#pharmaceutical manufacturing process
Explore tagged Tumblr posts
Text
Empowering the Pharma Ecosystem with Precision | Chemxpert Database
Chemxpert Database is your ultimate partner in navigating the pharmaceutical landscape. From supporting API manufacturers in India to connecting with top pharmaceutical software companies and leading pharmacovigilance companies, we provide robust insights. Our platform streamlines discovery, enabling access to trusted pharma software companies offering advanced solutions. With a focus on compliance, we help businesses meet Food Drug Administration standards while leveraging expert pharmacovigilance services. Stay ahead with Chemxpert Database, your gateway to excellence in pharma innovation.
#top pharma companies in Canada#pharmaceutical manufacturing process#pharmaceutical development#nitrosamine risk assessment#pharmaceutical companies in Netherlands#pharmaceutical compliance
1 note
·
View note
Text
High-Speed Tablet Press Machines for Pharma Manufacturing in the USA: A Complete Guide
Discover high-speed tablet press machines designed for pharmaceutical manufacturing in the USA. Our comprehensive guide covers features, benefits, and optimization tips for your production line.
#Tablet Press Machines#Tablet Compression Machine#High-Speed Tablet Press#Pharmaceutical Manufacturing Equipment#Tablet Press for Pharma Industry#Tablet Press Machine for Pharmaceuticals#Automatic Tablet Press Machine#Tablet Pressing Process#Pharmaceutical Tablet Manufacturing In USA#High-Speed Tablet Press USA#Tablet Press Machines for Large Production USA#Pharmaceutical Compression Machine USA#Tablet Compression Machine supplier in USA#Best High-Speed Tablet Press Machines for Pharmaceutical Manufacturing#How to Choose a Tablet Press Machine for Pharmaceuticals#Automatic Pharmaceutical Tablet Press for High-Volume Production#Tablet Press Machines for Efficient Pharmaceutical Manufacturing#Top Tablet Press Machines for Pharma Production in USA#Tablet Press Machine Features for Pharmaceutical Industry#Cost of Tablet Press Machines for Pharmaceutical Manufacturing
0 notes
Text

#Pharmaceutical Product#Processed Food Products#premium pharmaceutical product#premium processed food products#Pharmaceutical Product Exporter#Processed Food Products Exporter#Pharmaceutical Product Manufacture#India
0 notes
Text
Innovations in Water for Injection Production for Biopharma Applications
The pharmaceutical and biotech sectors are witnessing rapid advancements in Water for Injection (WFI) production. As regulatory requirements evolve and sustainability becomes a priority, innovative approaches are shaping the future of WFI systems.
Transition to Membrane-Based Technologies
Traditionally, WFI has been produced through distillation, a process known for its reliability but also its high energy consumption. Recent shifts towards membrane-based technologies, such as reverse osmosis (RO) combined with ultrafiltration and electrodeionization (EDI), offer significant advantages. These methods provide:
Energy Efficiency: Membrane systems consume less energy, aligning with industry sustainability goals.
Cost-Effectiveness: Lower operational costs make them attractive for facilities aiming to optimize budgets.
Scalability: These systems are easily scalable, accommodating diverse production needs.
Real-Time Monitoring and Automation
Advanced WFI systems now incorporate real-time monitoring and automation to enhance reliability and compliance. Features include:
Continuous Quality Monitoring: Sensors track key parameters such as conductivity, Total Organic Carbon (TOC), and microbial load in real-time.
Automated Cleaning: Automated Clean-in-Place (CIP) and Steam-in-Place (SIP) systems ensure consistent sterility and minimize downtime.

These technologies reduce human intervention, lowering the risk of errors and ensuring adherence to Good Manufacturing Practices (GMP).
Sustainable Practices
The push towards sustainability has led to innovations that minimize resource consumption in WFI production. Reclaiming water from other processes, optimizing energy use in heating systems, and adopting recyclable materials in equipment construction are just a few examples.
Regulatory Adaptations
Regulatory bodies are recognizing membrane-based WFI production as equivalent to distillation. This shift enables facilities to adopt newer technologies without compromising compliance. It also reduces the burden on resources while maintaining the same high standards.
Conclusion
Innovation in Water for Injection production is redefining operational efficiency and sustainability in the pharmaceutical and biotech sectors. As technologies evolve, they provide robust solutions to meet regulatory requirements and environmental challenges, securing WFI's critical role in future biopharma advancements.
#water for injection manufacturers#high purity water#water treatment plant#industrial water treatment plant#pharmaceutical water treatment#biotech#swjal process
0 notes
Text
https://www.hindustanprocess.com/spin-flash-dryer-manufacturers.php
#Spin Flash Dryer Manufacturers#Spin Flash Dryer Suppliers#Spin Flash Dryer in Pune#Spin Flash Dryer Consultant#Spin Flash Dryer Consultancy Services#Spin Flash Dryer Services#Spin Flash Dryer Manufacturers in Pune#Spin Flash Dryer Suppliers in Pune#Spin Flash Dryer Consultant in Pune#Spin Flash Dryer Consultancy Services in Pune#Spin Flash Dryer Services in Pune#Spin flash dryer for food industry#Spin flash dryer for chemical industry#Spin flash dryer for pharmaceutical industry#Spin flash dryer for agricultural products#Spin flash dryer for dye industry#Spin flash dryer for mineral processing#Spin flash dryer for waste management#Spin flash dryer for sludge drying#Spin flash dryer for biomass drying
0 notes
Text

We are an innovative company centered on chemistry, excelling in the development of advanced processes for the synthesis and production of various compounds across the Pharma, Agro, Fine, and Specialty Chemicals, as well as the CRO and CDMO industries. Offering Custom Development and CRAMS, we serve both local and global markets. Our expansion is driven by strong partnerships with like-minded clients. Delivering top-tier quality remains our primary focus.
#cdmo#chemicals#chemistry#cro#healthcare#science#cdmo companies in india#cdmo services#chemical synthesis#cro services#custom synthesis#process development#contract research#contract manufacturing#pharma company#pharmaceutical#big pharma
0 notes
Text
Opinion Here’s how to get free Paxlovid as many times as you need it
When the public health emergency around covid-19 ended, vaccines and treatments became commercial products, meaning companies could charge for them as they do other pharmaceuticals. Paxlovid, the highly effective antiviral pill that can prevent covid from becoming severe, now has a list price of nearly $1,400 for a five-day treatment course.
Thanks to an innovative agreement between the Biden administration and the drug’s manufacturer, Pfizer, Americans can still access the medication free or at very low cost through a program called Paxcess. The problem is that too few people — including pharmacists — are aware of it.
I learned of Paxcess only after readers wrote that pharmacies were charging them hundreds of dollars — or even the full list price — to fill their Paxlovid prescription. This shouldn’t be happening. A representative from Pfizer, which runs the program, explained to me that patients on Medicare and Medicaid or who are uninsured should get free Paxlovid. They need to sign up by going to paxlovid.iassist.com or by calling 877-219-7225. “We wanted to make enrollment as easy and as quick as possible,” the representative said.
Indeed, the process is straightforward. I clicked through the web form myself, and there are only three sets of information required. Patients first enter their name, date of birth and address. They then input their prescriber’s name and address and select their insurance type.
All this should take less than five minutes and can be done at home or at the pharmacy. A physician or pharmacist can fill it out on behalf of the patient, too. Importantly, this form does not ask for medical history, proof of a positive coronavirus test, income verification, citizenship status or other potentially sensitive and time-consuming information.
But there is one key requirement people need to be aware of: Patients must have a prescription for Paxlovid to start the enrollment process. It is not possible to pre-enroll. (Though, in a sense, people on Medicare or Medicaid are already pre-enrolled.)
Once the questionnaire is complete, the website generates a voucher within seconds. People can print it or email it themselves, and then they can exchange it for a free course of Paxlovid at most pharmacies.
Pfizer’s representative tells me that more than 57,000 pharmacies are contracted to participate in this program, including major chain drugstores such as CVS and Walgreens and large retail chains such as Walmart, Kroger and Costco. For those unable to go in person, a mail-order option is available, too.
The program works a little differently for patients with commercial insurance. Some insurance plans already cover Paxlovid without a co-pay. Anyone who is told there will be a charge should sign up for Paxcess, which would further bring down their co-pay and might even cover the entire cost.
Several readers have attested that Paxcess’s process was fast and seamless. I was also glad to learn that there is basically no limit to the number of times someone could use it. A person who contracts the coronavirus three times in a year could access Paxlovid free or at low cost each time.
Unfortunately, readers informed me of one major glitch: Though the Paxcess voucher is honored when presented, some pharmacies are not offering the program proactively. As a result, many patients are still being charged high co-pays even if they could have gotten the medication at no cost.
This is incredibly frustrating. However, after interviewing multiple people involved in the process, including representatives of major pharmacy chains and Biden administration officials, I believe everyone is sincere in trying to make things right. As we saw in the early days of the coronavirus vaccine rollout, it’s hard to get a new program off the ground. Policies that look good on paper run into multiple barriers during implementation.
Those involved are actively identifying and addressing these problems. For instance, a Walgreens representative explained to me that in addition to educating pharmacists and pharmacy techs about the program, the company learned it also had to make system changes to account for a different workflow. Normally, when pharmacists process a prescription, they inform patients of the co-pay and dispense the medication. But with Paxlovid, the system needs to stop them if there is a co-pay, so they can prompt patients to sign up for Paxcess.
Here is where patients and consumers must take a proactive role. That might not feel fair; after all, if someone is ill, people expect that the system will work to help them. But that’s not our reality. While pharmacies work to fix their system glitches, patients need to be their own best advocates. That means signing up for Paxcess as soon as they receive a Paxlovid prescription and helping spread the word so that others can get the antiviral at little or no cost, too.
{source}
29K notes
·
View notes
Text
Fill Finish Manufacturing Market is Estimated to Witness High Growth Owing to Increasing Demand

The fill finish manufacturing market involves various downstream processing methods in the production of pharmaceutical products and medical devices which includes pre-filling inspection and labeling. The fill finish processes encompass liquid filling as well as lyophilization, assembly, labeling and packaging of vials, syringes, and cartridges. It ensures optimal product quality, safety and integrity for final use. The fill finish manufacturing plays a crucial role in bringing biologics and vaccines to patients requiring strict adherence to regulations. The need for personalized medicine and shortage of vaccines during the COVID-19 pandemic has also propelled the demand in recent times. The fill finish manufacturing market is estimated to be valued at USD 16.41 Bn in 2024 and is expected to reach USD 30.36 Bn by 2031, exhibiting a compound annual growth rate (CAGR) of 9.2% from 2024 to 2031.
Key Takeaways Key players operating in the fill finish manufacturing market are Asymchem Inc., Syntegon Technology GmbH, I.M.A. INDUSTRIA MACCHINE AUTOMATICHE S.P.A, West Pharmaceutical Services, Inc., Gerresheimer AG, AptarGroup, Inc., Dätwyler Holding Inc., Stevanato Group, OPTIMA, SGD Pharma, Nipro Corporation, Bausch Advanced Technology Group, and Berry Global Inc. The key opportunities in the market include increased outsourcing activities by pharmaceutical companies and advanced technologies providing flexibility, connectivity and efficiency. Emerging economies in Asia Pacific and Latin America present lucrative growth prospects owing to rising healthcare expenditures and increasing biologics production. The Fill Finish Manufacturing Market Share is witnessing increased global expansion strategies by key market players through mergers, acquisitions and partnerships. This allows companies to broaden their service offerings and geographic footprints to tap high growth markets. Market drivers The major market driver is the rising demand for biologics and vaccines. Biologics have revolutionized the treatment of complex diseases but require specialized fill finish facilities owing to their sensitivity. Furthermore, inadequate vaccine supplies during the pandemic underscored the need to boost local manufacturing capacities through technology transfers. This is expected to drive greater outsourcing of fill finish activities to specialized contract service providers globally.
PEST Analysis Political: Regulations regarding pharmaceutical packaging have become increasingly stringent over the years, requiring compliance. This drives demand for technologically advanced fill and finish manufacturing solutions. Economic: With the economy recovering post-COVID and healthcare expenditure rising worldwide, the Fill Finish Manufacturing Market Challenges And Opportunities inindustry is benefitting from higher outsourcing and greater uptake of advanced machinery. Social: An aging global population is driving higher demand for medicines and therapeutics. Further, greater health awareness is boosting pharmacy visits and medication consumption. Technological: Advanced technologies like automation, Internet of Things connectivity, and digital manufacturing are being integrated to enhance production efficiency, throughput, quality control, and regulatory compliance documentation in fill and finish plants. Geographical regions of concentration The North American and European fill and finish manufacturing markets currently account for the largest share of the global market value, driven by strong domestic pharmaceutical industries and stringent quality and regulatory standards in countries like the US, Germany, France, and UK. Proximity to key pharmaceutical markets and customers provides an inherent advantage to manufacturers located in these regions. Fastest growing region The Asia Pacific region, led by China and India, is poised to witness the fastest growth in the fill and finish manufacturing market over the forecast period. This can be attributed to rising generic and biologics manufacturing in the region coupled with increasing localization requirements. Additionally, lower operating costs and a rapidly expanding local pharmaceutical customer base are encouraging global players to set up or expand operations in Asia Pacific.
PEST Analysis Political: Regulations regarding pharmaceutical packaging have become increasingly stringent over the years, requiring compliance. This drives demand for technologically advanced fill and finish manufacturing solutions. Economic: With the economy recovering post-COVID and healthcare expenditure rising worldwide, the fill and finish manufacturing industry is benefitting from higher outsourcing and greater uptake of advanced machinery. Social: An aging global population is driving higher demand for medicines and therapeutics. Further, greater health awareness is boosting pharmacy visits and medication consumption. Technological: Advanced technologies like automation, Internet of Things connectivity, and digital manufacturing are being integrated to enhance production efficiency, throughput, quality control, and regulatory compliance documentation in fill and finish plants. The North American and European fill and finish manufacturing markets currently account for the largest share of the global market value, driven by strong domestic pharmaceutical industries and stringent quality and regulatory standards in countries like the US, Germany, France, and UK. Proximity to key pharmaceutical markets and customers provides an inherent advantage to manufacturers located in these regions. The Asia Pacific region, led by China and India, is poised to witness the fastest growth in the fill and finish manufacturing market over the forecast period. This can be attributed to rising generic and biologics manufacturing in the region coupled with increasing localization requirements. Additionally, lower operating costs and a rapidly expanding local pharmaceutical customer base are encouraging global players to set up or expand operations in Asia Pacific.
Get more insights on Fill Finish Manufacturing Market
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
#Coherent Market Insights#Fill Finish Manufacturing Market#Fill Finish Manufacturing#Pharmaceutical Manufacturing#Biopharmaceuticals#Drug Production#Sterile Filling#Aseptic Processing#Vial Filling#Syringe Filling
0 notes
Text
Diesel Fired Thermic Fluid Heater
Introducing our cutting-edge Diesel Fired Thermic Fluid Heater – an epitome of innovation in industrial heating solutions. This advanced heater redefines heat transfer, offering a seamless blend of speed, reliability, durability, and precision temperature control for diverse industrial applications.

Key Features:
Experience the efficiency of quick and uniform heat transfer using thermic fluid with Avon engineering Diesel Fired Thermic Fluid Heater. Rapid Heat Transfer ensures a reliable exchange of heat for various operational needs. Additionally, it adeptly converts diesel fuel into heat, providing seamless and efficient energy utilization.
Moreover, Powered by Reliable Diesel Fuel, our heater harnesses the reliability of diesel fuel as an energy source. It utilizes this dependable fuel type, offering continuous and dependable heat for a wide range of applications, ensuring uninterrupted operation.
Furthermore, Designed with longevity in mind, our heater boasts Durable Construction for Lasting Use. Its robust construction can withstand the demands of industrial environments, ensuring consistent performance and reliability over time.
Lastly, Achieve accurate temperature control within your processes with our product. Precise Temperature Control is enabled through its advanced control systems, allowing you to maintain desired temperature ranges crucial for applications requiring specific heat levels.
#Diesel fired thermic fluid heater#Thermic fluid heater#Diesel heater for heat transfer#Rapid heat transfer fluid heater#Efficient diesel-fired heater#Industrial thermic fluid heater#Uniform heat transfer heater#Reliable diesel fuel heater#Durable construction heater#Precise temperature control heater#Process heating solutions#Heat exchange equipment#Thermic fluid heating system#Quick heat transfer heater#Uniform heat distribution heater#Reliable diesel fuel utilization heater#Robust construction for industrial use#Accurate temperature control heater#Dependable heat source heater#Energy-efficient heating solution#Industrial-grade thermic fluid heater#Long-term reliability heater#Versatile heat transfer equipment#Industrial heating solution for manufacturing#Heater for process industries#Heat transfer equipment for textile industry#Heater for food processing#Heater for pharmaceutical industry#Heater for chemical industry#Benefits of diesel-fired thermic fluid heaters
0 notes
Text
QVF Glassware | Canada | Goel Scientific | USA
Goel Scientific provides QVF Glassware manufacturing for many industries like pharmaceuticals, petrochemicals, chemicals, etc. We Supplier QVF beaded process pipe in Canada and USA. for more information call now.

#QVF Glassware#QVF Glassware Manufacture#QVF Glassware Supplier#QVF Glassware for pharmaceuticals#QVF Glassware for petrochemicals#QVF Glassware for speciality chemicals#QVF beaded process pipe#QVF Glassware Manufacture in Canada#QVF Glassware Manufacture in USA
0 notes
Text
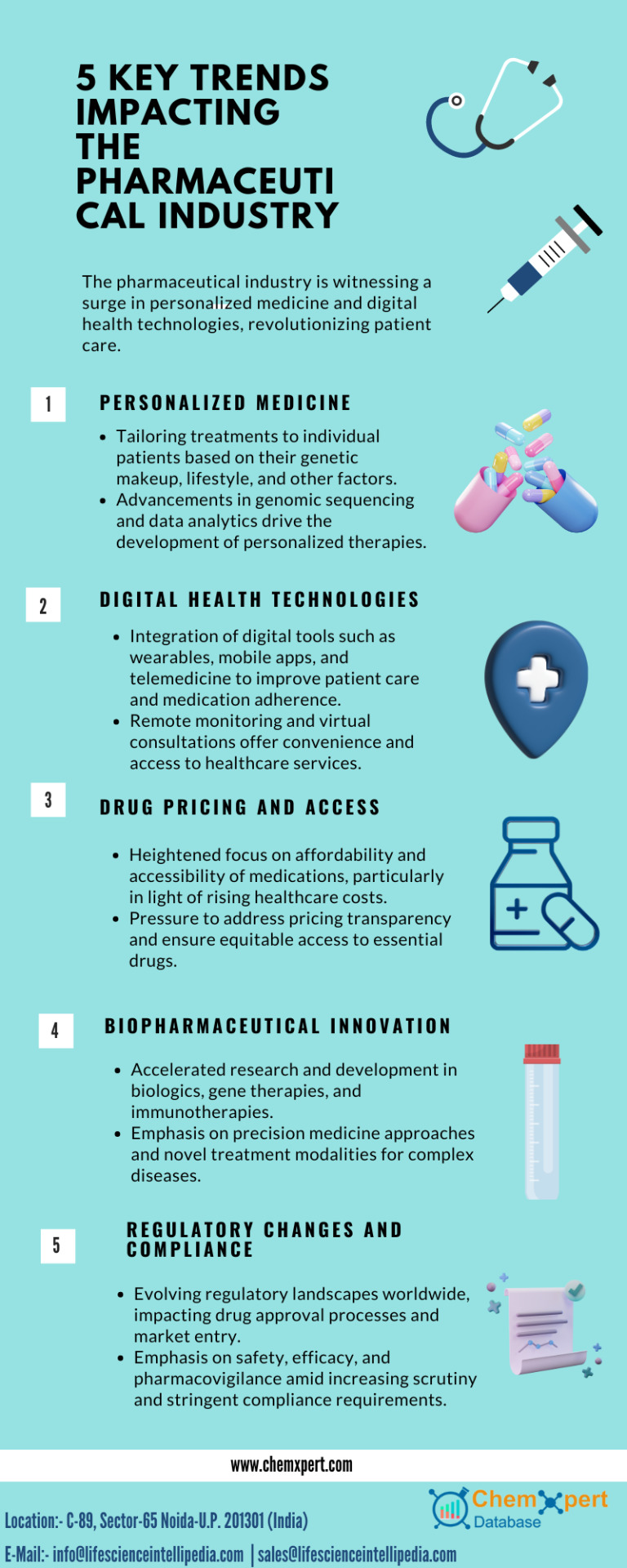
Comprehensive Pharmaceutical Solutions with Chemxpert Database
Chemxpert Database offers robust solutions for pharmaceutical online needs, facilitating cutting-edge pharmaceutical research and seamless connections with third-party medicine manufacturers. Our platform streamlines the drug manufacturing process, ensuring quality and efficiency. Additionally, we provide targeted pharma advertising opportunities to enhance your brand's reach. Trust Chemxpert Database for a comprehensive approach to all your pharmaceutical requirements, from research and manufacturing to marketing.
#pharmaceutical manufacturing process#pharmaceutical development#nitrosamine risk assessment#pharmaceutical companies in Netherlands#pharmaceutical compliance
1 note
·
View note
Text
The Importance of Discrete Element Modeling (DEM) Studies and What Problems It Can Solve
In today's rapidly advancing world of science and engineering, the need for accurate and efficient simulation tools has never been greater. One such tool that has gained significant prominence in recent years is Discrete Element Modeling (DEM). DEM is a numerical technique used to simulate the behavior of granular materials, such as powders, grains, and particles, on a microscale level. This modeling approach has proven to be invaluable in a wide range of industries, from pharmaceuticals to civil engineering. In this article, we will explore the importance of DEM studies and delve into the various problems it can solve, demonstrating its versatility and impact across diverse fields.
I. Understanding Discrete Element Modeling (DEM)
Before we dive into the importance of DEM studies, it's essential to grasp the fundamentals of Discrete Element Modeling itself. DEM is a computational technique that simulates the behavior of a large number of individual particles. Each particle is treated as a discrete entity and follows specific rules and interactions with other particles. These interactions are governed by various force laws, including contact forces, friction, and collision dynamics. By tracking the motion and interactions of these particles over time, DEM can provide valuable insights into the behavior of granular materials.
DEM Fundamentals
At the core of DEM lies the discrete nature of particles. Unlike continuum-based methods, DEM models materials as a collection of individual particles, each with its own properties and interactions. These particles move within a virtual space and collide with one another, creating complex dynamics that mirror real-world granular materials.
The essential components of a DEM simulation include:
Particles: These represent the individual grains or particles within the material.
Interactions: DEM defines the rules governing how particles interact with each other, including contact forces, friction, and restitution coefficients.
Time Integration: DEM calculates the motion of particles over discrete time steps, accounting for forces and interactions at each step.
Boundaries and Constraints: The simulation environment often includes boundaries and constraints to model specific scenarios accurately.
DEM Applications
The versatility of DEM has led to its adoption in various fields and industries. Some notable applications of DEM include:
Geotechnical Engineering: DEM is used to study soil mechanics, soil-structure interactions, and landslide prediction.
Pharmaceutical Manufacturing: DEM helps optimize drug formulation, tablet compression, and powder flow in pharmaceutical processes.
Mining and Minerals Processing: DEM is employed to understand the behavior of ore materials during crushing, grinding, and transport.
Food Processing: DEM studies can improve the design of food processing equipment and optimize the handling of food particles.
Civil Engineering: DEM is applied to simulate granular materials in construction, such as concrete mixing and soil compaction.
Powder Technology: In industries like powder metallurgy and ceramics, DEM assists in optimizing powder compaction and sintering processes.
Now that we have a fundamental understanding of DEM, let's explore the significance of DEM studies and the diverse range of problems it can solve across these industries.
II. The Importance of DEM Studies
DEM studies have become increasingly important in various fields, offering valuable insights, solutions, and advancements. Here, we will delve into the significance of DEM studies by examining the critical problems it addresses across industries.
Geotechnical Engineering
a. Soil Mechanics
In geotechnical engineering, understanding the behavior of soils is paramount for infrastructure design and construction. DEM studies provide insights into soil mechanics by simulating the interaction between soil particles under various loading conditions. This allows engineers to predict soil settlement, shear strength, and bearing capacity, all of which are crucial for designing stable foundations for buildings, bridges, and other structures.
b. Landslide Prediction
Landslides pose a significant threat in hilly and mountainous regions. DEM can simulate the movement of soil and rocks on slopes, aiding in landslide prediction and risk assessment. By analyzing factors like particle size, shape, and cohesion, DEM models can help identify areas prone to landslides and develop mitigation strategies.
Pharmaceutical Manufacturing
a. Tablet Compression
In the pharmaceutical industry, tablet compression is a critical process in drug manufacturing. DEM studies help optimize tablet formulation by simulating the compaction of powder blends. By varying particle properties and compaction conditions, researchers can predict tablet properties like hardness, friability, and dissolution rate, leading to improved drug formulations and reduced development costs.
b. Powder Flow and Mixing
Powder flow and mixing are crucial steps in pharmaceutical manufacturing. DEM models can simulate the flow of powders through equipment like hoppers, silos, and blenders. This enables the identification of potential flow problems, such as segregation or arching, and the design of equipment modifications to enhance powder handling and mixing efficiency.
Mining and Minerals Processing
a. Crushing and Grinding
In mining and minerals processing, the efficient comminution of ore materials is essential for resource extraction. DEM studies simulate the crushing and grinding of ore particles in crushers and mills, allowing engineers to optimize equipment design and operating conditions. This leads to improved energy efficiency and increased mineral recovery rates.
b. Material Handling
The transport of bulk materials within mining and processing facilities can be challenging. DEM helps analyze conveyor belt behavior, chute design, and transfer point performance. By studying particle trajectories and interaction forces, engineers can minimize material spillage, dust generation, and equipment wear, ultimately reducing operational costs.
Food Processing
a. Mixing and Blending
In the food processing industry, achieving uniform mixing and blending of ingredients is critical for product quality. DEM simulations of mixing processes help optimize equipment design and operating parameters. By visualizing particle distribution and movement, manufacturers can ensure consistent product quality and reduce waste.
b. Powder Handling
Powder handling in the food industry can be complex due to the diverse properties of food powders. DEM studies assist in designing equipment such as pneumatic conveyors and feeders. By predicting powder flow behavior and potential issues like segregation, DEM helps ensure the efficient and hygienic handling of food ingredients.
Civil Engineering
a. Concrete Mixing and Placement
In civil engineering, the proper mixing and placement of concrete are essential for constructing durable structures. DEM can model the behavior of concrete constituents, such as aggregates and cement particles, during mixing and placement processes. This allows engineers to optimize concrete mix designs and construction techniques, leading to improved performance and longevity of concrete structures.
b. Soil Compaction
Achieving adequate soil compaction is crucial for road construction, embankment construction, and foundation preparation. DEM simulations can replicate the compaction process, considering factors like soil particle properties, compactor geometry, and dynamic loading. Engineers can use DEM to optimize compaction equipment and procedures, ensuring the desired level of soil compaction is achieved.
III. Challenges and Advances in DEM Studies
While DEM has proven to be a valuable tool in addressing various problems, it is not without its challenges and limitations. Researchers continue to work on improving DEM techniques and expanding their capabilities. Let's explore some of the challenges and recent advances in DEM studies:
Computational Intensity
DEM simulations involving a large number of particles can be computationally intensive and time-consuming. To address this challenge, researchers have developed parallel algorithms and utilized high-performance computing clusters to accelerate simulations. Additionally, advancements in graphics processing units (GPUs) have significantly improved the efficiency of DEM simulations.
Particle-Particle Interactions
Accurately modeling complex particle-particle interactions, including adhesive forces and agglomeration, remains a challenge in DEM. Recent research has focused on refining contact models to better capture these interactions, allowing for more realistic simulations of cohesive and adhesive materials.
Scale-Up and Scale-Down
Scaling DEM simulations from laboratory-scale experiments to real-world applications can be challenging due to differences in length and time scales. Researchers are developing multiscale modeling approaches to bridge this gap, enabling more accurate predictions in practical engineering applications.
Integration with Other Simulation Techniques
In some cases, it is necessary to combine DEM with other simulation techniques, such as Computational Fluid Dynamics (CFD) or Finite Element Analysis (FEA), to study complex multiphysics problems. Integrating DEM with these techniques and developing robust coupling methods are active areas of research.
Calibration and Validation
Calibrating DEM models to match real-world behavior and validating simulations against experimental data are crucial for model accuracy. Researchers are developing techniques for parameter calibration and validation, including advanced imaging and tracking technologies for particle characterization.
GPU Acceleration and Cloud Computing
As computing power continues to advance, the use of GPUs and cloud computing resources has become more accessible for DEM simulations. These technologies enable researchers and engineers to perform more extensive and detailed simulations, opening new possibilities for problem-solving and optimization.
Machine Learning and AI Integration
The integration of machine learning and artificial intelligence (AI) with DEM is a promising avenue for advancing the field. These techniques can aid in data analysis, model parameterization, and real-time decision-making in DEM simulations.
IV. Conclusion
Discrete Element Modeling (DEM) has emerged as a powerful and versatile tool for simulating the behavior of granular materials in various industries. Its ability to address critical problems in geotechnical engineering, pharmaceutical manufacturing, mining, food processing, and civil engineering has led to its widespread adoption and continued development.
DEM studies have provided engineers and researchers with valuable insights into the behavior of granular materials, enabling them to optimize processes, design equipment, and make informed decisions. Despite its challenges, ongoing advancements in computational methods, particle interactions, and multiscale modeling are expanding the capabilities of DEM and enhancing its accuracy.
As industries continue to evolve and face new challenges, DEM will likely play an increasingly vital role in solving complex problems and driving innovation. Its integration with emerging technologies like machine learning and AI holds promise for further enhancing its capabilities and broadening its application areas.
In conclusion, Discrete Element Modeling stands as a testament to the power of computational simulations in shaping the future of science and engineering. Its importance in solving real-world problems cannot be overstated, and its continued development promises to revolutionize the way we understand and manipulate granular materials in the years to come.
V. The Capabilities of Newton DEM Software
In the realm of Discrete Element Modeling (DEM), the choice of software is paramount to achieving accurate and insightful simulations. One software package that has gained recognition for its capabilities and versatility in solving complex granular material problems is Newton DEM Software. In this section, we will explore the unique features and advantages that Newton DEM Software offers in the context of DEM studies.
High-Performance Simulations
Newton DEM Software is renowned for its high-performance capabilities. It leverages advanced algorithms and efficient parallel processing to handle simulations involving a vast number of particles seamlessly. This makes it suitable for tackling large-scale industrial problems, such as those encountered in mining, pharmaceuticals, and construction.
Comprehensive Material Models
One of the standout features of Newton DEM Software is its extensive library of material models. It provides users with the flexibility to simulate a wide range of granular materials, including various shapes, sizes, and properties. This enables researchers and engineers to model materials accurately, whether they are dealing with cohesive powders, irregularly shaped particles, or even mixtures of different materials.
Advanced Contact Mechanics
Accurate modeling of particle-particle interactions is crucial for DEM simulations. Newton DEM Software employs advanced contact mechanics algorithms to precisely capture complex interactions, such as rolling, sliding, and friction. Additionally, it allows users to define custom contact models, ensuring that simulations closely mirror real-world behavior.
Multiscale Modeling Capabilities
Newton DEM Software recognizes the importance of bridging the gap between laboratory-scale experiments and practical engineering applications. It offers multiscale modeling capabilities that enable users to perform simulations at various length and time scales. This flexibility is particularly valuable when dealing with materials that exhibit different behaviors under different conditions.
Coupling with Other Simulation Techniques
Many real-world problems require a multiphysics approach, combining DEM with other simulation techniques like Computational Fluid Dynamics (CFD) or Finite Element Analysis (FEA). Newton DEM Software supports seamless coupling with these techniques, allowing users to investigate complex interactions between granular materials and fluid flows or structural elements.
User-Friendly Interface
Usability is a key consideration in software tools, and Newton DEM Software excels in this regard. Its user-friendly interface streamlines the simulation setup and visualization processes, making it accessible to both seasoned researchers and newcomers to DEM. The software provides an intuitive environment for defining particle properties, boundary conditions, and analysis parameters.
Visualization and Data Analysis
Newton DEM Software offers robust visualization and data analysis tools. Users can visualize simulation results in real-time, enabling immediate insights into particle behavior. Additionally, the software provides tools for post-processing and data analysis, allowing users to extract valuable information from their simulations and make informed decisions.
Integration with Machine Learning and AI
To stay at the forefront of technological advancements, Newton DEM Software has embraced the integration of machine learning and artificial intelligence (AI). Users can leverage these capabilities to enhance their DEM simulations, from automating parameter tuning to making real-time predictions based on simulation data.
Scalability and Cloud Computing
Recognizing the growing demand for scalability and accessibility, Newton DEM Software is compatible with cloud computing platforms. This facilitates the execution of resource-intensive simulations on remote clusters, reducing computational bottlenecks and accelerating research and development efforts.
Comprehensive Support and Training
Effective use of DEM software requires proper training and support. Newton DEM Software provides comprehensive training materials, documentation, and customer support to assist users at every stage of their simulations. This ensures that users can leverage the full potential of the software and achieve meaningful results.
Incorporating Newton DEM Software into DEM studies enhances the capabilities of researchers and engineers, enabling them to tackle increasingly complex granular material problems across a spectrum of industries. Its combination of high-performance simulations, advanced contact mechanics, multiscale modeling, and integration with other simulation techniques makes it a valuable asset for those seeking to push the boundaries of DEM.
In conclusion, the capabilities of Newton DEM Software exemplify the ongoing evolution of computational tools in solving real-world problems. Its user-friendly interface, extensive material models, and support for multiscale modeling and coupling with other simulation techniques empower researchers and engineers to explore the behavior of granular materials with unparalleled accuracy and efficiency. As industries continue to advance, Newton DEM Software stands as a reliable and indispensable tool in the realm of Discrete Element Modeling.
Read more about:
Little P.Eng. for Discrete Element Modeling (DEM) Services: Unveiling the Power of Simulation
Little P.Eng. for Discrete Element Modeling (DEM) Services
Tags:
Artificial Intelligence
Discrete Element Modeling
Mixing
Geotechnical Engineering
Granular Materials
DEM Studies
Simulation
Particle Interaction
Pharmaceutical Manufacturing
Mining
Food Processing
Civil Engineering
Soil Mechanics
Landslide Prediction
Tablet Compression
Powder Flow
Crushing
Material Handling
Concrete Mixing
Soil Compaction
Computational Intensity
Particle-Particle Interactions
Multiscale Modeling
Machine Learning
GPU Acceleration
High-Performance Computing
Newton DEM Software
Contact Mechanics
Cloud Computing
Validation
Bulk Material Handling & Processing
Engineering Services
Located in Calgary, Alberta; Vancouver, BC; Toronto, Ontario; Edmonton, Alberta; Houston Texas; Torrance, California; El Segundo, CA; Manhattan Beach, CA; Concord, CA; We offer our engineering consultancy services across Canada and United States. Meena Rezkallah.
#•#Artificial Intelligence#Discrete Element Modeling#Mixing#Geotechnical Engineering#Granular Materials#DEM Studies#Simulation#Particle Interaction#Pharmaceutical Manufacturing#Mining#Food Processing#Civil Engineering#Soil Mechanics#Landslide Prediction#Tablet Compression#Powder Flow#Crushing#Material Handling#Concrete Mixing#Soil Compaction#Computational Intensity#Particle-Particle Interactions#Multiscale Modeling#Machine Learning#GPU Acceleration#High-Performance Computing#Newton DEM Software#Contact Mechanics#Cloud Computing
0 notes
Text
#Pharmaceutical Product#Processed Food Products#premium pharmaceutical product#premium processed food products#Pharmaceutical Product Exporter#Processed Food Products Exporter#Pharmaceutical Product Manufacture#India
1 note
·
View note
Text
Equipment Used in the Pharmaceutical Industry
The pharmaceutical industry relies on a vast range of specialized equipment to ensure that the production processes for medicines, vaccines, and other health products are safe, efficient, and compliant with regulatory standards. From raw material handling to the packaging of the final product, equipment plays a crucial role in ensuring quality and sterility. This article explores the essential equipment used in pharmaceutical manufacturing, highlighting the importance of each category and its application in the production process.
1. Mixing and Blending Equipment
Mixers and blenders are critical in combining raw materials and active pharmaceutical ingredients (APIs) into homogeneous mixtures. This equipment ensures that the components are evenly distributed, which is vital for dosage consistency in tablets, capsules, and other pharmaceutical forms. Different types of mixing equipment are used depending on the properties of the ingredients:
Ribbon Blenders: Used for dry powder mixing, commonly in tablet production.
High-Shear Mixers: Ideal for wet granulation processes where liquid binding agents are added to powders.
2. Granulation Equipment
Granulation is a key step in the production of tablets. This process converts fine powders into larger, free-flowing granules, making it easier to compress them into solid dosage forms. The two main types of granulation processes are wet granulation and dry granulation. Equipment used for this purpose includes:
Fluid Bed Granulators: Used in wet granulation to spray binder solutions onto powder particles.
Roller Compactors: Used in dry granulation, where powders are compacted between rollers to form granules without adding liquids.
3. Tableting and Encapsulation Machines
Tableting and encapsulation are the processes of converting granules into solid dosage forms. Specialized machines are required to compress powders into tablets or encapsulate them in capsules:
Tablet Presses: These machines compress powders or granules into tablets of uniform size and weight. Single-punch or rotary tablet presses are commonly used, depending on production scale.
Capsule Fillers: Capsule filling machines automatically fill empty gelatin or HPMC capsules with powders, granules, or liquid formulations, ensuring precise dosage in each capsule.
4. Coating Machines
Pharmaceutical tablets often require coatings for several reasons, such as improving taste, protecting the active ingredients, or controlling drug release. Coating machines apply a thin layer of polymer or sugar-based coating onto the tablet's surface. The most common equipment used for this purpose includes:
Pan Coaters: These machines rotate tablets in a pan while spraying the coating solution.
Fluidized Bed Coaters: Used for applying coatings to particles or small tablets by suspending them in an air stream while applying the coating material.
5. Sterilization Equipment
In pharmaceutical production, sterility is paramount, especially in the manufacturing of injectable drugs, vaccines, and other sterile products. Sterilization equipment ensures that any microbial contamination is eliminated. The common sterilization methods and equipment include:
Autoclaves: Use steam under pressure to sterilize equipment and pharmaceutical products. They are widely used for sterilizing surgical instruments, glassware, and some types of media.
Dry Heat Sterilizers: Ideal for sterilizing equipment that can withstand high temperatures but not moisture, such as glass bottles and metal equipment.
Gamma Irradiation: Used for sterilizing pharmaceutical products that are sensitive to heat or moisture.
6. Filtration Systems
Filtration is critical in ensuring that liquids and gases used in pharmaceutical manufacturing are free from particulates, bacteria, and other contaminants. Filtration systems are essential in the production of injectable drugs, vaccines, and sterile products:
Membrane Filters: Used to remove bacteria and particulates from liquid formulations. These filters are commonly used in the preparation of sterile solutions.
HEPA Filters: High-Efficiency Particulate Air (HEPA) filters are used in cleanrooms and HVAC systems to ensure the air is free from particulates and microorganisms.
7. Water Purification Systems
Water is a fundamental ingredient in the pharmaceutical industry, used in various forms, such as Purified Water (PW), Water for Injection (WFI), and Ultra-Pure Water (UPW). Water purification systems ensure that the water used in pharmaceutical production meets strict purity standards:
Reverse Osmosis (RO) Systems: Remove dissolved solids, bacteria, and organic impurities from water.
Electrodeionization (EDI) Systems: Used for the continuous production of ultra-pure water by removing ionic contaminants.
Water for Injection (WFI) Systems: Produce highly purified, sterile water used in the production of injectables and other sterile pharmaceutical products.
8. Packaging Equipment
Pharmaceutical packaging must ensure the product’s safety, integrity, and compliance with regulatory requirements. The packaging process also protects the product from contamination, tampering, and environmental factors. Common types of packaging equipment include:
Blister Packaging Machines: Used for packaging tablets and capsules in pre-formed cavities made of plastic or aluminum.
Bottle Filling Machines: Automatically fill liquid pharmaceuticals into bottles or vials, ensuring precise measurement and sealing.
Labeling Machines: Apply labels to pharmaceutical containers, ensuring that they contain the correct product information and batch details.
9. Quality Control Equipment
Pharmaceutical production requires stringent quality control to ensure that products meet regulatory standards. Quality control equipment is used for testing various attributes of the product, such as potency, purity, dissolution rate, and more:
HPLC (High-Performance Liquid Chromatography): Used to analyze the chemical composition of drugs and ensure that they contain the correct concentrations of active ingredients.
Dissolution Testers: Measure the rate at which tablets or capsules dissolve in liquids, ensuring that they meet specified release profiles.
Spectrophotometers: Analyze the absorbance of light in drug samples, helping to identify impurities or verify the concentration of active ingredients.
10. Cleanroom Equipment
Pharmaceutical manufacturing often takes place in cleanrooms, which are controlled environments designed to minimize contamination from airborne particles. Cleanroom equipment includes:
Air Showers: Used at the entrances to cleanrooms to blow off dust and contaminants from personnel before entering the sterile environment.
Laminar Flow Hoods: Ensure a sterile working environment by providing a stream of filtered air over work surfaces, minimizing the risk of contamination.
Conclusion
The pharmaceutical industry relies on a wide variety of specialized equipment to ensure the safe, efficient, and compliant production of medicines and other health products. From mixing and granulation to sterilization, packaging, and quality control, every stage of pharmaceutical manufacturing is supported by advanced technologies designed to maintain product integrity and meet the highest purity standards. With the demand for pharmaceuticals constantly increasing, the role of equipment in ensuring product safety, quality, and regulatory compliance cannot be overstated.
SWJAL PROCESS Pvt. Ltd. offers high-quality pharmaceutical equipment to ensure safe, efficient, and compliant production processes across the pharmaceutical industry.
0 notes
Text
Spray Dryer manufacturers in India
Ronetech: Leading Spray Dryer Manufacturers in India. Expert solutions for efficient drying processes. Explore now!
https://ronetech.co.in/spray-dryer/
#Spray Dryer#Spray Dryer India#Industrial Dryers#Spray Drying Technology#Indian Manufacturing#Drying Equipment#Chemical Processing#Pharmaceutical Dryers#Food Processing Dryers
0 notes
Text
#Mullen Equipment Corporation is a distributor and representative for many leading process solution equipment manufacturers in various sector#including chemical#industrial#pharmaceutical#and municipal.#Pharmaceutical Equipment#equipment manufacturers#replacement equipment parts#pharmaceutical equipment
0 notes