections, and enforcement.
Consultant Selection for Pharmaceutical Regulatory Affairs
#pharma regulatory services#drugapprovalstrategies#regulatorynavigators#complianceconsulting#artists on tumblr#pharmaregulationguidance#attack on titan
0 notes
Text
Research, Regulation, Export: Your Pharmaceutical Regulatory Affairs One-Stop Shop
Pharmaceutical research, regulation, and export are complex, especially in the dynamic and highly regulated business. From idea to market launch, every step requires painstaking attention to detail, strict compliance with rules, and perfection. Frontro Pharma provides comprehensive pharmaceutical regulatory affairs services as a one-stop solution for pharmaceutical firms since we understand their issues.
Frontro Pharma, a major pharmaceutical regulatory consultant in India, provides regulatory, CRO, and worldwide export solutions to the pharmaceutical, food, and cosmetic industries. Our team prioritizes human well-being and delivers high-quality, safe, and effective goods that satisfy worldwide standards.
Pharma Regulatory Services:
Compliance is essential in a changing regulatory environment. To help companies negotiate the complex regulatory landscape, Frontro Pharma offers many regulatory services. Our experts advise on CDSCO, FSSAI, and DGFT services to ensure regulatory compliance during product development.
Development of Products:
The CRO team specializes at product creation using scientific skills and cutting-edge technologies. In designing complicated generics, AYUSH products, nutraceuticals, and cosmetics, we optimize nano and liposome drug delivery systems. Our rigorous scientific technique produces cutting-edge formulas that guarantee product efficacy and excellence.
Pre-clinical research:
Trust Frontro Pharma for extensive preclinical safety and efficacy investigations. Modern facilities and in vivo models ensure accurate and trustworthy findings for product development and market approval.
Methods of Analysis:
Trust our one-stop shop for product development and evaluation analytical methods. Our expertise provide fast, accurate analytical support for method development, validation, stability studies, and impurity profiling, ensuring product quality and integrity.
Scientific and Regulatory Writing:
Our skilled regulatory and scientific writing services simplify regulatory standards. Our experts explain your research effectively and in accordance with worldwide regulations via regulatory narratives, systemic reviews, meta-analyses, and grants.
Frontro Pharma combines scientific understanding with quality to create a smooth concept-to-market process. Experience revolutionary CRO with us and unleash the worldwide potential of your breakthrough products. You may trust Frontro Pharmaceuticals Pvt Ltd for pharmaceutical regulatory affairs.
#pharma regulatory services#regulatoryaffairsexperts#pharmaceutical regulatory affairs services#complianceconsulting#drugapprovalstrategies#regulatorynavigators#pharmaregulationguidance
0 notes